Abstract
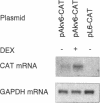
Identical transcription units inserted at different positions of mammalian chromosomes may vary widely in transcriptional activity. We have used a set of ten cell clones with random unselected single integrations of retroviral vectors to study such position effects. The vector used carries a neo gene driven by the Akv murine leukemia virus long terminal repeat that has only a weak promoter-enhancer activity in the target cell, the lymphoid cell line L691. Under transient expression conditions, the strength of the Akv promoter-enhancer in the L691 cells is increased by dexamethasone. In cell clones with single vector integrations, a correlation is observed between the non-induced expression levels and the degree of dexamethasone induction. The strongest relative induction is found for the integrated vectors with the lowest non-induced expression levels and approaches the inducibility under transient expression. These results indicate that expression levels are composed of distinct contributions from the integrated vector and from the site of integration and are best explained in terms of a model in which the sites of chromosomal integration exert variable positive enhancer effects upon vector transcription.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck E., Ludwig G., Auerswald E. A., Reiss B., Schaller H. Nucleotide sequence and exact localization of the neomycin phosphotransferase gene from transposon Tn5. Gene. 1982 Oct;19(3):327–336. doi: 10.1016/0378-1119(82)90023-3. [DOI] [PubMed] [Google Scholar]
- Bhat K., McBurney M. W., Hamada H. Functional cloning of mouse chromosomal loci specifically active in embryonal carcinoma stem cells. Mol Cell Biol. 1988 Aug;8(8):3251–3259. doi: 10.1128/mcb.8.8.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifer C., Vidal M., Grosveld F., Sippel A. E. Tissue specific and position independent expression of the complete gene domain for chicken lysozyme in transgenic mice. EMBO J. 1990 Sep;9(9):2843–2848. doi: 10.1002/j.1460-2075.1990.tb07473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boral A. L., Okenquist S. A., Lenz J. Identification of the SL3-3 virus enhancer core as a T-lymphoma cell-specific element. J Virol. 1989 Jan;63(1):76–84. doi: 10.1128/jvi.63.1.76-84.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celander D., Haseltine W. A. Glucocorticoid regulation of murine leukemia virus transcription elements is specified by determinants within the viral enhancer region. J Virol. 1987 Feb;61(2):269–275. doi: 10.1128/jvi.61.2.269-275.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celander D., Haseltine W. A. Tissue-specific transcription preference as a determinant of cell tropism and leukaemogenic potential of murine retroviruses. Nature. 1984 Nov 8;312(5990):159–162. doi: 10.1038/312159a0. [DOI] [PubMed] [Google Scholar]
- Celander D., Hsu B. L., Haseltine W. A. Regulatory elements within the murine leukemia virus enhancer regions mediate glucocorticoid responsiveness. J Virol. 1988 Apr;62(4):1314–1322. doi: 10.1128/jvi.62.4.1314-1322.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dai H. Y., Etzerodt M., Baekgaard A. J., Lovmand S., Jørgensen P., Kjeldgaard N. O., Pedersen F. S. Multiple sequence elements in the U3 region of the leukemogenic murine retrovirus SL3-2 contribute to cell-dependent gene expression. Virology. 1990 Apr;175(2):581–585. doi: 10.1016/0042-6822(90)90445-w. [DOI] [PubMed] [Google Scholar]
- Doerfler W. Patterns of DNA methylation--evolutionary vestiges of foreign DNA inactivation as a host defense mechanism. A proposal. Biol Chem Hoppe Seyler. 1991 Aug;372(8):557–564. [PubMed] [Google Scholar]
- Duch M., Paludan K., Pedersen L., Jørgensen P., Kjeldgaard N. O., Pedersen F. S. Determination of transient or stable neo expression levels in mammalian cells. Gene. 1990 Nov 15;95(2):285–288. doi: 10.1016/0378-1119(90)90373-y. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Feinstein S. C., Ross S. R., Yamamoto K. R. Chromosomal position effects determine transcriptional potential of integrated mammary tumor virus DNA. J Mol Biol. 1982 Apr 15;156(3):549–565. doi: 10.1016/0022-2836(82)90266-2. [DOI] [PubMed] [Google Scholar]
- Fort P., Marty L., Piechaczyk M., el Sabrouty S., Dani C., Jeanteur P., Blanchard J. M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985 Mar 11;13(5):1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosveld F., van Assendelft G. B., Greaves D. R., Kollias G. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell. 1987 Dec 24;51(6):975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- Günzburg W. H., Groner B. The chromosomal integration site determines the tissue-specific methylation of mouse mammary tumour virus proviral genes. EMBO J. 1984 May;3(5):1129–1135. doi: 10.1002/j.1460-2075.1984.tb01941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallberg B., Schmidt J., Luz A., Pedersen F. S., Grundström T. SL3-3 enhancer factor 1 transcriptional activators are required for tumor formation by SL3-3 murine leukemia virus. J Virol. 1991 Aug;65(8):4177–4181. doi: 10.1128/jvi.65.8.4177-4181.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada H. Activation of an enhancerless gene by chromosomal integration. Mol Cell Biol. 1986 Dec;6(12):4179–4184. doi: 10.1128/mcb.6.12.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada H. Random isolation of gene activator elements from the human genome. Mol Cell Biol. 1986 Dec;6(12):4185–4194. doi: 10.1128/mcb.6.12.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeben R. C., Migchielsen A. A., van der Jagt R. C., van Ormondt H., van der Eb A. J. Inactivation of the Moloney murine leukemia virus long terminal repeat in murine fibroblast cell lines is associated with methylation and dependent on its chromosomal position. J Virol. 1991 Feb;65(2):904–912. doi: 10.1128/jvi.65.2.904-912.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly D. J., Willis R. C., Friedmann T. Variable stability of a selectable provirus after retroviral vector gene transfer into human cells. Mol Cell Biol. 1986 Apr;6(4):1141–1147. doi: 10.1128/mcb.6.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jähner D., Jaenisch R. Chromosomal position and specific demethylation in enhancer sequences of germ line-transmitted retroviral genomes during mouse development. Mol Cell Biol. 1985 Sep;5(9):2212–2220. doi: 10.1128/mcb.5.9.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellum R., Schedl P. A position-effect assay for boundaries of higher order chromosomal domains. Cell. 1991 Mar 8;64(5):941–950. doi: 10.1016/0092-8674(91)90318-s. [DOI] [PubMed] [Google Scholar]
- Lenz J., Celander D., Crowther R. L., Patarca R., Perkins D. W., Haseltine W. A. Determination of the leukaemogenicity of a murine retrovirus by sequences within the long terminal repeat. 1984 Mar 29-Apr 4Nature. 308(5958):467–470. doi: 10.1038/308467a0. [DOI] [PubMed] [Google Scholar]
- Lovmand S., Kjeldgaard N. O., Jørgensen P., Pedersen F. S. Enhancer functions in U3 of Akv virus: a role for cooperativity of a tandem repeat unit and its flanking DNA sequences. J Virol. 1990 Jul;64(7):3185–3191. doi: 10.1128/jvi.64.7.3185-3191.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Paludan K., Dai H. Y., Duch M., Jørgensen P., Kjeldgaard N. O., Pedersen F. S. Different relative expression from two murine leukemia virus long terminal repeats in unintegrated transfected DNA and in integrated retroviral vector proviruses. J Virol. 1989 Dec;63(12):5201–5207. doi: 10.1128/jvi.63.12.5201-5207.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paludan K., Duch M., Jørgensen P., Kjeldgaard N. O., Pedersen F. S. Graduated resistance to G418 leads to differential selection of cultured mammalian cells expressing the neo gene. Gene. 1989 Dec 28;85(2):421–426. doi: 10.1016/0378-1119(89)90435-6. [DOI] [PubMed] [Google Scholar]
- Panganiban A. T., Temin H. M. The retrovirus pol gene encodes a product required for DNA integration: identification of a retrovirus int locus. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7885–7889. doi: 10.1073/pnas.81.24.7885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen F. S., Paludan K., Dai H. Y., Duch M., Jørgensen P., Kjeldgaard N. O., Hallberg B., Grundström T., Schmidt J., Luz A. The murine leukemia virus LTR in oncogenesis: effect of point mutations and chromosomal integration sites. Radiat Environ Biophys. 1991;30(3):195–197. doi: 10.1007/BF01226617. [DOI] [PubMed] [Google Scholar]
- Pedersen K., Lovmand S., Jørgensen E. C., Pedersen F. S., Jørgensen P. Efficient replication and expression of murine leukemia virus with major deletions in the enhancer region of U3. Virology. 1992 Apr;187(2):821–824. doi: 10.1016/0042-6822(92)90486-9. [DOI] [PubMed] [Google Scholar]
- Razin A., Cedar H. DNA methylation and gene expression. Microbiol Rev. 1991 Sep;55(3):451–458. doi: 10.1128/mr.55.3.451-458.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy S., DeGregori J. V., von Melchner H., Ruley H. E. Retrovirus promoter-trap vector to induce lacZ gene fusions in mammalian cells. J Virol. 1991 Mar;65(3):1507–1515. doi: 10.1128/jvi.65.3.1507-1515.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D. L. Dexamethasone-stimulated expression of a proviral copy of mouse mammary tumor virus env mRNA. J Virol. 1984 May;50(2):632–635. doi: 10.1128/jvi.50.2.632-635.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Shih C. C., Stoye J. P., Coffin J. M. Highly preferred targets for retrovirus integration. Cell. 1988 May 20;53(4):531–537. doi: 10.1016/0092-8674(88)90569-7. [DOI] [PubMed] [Google Scholar]
- Short M. K., Okenquist S. A., Lenz J. Correlation of leukemogenic potential of murine retroviruses with transcriptional tissue preference of the viral long terminal repeats. J Virol. 1987 Apr;61(4):1067–1072. doi: 10.1128/jvi.61.4.1067-1072.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen M. S., Duch M., Paludan K., Jørgensen P., Pedersen F. S. Measurement of hygromycin B phosphotransferase activity in crude mammalian cell extracts by a simple dot-blot assay. Gene. 1992 Mar 15;112(2):257–260. doi: 10.1016/0378-1119(92)90386-4. [DOI] [PubMed] [Google Scholar]
- Truss M., Chalepakis G., Piña B., Barettino D., Brüggemeier U., Kalff M., Slater E. P., Beato M. Transcriptional control by steroid hormones. J Steroid Biochem Mol Biol. 1992 Mar;41(3-8):241–248. doi: 10.1016/0960-0760(92)90350-r. [DOI] [PubMed] [Google Scholar]
- Ucker D. S., Firestone G. L., Yamamoto K. R. Glucocorticoids and chromosomal position modulate murine mammary tumor virus transcription by affecting efficiency of promoter utilization. Mol Cell Biol. 1983 Apr;3(4):551–561. doi: 10.1128/mcb.3.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger J., Baltimore D., Sharp P. A. Distinct factors bind to apparently homologous sequences in the immunoglobulin heavy-chain enhancer. 1986 Aug 28-Sep 3Nature. 322(6082):846–848. doi: 10.1038/322846a0. [DOI] [PubMed] [Google Scholar]
- Wilson C., Bellen H. J., Gehring W. J. Position effects on eukaryotic gene expression. Annu Rev Cell Biol. 1990;6:679–714. doi: 10.1146/annurev.cb.06.110190.003335. [DOI] [PubMed] [Google Scholar]
- Xu L., Yee J. K., Wolff J. A., Friedmann T. Factors affecting long-term stability of Moloney murine leukemia virus-based vectors. Virology. 1989 Aug;171(2):331–341. doi: 10.1016/0042-6822(89)90600-4. [DOI] [PubMed] [Google Scholar]
- von Melchner H., Ruley H. E. Identification of cellular promoters by using a retrovirus promoter trap. J Virol. 1989 Aug;63(8):3227–3233. doi: 10.1128/jvi.63.8.3227-3233.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]