Abstract
The majority of smokers relapse during the acute withdrawal phase when withdrawal symptoms are most severe. The goal of the present studies was to investigate the role of corticotropin-releasing factor (CRF) and noradrenergic transmission in the central nucleus of the amygdala (CeA) in the dysphoria associated with smoking cessation. It was investigated if blockade of CRF1 receptors, blockade of α1-adrenergic receptors, or stimulation of α2-adrenergic receptors in the CeA diminishes the deficit in brain reward function associated with nicotine withdrawal in rats. Nicotine dependence was induced by implanting minipumps that delivered a nicotine solution. Withdrawal was precipitated with the nicotinic acetylcholine receptor antagonist mecamylamine. A discrete-trial intracranial self-stimulation procedure was used to assess the negative affective aspects of nicotine withdrawal. Elevations in brain reward thresholds are indicative of a deficit in brain reward function. In all the experiments, mecamylamine elevated the brain reward thresholds of the rats chronically treated with nicotine and did not affect the brain reward thresholds of the saline-treated control rats. Intra-CeA administration of the CRF1 receptor antagonist R278995/CRA0450 completely prevented the mecamylamine-induced elevations in brain reward thresholds in the nicotine-treated rats and did not affect the brain reward thresholds of the saline-treated control rats. R278995/CRA0450 has also been shown to block sigma-1 receptors but there is no evidence that this could affect negative mood states. Intra-CeA administration of the α1-adrenergic receptor antagonist prazosin or the α2-adrenergic receptor agonist clonidine did not affect the brain reward thresholds of the nicotine or saline-treated rats. These studies suggest that CRF1 receptor antagonists may diminish the dysphoria associated with smoking cessation by blocking CRF1 receptors in the CeA.
Keywords: Nicotine, CRF, norepinephrine, central amygdala, withdrawal, ICSS, rats
1. Introduction
Tobacco addiction is a chronic disorder that is characterized by compulsive smoking and relapse after periods of abstinence (American Psychiatric Association, 2000). The positive reinforcing effects of cigarettes have been suggested to play a pivotal role in the initiation of smoking (Chen et al., 2003; Finkenauer et al., 2009). The positive reinforcing effects of smoking include mild euphoria, relaxation, and improved cognitive functioning (Ague, 1973; Benowitz, 1988; Wesnes and Warburton, 1983). Smoking induces adaptations in the brain that may cause the negative mood state, increased anxiety, and impaired cognitive function associated with smoking cessation (Bruijnzeel, 2009; Bruijnzeel and Gold, 2005; Hughes et al., 1991). The negative affective symptoms associated with smoking cessation have been suggested to play an important role in relapse to smoking (Koob and Le Moal, 2005).
Corticotropin-releasing factor (CRF) and norepinephrine play a pivotal role in the regulation of mood states. CRF levels are elevated in depressed patients and treatment with antidepressant drugs improves mood states and decreases brain CRF levels (De Bellis et al., 1993; Nemeroff et al., 1984). Furthermore, chronic treatment with noradrenergic reuptake inhibitors improves mood states in depressed patients (Nelson, 1999). More recent studies have provided evidence for a role of CRF and norepinephrine in drug addiction. The administration of the non-specific CRF1/CRF2 receptor antagonist D-Phe CRF(12–41) and the CRF1 receptor antagonist R278995/CRA0450 into the lateral ventricles attenuates the negative mood state associated with nicotine withdrawal (Bruijnzeel et al., 2009; Bruijnzeel et al., 2007). Blockade of CRF1 receptors also attenuates stress-induced reinstatement of nicotine seeking and increased nicotine intake after a period of abstinence (Bruijnzeel et al., 2009; George et al., 2007). Numerous studies have provided evidence for a role of noradrenergic transmission in drug addiction. The α1-adrenergic receptor antagonist prazosin attenuates the negative mood state associated with nicotine withdrawal in rats (Bruijnzeel et al., 2010). Prazosin also reduces heroin and cocaine self-administration in rats with extended access (6–12 hours/day) to these drugs (Greenwell et al., 2009; Wee et al., 2008). The α2-adrenergic receptor agonist clonidine attenuates the morphine withdrawal-induced decrease in operant responding for food and prevents naloxone-induced conditioned place aversion in morphine dependent animals (Kosten, 1994; Sparber and Meyer, 1978). Clonidine also improves smoking cessation rates (Glassman et al., 1988; Gourlay et al., 2004) and attenuates opioid withdrawal symptoms in humans (Gold et al., 1978).
Although the aforementioned studies indicate that increased CRF and noradrenergic transmission plays a role in drug withdrawal, very few studies have investigated the role of CRF and noradrenergic transmission in the central nucleus of the amygdala (CeA) in nicotine dependence. The present studies investigated the effect of the CRF1 receptor antagonist R278995/CRA0450 (Ki CRF1 = 53.2 nM; CRF2 >10,000 nM), the α1-adrenergic receptor antagonist prazosin (IC50 α1 = 0.6 nM, α2 = 5,000 nM), and the α2-adrenergic receptor agonist clonidine (Ki α1 = 713 nM, α2 = 3.2 nM) in the CeA on precipitated nicotine withdrawal (Chaki et al., 2004; van Meel et al., 1981; Virtanen et al., 1988). Both prazosin and clonidine inhibit noradrenergic transmission in the brain; prazosin by blocking postsynaptic α1-adrenergic receptors and clonidine by stimulating presynaptic α2-adrenergic receptors. In a separate experiment, R278995/CRA0450 was administered into the basolateral nucleus of the amygdala (BLA) before precipitating nicotine withdrawal. This control experiment was conducted to rule out the possibility that R278995/CRA0450 affected nicotine withdrawal by blocking CRF1 receptors in brain sites in close proximity to the CeA. The negative mood state associated with nicotine withdrawal was investigated using a discrete-trial intracranial self-stimulation (ICSS) procedure. Previous studies have shown that this ICSS procedure provides a quantitative measure of the emotional aspects of drug withdrawal (Bruijnzeel et al., 2006; Schulteis et al., 1995; Wise and Munn, 1995).
2. Methods
2.1. Subjects
Male Wistar rats (Charles River, Raleigh, NC, USA) weighing 250–300 gram at the beginning of the experiments were used. Animals were group-housed (two per cage) in a temperature and humidity-controlled vivarium and maintained on a 12-hour light–dark cycle (lights off at 8 AM). All testing occurred at the beginning of the dark cycle. Food and water were available ad libitum in the home cages. All subjects were treated in accordance with the National Institutes of Health guidelines regarding the principles of animal care. Animal facilities and experimental protocols were in accordance with the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC) and approved by the University of Florida Institutional Animal Care and Use Committee (IACUC).
2.2. Drugs
Nicotine hydrogen tartrate salt, mecamylamine hydrochloride, clonidine hydrochloride, prazosin hydrochloride, and pentobarbital sodium salt were purchased from Sigma (Sigma–Aldrich, St. Louis, MO, USA). R278995/CRA0450 (1-[8-(2,4-dichlorophenyl)-2-methylquinolin-4-yl]-1,2,3,6-tetrahydropyridine-4-carboxamide benzenesulfonate) was synthesized by Taisho Pharmaceutical Co. (Saitama, Japan). Nicotine, mecamylamine, and pentobarbital were dissolved in saline (0.9 % sodium chloride). R278995/CRA0450, clonidine, and prazosin were dissolved in distilled water. Prazosin was dissolved by the application of heat. Mecamylamine was administered subcutaneously (sc) in a volume of 1 ml/kg body weight and pentobarbital was administered intraperitoneally (ip) in a volume of 2 ml. Drug doses refer to the salt form.
2.3. Cannulae and electrode implantations
At the beginning of the intracranial surgeries, the rats were anesthetized with an isoflurane/oxygen vapor mixture (1–3% isoflurane) and placed in a Kopf stereotaxic frame (David Kopf Instruments, Tujunga, CA, USA) with the incisor bar set 3.3 mm below the interaural line (flat skull). The rats were prepared stainless steel cannulae (11 mm long, 23 gauge) above the CeA or BLA using flat skull coordinates according to Paxinos and Watson, 1998, and previous studies by our research group (Marcinkiewcz et al., 2009; Yamada and Bruijnzeel, 2011) and others (Campolongo et al., 2009; Funk et al., 2006). Bilateral cannulae were implanted 2.5 mm above the CeA (anterior-posterior [AP] - 2.3, medial lateral [ML] ± 4.0 mm, dorsal-ventral [DV] - 4.7 from dura) or 1.5 mm above the BLA (AP - 2.8, ML ± 5.0 mm, DV - 6.5 from skull). For the electrode implantations, the incisor bar was set 5 mm above the interaural line. The rats were prepared with 11 mm long stainless steel bipolar electrodes (model MS303/2 Plastics One, Roanoke, VA, USA) in the medial forebrain bundle at the level of the posterior lateral hypothalamus (AP - 0.5 mm, ML ± 1.7 mm, DV - 8.3 mm from dura). The electrodes and cannulae were permanently secured to the skull using dental cement anchored with four skull screws. At the end of the surgery, 11 mm removable 30 gauge wire stylets were inserted in the cannulae to maintain patency.
2.4. Osmotic minipump implantations
The rats were prepared with osmotic minipumps (model 2ML4, 28 day pumps, Durect Corporation, Cupertino, CA, USA) filled with either saline or nicotine. The pumps were implanted subcutaneously under isoflurane/oxygen (1–3% isoflurane) anesthesia. The nicotine concentration was adjusted to compensate for differences in body weight and to deliver 9 mg/kg/day of nicotine salt (3.16 mg/kg/day nicotine base). This dose of nicotine does not have a long-term effect on brain reward thresholds or response latencies of rats in the ICSS procedure (Harrison et al., 2001).
2.5. Apparatus
The experimental apparatus consisted of twelve Plexiglas chambers (30.5 × 30 × 17 cm; Med Associates, Georgia, VT, USA), each housed in a sound-attenuating melamine chamber (Med Associates). The operant chambers consisted of a metal grid floor and a metal wheel (5 cm wide) centered on a sidewall. A photobeam detector was attached next to the response wheel and recorded every 90 degrees of rotation. Brain stimulation was delivered using constant current stimulators (Model 1200C, Stimtek, Acton, MA, USA). Subjects were connected to the stimulation circuit through bipolar leads (Plastics One, Roanoke, VA, USA) attached to gold-contact swivel commutators (model SL2C, Plastics One). A computer controlled the stimulation parameters, data collection, and all test session functions.
2.6. Intracranial self-stimulation procedure
Rats were trained on a modified discrete-trial ICSS procedure (Kornetsky and Esposito, 1979), as described previously (Bruijnzeel et al., 2007; Markou and Koob, 1992). The subjects were trained to turn the wheel on a fixed ratio 1 (FR1) schedule of reinforcement. Each quarter turn resulted in the delivery of a 0.5 second train of 0.1 millisecond cathodal square-wave pulses at a frequency of 100 Hz. After the successful acquisition of responding, defined as 100 reinforcements within 10 minutes, the rats were gradually trained on a discrete-trial current-threshold procedure. Each trial began with the delivery of a non-contingent electrical stimulus, followed by a 7.5 second response window within which the animal could respond to receive a second contingent stimulus identical to the initial non-contingent stimulus. A response during this 7.5 second response window was labeled as a positive response and the lack of a response was labeled as a negative response. During a 2-second period immediately after a positive response, additional responses had no scheduled consequences. The inter-trial interval (ITI), which followed either a positive response or the end of the response window, had an average duration of 10 seconds (7.5 – 12.5 seconds). Responses that occurred during the ITI resulted in an additional 12.5 second delay of the onset of the next trial. During training on the discrete-trial procedure, the duration of the ITI period was gradually increased until animals performed consistently at standard test parameters. The rats were subsequently tested on the current-threshold procedure in which stimulation intensities varied according to the classical psychophysical method of limits. A test session consisted of four alternating series of descending and ascending current intensities starting with a descending series. Blocks of three trials were presented to the subject at a given stimulation intensity, and the intensity was altered systematically between blocks by 5 μA steps. The initial stimulus intensity was set 40 μA above the baseline current-threshold for each animal. Each test session typically lasted 30–40 minutes and provided two variables: brain reward thresholds and response latencies. The brain reward threshold for a descending series was defined as the midpoint between stimulation intensities that supported responding (i.e., positive responses on at least two of the three trials), and current intensities that failed to support responding (i.e., positive responses on fewer than two of the three trials for two consecutive blocks of trials). The threshold for an ascending series was defined as the midpoint between stimulation intensities that did not support responding and current intensities that supported responding for two consecutive blocks of trials. Four threshold estimates were recorded and the mean of these values was taken as the brain reward threshold for a specific subject. The response latency was defined as the time interval between the beginning of the non-contingent stimulus and a positive response. The response latency for each test session was defined as the mean response latency on all trials during which a positive response occurred.
2.7. Intracerebral microinjections
The bilateral injections were administered by using 30 gauge stainless steel injectors that extended 2.5 mm (length of injector tip, 13.5 mm) beyond the guide cannulae. The injection volume was 0.5 μl / side and this was infused over 66 seconds. The infusion speed was regulated by a Harvard Apparatus syringe pump (model 975) and the pump was equipped with 10 μl syringes (Hamilton, Rena, NE). The syringes were connected to the injectors with Tygon microbore PVC tubing (0.25 mm ID × 0.76 mm OD). The injectors were left in place for 10 seconds post-injection to allow diffusion from the injector tip. The 11-mm dummy stylets were re-inserted immediately after the injectors were removed.
2.8. Histology
The placement of the injection sites was verified as in previous studies by our research group in which the role of the CeA in nicotine addiction was investigated (Marcinkiewcz et al., 2009; Yamada and Bruijnzeel, 2011). The rats were euthanized with an overdose of sodium pentobarbital (150 mg, ip) and perfused via the ascending aorta with physiological saline (100 ml) followed by a 10% phosphate buffered formalin solution (150 ml). Brains were postfixed for 24 hours and cryoprotected in 10% sucrose in phosphate buffered saline for 48 hours. Sections were cut on a Leica CM3050 S cryostat (coronal sections of 40 μm at −15 °C). The sections were mounted on Fisher Superfrost Plus slides and stained with cresyl violet. The locations of the guide cannulae and injections sites were verified with light microscopy and with reference to a stereotaxic atlas of the rat brain (Paxinos and Watson, 1998). Histological verification indicated that all the injector tracts ended within or at the boundaries of the CeA (− 1.88 to − 2.56 AP) or the BLA (− 2.56 to − 3.30 AP).
2.9. Experimental design
The studies investigated the effects of the intra-CeA administration of R278995/CRA0450, clonidine, and prazosin on precipitated nicotine withdrawal in rats. In an additional control experiment, the effect of the administration of R278995/CRA0450 into the BLA on precipitated nicotine withdrawal was investigated. Drug naïve rats were used for all the experiments. After recovery from the cannulae and electrode implantations, the rats were trained on the ICSS procedure. When stable baseline brain reward thresholds were achieved (defined as less than 10% variation within a 5 day period), the rats were prepared with 28-day osmotic minipumps that contained either saline or nicotine dissolved in saline (Exp. 1A, intra-CeA R278995/CRA0450: saline n = 9, nicotine n = 8; Exp. 1B, intra-BLA R278995/CRA0450: nicotine n = 6; Exp. 2, intra-CeA prazosin: saline n = 10, nicotine n = 10; Exp. 3, intra-CeA clonidine: saline n = 8, nicotine n = 13). All drugs were administered according to a Latin-square design and separate groups of rats were used for each experiment. Brain reward thresholds and response latencies were assessed daily throughout the experiment between 9:00 AM and 12:00 noon. The nicotinic acetylcholine receptor (nAChR) antagonist mecamylamine (3 mg/kg, sc) was used to precipitate nicotine withdrawal. Mecamylamine injections started at least 6 days after the implantation of the minipumps to allow time for the development of nicotine dependence. The bilateral intra-CeA and intra-BLA infusions (CeA and BLA R278995/CRA0450, 0.005 – 0.5 μg; CeA Prazosin 0.3 – 1.5 μg; CeA Clonidine 0.25 – 1 μg, unilateral doses) were conducted 10 minutes prior to treatment with mecamylamine. The rats were placed in the ICSS test chambers 5 minutes after mecamylamine administration. The minimum time interval between the mecamylamine injections was at least 72 hours to allow the brain reward thresholds to return to baseline levels. The serum elimination half-life of mecamylamine is approximately 1 hour (Debruyne et al., 2003). At the end of the experiments the rats were perfused under pentobarbital anesthesia and the brains were removed for histological verification of the injection sites.
2.10. Statistical analyses
One-way analyses of variance (ANOVA) were used to verify that the ICSS parameters (absolute brain reward thresholds and response latencies) between the saline rats and nicotine rats did not differ prior to the implantation of the minipumps or prior to the onset of the injections with mecamylamine. One way ANOVA's were also used to compare the absolute brain reward thresholds and response latencies between experiments in which drugs were administered into the CeA. For all the experiments, brain reward thresholds and response latencies were expressed as percentages of the values obtained on the day prior to each test day. Percent changes in brain reward thresholds and response latencies were analyzed by using two-way repeated-measures ANOVA's with the dose of R278995/CRA0450, clonidine, or prazosin as the within-subjects factor and pump content (saline or nicotine) as the between-subjects factor. One way ANOVA's were used to compare the effects of mecamylamine on brain reward thresholds and response latencies between experiments in rats treated with vehicle in the CeA. Statistically significant interactions in the ANOVA were followed by the Newman-Keuls post-hoc test.
3. Results
3.1. Experiments 1A and B: Intra-CeA and intra-BLA administration of R278995/CRA0450 and nicotine withdrawal
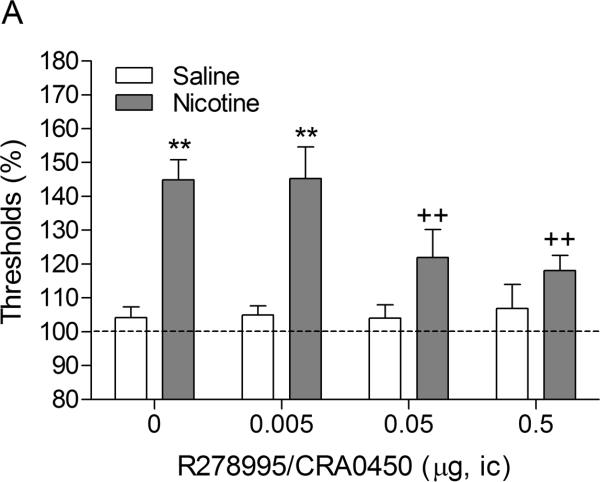
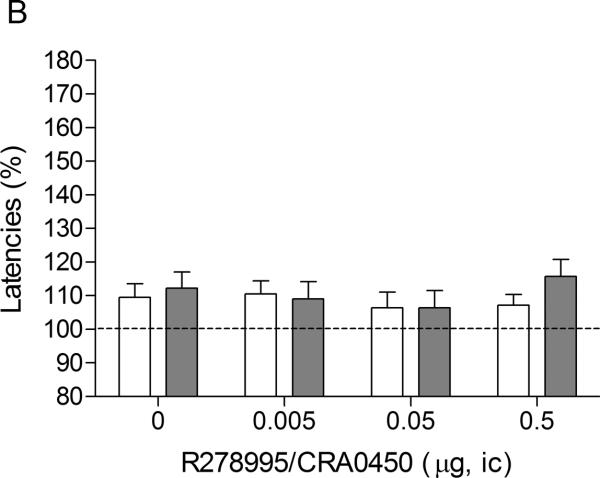
There were no differences in absolute brain reward thresholds and response latencies between the saline group and the nicotine group on the test-day prior to the minipump implantations or on the test-day prior to the first mecamylamine / R278995/CRA0450 injections (Experiment 1A, Table 1). Mecamylamine elevated the brain reward thresholds of the nicotine-treated rats, 145%, and did not affect the brain reward thresholds of the control rats (Figure 1A, Treatment: F1,15–43.98, P<0.0001). Mecamylamine did not affect the response latencies of the nicotine-treated rats or the saline-treated rats (Figure 1B, Treatment: F1,15–0.61, n.s.). The administration of R278995/CRA0450 into the CeA prevented the mecamylamine-induced elevations in brain reward thresholds in the nicotine dependent rats and did not affect the brain reward thresholds of the rats that were chronically treated with saline (Figure 1A; Dose × Treatment interaction: F3,45=3.27, P<0.03). Newman-Keuls post-hoc comparisons indicated that the administration of 0.05 and 0.5 μg of R278995/CRA0450 into the CeA prevented the elevations in brain reward thresholds associated with precipitated nicotine withdrawal. R278995/CRA0450 did not affect the response latencies (Figure 1B; Dose: F3,45–0.51, n.s.; Dose × Treatment interaction: F3,45=0.49, n.s.).
Table 1.
Absolute baseline brain reward thresholds and response latencies.
| Thresholds (μA) | Latencies (s) | |||
|---|---|---|---|---|
| Saline | Nicotine | Saline | Nicotine | |
| Exp. 1A R278995/CRA0450-CeA (saline, n = 9; nicotine, n = 8) | ||||
| Prior pump implantation | 123.3 ± 14.5 | 119.0 ± 13.2 | 3.1 ± 0.1 | 3.0 ± 0.2 |
| Prior first injection | 113.8 ± 15.3 | 108.8 ± 13.4 | 3.2 ± 0.2 | 3.0 ± 0.2 |
| Exp. 1B R278995/CRA0450-BLA (nicotine, n = 6) | ||||
| Prior pump implantation | N/A | 85.8 ± 9.3 | N/A | 3.5 ± 0.1 |
| Prior first injection | N/A | 82.7 ± 11.3 | N/A | 2.9 ± 0.1 |
| Exp. 2 Prazosin-CeA (saline, n = 10; nicotine, n = 10) | ||||
| Prior pump implantation | 96.3 ± 7.1 | 88.8 ± 8.5 | 3.3 ± 0.1 | 3.3 ± 0.1 |
| Prior first injection | 100.2 ± 6.6 | 94.4 ± 8.9 | 3.5 ± 0.1 | 3.4 ± 0.1 |
| Exp. 3 Clonidine-CeA (saline, n = 8; nicotine, n = 13) | ||||
| Prior pump implantation | 100.7 ± 7.7 | 112.5 ± 10.6 | 3.1 ± 0.1 | 3.6 ± 0.2* |
| Prior first injection | 101.1 ± 8.1 | 115.7 ± 11.4 | 3.2 ± 0.1 | 3.5 ± 0.2 |
Prior refers to the test-day immediately before the implantation of the osomotic minipumps or the start of the mecamylamine injections. Asterisk (P<0.05) indicates that the latency of the nicotine group is longer than the latency of the saline group. Abbreviations: CeA, central nucleus of the amygdala; BLA, basolateral nucleus of the amygdala.
Fig. 1.
Effect of intra-CeA administration of the CRF1 receptor antagonist R278995/CRA0450 on the elevations in ICSS thresholds associated with precipitated nicotine withdrawal (A; saline n = 9, nicotine n = 8). Effect of intra-CeA R278995/CRA0450 on the response latencies of rats chronically treated with saline or nicotine and acutely treated with mecamylamine (B; saline n = 9, nicotine n = 8). ICSS thresholds and response latencies are expressed as a percentage of the pre-test day values. R278995/CRA0450 was administered bilaterally and the figures depict the unilateral dose. Asterisks (** P<0.01) indicate elevations in ICSS thresholds compared to those of the corresponding saline-treated control group. Plus signs (++ P<0.01) indicate lower ICSS thresholds compared to those of rats chronically treated with nicotine and acutely treated with mecamylamine and vehicle (0 μg of R278995/CRA0450). Abbreviation: ic, intracranial.
In a separate study, the effect of intra-BLA R278995/CRA0450 on nicotine withdrawal was investigated (Experiment 1B). The administration of R278995/CRA0450 into the BLA did not affect the brain reward thresholds (Table 2, Dose: F3,15–0.43, n.s.) or the response latencies (Dose: F3,15–1.58, n.s.) of the nicotine dependent rats treated with mecamylamine. This suggests that in the first experiment (Experiment 1A), R278995/CRA0450 prevented the elevations in brain reward thresholds by blocking CRF1 receptors in the CeA and not by blocking CRF1 receptors in brain sites in close proximity to the CeA.
Table 2.
Intra-BLA administration of the CRF1 receptor antagonist R278995/CRA0450 does not affect the ICSS thresholds and response latencies of nicotine withdrawing rats.
| R278995/CRA0450 | ||
|---|---|---|
| Dose (μg, ic) | Thresholds | Latencies |
| 0 | 145.6 ± 5.9 | 120.1 ± 3.1 |
| 0.005 | 139.2 ± 8.4 | 107.5 ± 4.1 |
| 0.05 | 146.4 ± 10.2 | 118.8 ± 7.5 |
| 0.5 | 137.4 ± 9.4 | 107.9 ± 5.6 |
ICSS thresholds and response latencies are expressed as a percentage of the pre-test day values (n = 6). R278995/CRA0450 was administered bilaterally and the unilateral dose is depicted in the table. Abbreviation: ic, intracranial.
3.2. Experiment 2: Intra-CeA administration of prazosin and nicotine withdrawal
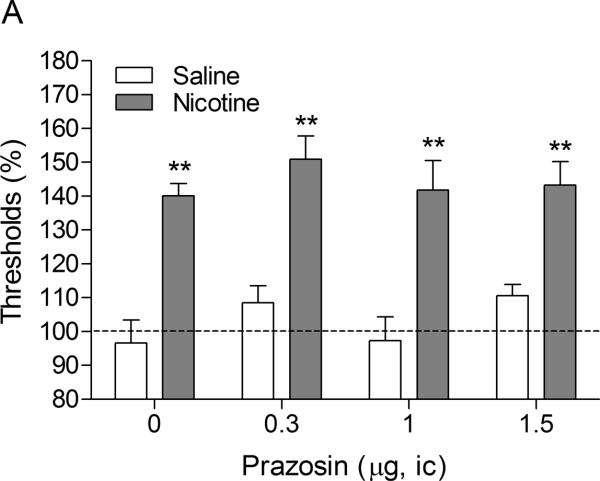
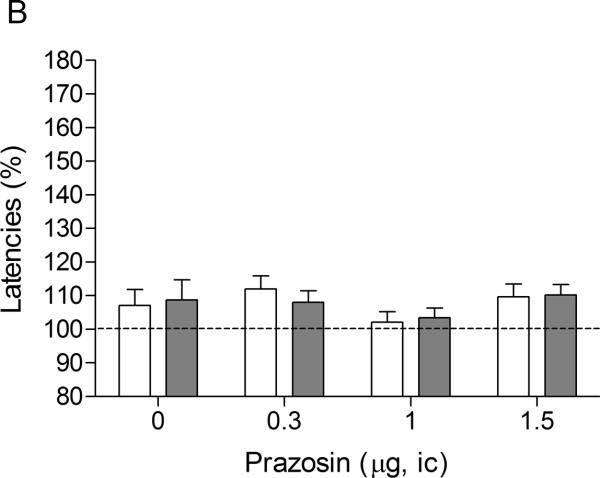
There were no differences in absolute brain reward thresholds and response latencies between the saline group and the nicotine group on the test-day before the minipump implantations or on the test-day before the first mecamylamine / prazosin injections (Table 1). Similar to the first experiment, mecamylamine elevated the brain reward thresholds of the rats that were chronically treated with nicotine and did not affect the brain reward thresholds of the saline-treated rats (Figure 2A, Treatment: F1,18–83.55, P<0.0001). Mecamylamine did not affect the response latencies of the nicotine-treated rats or the saline-treated rats (Figure 2B, Treatment: F1,18–0.0009, n.s.). Intra-CeA administration of prazosin did not affect the brain reward thresholds (Dose: F3,54=1.55, n.s.; Dose × Treatment: F3,54–0.38, n.s.) or the response latencies (Dose: F3,54–1.69, n.s.; Dose × Treatment: F3,54–0.25, n.s.) of the rats in the saline group or in the nicotine group.
Fig. 2.
Effect of intra-CeA administration of the α1-adrenergic receptor antagonist prazosin on the elevations in ICSS thresholds associated with precipitated nicotine withdrawal (A; saline, n = 10; nicotine, n = 10). Effect of intra-CeA prazosin on the response latencies of rats chronically treated with saline or nicotine and acutely treated with mecamylamine (B; saline n = 10, nicotine n = 10). ICSS thresholds and response latencies are expressed as a percentage of the pre-test day values. Prazosin was administered bilaterally and the figures depict the unilateral dose. Abbreviation: ic, intracranial.
3.3. Experiment 3: Intra-CeA administration of clonidine and nicotine withdrawal
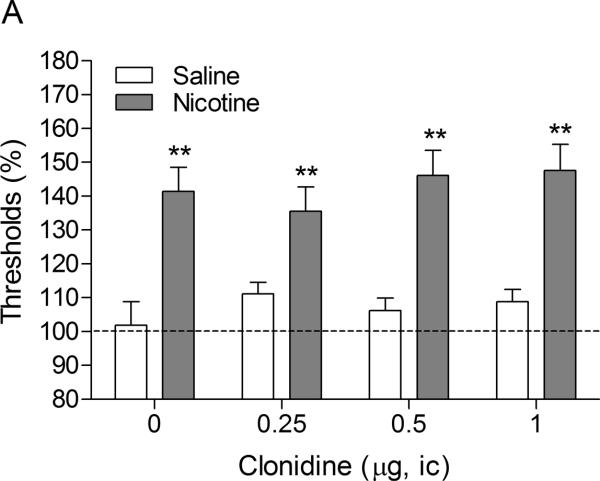
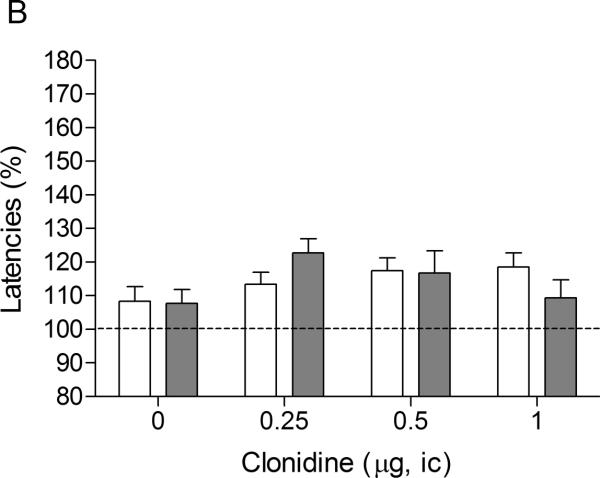
There were no differences in absolute brain reward thresholds between the saline group and the nicotine group on the test-day prior to the minipump implantations or on the test-day prior to the first mecamylamine / clonidine injections (Table 1). The latency of the nicotine group was slightly longer than the latency of the control group on the test-day prior to the minipump implantations (F1,20–4.44, P<0.049). However, there was no difference between the latency of the nicotine group and the saline-treated control group on the test-day prior to the first mecamylamine / clonidine injections. The administration of mecamylamine led to an elevation in the brain reward thresholds of the nicotine-treated rats and did not affect the brain reward thresholds of the saline-treated rats (Figure 3A, Treatment: F1,19–34.85, P<0.0001). Mecamylamine did not affect the response latencies of the nicotine-treated rats or the saline-treated rats (Figure 3B, Treatment: F1,19–0.006, n.s.). The administration of clonidine into the CeA did not have an effect on the brain reward thresholds (Dose: F3,57–0.53, n.s.; Dose × Treatment: F3,57–0.88, n.s.) or the response latencies (Dose: F3,57=2.17, n.s.; Dose × Treatment: F3,57–4.52, n.s.) of the saline-treated rats or the nicotine-treated rats.
Fig. 3.
Effect of intra-CeA administration of the α2-adrenergic receptor agonist clonidine on elevations in ICSS thresholds associated with precipitated nicotine withdrawal (A; saline, n = 8; nicotine, n = 13). Effect of intra-CeA clonidine on the response latencies of rats chronically treated with saline or nicotine and acutely treated with mecamylamine (B; saline n = 8, nicotine n = 13). ICSS thresholds and response latencies are expressed as a percentage of the pre-test day values. Clonidine was administered bilaterally and the figures depict the unilateral dose. Abbreviation: ic, intracranial.
Additional analyses were conducted to rule out the possibility that some of the differences in the effects of the drugs (intra-CeA: R278995/CRA0450, prazosin, and clonidine) were due to baseline differences between the experiments. There were no baseline differences in absolute brain reward thresholds (Experiment: F2,57–1.03, n.s.) and response latencies (Experiment: F2,57–1.96, n.s.) between the three experiments. In addition, mecamylamine had the same effect on the brain reward thresholds (Nicotine groups, Experiment: F2,30=0.16, n.s.; Saline groups, Experiment: F2,26=0.42, n.s.) and the response latencies (Nicotine groups, Experiment: F2,30=0.22, n.s.; Saline groups, Experiment: F2,26=0.07, n.s.) of the control animals (nicotine or saline animals treated with vehicle in the CeA) in all three experiments.
4. Discussion
The aim of the present studies was to investigate the role of CRF and noradrenergic transmission in the CeA in the negative mood state associated with nicotine withdrawal in rats. In all the experiments, the nAChR antagonist mecamylamine elevated the brain reward thresholds of the nicotine-treated rats and did not affect the brain reward thresholds of the saline-treated control rats. Intra-CeA administration of the CRF1 receptor antagonist R278995/CRA0450 prevented the elevations in brain reward thresholds associated with nicotine withdrawal. R278995/CRA0450 did not affect the response latencies and therefore it is unlikely that this drug induced sedative effects or motor impairments. Pretreatment with the α1-adrenergic receptor antagonist prazosin and the α2-adrenergic receptor agonist clonidine in the CeA did not affect the elevations in brain reward thresholds associated with precipitated nicotine withdrawal. Taken together, these findings would suggest that CRF transmission, but not noradrenergic transmission, in the CeA plays a critical role in the negative mood state associated with tobacco smoking cessation.
The lowest intra-CeA dose of R278995/CRA0450 that prevented the elevations in brain reward thresholds associated with nicotine withdrawal was 0.05 μg/side (0.1 μg total bilateral dose). The total bilateral dose in the present study is 100 times lower than the lowest icv dose of R278995/CRA0450, 10 μg, that prevents the elevations in brain reward thresholds associated with nicotine withdrawal (Bruijnzeel et al., 2009). Therefore, the amount of R278995/CRA0450 that was infused into the CeA would not have prevented the elevations in brain reward thresholds when administered into the lateral ventricles. This excludes the possibility that R278995/CRA0450 diffused into the lateral ventricles and attenuated nicotine withdrawal by acting upon distant brain sites. We also investigated the effect of the administration of R278995/CRA0450 into a brain site in close proximity to the CeA, namely the BLA. The administration of R278995/CRA0450 into the BLA did not affect the brain reward thresholds of nicotine withdrawing rats. This suggests that in the first experiment, R278995/CRA0450 mediated its effects on nicotine withdrawal by blocking CRF1 receptors in the CeA and not by blocking CRF1 receptors in brain sites in close proximity to the CeA such as the BLA.
The administration of a nAChR antagonist to nicotine dependent animals leads to elevations in brain reward thresholds in the ICSS procedure and an increased release of CRF in the CeA (Epping-Jordan et al., 1998; George et al., 2007). The data presented here indicate that blockade of CRF1 receptors in the CeA prevents the elevation in brain reward thresholds associated with nicotine withdrawal. This study extends and corroborates a previous study that showed that the administration of the nonspecific CRF1/CRF2 receptor antagonist D-Phe CRF(12–41) into the CeA prevents the elevations in brain reward thresholds associated with nicotine withdrawal (Marcinkiewcz et al., 2009). The icv administration of the CRF2 receptor antagonist astressin-2B does not affect the elevations in brain reward thresholds associated with precipitated nicotine withdrawal (Bruijnzeel et al., 2009). Therefore these studies strongly suggest that precipitated nicotine withdrawal leads to an increased release of CRF in the CeA which induces a negative mood state via the activation of CRF1, but not CRF2, receptors in this brain site. The negative emotional state associated with nicotine withdrawal may play a role in the development and maintenance of a tobacco addiction in humans. George et al. (2007) showed that the self-administration of nicotine in rats is increased after a period of abstinence (i.e., nicotine-deprivation effect). This deprivation effect is prevented by pretreatment with a specific CRF1 receptor antagonist (George et al., 2007). CRF1 receptor antagonists also prevent the dysphoria and anxiety-like behavior associated with nicotine withdrawal (Bruijnzeel et al., 2009; George et al., 2007). Therefore, these findings suggest that after a period of abstinence, rats may increase their nicotine intake to diminish the CRF-induced dysphoria and anxiety-like behavior.
Pharmacological evidence suggests that the CRF1 receptor antagonist R278995/CRA0450 also blocks sigma-1 receptors (Chaki et al., 2004). Although sigma-1 receptors have been detected in the amygdala, it is unlikely that R278995/CRA0450 prevented the nicotine withdrawal-induced elevations in brain reward thresholds by blocking these receptors (Alonso et al., 2000). Animal studies suggest that stimulation of sigma-1 receptors has antidepressant and anxiolytic-like effects (Kamei et al., 1996; Ukai et al., 1998; Wang et al., 2007). In contrast, blockade of sigma-1 receptors does not affect the behavior of rats and mice in a wide variety of anxiety and depression tests (Chaki et al., 2004; Noda et al., 2000; Ukai et al., 1998).
The present study showed that blockade of α1-adrenergic receptors and stimulation of α2-adrenergic receptors in the CeA does not affect the elevations in brain reward thresholds associated with precipitated nicotine withdrawal. It is unlikely that the lack of effect of prazosin or clonidine on nicotine withdrawal was due to the absence of α1 or α2-adrenergic receptors in the CeA. Previous studies have shown that α1 and α2-adrenergic receptors are expressed in this brain site (Rainbow and Biegon, 1983; Young, III and Kuhar, 1980). It is also unlikely that the doses of clonidine or prazosin were too low. Previous studies by our group and others have shown that lower doses than those used in the present studies affect the behavior of rats (Ferry et al., 1999; Yamada and Bruijnzeel, 2011). The highest dose of clonidine in the present study was 1 μg/side. In a previous study, we reported that the bilateral administration of the same dose of clonidine into the CeA attenuates stress-induced reinstatement of nicotine-seeking behavior in rats (Yamada and Bruijnzeel, 2011). At this point, we are not aware of any studies that investigated the behavioral effects of the administration of prazosin in the CeA. However, one study investigated the effects of the administration of prazosin into the BLA in a learning and memory test. It was shown that the administration of 0.1 – 1 μg/side of prazosin produces deficits in shock-induced avoidance learning (Ferry et al., 1999). These doses fall in the same dose-range as the prazosin doses, 0.3 – 1.5 μg/side, which were used in the present nicotine withdrawal study.
In a previous study we reported that the systemic administration of the α1-adrenergic receptor antagonist prazosin attenuates the deficit in brain reward function associated with precipitated nicotine withdrawal (Bruijnzeel et al., 2010). One of the aims of the present experiments was to investigate if the effects of prazosin on nicotine withdrawal were mediated by blocking α1-adrenergic receptors in the CeA. This study demonstrated that the effects of systemically administered prazosin on nicotine withdrawal were not mediated by α1-adrenergic receptors in the CeA. Future studies will explore the role of α1-adrenergic receptors in other brain sites in nicotine withdrawal. A binding study with the selective α1-adrenergic receptor agonist HEAT has shown that high levels of α1-adrenergic receptors are expressed in the bed nucleus of the stria terminalis (Jones et al., 1985). In addition, noradrenergic projections from the A1 and A2 noradrenergic cell groups in the brainstem to the BNST have been implicated in opioid withdrawal-induced place aversion (Delfs et al., 2000). Therefore, it might be possible that α1-adrenergic receptors in the BNST play a role in in nicotine withdrawal.
In conclusion, the present findings indicate that the activation of CRF1 receptors in the CeA mediates the elevations in brain reward thresholds associated with nicotine withdrawal. This would suggest that CRF1 receptor antagonists may attenuate the dysphoria associated with smoking cessation by blocking CRF1 receptors in the CeA. The dysphoria associated with smoking cessation increases craving for cigarettes and contributes to relapse to smoking (Doherty et al., 1995; West et al., 1989). Therefore, CRF1 receptor antagonists may improve smoking cessation rates by attenuating the negative mood state associated with smoking cessation.
Acknowledgements
This research was funded by a National Institute on Drug Abuse grant (DA023575) to A. Bruijnzeel. J. Alexander was supported by a Postdoctoral Research Fellowship from the James & Esther King Biomedical Research Program. The authors are grateful to Dr. Shigeyuki Chaki (Taisho Pharmaceutical Co., Saitama, Japan) and Dr. Thomas Steckler (Johnson & Johnson Pharmaceuticals Research & Development, Beerse, Belgium) for generously providing R278995/CRA0450.
References
- Ague C. Nicotine and smoking: effects upon subjective changes in mood. Psychopharmacologia. 1973;30:323–328. doi: 10.1007/BF00429191. [DOI] [PubMed] [Google Scholar]
- Alonso G, Phan V, Guillemain I, Saunier M, Legrand A, Anoal M, Maurice T. Immunocytochemical localization of the sigma(1) receptor in the adult rat central nervous system. Neuroscience. 2000;97:155–170. doi: 10.1016/s0306-4522(00)00014-2. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Press; Washington, DC: 2000. [Google Scholar]
- Benowitz NL. Drug therapy. Pharmacologic aspects of cigarette smoking and nicotine addition. N. Engl. J. Med. 1988;319:1318–1330. doi: 10.1056/NEJM198811173192005. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW. kappa-Opioid receptor signaling and brain reward function. Brain Res. Rev. 2009;62:127–146. doi: 10.1016/j.brainresrev.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Bishnoi M, van Tuijl IA, Keijzers KF, Yavarovich KR, Pasek TM, Ford J, Alexander JC, Yamada H. Effects of prazosin, clonidine, and propranolol on the elevations in brain reward thresholds and somatic signs associated with nicotine withdrawal in rats. Psychopharmacology (Berl) 2010;212:485–499. doi: 10.1007/s00213-010-1970-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Gold MS. The role of corticotropin-releasing factor-like peptides in cannabis, nicotine, and alcohol dependence. Brain Res. Brain Res. Rev. 2005;49:505–528. doi: 10.1016/j.brainresrev.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Lewis B, Bajpai LK, Morey TE, Dennis DM, Gold M. Severe deficit in brain reward function associated with fentanyl withdrawal in rats. Biol. Psychiatry. 2006;59:477–480. doi: 10.1016/j.biopsych.2005.07.020. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Prado M, Isaac S. Corticotropin-Releasing Factor-1 Receptor Activation Mediates Nicotine Withdrawal-Induced Deficit in Brain Reward Function and Stress-Induced Relapse. Biol. Psychiatry. 2009;66:110–117. doi: 10.1016/j.biopsych.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Zislis G, Wilson C, Gold MS. Antagonism of CRF receptors prevents the deficit in brain reward function associated with precipitated nicotine withdrawal in rats. Neuropsychopharmacology. 2007;32:955–963. doi: 10.1038/sj.npp.1301192. [DOI] [PubMed] [Google Scholar]
- Campolongo P, Roozendaal B, Trezza V, Hauer D, Schelling G, McGaugh JL, Cuomo V. Endocannabinoids in the rat basolateral amygdala enhance memory consolidation and enable glucocorticoid modulation of memory. Proc. Natl. Acad. Sci. U. S. A. 2009;106:4888–4893. doi: 10.1073/pnas.0900835106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaki S, Nakazato A, Kennis L, Nakamura M, Mackie C, Sugiura M, Vinken P, Ashton D, Langlois X, Steckler T. Anxiolytic- and antidepressant-like profile of a new CRF1 receptor antagonist, R278995/CRA0450. Eur. J. Pharmacol. 2004;485:145–158. doi: 10.1016/j.ejphar.2003.11.032. [DOI] [PubMed] [Google Scholar]
- Chen X, Stacy A, Zheng H, Shan J, Spruijt-Metz D, Unger J, Gong J, Gallaher P, Liu C, Azen S, Shakib S, Ph DA. Sensations from initial exposure to nicotine predicting adolescent smoking in China: a potential measure of vulnerability to nicotine. Nicotine. Tob. Res. 2003;5:455–463. doi: 10.1080/14622200307239. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Gold PW, Geracioti TD, Jr., Listwak SJ, Kling MA. Association of fluoxetine treatment with reductions in CSF concentrations of corticotropin-releasing hormone and arginine vasopressin in patients with major depression. Am. J. Psychiatry. 1993;150:656–657. doi: 10.1176/ajp.150.4.656. [DOI] [PubMed] [Google Scholar]
- Debruyne D, Sobrio F, Hinschberger A, Camsonne R, Coquerel A, Barre L. Short-term pharmacokinetics and brain distribution of mecamylamine as a preliminary to carbon-11 labeling for nicotinic receptor investigation. J. Pharm. Sci. 2003;92:1051–1057. doi: 10.1002/jps.10302. [DOI] [PubMed] [Google Scholar]
- Delfs JM, Zhu Y, Druhan JP, Aston-Jones G. Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature. 2000;403:430–434. doi: 10.1038/35000212. [DOI] [PubMed] [Google Scholar]
- Doherty K, Kinnunen T, Militello FS, Garvey AJ. Urges to smoke during the first month of abstinence: relationship to relapse and predictors. Psychopharmacology (Berl) 1995;119:171–178. doi: 10.1007/BF02246158. [DOI] [PubMed] [Google Scholar]
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- Ferry B, Roozendaal B, McGaugh JL. Involvement of alpha1-adrenoceptors in the basolateral amygdala in modulation of memory storage. Eur. J. Pharmacol. 1999;372:9–16. doi: 10.1016/s0014-2999(99)00169-7. [DOI] [PubMed] [Google Scholar]
- Finkenauer R, Pomerleau CS, Snedecor SM, Pomerleau OF. Race differences in factors relating to smoking initiation. Addict. Behav. 2009;34:1056–1059. doi: 10.1016/j.addbeh.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, O'Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J. Neurosci. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Ghozland S, Azar MR, Cottone P, Zorrilla EP, Parsons LH, O'Dell LE, Richardson HN, Koob GF. CRF CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proc. Natl. Acad. Sci. U. S. A. 2007;104:17198–17203. doi: 10.1073/pnas.0707585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glassman AH, Stetner F, Walsh BT, Raizman PS, Fleiss JL, Cooper TB, Covey LS. Heavy smokers, smoking cessation, and clonidine. Results of a double-blind, randomized trial. JAMA. 1988;259:2863–2866. [PubMed] [Google Scholar]
- Gold MS, Redmond DE, Jr., Kleber HD. Clonidine blocks acute opiate-withdrawal symptoms. Lancet. 1978;2:599–602. doi: 10.1016/s0140-6736(78)92823-4. [DOI] [PubMed] [Google Scholar]
- Gourlay SG, Stead LF, Benowitz NL. Clonidine for smoking cessation. Cochrane. Database. Syst. Rev. 2004:CD000058. doi: 10.1002/14651858.CD000058.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwell TN, Walker BM, Cottone P, Zorrilla EP, Koob GF. The alpha1 adrenergic receptor antagonist prazosin reduces heroin self-administration in rats with extended access to heroin administration. Pharmacol. Biochem. Behav. 2009;91:295–302. doi: 10.1016/j.pbb.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison AA, Liem YT, Markou A. Fluoxetine combined with a serotonin-1A receptor antagonist reversed reward deficits observed during nicotine and amphetamine withdrawal in rats. Neuropsychopharmacology. 2001;25:55–71. doi: 10.1016/S0893-133X(00)00237-2. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Gust SW, Skoog K, Keenan RM, Fenwick JW. Symptoms of tobacco withdrawal. A replication and extension. Arch. Gen. Psychiatry. 1991;48:52–59. doi: 10.1001/archpsyc.1991.01810250054007. [DOI] [PubMed] [Google Scholar]
- Jones LS, Gauger LL, Davis JN. Anatomy of brain alpha 1-adrenergic receptors: in vitro autoradiography with [125I]-heat. J. Comp Neurol. 1985;231:190–208. doi: 10.1002/cne.902310207. [DOI] [PubMed] [Google Scholar]
- Kamei H, Kameyama T, Nabeshima T. (+)-SKF-10,047 and dextromethorphan ameliorate conditioned fear stress via dopaminergic systems linked to phenytoin-regulated sigma 1 sites. Eur. J. Pharmacol. 1996;309:149–158. doi: 10.1016/0014-2999(96)00346-9. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the 'dark side' of drug addiction. Nat. Neurosci. 2005;8:1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Kornetsky C, Esposito RU. Euphorigenic drugs: effects on the reward pathways of the brain. Fed. Proc. 1979;38:2473–2476. [PubMed] [Google Scholar]
- Kosten TA. Clonidine attenuates conditioned aversion produced by naloxone-precipitated opiate withdrawal. Eur. J. Pharmacol. 1994;254:59–63. doi: 10.1016/0014-2999(94)90370-0. [DOI] [PubMed] [Google Scholar]
- Marcinkiewcz CA, Prado MM, Isaac SK, Marshall A, Rylkova D, Bruijnzeel AW. Corticotropin-releasing factor within the central nucleus of the amygdala and the nucleus accumbens shell mediates the negative affective state of nicotine withdrawal in rats. Neuropsychopharmacology. 2009;34:1743–1752. doi: 10.1038/npp.2008.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A, Koob GF. Construct validity of a self-stimulation threshold paradigm: effects of reward and performance manipulations. Physiol Behav. 1992;51:111–119. doi: 10.1016/0031-9384(92)90211-j. [DOI] [PubMed] [Google Scholar]
- Nelson JC. A review of the efficacy of serotonergic and noradrenergic reuptake inhibitors for treatment of major depression. Biol. Psychiatry. 1999;46:1301–1308. doi: 10.1016/s0006-3223(99)00173-0. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Widerlov E, Bissette G, Walleus H, Karlsson I, Eklund K, Kilts CD, Loosen PT, Vale W. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984;226:1342–1344. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- Noda Y, Kamei H, Kamei Y, Nagai T, Nishida M, Nabeshima T. Neurosteroids ameliorate conditioned fear stress: an association with sigma receptors. Neuropsychopharmacology. 2000;23:276–284. doi: 10.1016/S0893-133X(00)00103-2. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego: 1998. [DOI] [PubMed] [Google Scholar]
- Rainbow TC, Biegon A. Quantitative autoradiography of [3H]prazosin binding sites in rat forebrain. Neurosci. Lett. 1983;40:221–226. doi: 10.1016/0304-3940(83)90042-3. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Markou A, Cole M, Koob GF. Decreased brain reward produced by ethanol withdrawal. Proc. Natl. Acad. Sci. U. S. A. 1995;92:5880–5884. doi: 10.1073/pnas.92.13.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparber SB, Meyer DR. Clonidine antagonizes naloxone-induced suppression of conditioned behavior and body weight loss in morphine-dependent rats. Pharmacol. Biochem. Behav. 1978;9:319–325. doi: 10.1016/0091-3057(78)90292-7. [DOI] [PubMed] [Google Scholar]
- Ukai M, Maeda H, Nanya Y, Kameyama T, Matsuno K. Beneficial effects of acute and repeated administrations of sigma receptor agonists on behavioral despair in mice exposed to tail suspension. Pharmacol. Biochem. Behav. 1998;61:247–252. doi: 10.1016/s0091-3057(98)00093-8. [DOI] [PubMed] [Google Scholar]
- Van Meel JC, de JA, Timmermans PB, Van Zwieten PA. Selectivity of some alpha adrenoceptor agonists for peripheral alpha-1 and alpha-2 adrenoceptors in the normotensive rat. J. Pharmacol. Exp. Ther. 1981;219:760–767. [PubMed] [Google Scholar]
- Virtanen R, Savola JM, Saano V, Nyman L. Characterization of the selectivity, specificity and potency of medetomidine as an alpha 2-adrenoceptor agonist. Eur. J. Pharmacol. 1988;150:9–14. doi: 10.1016/0014-2999(88)90744-3. [DOI] [PubMed] [Google Scholar]
- Wang J, Mack AL, Coop A, Matsumoto RR. Novel sigma (sigma) receptor agonists produce antidepressant-like effects in mice. Eur. Neuropsychopharmacol. 2007;17:708–716. doi: 10.1016/j.euroneuro.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Mandyam CD, Lekic DM, Koob GF. Alpha 1-noradrenergic system role in increased motivation for cocaine intake in rats with prolonged access. Eur. Neuropsychopharmacol. 2008;18:303–311. doi: 10.1016/j.euroneuro.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesnes K, Warburton DM. Smoking, nicotine and human performance. Pharmacol. Ther. 1983;21:189–208. doi: 10.1016/0163-7258(83)90072-4. [DOI] [PubMed] [Google Scholar]
- West RJ, Hajek P, Belcher M. Severity of withdrawal symptoms as a predictor of outcome of an attempt to quit smoking. Psychol. Med. 1989;19:981–985. doi: 10.1017/s0033291700005705. [DOI] [PubMed] [Google Scholar]
- Wise RA, Munn E. Withdrawal from chronic amphetamine elevates baseline intracranial self-stimulation thresholds. Psychopharmacology (Berl) 1995;117:130–136. doi: 10.1007/BF02245178. [DOI] [PubMed] [Google Scholar]
- Yamada H, Bruijnzeel AW. Stimulation of alpha2-adrenergic receptors in the central nucleus of the amygdala attenuates stress-induced reinstatement of nicotine seeking in rats. Neuropharmacology. 2011;60:303–311. doi: 10.1016/j.neuropharm.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young WS, III, Kuhar MJ. Noradrenergic alpha 1 and alpha 2 receptors: light microscopic autoradiographic localization. Proc. Natl. Acad. Sci. U. S. A. 1980;77:1696–1700. doi: 10.1073/pnas.77.3.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]