Abstract
Although the thalamus and/or mammillary bodies are the primary sites of neuropathology in cases of diencephalic amnesia such as Wernicke Korsakoff Syndrome (WKS), there is also functional deactivation of certain cortical regions that contribute to the cognitive dysfunction. Acetylcholine (ACh) is a key neurotransmitter that modulates neural processing within the cortex and between the thalamus and cortex. In the pyrithiamine-induced thiamine deficiency (PTD) rat model of WKS there are significant reductions in cholinergic innervation and behaviorally-stimulated ACh efflux in the frontal (FC) and retrosplenial (RSC) cortices. In the present study, ACh released levels were site-specifically amplified with physostigmine (0.5 μg, 1.0 μg) in the FC and the RSC, which was confirmed with in vivo microdialysis. Although physostigmine sustained high ACh levels in both cortical regions, the effects on spatial memory, assessed by spontaneous alternation, were different as a function of region (FC, RSC) and treatment (PTD, pair-fed [PF]). Higher ACh levels in the FC recovered the rate of alternation in PTD rats as well as reduced arm-re-entry preservative errors. However, higher ACh levels within the FC of PF rats exacerbated arm-re-entry preservative errors without significantly affecting alternation rates. Maintaining high ACh levels in the RSC had no procognitive effects in PTD rats, but rather impaired alternation behavior in PF rats. These results demonstrate that diverse cortical regions respond differently to intensified ACh levels—and the effects are dependent on thalamic pathology. Thus, pharmacotherapeutics aimed at enhancing cognitive functions must account for the unique features of cortical ACh stimulation and the connective circuitry with the thalamus.
Keywords: Acetylcholine, Frontal cortex, Retrosplenial cortex, Spontaneous alternation, Rat
Introduction
The amnestic condition Wernicke-Korsakoff Syndrome (WKS) is produced by the neurotoxic events that follow thiamine deficiency ([TD] Langlais, 1995; Kopelman, Thomson, Guerrini & Marshall, 2009). WKS can be modeled in rodents by the prescription of a TD diet and injections of pyrithiamine (PTD), an inhibitor of thiamine pyrophosokinase. The critical neuropathology associated with cognitive dysfunction in WKS and PTD is neuronal loss in the anterior and midline thalamus as well as the mammillary bodies (Mair, 1994, Sullivan & Pfferbaum, 2009; Vetreno, Hall & Savage, 2011). However, cortical atrophy within the frontal (FC) and retrosplenial (RSC) regions is commonly associated with WKS and PTD and such neuropathology has been suggested to further impair cognitive functioning (Langlais & Savage, 1995: Pitkin & Savage, 2001; Reed et al 2003). It has been suggested that cortical degeneration and shrinkage is the result thalamic input loss to these regions as well as the direct demyelination that occurs from altered lipid synthesis accompanying TD (Langlais & Zhang, 1997).
Furthermore, cholinergic fiber innervation of the hippocampus, FC and RSC is reduced after PTD treatment and is also accompanied by reductions in behaviorally-stimulated ACh release within those regions (Anzalone, Vetreno, Ramos & Savage, 2010). It should be noted that the cholinergic deficits in ACh efflux seen in PTD rats are only seen when the system is taxed by behavioral demands, and are not a function of basal differences in ACh levels (Vetreno et al 2011). This “functional lesion” of the hippocampus and associated limbic cortices is a result from the reduction of excitatory input from the damaged diencephalic regions and decreased cholinergic innervation from the basal forebrain. It is proposed that the loss of diencephalic input results in a “disconnection syndrome” within the extended hippocampal system that includes the FC and RSC (Aggleton, 2008; Caulo et al, 2004; Jenkins, Dias, Amin, Brown & Aggleton, 2002; Vann & Albasser, 2009).
Wernicke-Korsakoff syndrome presents clinically as retrograde amnesia with antereograde declarative or explicit-type memory loss (Gold & Squire, 2006; Kopelman, Thompson Guerrini & Marshall, 2009; Mair, 1994). However, there are also behavioral deficits such as perseveration and working memory loss suggestive of frontal-cortical dysfunction (Oscar-Berman, Kirkley, Gansler, & Couture, 2004). Previous research has shown that in the PTD model there is a loss of tissue in the premotor area (M2) of the FC and this cortical region along with the prefrontal cortex display the greatest deficits in behaviorally-activated ACh efflux (Anzalone et al 2010; Vetreno et al, 2011). Human imaging studies as well as electro-physiological, gene expression, and neurochemical data from animal models demonstrate that discrete diencephalic damage has profound effects on the functioning of both the FC and RSC (Aggleton, 2008; Caulo et al, 2003; Jenkins et al, 2002; Vann & Albasser, 2009). Thus, WKS and the PTD model display a breakdown of the functional integration within several regions of the limbic system (thalamus, hippocampus, cortex).
Increasing cholinergic tone within the extended hippocampal circuit can lead to improvements in cognitive functioning in disorders of memory functioning even though cholinergic cell loss cannot fully account for the deficit (Chang & Gold, 2004; Roland, Mark, Vetreno & Savage, 2008; Roland & Savage, 2009; Roland, Levinson, Vetreno & Savage, 2010). Previous studies have shown that maintaining high hippocampal ACh levels recovers cognitive performance in PTD rats (Roland et al, 2008; Roland & Savage, 2009; Roland et al, 2010). The current set of experiments are designed to determine whether cholinergic stimulation of the FC and RSC, through the use of an acetylcholinesterase inhibitor (AChEI), would equally restore cognitive function in the PTD model. Such results suggest that regions that are “functionally” (septum, hippocampus, cortex) rather than “structurally” (thalamus, mammillary bodies) lesioned can be critical targets for neurochemical modulation to recover cognitive performance.
Methods
Subjects
Male Sprague–Dawley rats, initially 3 months old, were obtained from Harlan Corporation (Indianapolis, IN) and allowed 2-weeks to acclimate. Prior to surgery the rats were housed in pairs, but after surgery rats were individually housed in a colony room (temperature: 22 ± 1 °C; humidity: 50 ± 10%) with a 12- hour/ 12-hour light-dark cycle (onset at 7:00 am). Every effort was made to minimize animal suffering and the number of animals used. All experiments were conducted according to the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1996) and the APA ethical standard for treatment of animals. All experimental procedures were approved by the Binghamton University Committee on Animal Care and Use (IACUC).
PTD treatment
After a 2-week acclimation period, rats were randomly assigned to one of the following treatments: (i) pair-fed (PF, n =20), or (ii) pyrithiamine-induced thiamine deficiency (PTD, n = 20). Subjects in the PTD treatment group were free-fed thiamine-deficient chow (Teklad Diets, Madison, WI) and received daily injections (0.25 mg/kg, i.p.) of pyrithiamine hydrobromide (Sigma-Aldrich, St. Louis, MO). On days 15–17 of treatment, animals developed signs of local tonoclonic movement of the front and hind limbs, and generalized convulsions (seizures). Some studies have implicated glutaminergic excitotoxicity in the formation of PTD-induced seizure-activity (Olney, 1985), but several thalamic nuclei are damaged prior to seizure activity (Zhang et al, 1995). Within 4 hours and 15 minutes of seizure onset, PTD treated animals were given an injection of thiamine hydrochloride (100 mg/kg, i.p., Sigma-Aldrich). The PF animals were fed an amount of thiamine-deficient chow equivalent to the average amount consumed by the PTD group but received daily injections of thiamine (0.4 mg/kg, i.p.) to ensure that the PF animals maintained normal levels of thiamine. Following treatment, all subjects were placed on regular Lab Diet (Richmond, VA) rat chow and allowed 14 days to regain the weight lost during treatment.
Cannula Surgery
Two-weeks after PTD/PF treatment, each subject was prepared for implantation of a total of two cortical cannulae in the brain: one cannula was a microdialysis/infusion cannula (MAB 6.14.IC, Scipro Inc, Sanborn, NY) and the other was a drug infusion cannula (31 gauge, Plastics One, Roanoke VA). Prior to surgery, animals were anesthetized with an i.p. injection (0.1 ml/kg) of a ketamine (83 mg/kg)/ Xylazine (17 mg/kg) mixture. After subjects were nonresponsive to a tail pinch, they were placed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA). Half of the subjects (10 PF/10 PTD) had one microdialysis cannula and one infusion cannula inserted into each hemisphere of the FC (1.6 mm anterior to Bregma, 2.0 mm lateral to the midline, and 1.20 mm DV) while the other 20 subjects (10 PF/10 PTD) had both a microdialysis cannula and infusion cannula inserted bilaterally into the RSC (6.3 mm AP, 1.0 mm lateral to the midline, and 1.25 mm DV). Acrylic cement and 2 skull screws were used to hold the cannulae in place. Immediately after surgery, subjects were placed in a warm incubator until they regained an upright posture. All subjects received the analgesic buprenorphine (0.3 ml, s.c., Buprenorphine hydrochloride, Hospira Inc, Lake Forest, IL) directly after surgery and another injection 24 h post surgery. All coordinates were derived using the atlas of Paxinos and Watson (2005). Cannulae were counterbalanced so that there was equal distribution of both cannula type in each hemisphere. After surgery, animals were allowed a four day recovery period, followed by five days of handling (5 min/day) prior to behavioral testing. All rats were allowed free access to food during the day, but were fasted the night before behavioral testing.
In-vivo microdialysis
In vivo microdialysis occurred across two sessions and each session included one saline infusion and one infusion of a specific dose of physostigmine (Sigma-Aldrich). The dose of physostigmine (1.0 μg/μl or 2.0 μg/μl) a subject received first was randomly determined, but counterbalanced across treatment condition.
Subjects were transported to the testing room and placed in a microdialysis holding cage (acrylic cage (30 cm × 40 cm, depth 35 cm) with wood shavings at the bottom. The rats were awake during infusion and during the drug state. A microdialysis/infusion probe (MAB 6.14.2.Inj 2mm, Scipro Inc, Sanborn, NY) was inserted into the guide cannula in one of the cortical targets ([i] FC or [ii] RSC). The probe was connected to plastic tubing and was perfused (CMA/400 pump, CMA Microdialysis Inc., North Chelmsford, MA) continuously at a rate of 2.0 μl/min with artificial CSF (in mM: 128 NaCL, 2.5 KCL, 1.3 CaCL, 2.1 MgCL, NaHPO, and 1.0 glucose, brought to a pH of 7.4) that contained the acetylcholinesterase inhibitor neostigmine (500 nM, Sigma-Aldrich). After 60 minutes of stabilization, dialysis samples (sample volume 30 μl) were collected every 15 minutes for a total of 105 minutes. After 30 minutes of baseline sampling (B1, B2), saline (0.5 μl) was directly infused into the injection port of the probe over a period of 5 min. Samples were collected for an additional hour. One hour after the saline infusion, physostigmine (1.0 μg/μl or 2.0 μg/μl in a volume of 0.5 μl in each hemisphere) was directly infused into the cortex via the injection port of the probe over a 5 min period. After 105 minutes, the probe was removed and placed in a 100 nm standard for ACh and choline and the animal was placed back into its home cage.
High Performance Liquid Chromatography
Acetylcholine output was assayed by an Epison HPLC system (BioAnaltical Systems, West Lafayette, IN) with an enzyme reactor. The assay system included an ion exchange microbore analytical column (BAS, MR-8904), microbore ACh/choline immobilized enzyme reactor containing acetylcholinesterase and choline oxidase (BAS MF-8903), an auxillary electrode with a radial flow electrochemical thin-layer cell and 13 mm thin layer gasket, a wired enzyme electrode kit (a redox polymer film containing horseradish peroxidase coated in the surface of a 3 mm glassy carbon working electrode) and a low dispersion injected value with a 10 μl polyether-etherketone loop. Standards (5 μl of 20 and 100 nM of ACh + Ch) were injected before and after samples to determine stability of detection. The detection level of ACh was approximately 10 fmol.
Apparatus and spontaneous alternation testing
The testing apparatus used for spontaneous alternation testing was a plus maze with clear Plexiglas sidewalls that were 12 cm high and a painted black wood floor. Each of the four arms was 55 cm from the center of the maze and the maze was elevated 80 cm from the floor. The maze was located in a moderately lit room that contained several extraneous cues such as black geometric shapes, posters, chairs and a lamp.
Drug infusion and behavioral testing
Animals were behaviorally tested 2–3 days after in vivo microdialysis to ensure that the previous drug injections did not interfere with behavioral testing. Behavioral testing took place over three consecutive days. On a given session, a rat was administered a 0.5 μl infusion of either saline, 1.0 μg/μl or 2 μg μg/μl doses of physostigmine into each hemisphere (microdialysis-infusion probe =MAB 6.14.2.Inj 2mm, Scipro Inc; 28 gauge infusion needle, Plastics One). These doses were counterbalanced across days to control for order effects. We ran an additional group of normal rats to assess changes in behavior as a function of repeated testing. Neither the rate of alternation (32.3% ± 3.2; p > 0.29) or the number of arms entered (34.8 ± 1.18; p > 0.6) was altered across three successive sessions of spontaneous alternation testing.
Ten minutes before behavioral testing rats were gently restrained and received bilateral infusions (of either 0.5 μl of saline or the physostigmine doses over a course of 5 minutes). The rat was placed back into the holding cage for an additional 5 minutes. After the 5 minutes had passed, the rat was picked up and placed on the center of the maze. The rat was allowed to traverse the maze freely for an 18-minute period and the number and sequence of arms entered were recorded to determine alternation scores. An alternation score was determined by recording the number of different arms (minimum = 1, maximum = 4) entered during a four-choice sequence, dividing the number of different arms entered by 4 and multiplying that fraction by 100. Two types of perseveration behavior were recorded: (1) Re-entry perseveration and (2) Response perseveration. An arm re-entry perseveration was defined as when a rat re-entered a previously explored arm within 2 choices and it is an index of cognitive inflexibility (see Vetreno et al., 2008). The arm re-entry perseveration percentage was equal to: (A sequence of nonoverlapping entries into three arms in which there was a repeat entry/[total arm entries – 2]) × 100. A response perseveration was defined as a rat making the same body turn (left or right) as the previous trial. A response perseveration percentage was determined by counting the number of trials in which a rat made the same consecutive body turn across two trials/[total arm entries –1]) × 100. Upon completion of 18 min of maze testing, rats were transferred back to their home cage and returned to the colony room.
Histology
After completion of the behavioral testing, animals were anesthetized with 0.5 mg/kg, i.p. injection of Sleep-away (26% sodium pentobarbital in 7.8% isopropyl alcohol and 20.7% propylene glycol solution; Fort Dodge Animal Health, Fort Dodge, IA). Animals’ brains were removed, post-fixed in a 10% formalin solution (Sigma-Aldrich) for at least 72 h and then transferred to a 30% sucrose solution. Coronal sections from the brains were cut (60 μm thick) on a sliding microtome (Sm2000r; Lecia Instruments, Germany) and later stained with cresyl violet for assessment of probe location.
The extent of midline lesions was determined immunohistochemistry for the neuronal marker NeuN. Briefly, free floating sections were agitated in Tris-buffered saline (TBS) and then rinsed in 0.3% hydrogen peroxide. After three TBS washes, slices were blocked and permeabilized using a blocking solution containing 10% normal horse serum in TBS. Slices were then incubated in primary NeuN antibody (monoclonal mouse anti-NeuN antibody, 1:500; Millapore, Billerica, MA) for 48 h and then incubated again in the secondary antibody on the third day (biotinylated horse anti-mouse IgG, 1:500; Vector Labs Burlingame, CA). After several TBS washes, the sections were incubated for 2 h in 1:200 avidin–biotin– horseradish peroxidase complex (ABC elite; Vector Laboratories) and visualized using 0.001% diamino-benzidine in 0.0004% H2O2 solution (Sigma-Aldrich). The reaction was stopped by TBS rinses and sections were mounted and dried on gelatin-coated slides.
Statistics
A single factor (Treatment: PTD vs. PF) ANOVA assessed basal concentrations of ACh in each cortical region. To assess the effects of drug dose on ACh release a mixed model ANOVA design of 1-between subjects factor (Treatment: PTD vs. PF) and 2-within subjects factors (Dose [saline, 0.5 μg/μl, 1 μg], sample Time [1–4]) ANOVA was used. Behavioral measures were analyzed with a mixed model ANOVA design of 1-between subjects factor (Treatment) and 1-within subjects factor (Dose). When there was a difference in activity scores (number of arms entered) an ANCOVA was conducted to partial out the effect of activity scores on alternation scores.
Results
1. Histology
1.1. Histological analysis of cannulae placement in the FC showed that four subjects displayed inaccurate cannulae placement or abnormalities at the injection site. All other subjects displayed accurate cannulae placement in the FC within the M2 subregion extending through cortical layers 5 and 6 (see Figure 1AB; final n’s=8 PTD/8 PF).
Figure 1.
Panels A and C show representative cannulae placements in the frontal cortex (FC; A) and the retrosplenial cortex (RSC; C). The second row is a schematic demonstrating the location of all cannulae implanted into either the FC (B) or RSC (D). Images B and D are adapted from: The Rat Brain in Stereotaxic Coordinates (5th ed.), pages 38 and 40, by G. Paxinos & C. Watson, 2005, New York, NY: Academic Press. Copyright 2005 by Elsevier Academic Press. Adapted with permission.
1.2. Histological analysis cannulae placement in the RSC showed that cannula tips and sampling sites were localized within areas 29a/b, but passed through area 30. However, the cannulae in four subjects were lateral to the RSC entering the dorsal portion of the major corpus callosum. These subjects were removed from all analyses. All other subjects showed accurate cannulae placement in the RSC (see Figure 1CD; final n’s= 8 PTD/8 PF).
In most subjects the permanent dwelling cannulae caused disruption of cortical tissue. We did not see significant differences in cortical damage across groups or cortical regions. The spontaneous alternation behaviors were similar to rats that did not have implanted cannulae.
1.3. We used NeuN immunohistochemistry to document the diencephalic pathology in PTD-treated rats. The expression of NeuN is observed in most neuronal cell types and not in glial cells. Representative thalamic sections of both PF and PTD animals are shown in Figure 2. Marked cell loss was seen in the anteroventral ventrolateral nucleus (AVVL) with relative sparing of the anteroventral dorsal medial (AVDM) and anterodorsal nuclei (AD). In addition, several midline intralaminar nuclei display cell loss including the caudal portion of central medial (CM), paracentral (PC) and centrolateral (CL) nuclei. This lesion also extended into the posterior thalamic nucleus (Po). Although the majority of the medial dorsal complex (MD) was spared, there was some loss of neurons in the most ventral portion.
Figure 2.
Neuronal specific nuclear protein (NeuN) stained sections comparing the anterior and midline thalamus of PF and PTD rats. The top row (A and B) reveals the anterior thalamic nuclei in both PF and PTD rats. In PTD rats there is selective cell loss in the anteroventral ventrolateral (AVVL), with relative sparing of the anteroventral dorsal medial (AVDM) and anterodorsal nuclei (AD). The middle row (C and D) is images of midline and intralaminar thalamic structures. There is significant cell loss in the intralaminar nuclei (central medial (CM), paracentral (PC) and centrolateral (CL) nuclei) in PTD rats. However, the medial dorsal (MD) nucleus is spared—with the exception of the most ventral tip.
2.1 Frontal cortical ACh efflux
Basal levels of ACh (147.97±46.5 femtomole) in the FC did not vary as a function of Treatment (F<1). Figure 3 displays the changes in FC ACh efflux as a function of drug dose in both PF (A) and PTD (B) rats. Analyses of changes in ACh levels revealed both a significant main effects of Dose (F [2, 28]=16.93, p<0.0001, Time (F [3, 42]=36.33, p<0.0001) and a significant Dose X Time interaction (F [6, 84]=17.64, p<.00001). There were no significant differences as a function of Treatment for drug induced changes in ACh efflux (all F’s<1). Further analyses of Dose X Time interaction revealed that in both groups an infusion of 1.0 μg/μl of physostigmine led to a significant 80% rise in ACh efflux relative to saline treatment during the first 15 minutes of drug administration (F [1, 14]=45.2, p<0.0001) and then decreased back down to saline levels by 30 minutes post drug infusion (F <1). Furthermore, administration of 2.0 μg/μl dose of physostigmine caused ACh release to rise 200% above saline levels that endured for approximately 30 minutes after drug infusion in both PF and PTD animals (15 min: F [1, 14]=59.0, p<0.0001; 30 min: F [1, 14]=15.2, p<0.01). ACh levels did not return to saline levels until approximately 45 minutes after drug infusion (Saline vs. D3: F <1). In addition, the 2.0 μg/μl of physostigmine maintained a significantly higher rise in ACh during the first 15 min relative to the 1.0 μg/μl dose (F [1, 14]=14.0, p<0.01). Furthermore, the 2.0 μg/μl dose, relative to the 1.0 μg/μl dose, showed a trend for prolonged heightened ACh level during 30 min period (F [1, 14]=4.3, p<0.06). Since both the 1.0 μg/μl and 2.0 μg/μl doses of physostigmine induced significant elevations in ACh across groups these doses were chosen to be administered during behavioral testing in the second phase of the experiment.
Figure 3.
Profiles of ACh efflux (Mean percent rise above baseline ± SEM) from the frontal cortex of PF (Panel A) and PTD (Panel B) rats as a function of drug dose (saline, 1.0 and 2.0 μg/μl of physostigmine) and across time. Dialysis samples were collected for 30 minutes prior to drug infusion (B1, B2) and after infusion were samples were collected every 15 minutes for a total of 195 minutes. There was a significant dose-dependent rise in ACh efflux in both groups of rats (p<.0001). However, there were no Treatment differences in the percent increase in ACh levels as a function of dose (PF vs. PTD, p>.1). The gray bar represents the infusion time and the black bar represents the timing of behavioral testing that occurred across the following days.
2.2 The behavioral effect of sustaining high ACh level in the Frontal cortex
The main behavioral effect was that PTD rats have impaired alteration scores relative to PF rats in the saline condition (F [1, 14]=28.2, p<0.001), but the infusions of physostigmine into the FC recovered the cognitive deficit (see Figure 4A; Treatment X Dose interaction (F [2, 28]=6.3, p<0.01). Post-hoc analyses of Treatment X Dose effect showed that spontaneous alternation performance significantly improved as a function of dose in the PTD group (F [2, 16]=14.4, p<0.001), but increases in Dose did not improve performance in the PF group (F <1). Specifically, maze performance improved from saline levels when PTD animals were infused with either 1.0 μg/μl (F [1, 7]=57.8, p<0.001) or 2.0 μg/μl (F [1, 7]=27.5, p<0.01) of physostigmine. Although behavioral performance during the high dose was superior to that observed during the saline condition, peak performance occurred during the lower dose of physostigmine in PTD animals (low dose vs. high dose: F [1, 7]=7.0, p<0.05). However, PTD and PF rats had equivalent alternation scores following administration of both the low and high doses of physostigmine (both F’s< 1.4, p’s>0.10).
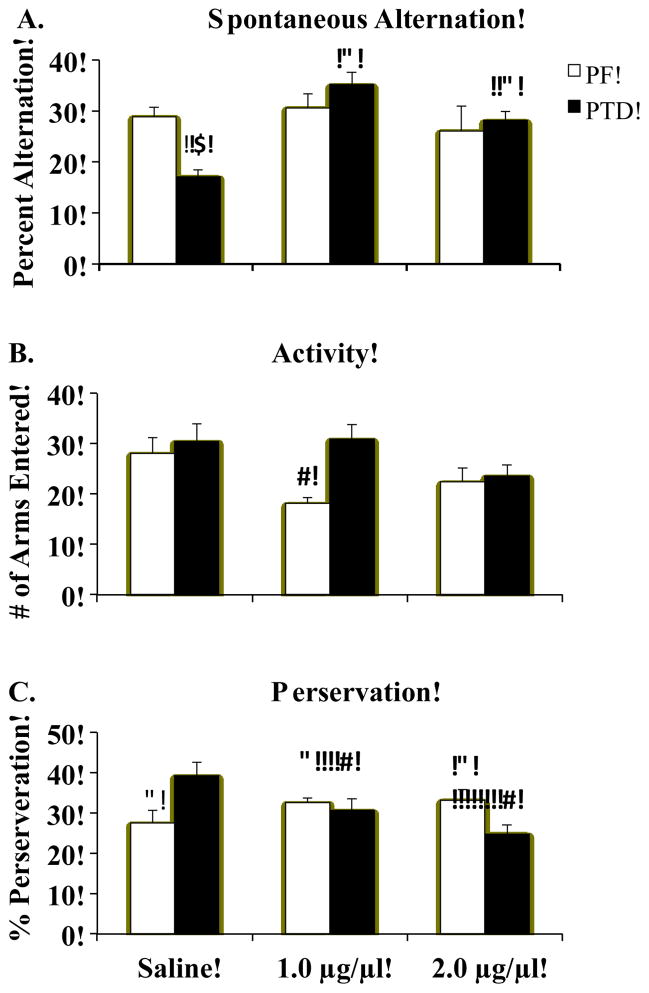
Figure 4.
Behavioral data (Mean ± SEM) from spontaneous alternation testing for PF (white bars) and PTD (solid bars) rats after infusions of saline or physostigmine (1.0 and 2.0 μg/μl) into the frontal cortex (FC). Panel A shows the PF rats had a higher rate of alternation than PTD rats after saline was infused (*). However, physostigmine infused into the FC selectively enhanced alternation rates (^) in the PTD group. Panel B reveals that activity was decreased (V) in the PF group when they received the 1.0 μg/μl dose. Panel C displays the Treatment X Dose interaction on arm re-entry preservative errors: Although the PTD rats had decreased re-entry perseverative errors (V) as a function of dose, the PF rats had an increase (^) in re-entry preservative errors as a function of dose.
It should be noted that, as shown in Figure 4B, the assessment of maze arm entries (activity) revealed significant main effects of Treatment (F [1, 14]=5.2, p<0.05) and Dose (F [2, 28]=3.4, p<0.05), but the Treatment X Dose interaction failed to reach significance (F [2, 28]=3.1, p<0.06). Given that activity levels varied as a function of both Treatment and Dose, we conducted an ANCOVA test on alternation scores with activity level as the covariate. We found that number of arm entries (covariate) did not affect alternation scores at any drug dose (all p’s>0.46). Furthermore, after the variance attributed to arm entries was partialled out, the significant interaction of Treatment X Dose for the alternation scores (p<0.02) prevailed in the ANCOVA analysis.
The analysis of arm re-entry perseveration behavior revealed a Treatment X Drug interaction (F[2, 28]=4.49, p<0.05). The PTD group had a higher level of this type of perseveration, relative to the PF rats, in the saline condition, but this type of perseverative behavior decreased in PTD rats when physostigmine was infused into the FC (see Figure 4C). In contrast, in the PF rats infusing either dose of physostigmine into the FC increased this type of perseveration behavior, relative to the saline condition. The repetition of body turns was not affected by Treatment, drug Dose of the interaction of those variables (all F’s<1, p’s>0.35). This type of response perseverations only made up about 18–19 (+ 3.06) % of the total behavioral choices in both groups regardless of drug condition.
A negative correlation between alternation scores and arm re-entry perseveration behavior were found in the saline condition (r=−0.64, p<.01) and the high dose condition (r=−0.71, p<.01). This effect was not observed when the low dose of physostigmine was infused into the FC (r=−0.44, p>.08).
3.1 Retrosplenial cortex ACh efflux
Basal levels of ACh (57.72±21.5 femtomole) did not differ as a function of Treatment condition (F <1). Statistical analyses revealed that ACh efflux was maintained at a high level as a function of Dose (F [1, 14]=7.32, p<0.01) and Time (F [3, 42]=14.35 p<0.0001). This is shown in Figure 5AB. In addition, there was a Dose X Time interaction (F [6, 84]=6.44, p<0.0001). Treatment was not significant and it did not interact with any other factors (all F’s <1.1).
Figure 5.
Profiles of ACh efflux (Mean percent rise above baseline ± SEM) from the retrosplenial cortex of PF (Panel A) and PTD (Panel B) rats as a function of drug dose (saline, 1.0 and 2.0 μg/μl of physostigmine) and as a function of time. Dialysis samples were collected for 30 minutes prior to drug infusion (B1, B2) and after infusion were samples were collected every 15 minutes for a total of 195 minutes. There was a significant dose-dependent rise in ACh efflux in both groups of rats (p<.0001), but the percent increase in ACh levels was not different as a function of Group (PF vs. PTD, p>.1). The gray bar represents the infusion time and the black bar represents the timing of behavioral testing that occurred across the following days
Post-hoc contrasts revealed that 1.0 μg/μl of physostigmine significantly sustained high ACh levels by approximately 30% from saline during the first 15 minutes of drug testing (F [1,14]=7.20, p<0.05) and peaked (100% above saline) at the 30 minute mark (F [1,14]=24.57, p<0.0001). However, ACh levels returned to baseline level 45 minutes following drug infusion (p>0.5). A similar timeline was observed for 2.0 μg/μl dose of physostigmine: ACh was maintained at a higher level (80%) during the first 15 minutes (F [1, 14]=10.33, p<0.01) and reached maximal levels of approximately 180% above baseline 30 minutes after drug infusion (F [1, 14]= 7.3, p<0.001). Comparable to that observed during the lower dose, ACh levels returned to baseline values 45 minutes after drug infusion (F [1,14]=1.37, p>0.1). Similar to the FC, both 1.0 and 2.0 μg/μl doses of physostigmine were administered into the RSC to assess changes in behavior.
3.2 The behavioral effect of sustaining high ACh levels in the Retrosplenial cortex
Again, we see that in the saline condition the PTD rats have impaired alternation scores relative to the PF rats (F [1, 14]=8.84, p<0.05). However, the main behavioral effect of infusing physostigmine into the RSC was it decreased alternation scores in the PF rats, but had no effect in the PTD rats (see Figure 6A). Furthermore, the analysis of alternation scores revealed a main effect of Treatment: PF animals, compared to PTD, performed better on the spontaneous alternation task regardless of drug condition (F [1,14]=16.82, p<0.01). A main effect of Dose was also observed (F [2, 28]=4.6, p<0.05): Alternation scores decreased as a result of RSC physostigmine infusions. Specifically, PF animals demonstrated an overall decrease in performance as a function of physostigmine administration (F [2, 14]=3.74, p<0.05). Post-hoc contrasts showed that the 2.0 μg/μl dose of physostigmine significantly decreased performance in PF animals relative to saline (F [1, 7]=5.89, p<0.05). Although not significant, a trend for 1.0 μg/μl of physostigmine to decrease performance in PF animals was also observed (F [1, 7]=4.34, p<0.08). In contrast, the alternation performance of PTD rats did not significantly change as a function of Dose (F [2, 14] = 1.19 p>0.20). In addition, the spontaneous alternation performance of PF rats was also better than PTD rats when rats received an infused of 2.0 μg/μl (F [1, 14] = 19.09, p<0.01, but not 1.0 μg/μl (F [1, 14] = 1.13 p>0.2) of physostigmine into the RSC.
Figure 6.
Behavioral data (Mean ± SEM) from spontaneous alternation testing for PF (white bars) and PTD (solid bars) rats after infusions of saline or physostigmine (1.0 and 2.0 μg/μl) into the retrosplenial cortex (RSC). Panel A shows the PF rats had a higher rate alternation than PTD rats after saline was infused (*). However, physostigmine infused into the RSC impaired alternation rates (V) in the PF group, but did not affect alternation scores in the PTD rats. Panels B and C reveal that neither activity (B) or re-entry perseveration (C) behaviors were affected by an infusion of physostigmine into the RSC.
The average number of arm entries revealed no effects of Treatment or drug Dose (all F’s <1.94, p’s >0.17; see Figure 6B). In addition, neither type of perseverative behavior was significantly affected when physostigmine was infused into the RSC in either Treatment condition (all F’s<1.13, p’s>0.15). Although there was a trend for the PTD rats to have higher re-entry preservation scores than the PF rats (see Figure 6C), the effect was not significant (F[1,14]=3.29, p=0.09). Response perseverations only made up about 16.7–20.2 % (+ 1.98) of the total behavioral choices in both groups regardless of drug condition.
A significant negative correlation was found between alternation scores and arm re-entry perseveration behavior in the saline condition (r=−0.64, p<.01). Thus, the higher the alternation scores, the lower the perseveration behavior. In contrast, these negative correlations were not significant when subjects received the low dose (r=−0.48, p>.06) or high dose (r=−0.45, p>.08) into the RSC.
Discussion
The present study used site-specific injections of physostigmine to prolong high cholinergic tone in two cortical regions (FC, RSC) that display hypocholinergic functioning selectively during behavioral testing after TD (see Anzalone et al 2010). The use of in vivo microdialysis allowed us to ensure that the drug doses that maintained high ACh levels were comparable across these diverse cortical regions. The goal of the experiment was to determine whether prolonging high ACh tone in either cortical region would restore spatial working memory in amnestic PTD rats. This is the first study to demonstrate a dissociable contribution of improving cholinergic tone in the FC versus the RSC. Furthermore, whether the effect was therapeutic or dysfunctional was dependent on brain pathology.
Similar to our previous study (Anzalone et al, 2010), basal levels of ACh were not affected by PTD treatment. Our prior work has consistently demonstrated that the dysfunction of ACh efflux is only observed when rats are cognitively challenged (Anzalone et al, 2010; Roland et al 2008; Savage et at, 2003; Vetreno et al, 2008). Similar to our previous work increasing ACh levels in the hippocampus, PTD rats respond similar to PF rats to the drug challenge in the cortex. Drug challenges sustain high local levels ACh, whereas the behavioral challenges activate multiple brain regions (see Anzalone et al, 2010). Thus, a behavioral challenge is likely to be more sensitive to circuit pathology than a local drug challenge.
Although cholinergic tone was increased in both regions regardless of brain pathology, this manipulation was only functionally effective in the FC after diencephalic pathology. Sustaining high ACh tone in the FC of PTD rats completely recovered their spontaneous alternation scores and suppressed their perseverative behavior. Although the high dose of physostigmine produced the greatest sustained level of ACh, the lower dose produced the greatest behavioral recovery. This suggests that there is an optimal level of ACh tone for restoration of behavior in brain damaged subjects and that there is likely to be an inverted U curve with excess cholinergic stimulation leading to cognitive impairment as seen in control rats (see Lalonde, 2002). In contrast, maintaining high cholinergic tone in the RSC in these amnestic rats was without any behavioral effect. This contrast of effects may be due to differences in thalamic pathology, which will discussed later.
An interesting pattern of impairment was found in normal control rats after cholinergic stimulation of these cortical regions. In the intact brain, increasing cholinergic activity in the FC increased perseverative behavior, whereas increasing ACh tone in the RSC impaired alternation behavior. Perseverative behavior can reflect a lack of inhibition, cognitive inflexibility or impaired working memory (Kolb, 1984). The type of perseverative behavior that was seen in the current study was return to recently visited arm, not an egocentric motor response pattern (i.e. turning left). Perseveration often occurs in situations with high working memory demands and after damage to the frontal cortex (Mogensen & Divac, 1984). It has been stated that the development of inflexible response strategies can make animals functionally inattentive to aspects in their environment (Stokes & Best, 1988). However, it is not possible to dissociate inattention, inflexibility and impaired working memory on this and many other maze tasks. It was interesting that in the nondrug condition there is a significant negative correlation between alternation score and perseveration to a recently visited arm. When physostigmine was infused into the FC, arm re-entry preservation decreased in PTD rats, but increased in PF rats without significantly affecting alternation scores. Thus, in the low dose condition the negative correlation between alternation and perseveration is no longer significant. There was also a decrease in activity, as measured by the number of arm entered, when physostigmine was infused into the FC of PF rats. However, this change in motor activity did not modify alternation behavior and only occurred in the low dose condition.
We believe these effects can be explained by the unique role these cortical regions play in different aspects of spatial behavior and by how ACh modulates the neural activity between each cortical region and the specific associated thalamic nuclei. First, we will briefly discuss the role of the FC and RSC in spatial behavior. This will set the stage for reviewing the nature of the behavioral improvement in PTD rats juxtaposed against the type of impairments observed in control rats. Finally, we discuss how the neuroanatomical relationships between that thalamus and cortex shape the behavioral pharmacological responses we observed. Damage to either the FC or RSC can impair spatial behavior (Dalley, Cardinal & Robbins, 2004; Harker & Whishaw, 2004; Pothuizen, Aggleton, Vann, 2008). Spontaneous alternation is an ethologically-based task that assays a rodents’ innate tendency to explore its environment. The tendency for a rodent to alternate between maze arms provides an index of working spatial memory. Many brain regions—such as the hippocampus, septum, basal forebrain, and frontal cortex—are involved in this task (Lalonde, 2002). Neural networks involving the frontal areas of M1, M2 and dorsomedial prefrontal area appear to be important for organizing sequential behavioral choices (Bailey & Mair, 2007). In the plus-maze version of spontaneous alternation, a correct alternation is defined as a behavioral sequence that requires a rodent to enter all four arms. Thus, on such tasks, successful performance is based on a rodent’s ability remember which arms it has already visited. Frontal dysfunction would result in a rat being unable to remember the sequence of arms it recently entered and therefore it would be more likely to re-visited an arm. This is exactly the type of behavior dysfunction observed in the untreated PTD rats: impaired alternation due to revisiting arms within 2 choices. Our previous study revealed that during spontaneous alternation testing, ACh efflux in the M2 region of the FC in PTD rats is dramatically declined (85%), relative to control rats (Anzalone et al, 2010). In the present study, when high cholinergic tone was maintained in the FC in PTD rats, there was a decrease in perseverative responding and improved alternation behavior. However, the current study also demonstrated that in control rats over stimulation of the FC by direct AChEI infusion increased perseverative behavior and such a deficit could be a result of excessive autoreceptor stimulation in the intact brain (see below).
The RSC is critical for spatial navigation as it mediates the processing of topographical relationships between visual cues in the environment (Harker & Whishaw, 2004). On a forced alternation task, retrosplenial cortex lesions impaired the use of direction cues (Pothuizen, Aggleton, Vann, 2008). Recently, we found that in normal rats there was a significant rise in ACh efflux in RSC area 29ab during spontaneous alternation testing, suggesting this region is important for alternation behavior (Anzalone, Vogt, Roland & Savage, 2009). Furthermore, ACh efflux in the RSC of PTD rats during spontaneous alternation testing was only 45% of control rats (Anzalone et al, 2010). The RSC is composed of two distinct cytoarchitectonic subregions, areas 29 (granular) and 30 (agranular). Our cannulae were aimed at area 29ab (see also Anzalone et al 2008; 2010), but passed through area 30. Lesion and early immediate gene mapping studies suggest that the subregions of RSC jointly support spatial learning and memory (Pothuizen, Davies, Aggleton & Vann, 2010). Given that the microdialysis data demonstrated that the doses chosen in the present study significantly prolonged high ACh levels in the RSC in both groups, the lack of behavioral effect after infusion of physostigmine in the PTD groups is likely caused by how their unique brain pathology disrupts ACh modulation of corticothalamic communication. In the PF rats, directly increasing ACh tone in the RSC lead to an impairment of alternation behavior. Again, this effect could be due to overstimulation of the cortex by ACh as discussed below.
In the cortex, ACh appears to have two actions: Local in suppression of intracortical inputs via muscaric receptors (mAChR) and stimulation of the afferents of the thalamocortical inputs via nicotinic receptors (nAChR; Gioanni et al, 1999). The actions of ACh in the cortex enhance the influence of feed-forward afferent input to the cortex while decreasing background activity due to excitatory feedback connections within cortical circuits: Specifically, activation of presynaptic M2-type receptors inhibits excitatory feedback synapses within cortical circuits, whereas activation of presynaptic nAChRs increases release of glutamate from thalamocortical afferents (Benarroch, 2010; Hasselmo, 2006; Hasselmo, Anderson & Bower, 1992; Hasselmo & Sarter, 2011). Acetylcholinesterase inhibitors, such as physostigmine increase the extracellular concentrations of ACh and thus are nondiscriminative in their activation of mAChR and nAChR. Thus, we can see both types of effects occurring after cortical applications of physostigmine—but these effects are modified by brain damage.
First in normal rats, the flood of ACh caused by a high dose of an AChEI can inhibit ACh release through the action of presynaptic mAChR and nAChR (Maelicke, Schrattenholz, Samachocki, Radina & Albuquerque, 2000; Samochocki et al, 2003). Specifically, the high stimulation of presynaptic M2 receptors can lead to an inhibition of ACh release and attenuate presynaptic signaling (Sarter, Parikh & Howe, 2009). Studies aimed at increasing the cholinergic levels in the septohippocampal pathway have found that in normal animals, high doses of cholinergic drugs produce amnestic effects (Bunce, Sabolek & Chrobak, 2004; Elvander et al. 2004; Lalonde 2002). Thus, the optimal level of cholinergic stimulation in the normal brain can be of a very limited range and vary as a function of local extracellular levels of ACh that are determined by a complex balance of both local and efferent circuitry (Hasselmo & Sarter, 2011).
In PTD rats that have a loss of forebrain cholinergic neurons as well as anterior and midline thalamic lesions, increasing cholinergic tone within the FC enhanced spatial memory performance and decreased arm re-entry perseverative responding. These results resemble previous studies in that drugs that increase hippocampal cholinergic levels facilitate behavioral performance in some rodent models of cognitive impairment (Barnes et al. 2000; Chang and Gold 2004; Mulder et al. 2005; Nakagawasai et al. 2000: Roland & Savage, 2009; Roland et al, 2010). In contrast, although the RSC is critical for the integration of spatial behavior in the intact rat (Dumont Petrides & Sziklas, 2010; Vann & Aggleton, 2004), the neuropathology associated with PTD treatment may impede the ability of the increased cholinergic tone within the RSC to restore behavior.
The diencephalic pathology in the PTD model and the unique inputs and outputs these two diverse cortical regions have with the thalamus are the likely cause for the differential drug effects observed in the present study. The medial prefrontal cortical region (mPFC) is innervated by the medial dorsal nucleus (MD; Hoover & Vertes, 2007) – which is relatively spared in the PTD model. The midline and intralaminar nuclei have a diverse pattern of innervation (see Van der Werf, Witter & Groenewegen, 2002). The rostal portion of central medial nucleus (CM) is relatively spared after PTD treatment and it innervates the mPFC and the premotor FC. In contrast, the caudal CM displays extensive cell loss after PTD treatment and it projects to the lateral orbital frontal region (OFC). Furthermore, the paracentral nucleus (PC) is also damaged after PTD treatment and it projects to the mPFC, FC, as well as the RSC. In addition, after PTD treatment the central lateral nucleus (CL) is lesioned and it projects to the lateral FC and RSC. Finally, the parafascicular nucleus (PFn) has significant cell loss after TD and it projects to the OFC and the more lateral motor cortices. Thus, after PTD treatment we see there is some sparing of midline thalamic regions (MD and rostral CM) and these regions have reciprocal connections with the mPFC and FC. In contrast, the intralaminar nuclei, such the caudal CM, PC, CL and PFn are lesioned in the PTD model, but their primary cortical targets are not the FC. Thus, direct cholinergic stimulation of the frontal cortex can activate a significant proportion of intact thalamic projection neurons in the PTD model.
The RSC has direct inputs/outputs to the anterior thalamic nuclei (both the dorsal and ventral nuclei) and ACh, in part, regulates the interaction between the RSC and the thalamus (Vogt & Burns, 1988). However, PTD treatment eliminates most of neurons in the anterior ventroventral nuclei (AVVL; see Figure 2; Anzalone et al, 2010; Vetreno et al, 2010) and thus increasing cholinergic tone in the RSC would have a limited effect on the activation of thalmacocortical neurons, given the paucity of neurons that remain in the AVVL after PTD treatment to respond to the increase in ACh.
Therefore, in the PTD model, one explanation for the enhancement of behavior after FC ACh levels are maintained at a high level and no behavioral reaction when high ACh levels are sustained in the RSC, is that some midline thalamus structures are relatively spared and increasing cholinergic tone in the FC could lead to stimulation of the relatively intact hippocampus and these spared thalamic nuclei. In contrast, the major input to the RSC the anterior nuclei, in particular the AVVL, that is void of neurons after PTD treatment and thus the increase in cholinergic tone does not re-activated thalamic-cortical interactions.
In summary, changes in cortical ACh levels are pivotal for arousal, attention, and cognitive processes. Loss or dysregulation of cholinergic inputs produces or exacerbates cognitive impairments in several neurodegenerative diseases such as WKS (Schliebs and Arendt, 2006). Such dysfunctions in the PTD model can be restored by selectively prolonging the action of ACh within the hippocampus and FC, but not the RSC. The differential behavioral response after restoration of ACh levels in these regions is a product of the intact or loss of thalamic circuits.
In future studies, it would be informative to determine whether direct stimulation of muscarinic or nicotinic ACh receptors within the FC results in differential effectiveness in ameliorating specific cognitive deficits. Neuropharmacological data suggest that in the cortex nictonic and muscarinic receptors have different roles: Activation of mAChRs causes heterosynaptic and presynaptic inhibition within cortical neurons and that reduces the spread of neural activity within a cortical region, but enhances the external input on cortical activity (Sarter, et al 2009). Whereas stimulation of nAChRs enhances glutamatergic transmission at thalamocortical synapses, which leads to the enhancement of thalamic activation of cortical neurons (Hasselmo, 2006; Hasselmo and Sarter, 2011; Hsieh, Cruikshank, Metherate, 2000). Such experiments may therefore shed light on the unique contributions for cortical and thalamic cholinergic stimulation in the recovery of cognitive function.
Acknowledgments
I would like to thank Steven Anzalone for his efforts in conducting the experiments and organization of the data. This research was funded by grants NINDS 054272 and ARRA NINDS 054272-S1 to LMS.
Abbreviations
- ACh
Acetylcholine
- AChEI
Acetylcholinesterase inhibitor
- ANOVA
Analysis of Variance
- ANCOVA
Analysis of Covariance
- FC
Frontal Cortex
- HPLC
High Performance Liquid Chromatography
- NBM
Nucleus Basalis Magnocellularis
- PTD
Pyrithiamine-induced Thiamine Deficiency
- PF
Pair-fed
- RSC
Retrosplenial Cortex
- TD
Thiamine Deficiency
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne
References
- Aggleton JP. Understanding anterograde amnesia: Disconnections and lesions. Quarterly Journal of Experimental Psychology. 2008;61:1441–1471. doi: 10.1080/17470210802215335. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, O’Mara SM, Vann SD, Wright NF, Tsanov M, Erichsen JT. Hippocampal- anterior thalamic pathways for memory: uncovering a network of direct and indirect actions. European Journal of Neuroscience. 2010;31:2292–2307. doi: 10.1111/j.1460-9568.2010.07251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Kurz J. An analyses of the origins of the cholinergic and non-cholinergic septal projections to the hippocampal formation of the rat. Journal of Comparative Neurology. 1985;240:37–59. doi: 10.1002/cne.902400104. [DOI] [PubMed] [Google Scholar]
- Anzalone S, Roland JJ, Vogt B, Savage LM. Acetylcholine efflux from retrosplenial areas and hippocampal sectors during maze exploration. Behavioral Brain Research. 2009;201:272–278. doi: 10.1016/j.bbr.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzalone S, Vetreno RP, Ramos RL, Savage LM. Cortical cholinergic abnormalities contribute to the amnesic state induced by pyrithiamine-induced thiamine deficiency in the rat. European Journal of Neuroscience. 2010;32:847–858. doi: 10.1111/j.1460-9568.2010.07358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey KR, Mair RG. Effects of frontal cortex lesions on action sequence learning in the rat. European Journal of Neuroscience. 2007;25:2905–2915. doi: 10.1111/j.1460-9568.2007.05492.x. [DOI] [PubMed] [Google Scholar]
- Barnes CA, Meltzer J, Houston F, Orr G, McGann K, Wenk GL. Chronic treatment of old rats with donepezil or galantamine: effects on memory, hippocampal plasticity and nicotinic receptors. Neuroscience. 2000;99:17–23. doi: 10.1016/s0306-4522(00)00180-9. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. Acetylcholine in the cerebral cortex: effects and clinical implications. Neurology. 2010;75:659–665. doi: 10.1212/WNL.0b013e3181ee267e. [DOI] [PubMed] [Google Scholar]
- Bunce JG, Sabolek HR, Chrobak JJ. Intraseptal Infusion of the Cholinergic Agonist Carbachol Impairs Delayed-Non-Match-to-Sample Radial Arm Maze Performance in the Rat. Hippocampus. 2004;14:450–459. doi: 10.1002/hipo.10200. [DOI] [PubMed] [Google Scholar]
- Carlesimo GA, Lombardi MG, Caltagirone C. Vascular thalamic amnesia: A reappraisal. Neuropsychologia. 2011;49:777–789. doi: 10.1016/j.neuropsychologia.2011.01.026. [DOI] [PubMed] [Google Scholar]
- Caulo M, Van Hecke J, Toma L, Ferretti A, Tartaro A, Colosimo C, Romani GL, Uncini A. Functional MRI study of diencephalic amnesia in Wernicke-Korsakoff syndrome. Brain. 2004;128:1584–1594. doi: 10.1093/brain/awh496. [DOI] [PubMed] [Google Scholar]
- Chang Q, Gold PE. Impaired and spared cholinergic functions in the hippocampus after lesions of the medial septum/vertical limb of the diagonal band with 192 IgG-saporin. Hippocampus. 2004;14:170–179. doi: 10.1002/hipo.10160. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neuroscience, Biobehavioral Reviews. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Dumont JR, Petrides M, Sziklas V. Fornix and retrosplenial contribution to a hippocampo-thalamic circuit underlying conditional learning. Behavioral Brain Research. 2010;209:13–20. doi: 10.1016/j.bbr.2009.12.040. [DOI] [PubMed] [Google Scholar]
- Dougherty KD, Turchin PI, Walsh TJ. Septocingulate and septohippocampal cholinergic pathways: involvement in working/episodic memory. Brain Research. 1991;810:59–71. doi: 10.1016/s0006-8993(98)00870-1. [DOI] [PubMed] [Google Scholar]
- Eckenstein FP, Baughman RW, Quinn J. An anatomical study of cholinergic innervation in rat cerebral cortex. Neuroscience. 1988;25:457–474. doi: 10.1016/0306-4522(88)90251-5. [DOI] [PubMed] [Google Scholar]
- Elvander E, Schöott PA, Sandin J, Bjelke B, Kehr J, Yoshitake T, Ogren SO. Intraseptal muscarinic ligands and galanin: influence on hippocampal acetylcholine and cognition. Neuroscience. 2004;126:541–557. doi: 10.1016/j.neuroscience.2004.03.058. [DOI] [PubMed] [Google Scholar]
- Gage SL, Keim SR, Simon JR, Low WC. Cholinergic innervation of the retrosplenial cortex via the fornix pathway as determined by high affinity choline uptake, choline acetyltransferase activity, and muscarinic receptor binding in the rat. Neurochemical Research. 1994;19:1379–1386. doi: 10.1007/BF00972466. [DOI] [PubMed] [Google Scholar]
- Gioanni Y, Rougeot C, Clarke PB, Lepousé C, Thierry AM, Vidal C. Nicotinic receptors in the rat prefrontal cortex: increase in glutamate release and facilitation of mediodorsal thalamo-cortical transmission. European Journal of Neuroscience. 1999;11:118–130. doi: 10.1046/j.1460-9568.1999.00403.x. [DOI] [PubMed] [Google Scholar]
- Gold JJ, Squire LR. The anatomy of amnesia: Neurohistological analysis of three new cases. Learning &Memory. 2006;13:699–710. doi: 10.1101/lm.357406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalo-Ruiz A, Morte L. Localization of amino acids, neuropeptides and cholinergic markers in neurons of the septum-diagonal band complex projecting to the retrosplenial granular cortex of the rat. Brain Research Bulletin. 2000;52:499–510. doi: 10.1016/s0361-9230(00)00287-2. [DOI] [PubMed] [Google Scholar]
- Harker KT, Whishaw IQ. A reaffirmation of the retrosplenial contribution to rodent navigation: reviewing the influences of lesion, strain, and task. Neuroscience, Biobehavioral Reviews. 2004;28:485–496. doi: 10.1016/j.neubiorev.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME. The role of acetylcholine in learning and memory. Current Opinion in Neurobiology. 2006;16:1–6. doi: 10.1016/j.conb.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Anderson BP, Bower JM. Cholinergic modulation of cortical associative memory function. Journal of Neurophysiology. 1992;67:1230–1246. doi: 10.1152/jn.1992.67.5.1230. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Sarter M. Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacology. 2011;36:52–73. doi: 10.1038/npp.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Structure & Function. 2007;212:149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Hsieh CY, Cruikshank SJ, Metherate R. Differential modulation of auditory thalamo-cortical and intracortical synaptic transmission by cholinergic agonist. Brain Research. 2000;880:51–64. doi: 10.1016/s0006-8993(00)02766-9. [DOI] [PubMed] [Google Scholar]
- Jenkins TA, Dias R, Amin E, Brown MW, Aggleton JP. Fos imaging reveals that lesions of the anterior thalamic nuclei produce widespread limbic hypoactivity in rats. Journal of Neuroscience. 2002;22:5230–5238. doi: 10.1523/JNEUROSCI.22-12-05230.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B. Functions of the frontal cortex of the rat: a comparative review. Brain Research. 1984;320:65–98. doi: 10.1016/0165-0173(84)90018-3. [DOI] [PubMed] [Google Scholar]
- Kopelman MD, Thomson AD, Guerrini I, Marshall EJ. The Korsakoff syndrome: clinical aspects, psychology and treatment. Alcohol & Alcoholism. 2009;44:148–154. doi: 10.1093/alcalc/agn118. [DOI] [PubMed] [Google Scholar]
- Lalonde R. The neurobiological basis of spontaneous alternation. Neuroscience Biobehavioral Reviews. 2002;26:91–104. doi: 10.1016/s0149-7634(01)00041-0. [DOI] [PubMed] [Google Scholar]
- Langlais PJ. Pathogenesis of diencephalic lesions in an experimental model of Wernicke’s encephalopathy. Metabolic Brain Disorders. 1995;10:31–44. doi: 10.1007/BF01991781. [DOI] [PubMed] [Google Scholar]
- Langlais PJ, Savage LM. Thiamine deficiency in rats produces cognitive and memory deficits on spatial tasks that correlate with tissue loss in diencephalon, cortex and white matter. Behavioral Brain Research. 1995;68:75–89. doi: 10.1016/0166-4328(94)00162-9. [DOI] [PubMed] [Google Scholar]
- Langlais PJ, Zhang SX. Cortical and subcortical white matter damage without Wernicke’s encephalopathy after recovery from thiamine deficiency in the rat. Alcoholism: Clinical & Experimental Research. 1997;21:434–443. doi: 10.1111/j.1530-0277.1997.tb03788.x. [DOI] [PubMed] [Google Scholar]
- Maelicke A, Schrattenholz A, Samochocki M, Radina M, Albuquerque EX. Allosterically potentiating ligands of nicotinic receptors as a treatment strategy for Alzheimer’s disease. Behavioral Brain Research. 2000;113:199–206. doi: 10.1016/s0166-4328(00)00214-x. [DOI] [PubMed] [Google Scholar]
- Mair RG. On the role of thalamic pathology in diencephalic amnesia. Reviews in Neuroscience. 1994;5:105–140. doi: 10.1515/revneuro.1994.5.2.105. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Levey AI, Wainer BH. Cholinergic Innervation of cortex by the basal forebrain: Cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. Journal of Comparative Neurololgy. 1983;214:170–197. doi: 10.1002/cne.902140206. [DOI] [PubMed] [Google Scholar]
- Mogensen J, Divac I. Behavioural changes after ablation of subdivisions of the rat prefrontal cortex. Acta Neurobiology Experimantal. 1993;53:439–49. [PubMed] [Google Scholar]
- Mulder J, Harkany T, Czollner K, Cremers TI, Keijser JN, Nyakas C, Luiten PG. Galantamine-induced behavioral recovery after sublethal excitotoxic lesions to the rat medial septum. Behavioral Brain Research. 2005;163:33–41. doi: 10.1016/j.bbr.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Nakagawasai O, Tadano T, Hozumi S, Tan-No K, Niijima F, Kisara K. Immunohisto-chemical estimation of brain choline acetyltransferase and somatostatin related to the impairment of avoidance learning induced by thiamine deficiency. Brain Research Bulletin. 2000;52:189–96. doi: 10.1016/s0361-9230(00)00248-3. [DOI] [PubMed] [Google Scholar]
- Olney JW. Excitatory transmitters and epilepsy-related brain damage. International Review of Neurobiology. 1985;27:337–362. doi: 10.1016/s0074-7742(08)60561-9. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M, Kirkley SM, Gansler DA, Couture A. Comparisons of Korsakoff and non-Korsakoff alcoholics on neuropsychological tests of prefrontal brain functioning. Alcoholism: Clinical & Experimental Research. 2004;28:667–675. doi: 10.1097/01.alc.0000122761.09179.b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 5. New York, NY: Elsevier Academic Press; 2005. [Google Scholar]
- Pitkin SR, Savage LM. Aging potentiates the acute and chronic neurological symptoms of pyrithiamine-induced thiamine deficiency in the rodent. Behavioral Brain Research. 2001;119:167–177. doi: 10.1016/s0166-4328(00)00350-8. [DOI] [PubMed] [Google Scholar]
- Pothuizen HH, Aggleton JP, Vann SD. Do rats with retrosplenial cortex lesions lack direction? European Journal of Neuroscience. 2008;28:2486–2498. doi: 10.1111/j.1460-9568.2008.06550.x. [DOI] [PubMed] [Google Scholar]
- Pothuizen HH, Davies M, Aggleton JP, Vann SD. Effects of selective granular retrosplenial cortex lesions on spatial working memory in rats. Behavioral Brain Research. 2010;208:566–575. doi: 10.1016/j.bbr.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Reed LJ, Lasserson D, Marsden P, Stanhope N, Stevens T, Bello F, Kingsley D, Colchester A, Kopelman MD. FDG-PET findings in Wernicke-Korsakoff syndrome. Cortex. 2003;39:1027–1045. doi: 10.1016/s0010-9452(08)70876-1. [DOI] [PubMed] [Google Scholar]
- Roland JJ, Levinson M, Vetreno RP, Savage LM. Differential effects of systemic and intraseptal administration of the acetylcholinesterase inhibitor tacrine on the recovery of spatial behavior in an animal model of diencephalic amnesia. European Journal of Pharmacology. 2010;629:31–39. doi: 10.1016/j.ejphar.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland JJ, Mark K, Vetreno RP, Savage LM. Increasing hippocampal acetylcholine levels enhance behavioral performance in an animal model of diencephalic amnesia. Brain Research. 2008;1234:116–127. doi: 10.1016/j.brainres.2008.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland JJ, Savage LM. Blocking GABA-A receptors in the medial septum enhances hippocampal acetylcholine release and behavior in a rat model of diencephalic amnesia. Phamacology Biochemistry & Behavioral. 2009;92:480–487. doi: 10.1016/j.pbb.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samochocki M, Höffle A, Fehrenbacher A, Jostock R, Ludwig J, Christner C, Radina M, Zerlin M, Ullmer C, Pereira EF, Lübbert H, Albuquerque EX, Maelicke A. Galantamine is an allosterically potentiating ligand of neuronal nicotinic but not of muscarinic acetylcholine receptors. Journal of Pharmacology & Experimental Therapeutics. 2003;305:1024–1036. doi: 10.1124/jpet.102.045773. [DOI] [PubMed] [Google Scholar]
- Sarter M, Parikh V, Howe WM. Phasic acetylcholine release and the volume transmission hypothesis: time to move on. Nature Review Neuroscience. 2009;10:383–390. doi: 10.1038/nm2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliebs R, Arendt T. The significance of the cholinergic system in the brain during aging and alzheimer’s disease. Journal of Neural Transmission. 2006;113:1625–1644. doi: 10.1007/s00702-006-0579-2. [DOI] [PubMed] [Google Scholar]
- Stokes KA, Best PJ. Mediodorsal thalamic lesions impair radial maze performance in the rat. Behavioral Neuroscience. 1988;102:294–300. doi: 10.1037//0735-7044.102.2.294. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neuroimaging of the Wernicke-Korsakoff syndrome. Alcohol & Alcoholism. 2009;44:155–165. doi: 10.1093/alcalc/agn103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Research Reviews. 2002;39:107–140. doi: 10.1016/s0165-0173(02)00181-9. [DOI] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP. Testing the importance of the retrosplenial guidance system: effects of different sized retrosplenial cortex lesions on heading direction and spatial working memory. Behavioral Brain Research. 2004;155:97–108. doi: 10.1016/j.bbr.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Vann SD, Albasser MM. Hippocampal, retrosplenial, and prefrontal hypoactivity in a model of diencephalic amnesia: Evidence towards an interdependent subcortical-cortical memory network. Hippocampus. 2009;19:1090–102. doi: 10.1002/hipo.20574. [DOI] [PubMed] [Google Scholar]
- Vetreno RP, Anzalone SJ, Savage LM. Impaired, spared, and enhanced ACh efflux across the hippocampus and striatum in diencephalic amnesia is dependent on task demands. Neurobiology of Learning Memory. 2008;90:237–244. doi: 10.1016/j.nlm.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP, Hall JM, Savage LM. Alcohol-related amnesia and dementia: Animal models have revealed the contributions of different etiological factors on neuropathology, neurochemical dysfunction and cognitive impairment. Neurobiology of Learning Memory. 2011 Jan 21; doi: 10.1016/j.nlm.2011.01.003. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Burns DL. Experimental localization of muscarinic receptor subtypes to cingulate cortical afferents and neurons. Journal of Neuroscience. 1988;8:643–652. doi: 10.1523/JNEUROSCI.08-02-00643.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SX, Weilersbacher GS, Henderson SW, Corso T, Olney JW, Langlais PJ. Excitotoxic cytopathology, progression, and reversibility of thiamine deficiency-induced diencephalic lesions. Journal of Neuropathology & Experimental Neurology. 1995;54:255–267. doi: 10.1097/00005072-199503000-00012. [DOI] [PubMed] [Google Scholar]