Extremely sensitive and accurate clinical measurements of biomarker proteins for early detection and monitoring of cancer pose a formidable challenge. However, successful inexpensive devices for reliable on-the-spot cancer diagnosis promise to lead to improved therapeutic outcomes with lower cost, decreased patient stress, and new targeted therapies.[1–3] Such devices will also provide tools for a better fundamental understanding of disease progression, and enable biomarker-based monitoring of therapy.[4] Herein, we report an ultra-sensitive immunosensor based on a glutathione-protected gold nanoparticle (GSH-AuNP) sensor surface. When combined with novel massively labeled paramagnetic particles for the electrochemical detection of cancer biomarker interleukin 8 (IL-8), we obtained an unprecedented detection limit (DL) of 1 fgmL−1 (100 aM) for IL-8, the lowest protein level yet detected in serum. Accuracy was demonstrated by determining IL-8 in conditioned media from head and neck squamous cell carcinoma (HNSCC) cells.
Our DL is lower than that of any method reported to date for direct biomarker protein detection in serum. It is similar to that of a DNA barcode method that used PCR amplification to achieve a DL of 1 fgmL−1 (30 aM) in goat serum.[5] Alternatively, fluorescence-based immunoassays were used to detect IL-6, IL-8 (DL 10 fM),[6] and prostate-specific antigen (PSA, DL 3 fM) in serum.[7] Surface plasmon resonance (SPR) using nanobead amplification has been used to detect brain natriuretic peptide (DL 25 pgmL−1)[8] in plasma, while our group used clustered superparamagnetic bead labels in SPR to detect PSA (DL 10 fgmL−1) in serum.[9] Other modern strategies include commercial immunobead-based assays for multiplexed protein detection (DL ≈ 10 pgmL−1),[3,10] a dendrimer/conducting-polymer array for IL-8 mRNA (DL ≈ 4 fM) and IL-8 (DL 7.4 pgmL−1)[11] in saliva, and nanowire nanotransitors with DLs of 0.9 pgmL−1 for various proteins in serum.[12]
Herein, we report a simple nanostructured amperometric sensor electrode coated with a dense film of GSH-AuNP with attached primary antibodies (Ab1) that capture human IL-8 from the sample. When coupled to superparamagnetic beads massively loaded with about 500000 horseradish peroxidase (HRP) labels and secondary antibodies (Ab2), an ultralow DL and very high sensitivity were achieved in sandwich immunoassays for IL-8 in serum. The enzyme labels are activated using hydrogen peroxide and hydroquinone to develop a steplike catalytic amperometric signal proportional to the amount of IL-8 in the sample.
We chose IL-8 as a model analyte protein because it is elevated in blood during inflammation and in the presence of several types of cancers including HNSCC.[13–15] Approximately 44000 patients are diagnosed with HNSCC each year in the USA with about 11000 cases resulting in death.[16] This high mortality rate is due to the difficulty of monitoring biomarker proteins for early detection. Current diagnoses rely on visual identification of lesions, often resulting in detection of cancers at advanced stages when prognosis is poor.[16] The average concentration of IL-8 in serum of a healthy individual is ≤ 13 pgmL−1, compared to elevated levels of ≥ 20 pgmL−1 in patients with HNSCC.[17] This provides a challenging application, since reliable diagnoses and active monitoring of HNSCC during therapy requires that changes in both normal and elevated levels of IL-8 be measured accurately. Furthermore, ultrasensitive detection methods will facilitate detecting recurrent cancer, and also enable sample dilution that can minimize interferences if ultrahigh sensitivity is not required.
A single biomarker found at an elevated level, however, does not give sufficient diagnostic accuracy. For example, PSA, the most widely used serum biomarker for prostate cancer, has a positive predictive value of only around 75%.[18] Recent studies have shown that nearly 100% predictive success can be achieved by measuring panels of four to ten biomarkers of a particular cancer.[19–21] Thus, sensitive multi-protein arrays are needed for point-of-care detection and monitoring. The ultrasensitive immunosensor development for IL-8 reported herein serves as the starting point for electrochemical immunoarrays for panels of biomarker proteins.
Previously, we reported a AuNP immunosensor for detection of IL-6 with a DL of 10 pgmL−1 in calf serum without using labeled magnetic particles.[22] A DL of 0.5 pgmL−1 was achieved for PSA in serum using approximately 1 μm magnetic beads containing about 7500 HRP labels per nanoparticle.[23] Alternatively, we used single-walled carbon nanotube (SWNT) immunosensors coupled to multilabeled HRP–carbon nanotube (CNT)–HRP–Ab2 detection bioconjugates to obtain a DL of 0.5 pgmL−1 for IL-6[24] and 4 pgmL−1 for PSA[25] in serum. In another strategy we used 0.5 μm multilabeled polymeric beads, polybeads–HRP–Ab2, to achieve a DL of 10 pgmL−1 for matrix metal-loproteinase-3 (MMP-3)[26] in calf serum. These studies revealed that high sensitivity can be facilitated by using nanostructured sensor surfaces that contain large amounts of capture antibody in combination with multilabel particle strategies.
To demonstrate a wide dynamic range, two protocols were used: one to measure ultralow (≤500 fgmL−1) levels of IL-8 and another for more elevated levels. The highly sensitive immunosensor is achieved by use of the 5 nm GSH-AuNP nanostructured sensor platform, coupled with 1.0 μm massively loaded superparamagnetic beads (Ab2–MB–HRP) with ≥500000 HRP labels attached through avidin–biotin interaction. This approach provides a massive number of HRP labels per binding event, thus allowing extremely sensitive monitoring of any changes in serum concentration with a remarkably low DL of 1 fgmL−1. This ultrahigh sensitivity and low DL is also enabled by the nanostructured AuNP platform, which provides a large surface area allowing dense Ab1 coverage. For elevated-level assays, biotinylated Ab2 bound to streptavidin–HRP giving 14–16 labels per antigen used for detection gave a DL of 10 pgmL−1 (1.0 pM). We have used this strategy previously to detect IL-6 in serum.[22]
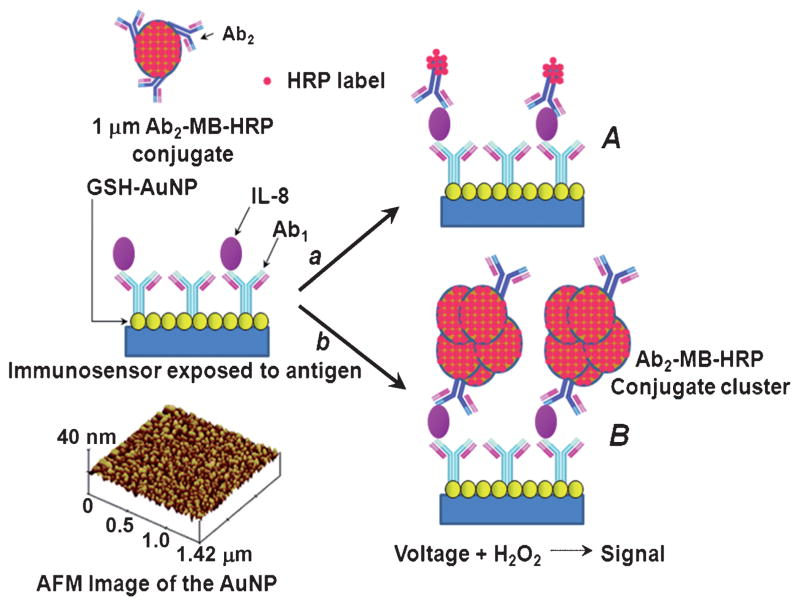
Figure 1 shows these two immunosensor approaches: the Ab2–HRP(14–16) protocol (A) and the signal amplification strategy using multienzyme label Ab2–MB–HRP (B) on GSH-AuNPs. Capture antibody, Ab1, is conjugated to the GSH-AuNP electrode surface, blocked with 1% bovine serum albumin (BSA), and then washed with blocking buffer, phosphate-buffered saline (PBS) with 0.05% Tween 20, before addition of calf serum (10 μL) containing IL-8. The electrode is then washed and incubated with either Ab2–HRP(14–16) or Ab2–MB–HRP. Finally, the electrode is washed and transferred to an electrochemical cell with PBS buffer (10 mL, pH 7.2) containing 1 mM hydroquinone mediator and then 0.4 mM H2O2 is injected to generate the amperometric signal.
Figure 1.
Detection principles of AuNP immunosensors. The sensor surface after protein capture is shown on the left in the center. On the bottom left is a tapping-mode AFM image of a AuNP film that serves as the immunosensor platform. A) The immunosensor after treatment with biotinylated Ab2 followed by streptavidin-modified HRP resulting in HRP–Ab2 and providing 14–16 labels per binding event. B) The immunosensor after treatment with massively labeled Ab2–MB–HRP particles to obtain amplification by providing around 500000 enzyme labels per binding event.
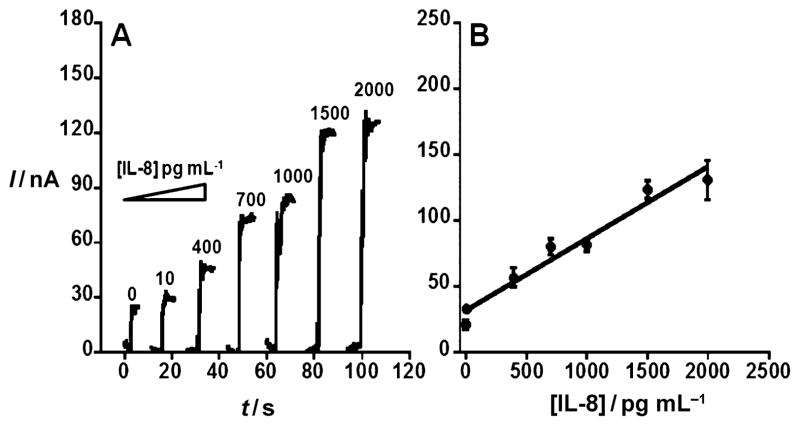
AFM images of the immunosensor platform (Figure 1, inset) show a dense packing of the approximately 5 nm AuNPs on a flat mica substrate, consistent with previous reports.[23] Our initial approach for elevated IL-8 antigen (Figure 1A) utilized the Ab2–biotin–streptavidin–HRP(14–16) label. Minimization of nonspecific binding (NSB) is critical to achieving the best sensitivity and DL,[27,28] and BSA and Tween 20 were found to effectively block NSB. Furthermore, capture antibody (Ab1; Figure S1A) and detection antibody (Ab2; Figure S1B) concentrations were optimized (see the Supporting Information). The best analytical performance was obtained using 10 μgmL−1 for Ab1 attachment and 0.05 μgmL−1 for Ab2 (Figure S1). These optimal conditions were used to obtain a calibration curve with a sensitivity of 0.34 nAmL(pg IL-8)−1cm−2 and a DL for the Ab2–HRP(14–16) of 10 pgmL−1 human IL-8 antigen (Figure 2). Figure 2A shows amperometric responses for IL-8 from 10 to 2000 pgmL−1. The calibration curve (Figure 2B) shows a linear relationship between concentration of IL-8 and change in the steady-state amperometric current. However, a more sensitive system is required to detect levels of IL-8 that fall below this DL.
Figure 2.
Amperometric response for AuNP immunosensor incubated with IL-8 in undiluted newborn calf serum (10 μL) for 1.25 h then conventional anti-IL-8–biotin in 0.05% Tween 20 for 1.25 h followed by 30 min of incubation with streptavidin-modified HRP (10 μL) at 1:200 dilution. A) Sensor current (I) response at −0.3 V and 2000 rpm after placing electrodes in buffer containing 1 mM hydroquinone mediator, then injecting H2O2 to 0.4 mM to develop the signal. The control shown on the left indicates full immunoassay with serum containing 0 pgmL−1 IL-8. B) Corresponding calibration curve of IL-8. Errors bars show device-to-device standard deviations (n =3). y =31.4 + 0.0543x; R =0.97817.
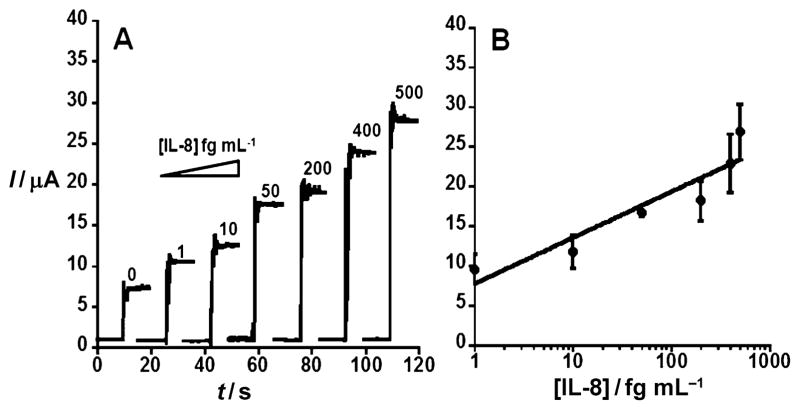
Amperometric responses using Ab2–MB–HRP with increasing concentrations of IL-8 (Figure 3A) range from 1 to 500 fgmL−1. Figure 3B presents a linear-logarithmic calibration curve with an extremely low DL of 1 fgmL−1. The slight nonlinear behavior at higher concentrations could be related to clustering of the Ab2–MB–HRP conjugate.[9] The 1 fgmL−1 DL is 10000-fold lower than that of our Ab2–biotin–streptavidin–HRP(14–16) protocol and 30000-fold lower than that of the standard ELISA[29] method.
Figure 3.
Amperometric response for AuNP immunosensor incubated with IL-8 (concentration in fgmL−1 labeled on curves) in undiluted calf serum (10 μL) for 1.25 h. A) Current at −0.3 V and 2000 rpm using Ab2–MB–HRP bioconjugate. Control shown on left with AuNP immunosensor with 0 pgmL−1 IL-8. B) Corresponding calibration curve of IL-8 immunosensor using Ab2–MB–HRP bioconjugate. Errors bars represent device-to-device standard deviations. y = 7.73 + 5.78log(x), R = 0.93199.
At very low IL-8 concentrations (1–50 fgmL−1) the sensitivity is 1060 nAmL(fg IL-8)−1cm−2. The massively labeled Ab2–MB–HRP bioconjugate particle allowed for ultrahigh sensitivity by virtue of the large number of HRP labels, which corresponds to the concentration of IL-8 in a given sample through the amperometric response. To make the detection particles, biotinylated HRP and Ab2 were bioconjugated to 1 μm streptavidin-coated magnetic beads with a reaction mixture having a 6800:1 HRP/Ab2 mole ratio. These mutilabeled magnetic particles with hundreds of thousands of HRP labels were used in place of the conventional Ab2–HRP(14–16) complex. The number of active HRP labels was estimated at 502700 per bead using the 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) HRP activity assay (see the Supporting Information, Figure S2). To reduce NSB in the amplified system, it was necessary to increase BSA in the blocking solution from 1 to 5% with the same amount of incubation time.
The sensitivity is six orders of magnitude better than that obtained with the Ab2–HRP(14–16) protocol. The large number of labels on the Ab2–MB–HRP bioconjugate significantly increases sensitivity and lowers DL compared to Ab2–HRP(14–16). This extremely high HRP label loading on the magnetic beads is achieved by taking advantage of interactions between the streptavidin-coated magnetic beads and biotinylated HRP labels. Streptavidin–biotin has a very large affinity constant (Ka = 1015M) and has four biotin binding sites for each streptavidin molecule.[30]
As a proof of concept to establish method accuracy, we then used the immunosensor to determine secreted levels of IL-8 in in-vitro cell preparations. Conditioned media from heterogeneous populations of six different cell lines were analyzed to test the validity of our immunosensor approach towards IL-8 detection in HNSCC.
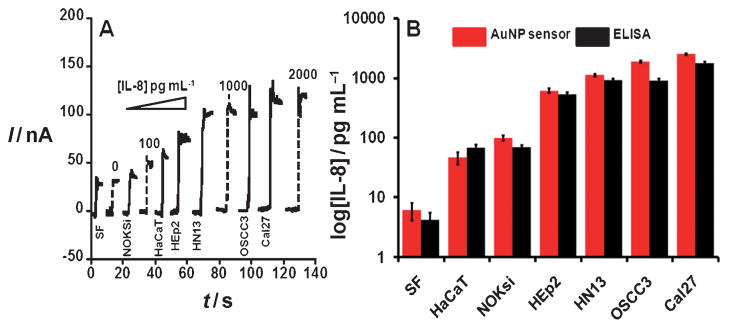
Figure 4A shows amperometric signals for different cell lines along with human IL-8 standards in serum at comparable levels. Most of the HNSCC cancer cell lines (HEp2, HN13, OSCC3, Cal27) were found to contain high levels of IL-8, ranging from 612 to 1760 pgmL−1, while HaCaT and NOKsi demonstrated low levels of the antigen, ranging from 46 to 99 pgmL−1. Spontaneously immortalized HaCaT and NOKsi cells were essentially established from normal epidermal and oral mucosal tissues, respectively, and maintained normal phenotypic features. Samples were also analyzed using the standard ELISA method, and gave excellent correlations with our AuNP immunosensor (Figure 4B). These results confirm the accuracy of this immunosensor for measurement of IL-8 in complex samples of a variety of cancer cell types.
Figure 4.
Amperometric response for AuNP immunosensor incubated with IL-8 (concentration labeled on curves) or conditioned media containing IL-8 secreted by human squamous cells. Conditioned media samples from HNSCC cell lines (HEp2, HN13, OSCC3, Cal27), immortalized epidermal (HaCaT) and mucosal (NOKsi) squamous cells, and serum-free (SF) media were analyzed using biotinylated Ab2 (10 μL, 0.05 μgmL−1) in 0.1% BSA in pH 7.2 PBS buffer and streptavidin-modified HRP (10 μL). A) Current at −0.3 V and 2000 rpm using hydroquinone mediator in PBS buffer, after injecting H2O2 to 0.4 mM. B) AuNP sensor results for conditioned media shown with results from ELISA of the samples.
In summary, we have reported a new electrochemical immunosensor protein detection method with ultrasensitive, selective, and reproducible measurement of IL-8. The streptavidin-modified magnetic bead amplification strategy gave a DL of 1.0 fgmL−1, to our knowledge the lowest yet reported for a protein in serum. This DL is 30000-fold lower than that of the conventional ELISA and about 1000-fold lower than that of commercial bead-based protein assays.[3,9] Accurate results were demonstrated for cell culture media samples representative of low cancer-free and high expression levels in cancer patients for a range of head and neck cancer cell lines. The strategy reported for these single-protein sensors has great promise for extension to arrays for clinical cancer screening and therapy monitoring.
Experimental Section
Monoclonal antihuman IL-8 antibody, biotinylated antihuman IL-8 antibody, recombinant IL-8 in calf serum, and streptavidin–HRP were from R&D Systems, Inc. HaCaT and the oral cancer cell lines were cultured as previously described[24] in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), at 37°C in 95% air/5% CO2, and the immortalized NOKsi cells were grown in defined keratinocyte serum-free medium KGM with the provided growth supplements (Invitrogen). Conditioned media from the different cells were prepared by incubating cells at 60–70% confluence, for 48 h in DMEM or KGM without supplements. Harvested conditioned media were then filtered to remove cellular debris and the samples were divided into aliquots and stored at −70°C prior to analysis. Streptavidin-coated superpara-magnetic beads were from Polyscience, Inc. Biotinylated HRP was from Invitrogen. HRP (molecular weight 44000 Da), lyophilized 99% BSA, Tween 20, methanol, acetic acid, sodium borohydride, gold(III) chloride trihydrate, poly(diallyldimethylammonium chloride) (PDDA; 20 wt.% in water), and L-glutathione were from Sigma–Aldrich. Immunoreagents were dissolved in PBS buffer (0.137M NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM NaH2PO4; pH 7.2). N-Hydroxysulfosuccinimide (NHS) and 1-[3-(dimethylamino)propyl]-3-ethyl carbodiimide hydrochloride (EDC) from Aldrich were dissolved in water immediately before use. A GSH-AuNP platform was assembled on the pyrolytic graphite (PG) tip of an electrode using a monolayer of PDDA. Capture antibody (Ab1) was attached on the GSH-AuNP platform followed by IL-8 standards in serum and/or conditioned media samples. Then Ab2–HRP(14–16) was added for electrochemical detection of elevated levels of IL-8. Alternatively, massively loaded 1 μm magnetic beads (Ab2–MB–HRP) with hundreds of thousands of HRP labels were used to detect ultralow levels of IL-8. Finally, the sensor was placed in an electrochemical cell containing PBS buffer (10 mL, pH 7.2) with 1 mM hydroquinone as a mediator. Amperometry was performed by rotating the disk at 2000 rpm and then injecting 0.4 mM H2O2 to generate the electrochemical signal. Details of GSH-AuNP platform fabrication, synthesis of Ab2–MB–HRP bioconjugate, and the immunosensor protocol are provided in the Supporting Information.
Supplementary Material
Footnotes
This research was financially supported by the NCRR/NIH (grant P20RR016457) awarded to B.S.M. and in part by the NIEHS/NIH (US PHS grant ES013557) awarded to J.F.R. Manuscript preparation was supported in part by the Intramural Research Program of NIDCR/NIH.
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.201102941.
Contributor Information
Prof. Bernard S. Munge, Email: bernard.munge@salve.edu, Department of Chemistry, Salve Regina University, 100 Ochre Point Avenue, Newport, RI 02840 (USA)
Amy L. Coffey, Department of Chemistry, Salve Regina University, 100 Ochre Point Avenue, Newport, RI 02840 (USA)
Jaimee M. Doucette, Department of Chemistry, Salve Regina University, 100 Ochre Point Avenue, Newport, RI 02840 (USA)
Brian K. Somba, Department of Chemistry, Salve Regina University, 100 Ochre Point Avenue, Newport, RI 02840 (USA)
Ruchika Malhotra, Department of Chemistry, University of Connecticut, 55 North Eagleville Road, Storrs, CT 06269 (USA) and Department of Cell Biology, University of Connecticut Health Center, Farmington, CT 06232 (USA).
Dr. Vyomesh Patel, Oral and Pharyngeal Cancer Branch, NIDCR/NIH, Bethesda, MD 20892 (USA)
Dr. J. Silvio Gutkind, Oral and Pharyngeal Cancer Branch, NIDCR/NIH, Bethesda, MD 20892 (USA)
Prof. James F. Rusling, Department of Chemistry, University of Connecticut, 55 North Eagleville Road, Storrs, CT 06269 (USA) and Department of Cell Biology, University of Connecticut Health Center, Farmington, CT 06232 (USA)
References
- 1.Manne U, Srivastava RG, Srivastava S. Drug Discovery Today. 2005;10:965–976. doi: 10.1016/S1359-6446(05)03487-2. [DOI] [PubMed] [Google Scholar]
- 2.Hanash SM, Baik CS, Kallioniemi O. Nat Rev Clin Oncol. 2011;8:142–150. doi: 10.1038/nrclinonc.2010.220. [DOI] [PubMed] [Google Scholar]
- 3.Rusling JF, Kumar CV, Gutkind JS, Patel V. Analyst. 2010;135:2496–2511. doi: 10.1039/c0an00204f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson MS, Nie WY. Anal Chem. 2006;78:6476–6483. doi: 10.1021/ac060843u. [DOI] [PubMed] [Google Scholar]
- 5.Nam JM, Thaxton CS, Mirkin CA. Science. 2003;301:1884–1886. doi: 10.1126/science.1088755. [DOI] [PubMed] [Google Scholar]
- 6.Konry T, Hayman RB, Walt DR. Anal Chem. 2009;81:5777–5782. doi: 10.1021/ac900694y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim D, Daniel WL, Mirkin CA. Anal Chem. 2009;81:9183–9187. doi: 10.1021/ac9018389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teramura Y, Arima Y, Iwata H. Anal Biochem. 2006;357:208–215. doi: 10.1016/j.ab.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 9.Krishnan S, Mani V, Wasalathanthri D, Kumar CV, Rusling JF. Angew Chem. 2011;123:1207–1210. doi: 10.1002/anie.201005607. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2011;50:1175–1178. doi: 10.1002/anie.201005607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luminex Corp. http://luminexcorp.com/technology/index.html.
- 11.Wei F, Patel P, Liao W, Chaudhry K, Zhang L, Arellano-Garcia M, Hu S, Elashoff D, Zhou H, Shukla S, Shah F, Ho C, Wong DT. Clin Cancer Res. 2009;15:4446–4452. doi: 10.1158/1078-0432.CCR-09-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng G, Patolsky F, Cui Y, Wang W, Lieber C. Nat Biotechnol. 2005;23:1294–1301. doi: 10.1038/nbt1138. [DOI] [PubMed] [Google Scholar]
- 13.Bigbee WL, Grandis JR, Siegfried JM. Clin Cancer Res. 2007;13:3107–3108. doi: 10.1158/1078-0432.CCR-07-0746. [DOI] [PubMed] [Google Scholar]
- 14.Cohen AN, Veena MS, Srivatsan ES, Wang MB. Arch Otolaryngol Head Neck Surg. 2009;135:190–197. doi: 10.1001/archotol.135.2.190. [DOI] [PubMed] [Google Scholar]
- 15.Cohen RF, Contrino J, Spiro JD, Mann EA, Chen LL, Kreutzer DL. Arch Otolaryngol Head Neck Surg. 1995;121:202–209. doi: 10.1001/archotol.1995.01890020064013. [DOI] [PubMed] [Google Scholar]
- 16.Jemal A, Siegel R, Xu J, Ward E. CA: Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 17.Gokhalea AS, Haddad RI, Cavacinia LA, Wirth L, Weeks L, Hallar M, Faucher J, Posner MR. Oral Oncol. 2005;41:70–76. doi: 10.1016/j.oraloncology.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Lilja H, Ulmert D, Vickers A. Nat Rev Cancer. 2008;8:268–278. doi: 10.1038/nrc2351. [DOI] [PubMed] [Google Scholar]
- 19.Hanash SM, Pitteri SJ, Faca VM. Nature. 2008;452:571–579. doi: 10.1038/nature06916. [DOI] [PubMed] [Google Scholar]
- 20.Stevens EV, Liotta LA, Kohn EC. Int J Gynecol Cancer. 2003;13:133–139. doi: 10.1111/j.1525-1438.2003.13358.x. [DOI] [PubMed] [Google Scholar]
- 21.Wagner PD, Verma M, Srivastava S. Ann N Y Acad Sci. 2004;1022:9–16. doi: 10.1196/annals.1318.003. [DOI] [PubMed] [Google Scholar]
- 22.Munge BS, Krause CE, Malhotra R, Patel V, Gutkind JS, Rusling JF. Electrochem Commun. 2009;11:1009–1012. doi: 10.1016/j.elecom.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mani V, Chikkaveeraiah BV, Patel V, Gutkind JS, Rusling JF. ACS Nano. 2009;3:585–594. doi: 10.1021/nn800863w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malhotra R, Patel V, Vaque PJ, Gutkind JS, Rusling JF. Anal Chem. 2010;82:3118–3123. doi: 10.1021/ac902802b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu X, Munge B, Patel V, Jensen G, Bhirde A, Gong JD, Kim SN, Gillespie J, Gutkind JS, Papadimitrakopoulos F, Rusling JF. J Am Chem Soc. 2006;128:11199–11205. doi: 10.1021/ja062117e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munge BS, Fisher J, Millord LN, Krause CE, Dowd RS, Rusling JF. Analyst. 2010;135:1345–1350. doi: 10.1039/c0an00028k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson DS, Nock S. Angew Chem. 2003;115:510–517. doi: 10.1002/anie.200390150. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2003;42:494–500. doi: 10.1002/anie.200390150. [DOI] [PubMed] [Google Scholar]
- 28.Ward AM, Catto JWF, Hamdy FC. Ann Clin Biochem. 2001;38:633–651. doi: 10.1258/0004563011901055. [DOI] [PubMed] [Google Scholar]
- 29.http://www.rndsystems.com/pdf/dy208.pdf
- 30.Diamandis EP, Christopoulos TK. Clin Chem. 1991;37:625–636. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.