Abstract
Objective
Brain enlargement has been observed in 2 year old children with autism but the underlying mechanisms are unknown. This longitudinal MRI study investigated early growth trajectories in brain volume and cortical thickness.
Method
Cerebral gray and white matter volumes and cortical thickness in children with autism spectrum disorder and controls were examined. Subjects were seen at approximately 2 years of age (autism = 59, controls = 38) and were rescanned approximately 24 months later at age 4–5 years (autism = 38, controls = 21).
Results
We observed generalized cerebral cortical enlargement in individuals with ASD at both 2 and 4 – 5 years of age. Rate of cerebral cortical growth across multiple brain regions and tissue compartments, in individuals with ASD, was parallel to that seen in controls, indicating that there was no increase in rate of cerebral cortical growth during this interval. No cerebellar differences were observed in ASD. After controlling for TBV, a disproportionate enlargement in temporal lobe white matter was observed in the ASD group. We found no differences in cortical thickness, but an increase in an estimate of surface area in the ASD group compared to controls for all cortical regions measured (temporal, frontal, and parietal-occipital).
Conclusions
Our longitudinal MRI study found generalized cerebral cortical enlargement in children with ASD, with a disproportionate enlargement in temporal lobe white matter. There was no difference from controls in the rate of brain growth for this age interval, indicating brain enlargement in ASD results from an increased rate of brain growth prior to age 2. The presence of increased cortical volume, but not cortical thickness, suggests that early brain enlargement may be associated with increased cortical surface area. Cortical surface area overgrowth in ASD may underlie brain enlargement and implicates a distinct set of pathogenic mechanisms.
Introduction
Autism is a neurodevelopmental disorder defined by the presence of social and communication deficits, stereotyped/repetitive behaviors, and a characteristic developmental course (1). The presence of brain enlargement on MRI in autism is now well established (2–5) and consistent with data showing enlarged head circumference (6–8) and increased brain weight (9–10). We previously reported brain enlargement on MRI in 51 two-year-olds with autistic disorder, the youngest cohort reported to date (11). These MRI data were consistent with retrospective, longitudinal head circumference data (from birth to age 3 years), that provide indirect evidence that increased brain growth may have its origins at the end of the first year of life (11). Recently, a longitudinal study of brain development (12) replicated and extended our finding of generalized brain enlargement present by age two in children with autism.
The timing of brain enlargement in autism is of particular importance given new evidence from prospective behavioral studies of infant siblings of autistic individuals showing typical social behaviors at 6 months of age followed by the onset of autistic social behavior at 12 months of age in infants who later meet criteria for autism at 36 months of age (13). Results from these behavioral studies suggest a period of typical development followed by the early post-natal onset of autistic disorder in the latter part of the first year or early second year of life. Direct evidence of the timing of early brain volume overgrowth in autism will focus future studies on this narrow window of brain development, providing important insights into potential underlying neural mechanisms and highlighting a potentially important period for early intervention/prevention.
The critical need for longitudinal brain imaging studies in conditions such as autism, characterized by clinical heterogeneity and the likelihood of non-linear development, has been established by the seminal paper of Giedd and colleagues (14). We present here a large longitudinal MRI data of brain volume and cortical thickness changes in two-year-olds with autism (the earliest date when valid diagnosis is considered possible), followed up at 4–5 years of age.
Methods
Sample
Subjects included 59 children with autism spectrum disorder (ASD) and 38 comparison cases who were enrolled in this longitudinal study between 18–35 months of age and received an initial behavioral assessment and brain MRI scan. Approximately two years later, at age 4–5 years old, this cohort of children received a repeat assessment and MRI. While we attempted to have all children return two years later in some cases this was not possible for some families and therefore some children were allowed to return up to 30 months later (age 5). There were no significant group differences in the interval for follow-up between timepoints (i.e., three months difference between ASD and controls). There were 38 children with ASD and 21 comparison cases seen for the follow-up visit. The comparison group was comprised of typically developing children (TYP) and children with developmental delay (DD) who had no evidence of a PDD. The group with ASD was observed to be lower functioning (estimated IQ in the 50s) while the TYP fell in the average range (estimated IQ ~100) and therefore the control group was enriched for lower functioning children (i.e., DD). The DD control group was included to enrich the comparison sample for low IQ non-autistic subjects. Autism is well known to include individuals with low IQ. Enrichment for low IQ non-autistic comparison subjects in the control group allowed us to take into account the effects of IQ on brain volumes.
See Table 1 for a description of subject characteristics. At time 1 there were a total of 38 children in the comparison group (26 TYP, 12 DD); at time 2 there were 21 cases (15 TYP, 6 DD). Cases and controls did not differ significantly on age, and gender ratios were comparable in both groups. Mean age (SD) for the TYP at time 1 was 2.49 (.54) and time 2 (4.59 (.34). Mean age (SD) for the DD group at time 1 was 2.83 (.4) and time 2 was 4.97 (.49). The TYP subjects were slightly younger than the other groups. There were no gender differences. At time 1, the TYP group was 74% male and the DD was 67% male.
Table 1.
Sample Characteristics
| Group | Time 1 N |
Age (yrs) M (SD) |
Time 2 N |
Age (yrs) M (SD) |
Percent Male at Time1* |
|---|---|---|---|---|---|
| ASD | 59 | 2.7 (.32) | 38 | 5.04 (.41) | 86% |
| Controls | 38 | 2.6 (.52) | 21 | 4.69 (.46) | 72% |
Percent male at Time 2: ASD 89%, Controls 71%
A full description of the ascertainment and inclusion criteria is detailed in Hazlett, et al (11). A brief summary only is included here. All subjects were enrolled between 18–35 months of age (time point 1) and seen for a repeat assessment approximately 24 months after their initial assessment (time point 2). At study enrollment, medical records and developmental history were reviewed and records were re-evaluated at time point 2. Children with ASD were referred after receiving a clinical diagnosis of autistic disorder. Subjects with DD were referred only if they had no known identifiable cause for their delay (e.g., prematurity, genetic or neurological disorder) and had no indication of a PDD. The TYP subjects were recruited from the community and were screened for ASD. All subjects were excluded for evidence of a medical condition thought to be associated with autism (15), including Fragile X Syndrome (FraX), Tuberous Sclerosis (TS), gross CNS injury (e.g., cerebral palsy, significant complications or perinatal/postnatal trauma, drug exposure), seizures, and significant motor or sensory impairments. Study approval was acquired from both the UNC and Duke Institutional Review Boards and written informed consent was obtained by getting parental (or custodial guardian) consent for each subject.
Clinical Assessment
At study entry, diagnosis for the subjects with autism was confirmed using the Autism Diagnostic Interview-Revised (ADI-R) (16) and the Autism Diagnostic Observation Schedule-G (ADOS-G) (17). Subjects were included in the autism group only if they met ADI-R algorithm criteria for autism (all domains), and obtained ADOS-G scores consistent with autism. The same assessments were used at time point 2 (age 4–5 years) and additionally all cases also met DSM-IV criteria (1) for autistic disorder. At the follow-up assessment, a small subset of subjects failed to meet the original study criteria for autistic disorder (e.g., ADI-R, ADOS-G, DSM-IV) but continued to show evidence of symptoms consistent with a PDD-NOS diagnosis. These subjects were classified as PDD. The ASD sample therefore included 52 autism and 7 PDD subjects at time 1, and 33 autism and 5 PDD subjects at time 2. For our primary analyses we included these children in the ASD group given that this approach has been used by many recent genetic studies of autism, but did examine them separately and have indicated comparisons where there are differences.
Subjects were given a largely identical battery of measures at both time points including the Mullen Scales of Early Learning (18), the Vineland Adaptive Behavior Scales (19), Preschool Language Scale 4th edition (20), behavioral rating scales, and a standardized neurodevelopmental examination to exclude subjects with any notable dysmorphology. At time point 2, subjects were also administered the Differential Abilities Scale (DAS) as an additional cognitive measure (21). All autistic and DD subjects received testing for Fragile X Syndrome (cytogenetics or molecular). DD and TYP children were screened for autism with the Childhood Autism Rating Scale (22) and excluded if they reached the cutoff for autism (≥ 30 total score). Medical records in the DD and TYP groups were also reviewed to exclude subjects for any possible evidence of an ASD.
Table 2 presents the cognitive and adaptive functioning characteristics of the sample. Many subjects with autism and DD failed to obtain a valid standard score on the DAS at time point 2, so we only provide estimates of cognitive functioning from their Mullen. Cognitive and adaptive functioning for those children classified as PDD was consistent with the autism sample, although the PDD group had slightly higher scores at time 2. At time 1, the IQ estimate (M/SD) was 56.1(7.1) and at time 2 was 74.8 (32.3). Mean (SD) adaptive functioning for the PDD group at time 1 was 62.9(6.4) and time 2 was 65.5(14.4). As noted above the controls were enriched for lower functioning children (DD) and there were significant differences in IQ and adaptive functioning. At time 1, the TYP group had a mean estimated IQ of 107.0(16.4) and adaptive functioning of 98.4(12.6). The children with DD had an estimated IQ of 55.5(6.7) and adaptive behavior of 65.8(13.9). These group differences remained at time 2. The TYP group had a mean IQ of 113.9(13.2) and adaptive behavior was 95.1(8.3). The DD group had a mean IQ of 58.5(12.7) and adaptive behavior 55.0(14.9).
Table 2.
Cognitive and adaptive functioning of sample
| Group | N | Age (M/SE) | Gender* | Mullena M (SD) |
Vinelandb M (SD) |
|
|---|---|---|---|---|---|---|
| ASD | ||||||
| time 1 | 59 | 2.7 (.32) | 86% | 54.32 (9.07) | 61.04 (6.05) | |
| time 2 | 38 | 5.0 (.41) | 89% | 58.97 (19.68) | 52.78 (13.49) | |
| Controls | ||||||
| time 1 | 38 | 2.6 (.52) | 74% | 90.74 (28.0) | 87.84 (20.1) | |
| time 2 | 21 | 4.7 (.46) | 71% | 97.25 (28.97) | 83.05 (21.45) | |
Percent Male
ASD = Autism and PDD subjects combined
Mullen Composite Standard Score
Vineland Adaptive Behavior Composite Standard Score
MRI Acquisition
All subjects were scanned at the Duke-UNC Brain Imaging and Analysis Center (BIAC), on a 1.5 Tesla GE Signa MRI scanner. Image acquisition was designed to maximize gray/white tissue contrast for the 18–35 month old child and included: (1) a coronal T1 IR Prepared: T1 300 msec, TR 12 msec, TE 5 msec, 20° flip angle, at 1.5 mm thickness with 1 NEX, 20 cm FOV; and 256 × 192 matrix; (2) a coronal PD/T2 2D dual FSE, TR 7200 msec, TE 17/75 msec, at 3.0 mm thickness with 1 NEX, 20 cm FOV, and 256 × 160 matrix. A localizer was included to monitor that the subjects were placed in the scanner in a systematic way. To evaluate scanner stability over time, we also collected geometric phantom data across the period of the study. Core brain measures (intracranial volume, gray matter, white matter) were graphed across the period of time for the study and evaluated for any significant changes.
Subjects with autism and DD were scanned using moderate sedation (combination of pentobarbital and fentanyl as per hospital sedation protocol) administered by a sedation nurse and under the supervision of a pediatric anesthesiologist in attendance. Physiological monitoring was conducted throughout the scan and recovery. TYP subjects were scanned without sedation. At age two all the TYP subjects were scanned in the evening, while sleeping. At age 4, some of the TYP subjects (N=5) were scanned while awake, after completing a behavioral training protocol to learn to lie still in the scanner. The remaining TYP subjects were scanned while sleeping for time point 2. All MRI scans were reviewed by a pediatric neuroradiologist and screened for significant abnormalities (e.g., malformations, lesions, etc.).
Image Processing
The image processing procedures for this data are identical to those described in the initial paper from this longitudinal study (11). The primary components are briefly reported here for reference. Scans first underwent quality control checks to determine if they were of sufficient quality to process. All subject scans were rated by an experienced image processor who was blind to group membership. Each case was reviewed on a variety of criteria (e.g., correct scan parameters used, motion artifact, flow artifact, etc.) and assigned a rating based on scan quality (1=poor, 2=mediocre, 3=good). No scans with ‘poor’ quality ratings were included in this report.
The T1 and PD/T2 scans were then registered and aligned into a standardized plane along an AC-PC (anterior-posterior commissure) axis (11). The co-registered and aligned images were then processed for tissue segmentation using the Expectation Maximization Segmentation (EMS) (23–24). An “averaged” pediatric probabilistic brain atlas serves as a spatial prior and was automatically aligned to each subject brain using a linear, affine transformation. The fully automatic EMS segmentation includes multi-channel registration, bias inhomogeneity correction, and non-brain stripping in one integrated tool. Gray, white, and CSF tissue segmentations were produced for each subject. Total brain volume (TBV) measures included total gray and white matter and all CSF. Total tissue volume (TTV) included all gray and white matter in the cerebrum (cerebral cortex), cerebellum, and brainstem.
Regional lobe volume measurements were obtained using a manually parcellated pediatric brain template (atlas) MRI developed by our group, which was then mapped onto each subject brain using a fluid high-dimensional deformation algorithm (described in Hazlett et al., 2005 (11)). Delineated regions included the frontal, temporal, parietal, and occipital lobes, cerebellum, corpus callosum, interhemispheric fissure, and a “subcortical area” (basal ganglia, thalamus, deep white matter, and brainstem). The insula and cingulate gyrus were also defined, but for the purposes of these analyses the insula was included in the cerebral cortex measure and the cingulate with the frontal/parietal lobes. Cortical label maps were combined with the EMS tissue classified images to produce gray/white/CSF volumes for each of these lobe compartments.
Regional cortical thickness maps appropriate for pediatric MRI data were created by our group using ARCTIC (Automatic Regional Cortical ThICkness) after attempts to use other available tools were unsuccessful. ARCTIC is a part of the 3D Slicer image processing toolkit, and is freely available to the public (www.nitrc.org/projects/arctic/). The computation uses the prior computed EMS tissue segmentation and lobar parcellation for a robust, image space derived cortical thickness measurement. Measures were obtained in native (not stereotaxic) space. In order to avoid extraction of topologically correct and precise cortical surfaces which is challenging in pediatric brains, our cortical thickness analysis method used a discrete distance transform method which results in sparse sets of distance measurements between cortical surface and white matter boundaries, along with detection of sulcal folds. The cortical thickness measurements were collected per lobe and average values are being reported as a regional cortical thickness. We did not directly measure surface area, but created an estimate of surface area (SA) using a ratio term (SA=regional cortical volume (CV)/ regional cortical thickness (CT)). Regional CV was defined as the total cortical gray matter volume for the lobar region of interest. The lobar regions used to generate the CT and SA measures are identical to those defined above for the lobe volumes, and do not include subcortical structures.
Statistical Analyses
A priori hypotheses were tested using general linear mixed models with repeated measures. In all models brain volume was the dependent variable and diagnostic group (ASD, DD, TYP), age, gender, and IQ were independent predictors. To account for the multiple ROIs included in each model (e.g. CSF, gray tissue, white tissue) a group of indicator variables were included which specified the ROI for each observation.
Group was entered as a 3-level categorical variable. All group differences were calculated using the model estimated coefficients. Comparisons with the controls used a weighted average of the two control groups (TYP+DD), which maximized the amount of variance that could be explained by group.
Age and IQ were scaled to aid interpretation of the results. Age was centered at 3.5 years which was close to the overall mean of 3.6 years. An IQ ratio was calculated by dividing the child’s age equivalent score on the Mullen Visual Reception subscale by the child’s actual age. This allows a more precise measure of children’s abilities who would otherwise score at the lower end of the standardized scale and be assigned values of ‘<49’. The IQ ratio was centered as the mean for all observations and all main effects were estimated at these values unless otherwise specified.
For each group of analyses (total brain, lobe) two models were fit to the data: (1) the first included only group, age, gender and IQ, (2) the second model added TBV as a covariate to evaluate whether any brain volume differences were disproportionate to differences observed for TBV.
Tables 1 and 2 provided above in the Methods describe the sample characteristics. Age differences were observed (the TYP subgroup was slightly younger than the other groups) and age was included as a covariate. Gender was unequally distributed across groups and included as a covariate. The number of females with autism was too small to perform separate analyses by group. The DD subjects were included in the control group to control for IQ differences. While IQ was not found to be a significant predictor between groups, comparisons were run both with and without IQ to be conservative. A difference in the retention rate for ASD subjects (64%) versus controls (55%) was observed at time 2, but the study results were unchanged when subjects who did not return were dropped from the analyses. No significant difference in age, developmental IQ, adaptive functioning, gender, and symptom severity as reported on the ADI-R (for the ASD group only) were found between subjects who completed the study (2 time points) versus those who dropped out (1 time point).
Differences between the groups controlling for age, gender, and IQ were examined. While both groups showed increases over time in brain volume in all areas measured, there was no difference in rate of brain growth over time between groups. Because age by group interactions were not significant, only the main effect of group (averaged over time) is reported. Interactions with side (right/left) were not significant, therefore results are reported as total volume (sides combined).
To assess regional cortical thickness, a linear mixed model similar to that used to assess volume differences was fit and included up to 12 measures per subject (3 lobes/side/timepoint). This model was fit to the unadjusted average cortical thickness for each lobe and hemisphere. Age, gender, and IQ were included as covariates in the models along with group, and a set of indicator variables that delineated hemisphere and region. A second identical model was fit to examine the estimate for surface area.
Results
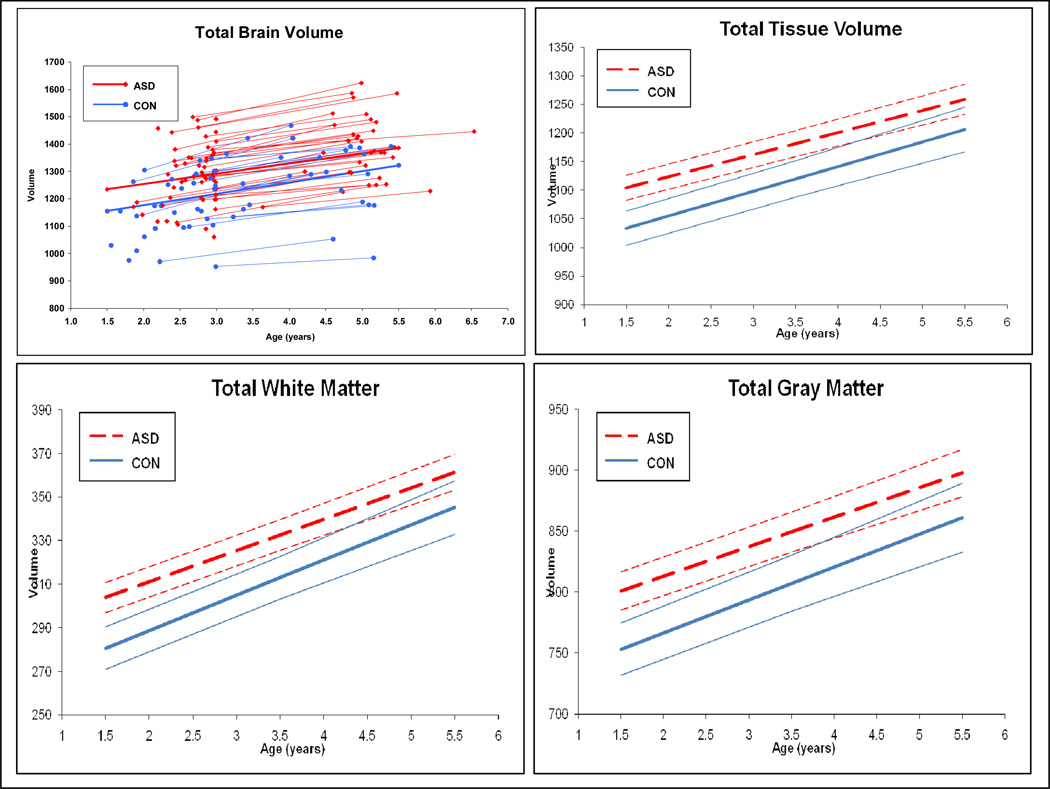
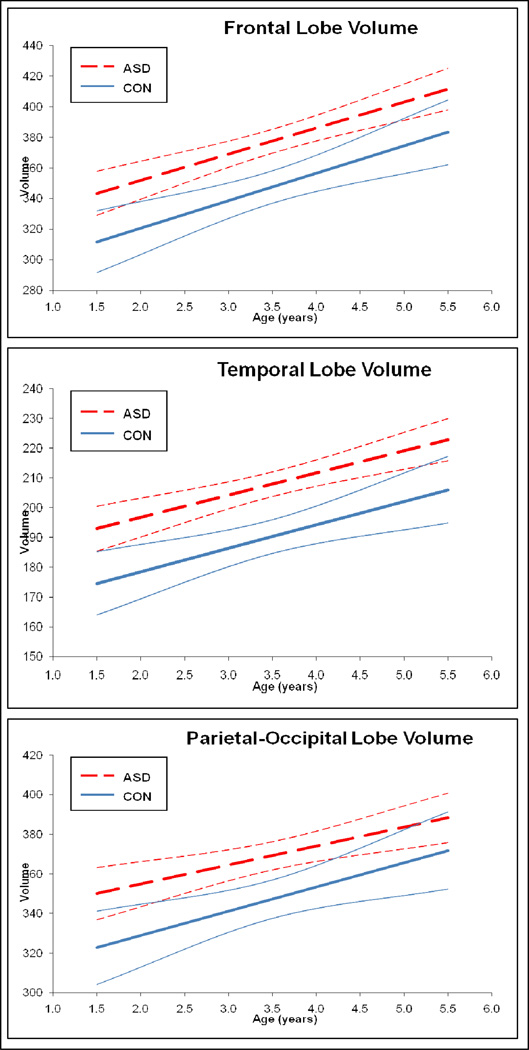
Growth trajectories for total brain (TBV), gray (TGV) and white matter (TWV) volumes are displayed in Figure 1. Trajectories of growth in cerebral cortical lobe regions (frontal, temporal, parietal-occipital) appear in Figure 2. While both the Autism Spectrum Disorder (ASD) and control groups show an increase over time in cerebral cortical volume in all compartments of the brain, there was no difference between groups in rate of brain growth during the 2 to 4–5 year old age interval. Brain volume enlargement observed in individuals with ASD at 2 years of age, continued to be present, to the same degree, at age 4–5 years.
Figure 1. Trajectory of Development: Total Brain, Total Tissue, Gray, and White Tissue Volumes.
Panels show the subject trajectories (scatterplot) for total brain volume, and mean group trajectories with confidence bands for total tissue volume, total gray matter and total white matter. Groups shown are ASD (color= red line, black & white= dashed line) and controls (CON) (color= blue line, black & white = solid line).
Figure 2. Trajectory of Development: Cerebral Cortical Lobe Volumes.
Panels show the mean group trajectories with confidence bands for the cortical lobe volumes. Cortical regions displayed include frontal, temporal, and parietal-occipital lobes. Combined volumes (right+left hemisphere) for each lobar region are shown. Groups shown are ASD (dashed line) and controls (CON) (solid line).
Mean group differences are reported in Table 3 for ASD versus controls, and in Table 4 for autistic subjects versus the TYP and DD control subgroups, respectively. Subjects with ASD had significant enlargement in TBV, total tissue volume (TGV+TWV), TGV, and TWV, with a 9% enlargement of cerebral cortex volume compared to controls. Cerebellar volume did not differ significantly between the ASD and control groups. Subjects with ASD had enlargement in both gray and white matter volume for all cortical lobes, but only temporal lobe white matter volume remained significantly enlarged in comparison to controls, after controlling for TBV. This same pattern of generalized volume enlargement in the ASD group for the cerebrum and cortical lobes was also seen in the TYP and DD subgroup comparisons (see Table 4).
Table 3.
Adjusted means* for brain volumes and group comparisons for ASD versus Controls
Table 3 presents the estimated adjusted means for total and lobar brain volumes at 3.5 years of age (the approximate mean age for our sample). Comparisons for brain volumes with enlargement (e.g., cerebrum, cortical lobes) that remained significantly enlarged after controlling for TBV are indicated with a +.
| Volume | ASD | Controls | Comparisons (ASD vs Controls)* | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Diff | SE | p | % | ||
| Total Brain Volume | 1310.4 | 13.0 | 1238.8 | 18.0 | 71.6 | 22.7 | .002 | 6% | |
| Total Tissue Volume | 1181.8 | 11.7 | 1120.1 | 16.2 | 61.7 | 20.4 | .003 | 6% | |
| Total Gray Volume | 849.2 | 8.4 | 807.2 | 11.7 | 42.1 | 14.6 | .005 | 5% | |
| Total White Volume | 332.6 | 3.6 | 312.9 | 5.0 | 19.6 | 6.3 | .002 | 6% | |
| Cerebral Spinal Fluid | 128.6 | 2.5 | 118.7 | 3.4 | 9.9 | 4.4 | .028 | 8% | |
| Cerebellum | 117.9 | 1.3 | 118.1 | 1.8 | −0.2 | 2.2 | .929 | 0% | |
| White | 18.5 | 0.3 | 19.1 | 0.4 | −0.7 | 0.5 | .174 | −3% | |
| Gray | 99.4 | 1.1 | 99.0 | 1.5 | 0.5 | 1.9 | .812 | 0% | |
| Cerebral Cortex | 986.0 | 8.6 | 908.3 | 11.9 | 77.7 | 15.1 | <.000 | 9% | |
| White | 280.9 | 2.9 | 253.3 | 3.9 | 27.6 | 5.2 | <.000 | 11% | |
| Gray | 705.1 | 6.0 | 655.0 | 8.3 | 50.1 | 10.3 | <.000 | 8% | |
| Parietal-Occipital | 369.2 | 3.6 | 347.2 | 5.0 | 21.9 | 6.7 | .001 | 6% | |
| White | 107.7 | 1.2 | 99.1 | 1.6 | 8.6 | 2.1 | <.000 | 9% | |
| Gray | 261.5 | 2.6 | 248.1 | 3.5 | 13.3 | 4.7 | .005 | 5% | |
| Temporal | 207.9 | 2.1 | 190.3 | 2.9 | 17.6 | 3.9 | <.000 | 9% + | |
| White | 44.6 | 0.6 | 39.8 | 0.8 | 4.8 | 1.0 | <.000 | 12% + | |
| Gray | 163.3 | 1.7 | 150.6 | 2.2 | 12.8 | 3.1 | <.000 | 8% | |
| Frontal | 377.5 | 4.0 | 347.5 | 5.4 | 30.0 | 7.3 | <.000 | 9% | |
| White | 114.9 | 1.4 | 104.1 | 1.9 | 10.8 | 2.6 | <.000 | 10% | |
| Gray | 262.6 | 2.7 | 243.4 | 3.6 | 19.2 | 4.9 | <.000 | 8% | |
controlling for age, gender, and IQ
Table 4.
Adjusted means* for brain volumes for subgroup comparisons
Table 4 presents the estimated adjusted means for total and lobar brain volumes at 3.5 years of age (the approximate mean age for our sample) for subjects in the ASD group compared to TYP and DD subjects. Comparisons for brain volumes with enlargement (e.g., cerebrum, cortical lobes) that remained significantly enlarged after controlling for TBV are indicated with a +.
| Volume | TYP | DD | Comparisons (ASD vs TYP)* |
Comparisons (ASD vs DD)* |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Diff | SE | p | Diff | SE | p | ||
| Total Brain Volume | 1258.0 | 21.2 | 1219.6 | 29.4 | 52.4 | 25.7 | .044 | 90.8 | 32.1 | .006 | |
| Total Tissue Volume | 1136.7 | 19.1 | 1103.5 | 26.5 | 45.1 | 23.1 | .053 | 78.3 | 28.9 | .008 | |
| Total Gray Volume | 816.6 | 13.8 | 797.7 | 19.0 | 32.6 | 16.7 | .053 | 51.5 | 20.7 | .015 | |
| Total White Volume | 320.1 | 5.9 | 305.8 | 8.1 | 12.5 | 7.2 | .086 | 26.8 | 8.8 | .003 | |
| Cerebral Spinal Fluid | 121.3 | 4.5 | 116.0 | 5.4 | 7.2 | 5.6 | .195 | 12.6 | 5.9 | .036 | |
| Cerebellum | 120.6 | 2.1 | 115.7 | 2.9 | −2.7 | 2.6 | .299 | 2.3 | 3.2 | .477 | |
| White | 19.4 | 0.5 | 18.9 | 0.5 | −0.9 | 0.6 | .152 | −0.4 | 0.6 | .492 | |
| Gray | 101.2 | 1.8 | 96.7 | 2.5 | −1.8 | 2.1 | .406 | 2.7 | 2.7 | .329 | |
| Cerebral Cortex | 919.9 | 14.8 | 896.7 | 19.0 | 66.1 | 17.7 | <.000+ | 89.3 | 20.8 | <.000 | |
| White | 256.3 | 5.2 | 250.3 | 6.1 | 24.6 | 6.5 | <.000 | 30.6 | 6.7 | <.000 | |
| Gray | 663.6 | 10.0 | 646.4 | 13.3 | 41.5 | 11.8 | <.001 | 58.7 | 14.6 | <.000 | |
| Parietal-Occipital | 350.9 | 6.9 | 343.6 | 7.6 | 18.3 | 8.6 | .035 | 25.6 | 8.4 | .003 | |
| White | 100.3 | 2.2 | 97.9 | 2.4 | 7.4 | 2.8 | .010 | 9.8 | 2.6 | <.000 | |
| Gray | 250.5 | 4.8 | 245.7 | 5.4 | 10.9 | 6.0 | .072 | 15.8 | 6.0 | .009 | |
| Temporal | 193.8 | 4.2 | 186.8 | 4.3 | 14.1 | 5.3 | .008 | 21.1 | 4.7 | <.000 | |
| White | 39.8 | 1.1 | 39.8 | 1.1 | 4.8 | 1.4 | <.001+ | 4.8 | 1.3 | <.000 | |
| Gray | 154.0 | 3.3 | 147.1 | 3.3 | 9.3 | 4.1 | .025 | 16.3 | 3.7 | <.000 | |
| Frontal | 354.0 | 7.7 | 341.0 | 8.2 | 23.5 | 9.6 | .016 | 36.5 | 9.0 | <.000 | |
| White | 105.7 | 2.7 | 102.6 | 2.9 | 9.2 | 3.5 | .009 | 12.3 | 3.2 | <.000 | |
| Gray | 248.3 | 5.1 | 238.4 | 5.5 | 14.3 | 6.4 | .027 | 24.2 | 6.1 | <.000 | |
controlling for age, gender, and IQ
Differences for all regions and tissues remained significant after removal of the subset of ASD subjects (N=7) who met criteria for autistic disorder at time 1 and pervasive developmental disorder not otherwise specified (PDDNOS) (but not autistic disorder) at time 2. Findings also remained the same after the removal of two controls observed to have the smallest TBV in Figure 1.
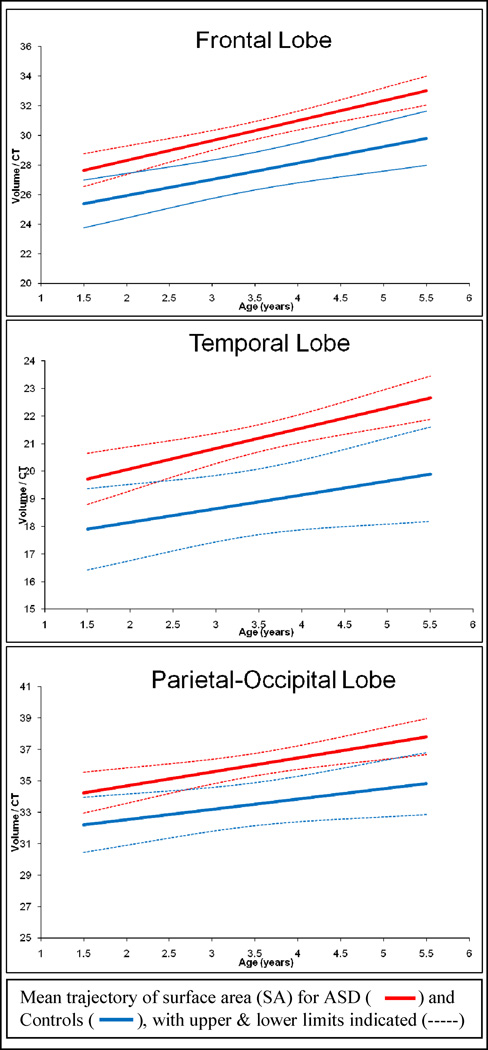
Average regional cortical thickness was measured in the frontal, temporal, and parietal-occipital lobe regions. Group raw means for CT by lobe region are provided in Table 5. We examined regional estimates of cortical thickness summarized over the cortical lobes. There was no significant interaction between group, age and regional brain volume. We observed no group differences in cortical thickness for any of the lobar regions measured (frontal: t = 0.1, p = 0.92; temporal: t =−0.33, p = 0.74; parietal-occipital: t = −0.05, p = 0.96). We then created an estimate of surface area using a ratio term (SA=regional CV/ regional CT) to examine whether there were differences in cortical thickness after adjusting for cortical volume. For this comparison, we found significantly increased estimates of SA in the ASD group compared to controls for all three cortical regions measured (frontal: t = 3.79, p <.000; temporal: t = 3.49, p <.001; and parietal-occipital: t = 3.18, p = .002). In summary, we find no differences in cortical thickness, but increased estimates of SA in children with ASD compared to controls. Trajectories of change over time in our estimate of surface area (CV/CT) appear in Figure 3.
Table 5.
Raw Means for Regional Cortical Thickness for ASD and Controls
| Group | Region | Side Left/Right |
Time 1 Mean /SD |
Time 2 Mean /SD |
||
|---|---|---|---|---|---|---|
| ASD | N = 54 | N = 35 | ||||
| Frontal | Left | 4.36 | 0.15 | 4.32 | 0.15 | |
| Right | 4.45 | 0.20 | 4.38 | 0.14 | ||
| Temporal | Left | 3.92 | 0.21 | 3.86 | 0.21 | |
| Right | 3.93 | 0.17 | 3.90 | 0.17 | ||
| Parietal-Occipital | Left | 3.68 | 0.24 | 3.64 | 0.25 | |
| Right | 3.57 | 0.19 | 3.60 | 0.15 | ||
| Controls | N = 36 | N = 18 | ||||
| Frontal | Left | 4.36 | 0.16 | 4.32 | 0.15 | |
| Right | 4.41 | 0.20 | 4.34 | 0.14 | ||
| Temporal | Left | 3.92 | 0.18 | 3.91 | 0.24 | |
| Right | 3.90 | 0.18 | 3.95 | 0.19 | ||
| Parietal-Occipital | Left | 3.62 | 0.15 | 3.65 | 0.17 | |
| Right | 3.57 | 0.15 | 3.58 | 0.19 | ||
Figure 3. Surface Area* Trajectory for ASD vs. Controls.
Panels show the mean group trajectories with confidence bands for an *estimate of surface area (SA = regional cortical volume (CV)/regional cortical thickness (CT)) in frontal, temporal, and parietal-occipital lobes. The measure of CV in this case refers to gray matter volume only. Groups shown are ASD (red dashed line) and controls (CON) (blue solid line) with lower (LL) and upper (UL) confidence bands.
Discussion
In this longitudinal MRI study of very early brain volume development in individuals with ASD we observed generalized cerebral cortical enlargement in children with ASD at both 2 and 4 – 5 years of age. Rate of cerebral cortical growth across multiple brain regions and tissue compartments, in children with ASD, parallels that seen in controls, indicating that there is no increased rate of cerebral cortical growth during this age interval. Our findings provide evidence that increased brain volume at age 2, largely due to increased cerebral cortical volume, results from an increased rate of brain growth occurring prior to two years of age. Together with previously reported findings from a longitudinal study of head circumference (11), and a recent longitudinal MRI study of early brain volume development (12), these data provide further evidence that brain overgrowth in autism occurs in the early post-natal period, before 2 years of age. In the cross-sectional analysis of two year olds (11), we previously reported that children with autism had significantly enlarged gray and white matter volumes compared to the DD subgroup, but only white matter volumes were enlarged compared to the TYP subgroup. The longitudinal analyses reveal increased white and gray matter volume in autism versus both the TYP and DD control subgroups. Given the fact that no differences in our data are seen between autistic and control subjects at either age point in this longitudinal study, we feel confident in our conclusion that volume increases are evident in this sample of autistic subjects. Of course, the small size of the control subgroups compels us to be most certain about our findings with respect to the total sample of controls.
The findings from the present study point to increased cerebral cortical surface area and not increased cortical thickness as the underlying factor in the increased cerebral cortical gray matter volume observed in very young children with ASD. Emerging literature on cortical maturation in older males with ASD has found evidence for decreased cortical thickness in adolescence (25, 26, 27), so it may be that a period of cortical thinning occurs in ASD after childhood. It is unclear at this point whether increased white matter results in enlarged gray matter and/or SA, or if instead a common etiology causes both increased white matter and SA. As we have learned in our study of the MAOA gene (28), where we find MAOA effects on both white and gray matter volumes, but not with the serotonin transporter, the biological mechanisms underlying cortical growth are complex. . Increased surface area results from an increase in the number and/or size of cerebral cortical gyri. Several studies suggest that such gyral abnormalities may be present in individuals with ASD. Nordahl et al. (29) observed ‘cortical folding abnormalities’ in autism, while Lenroot et al. (personal communication) has reported an increase in surface area in 4–5 year olds with ASD. Kates et al. (30) noted abnormal ‘gyrification’ in monozygotic twins discordant for autism. And, Raznahan et al. (31) reported that adults with ASD differ from controls in the relationship between a key genotype for determining regional cortical volume (Brain Derived Neurotrophic Factor, or BDNF val66met) and cortical volume and surface area (but not cortical thickness). Petropoulos et al. (32) reported prolonged T2 relaxation for cortical gray matter in a large sample of 2–4 year olds with ASD compared to typically-developing controls. Our findings and the observation by Petropoulos et al. both suggest that abnormal early development of gray matter is associated with ASD.
Human studies have suggested several candidate genes that may play a role in the increased cerebral cortical volume in ASD (28,33). The likely importance of epistasis in brain overgrowth in ASD is underscored by a mouse study of deletions in the serotonin transporter and PTEN genes showing an interactive effect, increasing both brain volume and autistic-like behaviors in mice (34). Family studies have revealed that both cortical surface area and cortical thickness are highly heritable but unrelated genetically, suggesting distinct genetic architecture underlying these phenomena (35). The finding of surface area but not cortical thickness differences provides a narrower phenotypic target for future studies exploring the genetic basis of autism; as distinct neurobiological mechanisms are thought to underlie these two determinants of cortical volume (36,37).
Surface area is thought to be determined by division of progenitor cells in the embryological periventricular area (with increased progenitor cells occurring in association with increased cortical surface area); whereas cortical thickness is thought to reflect variation in dendritic development (arborization and pruning) in gray matter (38,39) or myelination (40). Molecular studies in mice have demonstrated the role of β-catenin in regulating cerebral cortical size (and resultant increases in cortical surface area but not thickness) by controlling the generation of neural precurors (37). Glycogen Synthetase Kinase-3 (GSK) was recently shown to cause massive hyper-proliferaton of neural progenitor cells in mice resulting in large brains with increased convolutions. GSK interacts with the Phosphatidyl Inositol-3 (PI3) kinase pathway, implicated in several neurodevelopmental disorders (e.g., Fragile X Syndrome and tuberous sclerosis) that are characterized by having autistic behavior (41,42). GSK also interacts with the receptor tyrosine kinase (RTK) signaling system, which has been linked to idiopathic autism (43). These various pathways for brain overgrowth clearly point to areas that need further study in autism.
We previously reported retrospective head circumference data on a large sample of children with ASD compared to local controls from birth to age 3 years that suggested increased head size in ASD has its onset around 12 months of age (11). We hypothesized that this increased head size was the result of increased brain size and that brain overgrowth had its onset in the latter part of the first year of life. Longitudinal behavioral studies of infants at high genetic risk for ASD, who are later diagnosed with ASD at 36 months, report no difference in social behavior at 6 months of age in comparison to controls, whereas marked deficits in reciprocal social interaction are observed by 12−14 months of age (13, 44). These behavioral studies suggest that the onset of autistic behavior has its origins in the latter part of the first year of life. The temporal relationship between the onset of both autistic behavior and brain overgrowth at the end of the first year of life suggests a relationship between these two phenomena; and specifically that increased rate of brain growth may be linked to the onset of autistic symptoms.
It is possible that brain overgrowth directly results in the development of autistic behavior, perhaps through a physical disruption of neural circuitry. An alternative hypothesis is that brain overgrowth is a secondary response to a more proximal event that affects downstream remodeling of neuronal processes. Disruption in experience-dependent cortical refinements caused by impaired synaptic plasticity has been reported in a mouse model of Angelman Syndrome, a disorder thought to be associated with autistic behavior (45). Similarly, disruptions in normal synaptic plasticity and experience-dependent neuronal development have been observed in a mouse model of Fragile X syndrome (46), a disorder also associated with autism. Consistent with the idea that autism is linked to impaired experience-dependent cortical development, a recent study has observed a high number of diverse mutations known to cause defective expression of activity-driven genes, in a sample of autistic individuals (47). Alterations in synapse development have also been proposed as a common mechanism in a number of neurodevelopmental disorders, including autism (48).
A potential limitation of this study stems from our inability to measure surface area directly in very young children. As such, we were only able to obtain regional estimates of cortical thickness and an estimate of surface area, and the surface area findings should therefore be considered preliminary While mean cortical thickness in each lobar region is not necessarily indicative of uniformity of cortical thickness throughout the cerebral cortical lobes (there exists normal variation in cortical thickness, known to be increased for example in heteromodal association areas (49)), the convergence of cortical thickness findings across the three cortical regions measured supports the validity of our findings. Software to enable local cortical thickness and surface area measurement in the developing pediatric brain is currently under development in our lab, and will be an important future step in characterizing early brain volume changes in individuals with ASD. An additional potential limitation of our study was the use of sedation with some participants (ASD, DD) and not others (TYP). However, we have no reason to believe that sedation at the time of the scan had any significant effect on cortical volume as there is no evidence in the literature to suggest a state effect that would confound our results.
Studies currently underway by our group (http://www.ibis-network.org/) are prospectively (at 6, 12 and 24 months) examining MRI/DTI brain and behavior development in infants at high risk for ASD, further characterizing the timing of brain-behavior changes in this disorder. Given the findings in other brain disorders (e.g., Parkinson’s, Alzheimer’s, and Huntington’s Disease), where brain changes are well known to precede the cognitive and/or behavioral manifestation of symptoms (50–52), observations from the present study support future research aimed at identifying early (under 2 years of age) brain markers that may increase prediction of ASD risk (e.g., maturational differences in selected DTI fiber tracts in infants with high genetic risk for ASD). Future studies should continue the strategy of longitudinal imaging to more definitively characterize the pattern of brain changes as individuals with ASD age across the lifespan.
Acknowledgements
We express our appreciation for the assistance we received from the following: the TEACCH centers, the NDRC Autism Subject Registry, and NC Children's Developmental Services Agencies for assisting with recruitment; Matthieu Jomier, Paul Yushkevich for image processing support, research team members Nancy Garrett, Michael Graves for their years of hard work on this project; Ben Philpot, Ralph Adolphs, John Rubenstein, Alan Evans and William Snider for comments on the manuscript; and, the families who have participated in this study. This work was supported by NIH grants MH61696, MH64580 and P30 HD03110 to J. Piven and PHS-NIH EB005149-04 (PI: Kikinis) National Alliance for Medical Image Computing (NA-MIC).
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 2.Piven J, Nehme E, Simon J, Barta P, Pearlson G, Folstein SE. Magnetic resonance imaging in autism: Measurement of the cerebellum, pons, and fourth ventricle. Biol. Psychiatry. 1992;31:491–504. doi: 10.1016/0006-3223(92)90260-7. [DOI] [PubMed] [Google Scholar]
- 3.Piven J, Ardnt S, Bailey J, Andreasen N. Regional brain enlargement in autism: A magnetic resonance imaging study. J. Am. Acad. Child Adol. Psychiatry. 1996;35:530–536. doi: 10.1097/00004583-199604000-00020. [DOI] [PubMed] [Google Scholar]
- 4.Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, Chisum HJ, Moses P, Pierce K, Lord C, Lincoln AJ, Pizzo S, Schreibman L, Haas RH, Akshoomoff NA, Courchesne RY. Unusual brain growth patterns in early life in patients with autistic disorder: An MRI study. Neurology. 2001;57:245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- 5.Sparks BF, Friedman SD, Shaw DW, Aylward EH, Echelard D, Artru AA, Maravilla KR, Giedd JN, Munson J, Dawson G, Dager SR. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59(2):184–192. doi: 10.1212/wnl.59.2.184. [DOI] [PubMed] [Google Scholar]
- 6.Bailey A, Le Couteur A, Gottesman I, Bolton P, Simonoff E, Yuzda E, Rutter M. Autism as a strongly genetic disorder: Evidence from a British twin study. Psychol Med. 1995;25:63–77. doi: 10.1017/s0033291700028099. [DOI] [PubMed] [Google Scholar]
- 7.Lainhart JE, Piven J, Wzorek M, Landa R, Santegelo SL, Coon H, Folstein SE. Macrocephaly in children and adults with autism. J Am Academy Child Adol Psychiatry. 1997;36:282–289. doi: 10.1097/00004583-199702000-00019. [DOI] [PubMed] [Google Scholar]
- 8.Stevenson R, Schroer R, Skinner C, Fender D, Simensen R. Autism and macrocephaly. Lancet. 1997;349:1744–1745. doi: 10.1016/S0140-6736(05)62956-X. [DOI] [PubMed] [Google Scholar]
- 9.Bailey A, Luthert P, Bolton P, LeCouteur A, Rutter M. Autism and megalencephaly. Lancet. 1993;34:1225–1226. doi: 10.1016/0140-6736(93)91065-t. [DOI] [PubMed] [Google Scholar]
- 10.Bauman M, Kemper T. Histoanatomic observations of the brain in early infantile autism. Neurology. 1985;35:866–874. doi: 10.1212/wnl.35.6.866. [DOI] [PubMed] [Google Scholar]
- 11.Hazlett HC, Poe M, Gerig G, Smith RG, Provenzale J, Ross A, Gilmore J, Piven J. Magnetic resonance imaging and head circumference study of brain size in autism. Arch Gen Psychiatry. 2005;62:1366–1376. doi: 10.1001/archpsyc.62.12.1366. [DOI] [PubMed] [Google Scholar]
- 12.Schumann CM, Bloss CS, Barnes CC, Wideman GM, Carper RA, Akschoomoff N, Pierce K, Hagler D, Schork N, Lord C, Courchesne E. Longitudinal magnetic resonance imaging study of cortical development through early childhood in autism. J Neurosci. 2010;30(12):4419–4427. doi: 10.1523/JNEUROSCI.5714-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. Int J Devl Neuroscience. 2005;23:143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 15.Fombonne E. Epidemiological surveys of autism and other pervasive developmental disorders: An update. J. Aut. Dev. Disord. 2003;33(4):365–382. doi: 10.1023/a:1025054610557. [DOI] [PubMed] [Google Scholar]
- 16.Lord C, Rutter M, LeCouteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 17.DiLavore PC, Lord C, Rutter M. The pre-linguistic autism diagnostic observation schedule. J. Autism Dev. Disord. 1995;25(4):355–379. doi: 10.1007/BF02179373. [DOI] [PubMed] [Google Scholar]
- 18.Mullen EM. Mullen Scales of Early Learning AGS Edition. USA: American Guidance Service, Inc.; 1995. [Google Scholar]
- 19.Sparrow SS, Balla DA, Cicche HV. Vineland Adaptive Behavior Scales-Interview Edition Survey Form Manual. Circle Pines: American Guidance Service, Inc; 1984. [Google Scholar]
- 20.Zimmerman IL, Steiner VG, Pond RE. Preschool Language Scale-Fourth Edition (PLS-IV) Psychological Corporation; 2002. [Google Scholar]
- 21.Elliott CD. Differential abilities scales: Introductory and technical handbook. New York: The Psychological Corporation; 1990. [Google Scholar]
- 22.Mesibov GB, Schopler E, Schaffer B, Michal N. Use of the childhood autism rating scale with autistic adolescents and adults. J. Am. Acad. Child Adol. Psychiatry. 1989;28(4):538–541. doi: 10.1097/00004583-198907000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Van Leemput K, Maes F, Vandermeulen D, Suetens P. Automated model-based bias field correction of MR images of the brain. IEEE Trans. Med. Imaging. 1999;18:885–896. doi: 10.1109/42.811268. [DOI] [PubMed] [Google Scholar]
- 24.Park J, Gerig G, Chakos M, Vandermeulen D, Lieberman JA. Neuroimaging of psychiatry disease: Reliable and efficient automatic brain tissue segmentation for increased sensitivity. Schizophr. Res. 2001;49(1–2):163. [Google Scholar]
- 25.Wallace GL, Dankner N, Kenworthy L, Giedd JN, Martin A. Age-related temporal and parietal cortical thinning in autism spectrum disorders. Brain. 2010;133 doi: 10.1093/brain/awq279. 37-45-3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raznahan A, Toro R, Daly E, Robertson D, Murphy C, Deeley Q, Bolton PF, Paus T, Murphy DGM. Cortical anatomy in autism spectrum disorder: An in vivo MRI study on the effect of age. Cerebral Cortex. 2010;20:1332–1340. doi: 10.1093/cercor/bhp198. [DOI] [PubMed] [Google Scholar]
- 27.Hardan AY, Libove RA, Keshavan MS, Melhem NM, Minshew NJ. A preliminary longitudinal magnetic resonance imaging study of brain volume and cortical thickness in autism. Biol Psychiatry. 2009;66:320–326. doi: 10.1016/j.biopsych.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis LK, Hazlett HC, Librant AL, Nopoulos P, Sheffield VC, Piven J, Wassink TH. Cortical enlargement in autism is associated with a functional VNTR in the monamine oxidase A gene. Am J Med Genet: Neuropsychiatr Genet. 2008;147B(7):1145–1151. doi: 10.1002/ajmg.b.30738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nordahl CW, Dierkler D, Mostafavi I, Schumann CM, Rivera SM, Amaral DG, Van Essen DC. Cortical folding abnormalities in autism revealed by surface-based morphometry. J Neuroscience. 2007;27(43):11725–11735. doi: 10.1523/JNEUROSCI.0777-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kates WR, Ikuta I, Burnett CP. Gyrification patterns in monozygotic twin pairs varying in discordance for autism. Autism Res. 2009;2(5):267–278. doi: 10.1002/aur.98. [DOI] [PubMed] [Google Scholar]
- 31.Raznahan A, Toro R, Priotsi P, Powell J, Paus T, Bolton PF, Murphy DG. A functional polymorphism of the brain derived neurotrophic factor gene and cortical anatomy of autism spectrum disorder. J Neurodevelopmental Disorders. 2009;1(3):215–223. doi: 10.1007/s11689-009-9012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petroupolus H, Friedman SD, Shaw DWW, Artru AA, Dawson G, Dager SR. Gray matter abnormalities in autism spectrum disorder revealed by T2 relaxation. Neurology. 2006;67:632–636. doi: 10.1212/01.wnl.0000229923.08213.1e. [DOI] [PubMed] [Google Scholar]
- 33.Wassink TH, Hazlett HC, Epping EA, Arndt S, Dager SR, Schellenberg GD, Dawson G, Piven J. Cerebral cortical gray matter overgrowth and functional variation of the serotonin transporter gene in autism. Arch Gen Psychiatry. 2007;64(6):709–717. doi: 10.1001/archpsyc.64.6.709. [DOI] [PubMed] [Google Scholar]
- 34.Page DT, Kuti OJ, Prestia C, Sur M. Haploinsufficiency for Pten and serotonin transporter cooperatively influences brain size and social behavior. Proc Natl Acad Sci USA. 2009;106(6):1989–1994. doi: 10.1073/pnas.0804428106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winkler AM, Kochunov P, Blangero J, Almasy L, Zilles K, Fox PT, Duggirala R, Glahn DC. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. NeuroImage (Epub) 2009 doi: 10.1016/j.neuroimage.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rubenstein JL, Rakic P. Genetic control of cortical development. Cereb Cortex. 1999;9(6):521–523. doi: 10.1093/cercor/9.6.521. [DOI] [PubMed] [Google Scholar]
- 37.Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–369. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- 38.Huttenlocher PR, de Courten C. The development of synapses in striate in the cortex of man. Hum Neurobiol. 1987;6(1):1–9. [PubMed] [Google Scholar]
- 39.Huttenlocher PR. Morphometric study of human cerebral cortex development. Neuropsychologia. 1990;28(6):517–527. doi: 10.1016/0028-3932(90)90031-i. [DOI] [PubMed] [Google Scholar]
- 40.Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24(38):8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu H, Qin Y, Bochorishvili G, Zhu Y, van Aelst L, Zhu JJ. Ras signaling mechanisms underlying impaired GluR1-dependent plasticity associated with fragile X syndrome. J Neurosci. 2008;28(31):7847–7862. doi: 10.1523/JNEUROSCI.1496-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silva AJ, Ehninger D. Adult reversal of cognitive phenotypes in neurodevelopmental disorders. J Neurodevelop Disord. 2009;1:150–157. doi: 10.1007/s11689-009-9018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Campbell DB, Sutcliffe JS, Ebert PJ, Militerni R, Bravaccio C, Trillo S, Elia M, Schneider C, Melmed R, Sacco R, Persico AM, Levitt P. A genetic variant that disrupts MET transcription is associated with autism. Proc Natl Acad Sci USA. 2006;103(45):16834–16839. doi: 10.1073/pnas.0605296103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Landa R, Garrett-Mayer E. Development in infants with autism spectrum disorders: a prospective study. J Child Psychol Psychiatry. 2006;47(6):629–638. doi: 10.1111/j.1469-7610.2006.01531.x. [DOI] [PubMed] [Google Scholar]
- 45.Yashiro K, Riday TT, Condon KH, Roberts AC, Bernardo DR, Prakash R, Weinberg RJ, Ehlers MD, Philpot BD. Ube3a is required for experience-dependent maturation of the neocortex. Nat Neuroscience. 2009;12(6):777–783. doi: 10.1038/nn.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dolen G, Osterweil E, Rao BS, Smith GB, Auerbach BD, Chattarji S, Bear MF. Correction of fragile X syndrome in mice. Neuron. 2007;56(6):955–962. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morrow EM, Yoo SY, Flavell SW, Kim TK, Lin Y, Hill RD, Mukaddes NM, Balkhy S, Glascon G, Hashmi A, Al-Saad S, Ware J, Joseph RM, Greenblatt R, Gleason D, Ertelt JA, Apse KA, Bodell A, Partlow JN, Barry B, Yao H, Markianos K, Ferland RJ, Greenberg ME, Walsh CA. Identifying autism loci and genes by tracing recent shared ancestry. Science. 2008;321(5886):218–223. doi: 10.1126/science.1157657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zogbi HY. Postnatal neurodevelopmental disorders: meeting at the synapse? Science. 2003;302(5646):826–830. doi: 10.1126/science.1089071. [DOI] [PubMed] [Google Scholar]
- 49.Mesulam M. From sensation to cognition. Brain. 1998;121:1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- 50.Rodriguez-Oroz MC, Jahanshahi M, Krack P, Litvan I, Macias R, Bezard E, Obeso JA. Initial clinical manifestations of Parkinson’s disease: features and pathophysiological mechanisms. Lancet Neurol. 2009;8(12):1128–1139. doi: 10.1016/S1474-4422(09)70293-5. [DOI] [PubMed] [Google Scholar]
- 51.Mistur R, Mosconi L, Santi SD, Guzman M, Li Y, Tsui W, de Leon MJ. Current challenges for the early detection of Alzheimer’s disease: Brain imaging and CSF studies. J Clin Neurol. 2009;5(4):153–166. doi: 10.3988/jcn.2009.5.4.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tabrizi SJ, Langbehn DR, Leavitt BR, Roos RA, Durr A, Craufurd D, Kennard C, Hicks SL, Fox NC, Scahill RI, Borowsky B, Tobin AJ, Rosas HD, Johnson H, Reilmann R, Landwehrmeyer B, Stout JC. TRACK-HD investigators. Biological and clinical manifestations of Huntington’s disease in the longitudinal TRACK-HD study: cross-sectional analysis of baseline data. Lancet Neurol. 2009;8(9):791–801. doi: 10.1016/S1474-4422(09)70170-X. [DOI] [PMC free article] [PubMed] [Google Scholar]