Abstract
Prior reinforcement of a neutral stimulus often blocks subsequent conditioning of a new stimulus if a compound of the original and new cues is paired with the same reinforcer. However, if the value of the reinforcer is altered when the compound is presented, the new cue typically acquires conditioning, a result called unblocking. Blocking, unblocking and related phenomena have been attributed to variations in processing of either the reinforcer, for example, the Rescorla-Wagner (1972) model, or cues, for example, the Pearce-Hall (1980) model. Here, we examined the effects of lesions of the basolateral amygdala on the occurrence of unblocking when the food reinforcer was increased in quantity at the time of introduction of the new cue. The lesions had no effects on unblocking in a simple design (Experiment 1), which did not distinguish between unblocking produced by variations in reward or cue processing. However, in a procedure that distinguished between unblocking due to direct conditioning by the added reinforcer, consistent with the Rescorla-Wagner (1972) model, and that due to increases in conditioning to the original reinforcer, consistent with the Pearce-Hall (1980) and other models of learning, the lesions prevented unblocking of the latter type. These results were discussed in the context of roles of the basolateral amygdala in coding and using reward prediction error information in associative learning.
Keywords: amygdala, associability, Pearce-Hall model, Rescorla-Wagner model, reward prediction error, unblocking
Most theories of associative learning assert that violation of learned expectancies can produce alterations in processing of the cues or reinforcers present near the time of those surprising outcomes. A common arena for evaluating those theories is the blocking/unblocking paradigm. In blocking, prior conditioning of one cue is found to interfere with learning about a second cue if a compound of those two cues is subsequently paired with the same reinforcer. Because that reinforcer is already well-predicted by the first cue when the new cue is introduced, processing of the reinforcer itself (e.g., Rescorla & Wagner, 1972; Sutton & Barto,1981) or the added cue (e.g., Esber & Haselgrove, 2011; LePelley, 2004; Mackintosh, 1975; Pearce & Hall, 1980; Pearce & Mackintosh, 2010) is minimized, and so the association between that cue and the reinforcer is not learned. However, if the value of the reinforcer is altered when the new cue is added, such learning is once again evident. This observation, termed unblocking, has been observed with both increases (“upshifts”; Kamin, 1968; Holland, 1984, 1988) and decreases (“downshifts”; Dickinson & Mackintosh, 1976; Holland, 1984; 1988).
Much attention has been focused on the downshift case, because reinforcer and cue processing theories make opposite predictions. When reinforcer value is decreased, the reward prediction error is negative, and reinforcer-processing theories such as the Rescorla-Wagner (1972) model predict that the added cue will acquire inhibitory learning. By contrast, cue-processing models such as the Pearce-Hall (1980) model predict that excitatory learning will accrue to the added cue, because changes in cue effectiveness (“associability”) are assumed to be driven by an unsigned error term. Thus, within these later theories, any surprising outcome, yielding either positive or negative prediction error, could enhance or restore the associability of the added cue, enabling its association with the reinforcer presented on compound trials.
Research from our laboratory, using a number of experimental paradigms (Holland & Maddux, 2010) identified components of brain circuitry critical to the enhancement of cue associability after negative prediction error consequent to the omission of expected events, including the amygdala central nucleus (CeA), the substantia nigra pars compacta, the sublenticular substantia innominata, and the posterior parietal cortex. Investigations of unblocking with a downshift found that rats with lesions of CeA failed to show normal excitatory learning about the added cue (Holland & Gallagher, 1993b; Holland & Kenmuir, 2005), but were intact in their learning of inhibitory associations between the added cue and the omitted reinforcer (Holland & Kenmuir, 2005).
By contrast, CeA lesions had no effect on additional learning that accrued when reinforcer value was increased in an unblocking design (Holland & Gallagher, 1993b), even when steps were taken to insure that the unblocking obtained reflected enhanced cue associability rather than enhanced processing of the unexpected new reinforcer (Holland, 2006). Results such as these led us to conclude that the role of CeA in associability enhancements was limited to cases involving negative prediction errors (Holland & Maddux, 2010). Notably, these observations are consistent with the results of a recent electrophysiological study in which we observed CeA neurons that responded to downshifts in reward value, but did not find significant numbers of neurons that responded to upshifts (Calu et al., 2010). However, considerable evidence indicates that another amygdala subregion, the basolateral amygdale (BLA, comprising the lateral, basal, basolateral, basomedial, and accessory basal nuclei) encodes reinforcer value (e.g. Holland & Gallagher, 2004). Furthermore, a study that used methods similar to those of Calu et al. (2010) found a significant number of BLA neurons that responded to both decreases and increases in US value (Roesch et al., 2010). Thus, these neurons provided precisely the unsigned error signal specified by theories such as the Pearce-Hall (1980) model. From these observations it seems reasonable to suggest that the BLA might be critical to processing of unsigned reinforcer prediction error signals that enhance cue associability in unblocking.
The experiments reported here considered whether enhanced learning with upshifts in reinforcer value might be mediated by the BLA. In Experiment 1, following the experimental procedures used by Holland and Gallagher (1993b), we found no evidence for a BLA role in unblocking with upshifts in reinforcer value. However, because upshift unblocking could in principle result solely from the greater effectiveness of the reinforcer when its value was increased (as expected within theories like the Rescorla-Wagner model, 1972), it could be argued that this study did not provide a fair test of BLA’s involvement in the enhancement of cue associability after upshifts in reward value, as predicted by theories such as the Pearce-Hall (1980) model. Therefore, in Experiment 2, we examined the effects of BLA lesions on upshift unblocking in a more complex paradigm used previously to disentangle cue- and reinforcer-processing contributions to upshift unblocking (Holland, 1988, 2006).
Experiment 1
In Experiment 1, we examined the effects of BLA lesions on upshift unblocking by using two qualitatively similar reinforcers (food pellets) delivered to the same food cup, as in Holland and Gallagher’s (1993b) experiment. Rats in the unblocking condition (UNB) first received a visual cue paired with the delivery of a single food pellet, and then in a second phase received pairings of a compound of that light and a white noise with delivery of one pellet followed 5 s later by two more pellets, a serial “food→food” reinforcer. Rats in a control condition (CTL) received similar training, except that the food→food reinforcer was used in both phases. Unblocking was assessed by examining responding to the noise alone during test sessions interspersed in Phase 2 training. If upshifts in reinforcer value enable learning about the noise that would otherwise have been blocked by prior training of the light, then the rats in the unblocking condition should respond more to the noise alone than rats in the control condition.
Approximately half of the rats in each condition received excitotoxic lesions of the BLA prior to the start of the experiment, and the remaining rats received sham lesions. The primary question of Experiment 1 was whether these lesions interfered with the observation of unblocking in the test sessions.
Methods
Subjects
The subjects were male Long-Evans rats from Charles River Laboratories (Raleigh, NC), housed individually in a colony room which was illuminated from 7 AM to 7 PM daily. Rats had ad libitum access to food and water until two weeks after surgery. Then, the rats’ access to food was restricted, and they were maintained at 85% of their ad libitum body weights throughout the rest of the experiment. Water was always available in the home cage.
Surgical Procedures
Surgeries were performed under isoflurane anesthesia with aseptic conditions. Bilateral BLA lesions were made with 10 mg/ml N-methy-D-aspartate (NMDA; Sigma, St. Louis, MO) in a phosphate-buffered saline (PBS) solution. Injections were made using a 2.0 µl Hamilton syringe over a 2-minute period for each injection. The stereotaxic coordinates used for BLA lesions were 2.8 mm posterior of bregma, 5.1 mm from the midline, with infusions made at 8.7 mm (0.16 µl) and 8.4 mm (0.08 µl) ventral from the skull surface at bregma. For sham-lesioned rats, the syringe needle was lowered to the same stereotaxic coordinates as for BLA lesions, but no injections were made.
Apparatus
Eight individual chambers (22.9 cm X 20.3 cm X 20.3 cm) were used, each of which was encased in a sound-resistant shell. The chambers had aluminum front and back walls, clear acrylic sides and top, and the floor was made of 0.48-cm stainless steel rods spaced 1.90 cm apart. A food cup was located at one end of the chamber, and a sucrose cup (not used in Experiment 1) was located at the opposite end of the chamber. A 6-W flashing lamp mounted on the wall of the chamber, 10 cm above the food cup, was used as the visual cue. An 80-dB white noise from a speaker located outside the chamber along the back wall of each shell was used as the auditory cue. A 6-W house lamp, used as a second visual cue in Experiment 2, but not in Experiment 1, was mounted next to the speaker. A 6-W light behind a red lens located on the ceiling of each shell provided constant dim illumination during sessions. A video camera was mounted on the back wall of each shell for recording training sessions, but video data are not reported here. Ventilation fans provided masking noise (70 dB).
Procedure
Rats first were trained to eat from the food cup. In each of two 64-min sessions, rats received 16 deliveries of one 45-mg food pellet (Test Diets, Richmond, IN) followed 5 s later by two food pellets.
Table 1 provides an outline of the major procedures of Experiment 1. In each of twelve 64-min Phase 1 Pavlovian conditioning sessions, rats in Group UNB (unblocking condition) received eight 10-s presentations of the flashing panel light (V1) followed by a single food pellet (food 1). Rats in Group CTL (control) received pairings of V1 with a single pellet followed 5 s later by two more pellets (food 2).
Table 1.
Outline of Experimental Procedures
| Experiment 1 | |||
| Group | Phase 1 | Phase 2 | Test |
| UNB | V1→fd1 | V1N→fd1→fd2 | N |
| CTL | V1→fd1→fd2 | V1N→fd1→fd2 | N |
| Experiment 2 | |||
| Group | Phase 1 | Phase 2 | Test |
| UNB | V1→food | V1N→food→suc | N |
| V2→suc | V2→suc | ||
| CTL | V1→food→suc | V1N→food→suc | N |
| V2→suc | V2→suc | ||
UNB, Unblocking condition; CTL, control condition; V1 and V2, visual cues; N, white noise cue; fd1, 1 food pellet; fd2, 2 food pellets; suc, sucrose.
In each of eight 64-minute sessions of compound conditioning (Phase 2), rats in each group received eight 10-s presentations of V1 and an 80-dB white noise followed by food 1 and then 5 s later by food 2. Thus, when the noise was introduced, rats in Group UNB received an upshift in the value of the reward, while for rats in Group CTL the value of the reinforcer did not change.
On the day after sessions 4 and 8 of compound conditioning, rats received a test session to assess conditioning to the added noise. In each of these two 32-minute sessions, all rats received four 10-s, non-reinforced presentations of the noise alone.
Response measures and data analysis
The measure of conditioning was the amount of time spent with the head in the food cup during any designated time interval, divided by the duration of that interval (% time in food cup). Because previous studies (e.g., Holland, 1977) showed that food cup behaviors are typically timed to occur near the time of reinforcer delivery, we reported responding during the last 5 s of the cue interval. For the nonreinforced test sessions, we also reported responding during the first two empty 5-s periods after cue termination, corresponding to the times when the two food reinforcers were delivered in compound conditioning. All statistical analyses were conducted at the p < .050 level of significance using mixed ANOVAs, with treatment (UNB vs CTL) and lesion (BLA vs. sham) as between-subject variables, and 5-s interval and/or session as within-subject variables.
Histological Procedures
After behavioral testing was completed, all rats were deeply anesthetized with pentobarbital (150 mg/kg) and perfused with 0.1M PBS, followed by 10% (v/v) Formalin. Brains were removed and stored in 0.1M PBS and 20% (w/v) sucrose. Forty-µm sections were taken, which were mounted on slides and Nissl-stained for lesion verification. Lesions were evaluated by drawing lesions on corresponding plates from Paxinos and Watson (1998) and calculating the percentage of BLA’s area that showed lesion damage. Lesion drawings and damage assessments were made blind with respect to behavioral performance.
Results
Histological results
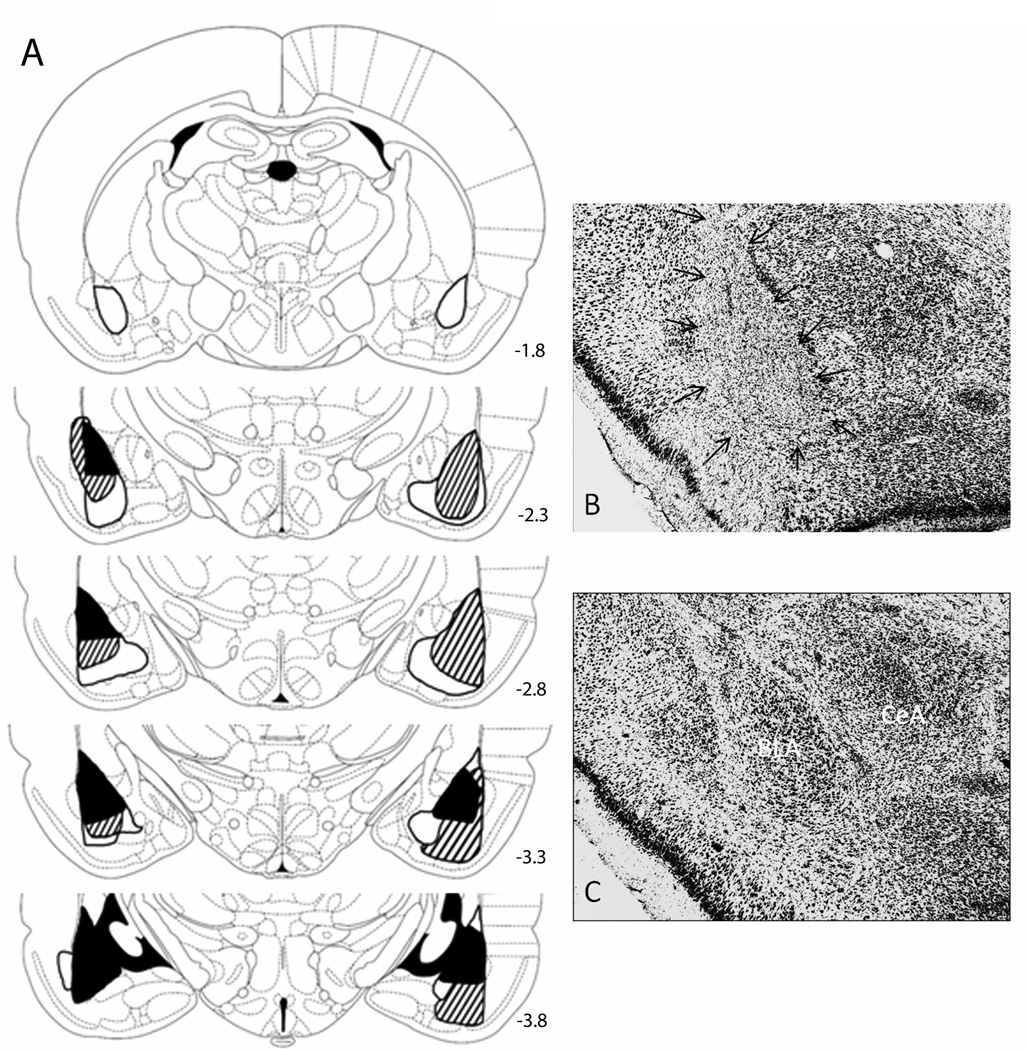
Of 25 BLA-lesioned rats, 13 (6 in Group CTL and 7 in Group UNB) had acceptable (greater than about 40%) bilateral BLA damage, with little bilateral damage to adjoining areas, including CeA. Two evaluators agreed on all accept/reject judgments. The mean (± sem) percentage of damage was 62.4 ± 5.9% in Group UNB and 64.8 ±6.3% in Group CTL, with most of the sparing in anterior areas. The 12 rejected lesions showed insufficient damage to one or both BLAs, or significant damage to CeA. Rats with sham lesions (CTL: 10; UNB: 9) had no visible damage except along the injector track. Figure 1 illustrates representative BLA and sham lesions.
Figure 1.
(A) Drawings of smallest (dark shading), largest (light shading) and median lesions (lined) of basolateral amygdala (BLA), and photomicrographs of representative neurotoxic (B) and sham (C) lesions. In excitotoxic lesions (arrows), extensive neuron loss marked by gliosis is confined to the BLA, sparing the amygdala central nucleus (CeA). The numbers on the atlas sections refer to stereotaxic distances posterior to bregma. The sections are from The Rat Brain in Stereotaxic Coordinates (4th ed.), Figures 26, 29, 31, 33, 35, by G. Paxinos & C. Watson, 1998, New York: Academic Press. Adapted by permission of Elsevier.
Behavioral results
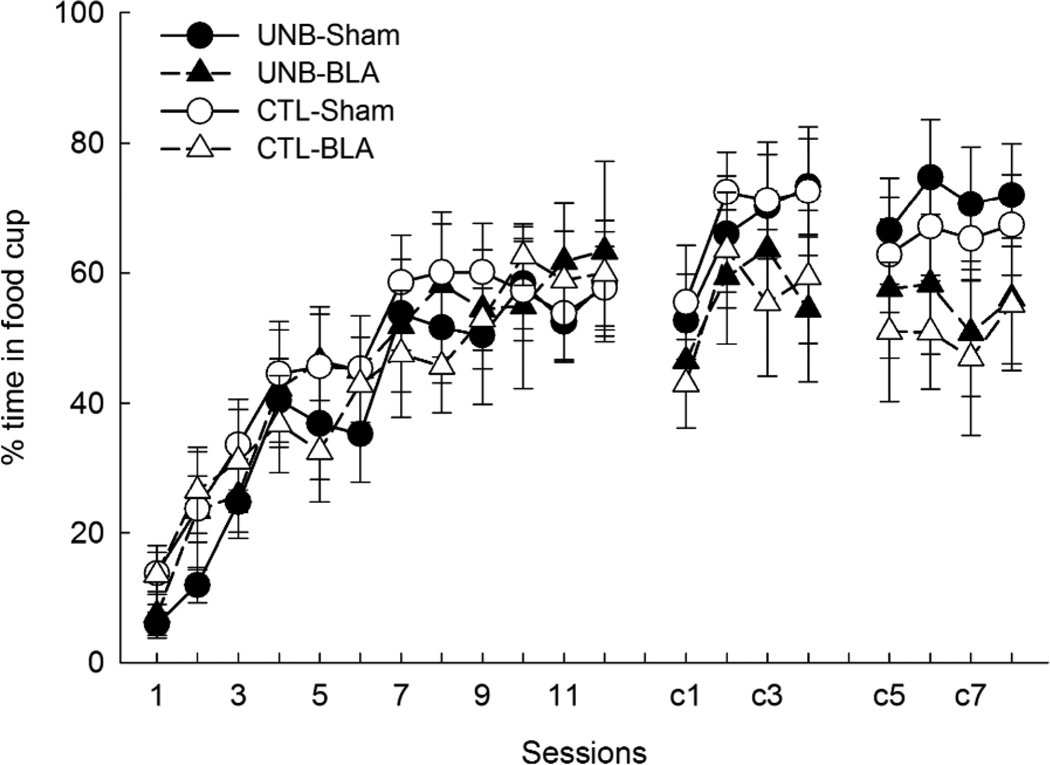
Figure 2 shows the acquisition of food cup behavior during V1 and the V1+noise compound in Phases 1 and 2. All rats rapidly acquired conditioned responding during Phase 1, regardless of lesion or treatment. ANOVA showed a significant effect of session, (F11,308 = 32.23; p < .001), but no other effects or interactions were significant, Fs < 1, ps > .48. After some initial disruption, responding was maintained at similar levels in Phase 2. ANOVA showed a main effect of session, (F7,196 = 8.02, p < .001), but no other effects or interactions were significant (largest F1,28 = 2.33, p > .138 [effect of lesion]).
Figure 2.
Mean (±sem) food cup behavior during the visual cue (V1) in phase 1 acquisition and during the V1 + noise compound in phase 2 of Experiment 1. Test sessions were administered after compound sessions c4 and c8. The dependent variable is the time spent in the food cup during the last 5 s of the cue, expressed as a percentage of that 5-s interval.
Test results
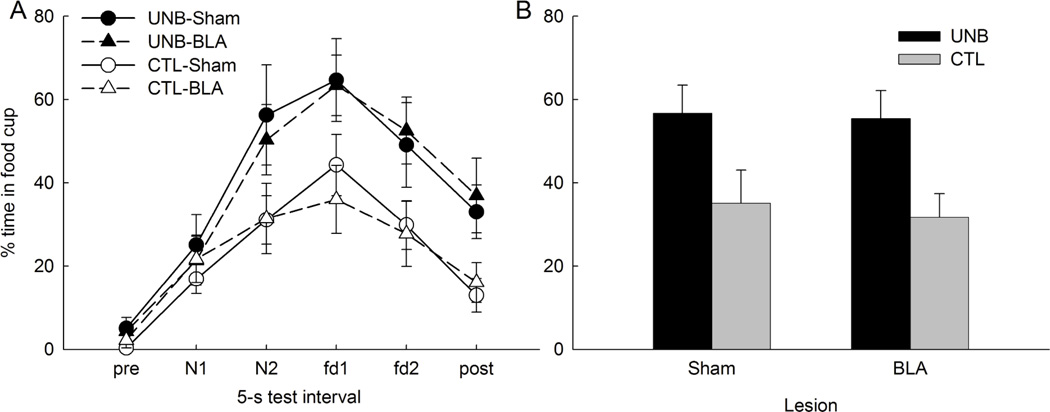
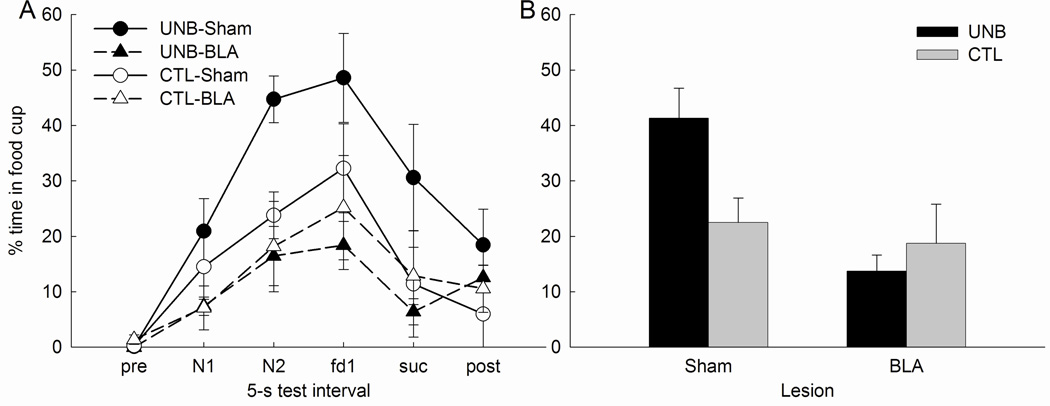
Rats that received an upshift in the reinforcer when the noise was introduced responded significantly more to that stimulus than rats that continued to receive the same reinforcer, regardless of lesion condition. Thus, unblocking occurred, but it was unaffected by the BLA lesion. Figure 3A shows food cup responding during the 5-s intervals that comprised the pre-CS period, the two 5-s CS periods, periods in which the first and second food USs were delivered in the compound conditioning phase, and a final 5-s period, combined across the two test sessions. Figure 3B shows that responding collapsed across the test intervals used to assess conditioning (the final 5-s CS period and the two US periods). A treatment X lesion X test X interval ANOVA showed a significant effect of treatment (F1,28 = 7.84; p = .009), but no effect of lesion (F1,28 = 0.93; p = .342) or treatment X lesion interaction (F1,28 = 0.51; p =.483). Planned comparisons showed that responding was greater in the UNB condition than in the CTL condition for both sham (F1,28 = 5.69, p = .024) and BLA-lesioned (F1,28 = 4.56, p = .042) rats. Finally, responding during the 5-s pre-CS periods did not differ among the groups (range = 1.0 ± 0.9% to 5.3 ± 3.5%; largest F1,28 = 1.72, p = .200).
Figure 3.
Mean (±sem) food cup responding during the Test phase of Experiment 1. Panel A shows responding during each of the 5-s test intervals, including the pre-CS interval, the first (N1) and second (N2) 5-s period of the noise cue, the 5-s period when food1 (fd1) would have been delivered in phase 2, the 5-s period when food2 (fd2) would have been delivered in phase 2, and the 5-s post-reinforcer period. Panel B shows average responding collapsed over the N2, fd1 and fd2 periods. No food was presented in the test sessions.
Discussion
Although all rats received the same pairings of a visual + noise compound with the food→food reinforcer in Phase 2, rats that originally received pairings of the visual cue with a single reinforcer learned significantly more about the added noise. One account of these data is that prior training of the visual cue with the foodfood reinforcer in the CTL condition blocked conditioning to the noise because that reinforcer was already well-expected when the noise was introduced. By contrast, because the visual cue had been trained with only a single pellet reinforcer in the UNB condition, presentations of the compound in those rats would be followed by an additional ‘surprising’ pair of pellets, enabling learning about the added noise stimulus.
Importantly, such additional learning is anticipated both within the Rescorla-Wagner (1972) model, because the unexpected delivery of the pair of pellets in the UNB condition would be a more effective reinforcer than the expected delivery of that same pair of pellets in the CTL condition, and the Pearce-Hall (1980) model, in which the surprising delivery of the new reinforcer would enhance the associability of the noise, allowing it to accrue more learning. Thus, our failure to observe an effect of BLA lesions in this experiment might simply indicate that BLA is not involved in modifications of reinforcer efficacy, as specified by the Rescorla-Wagner (1972) model, and hence may be uninformative about BLA’s role in changes in associability. Experiment 2 was designed to disentangle these two possibilities.
Experiment 2
Holland (1988, 2006) developed a procedure to distinguish between cue- and reinforcer-processing contributions to upshift unblocking. When the target cue was introduced, the original reinforcer was supplemented by the delivery of an additional, qualitatively different reinforcer to a separate location. In this way, additional learning about the first reinforcer as a result of the surprising delivery of the second (as specified by Pearce & Hall, 1980) could be distinguished from learning about the second reinforcer (as suggested by Rescorla & Wagner, 1972) by the nature of the response elicited by the target cue at test, that is, whether the rats approached the food cup associated with the first or second reinforcer.
Experiment 2 was similar to Experiment 1, except that two qualitatively different USs (food pellets and sucrose) were delivered to two different locations, as in Holland’s (1988, 2006) studies. In Phase 1, rats in the unblocking (UNB) condition received V1-food pairings, followed by Phase 2 pairings of a V1+N compound with a serial food→sucrose reinforcer. Rats in the CTL control condition received the same kinds of trials, but the food→sucrose reinforcer was delivered after V1 in the first phase as well. To insure that both groups had ample experience approaching the sucrose cup, rats in both groups received pairings of a second visual cue (V2) with sucrose, intermixed in each Phase 1 and Phase 2 training session. Finally, as in Experiment 1, responding to the noise alone was assessed in test sessions administered after Phase 2 compound sessions 4 and 8. If unblocking occurs according to Pearce-Hall (1980) cue processing mechanisms, then the surprising addition of sucrose should enhance the associability of the noise. In that case, addition of the sucrose reinforcer should result in learning (unblocking) of both noise-food and noise-sucrose associations, indexed by responding to the food and sucrose cups, respectively. If unblocking occurs according to Rescorla-Wagner (1972) reinforcer processing mechanisms, then the addition of sucrose should result only in noise-sucrose learning, because the food reinforcer was already predicted by V1, and hence would be ineffective as a reinforcer.
Methods
Subjects, apparatus, surgical, and histological procedures
The subjects were male Long-Evans from Charles River Laboratories (Raleigh, NC), housed and treated in the same manner as the rats in Experiment 1. The apparatus, surgical procedures, and histological procedures were the same as those used in Experiment 1.
Procedure
Rats first received training to eat from the food cup. In each of two 64-min sessions, they received 16 deliveries of one 45-mg food pellet. Next, the rats were trained to drink sucrose from the sucrose cups. In each of two 64-min sessions, they received sixteen 0.4-ml deliveries of 0.2M sucrose.
Table 1 provides an outline of the major procedures of Experiment 2. In each of twenty-eight 64-min Phase 1 Pavlovian conditioning sessions, rats in Group UNB (unblocking) received four 10-s presentations of V1 followed by a single food pellet, and rats in Group CTL (control) received pairings of V1 with a food pellet followed 5 s later by sucrose. In addition, all rats received four 10-s presentations of V2 followed by sucrose in each session. The identity of V1 and V2 (panel light or houselight) was counterbalanced across both groups, and the presentation order of V1 and V2 trials within each session was randomized.
In each of eight 64-minute sessions of compound conditioning (Phase 2), rats in each group received four 10-s presentations of V1 and an 80-dB white noise (N) followed by a food pellet and then 5 s later by sucrose. As in Phase 1, all rats received four V2-sucrose presentations in each of these sessions. Thus, rats in Group UNB received an upshift in the value of the reward at the time when N was introduced, while the value of the reinforcer for rats in Group CTL did not change.
On the day after compound conditioning sessions 4 and 8, rats received a test session to assess conditioning to N. In each of two 32-min sessions, all rats received four 10-s, non-reinforced presentations of N alone.
Response measures
The response measures used were the same as in Experiment 1, with the addition of the percentage of time spent in the sucrose cup.
Results
Histology
Out of 29 BLA-lesioned rats, 13 had acceptable bilateral BLA damage, following the same criteria as in Experiment 1. Two evaluators agreed on all accept/reject judgments. Percentage damage was 64.8 ± 4.2% and 67.3 ± 4.2% in Groups UNB and CTL, respectively. All rats with sham lesions had no visible brain damage other than minor cell loss along the injector tract. However, because one sham-lesioned rat showed nearly no food cup behavior during test sessions (5 sem below the overall mean and 9 sem below its group mean), all data from that rat were excluded from analysis. Thus, the final numbers of subjects were: UNB-Sham, 7; UNB-BLA, 8; CTL-Sham, 8; CTL-BLA, 5).
Phase 1 and Phase 2 training behavior
Food cup responding during V1
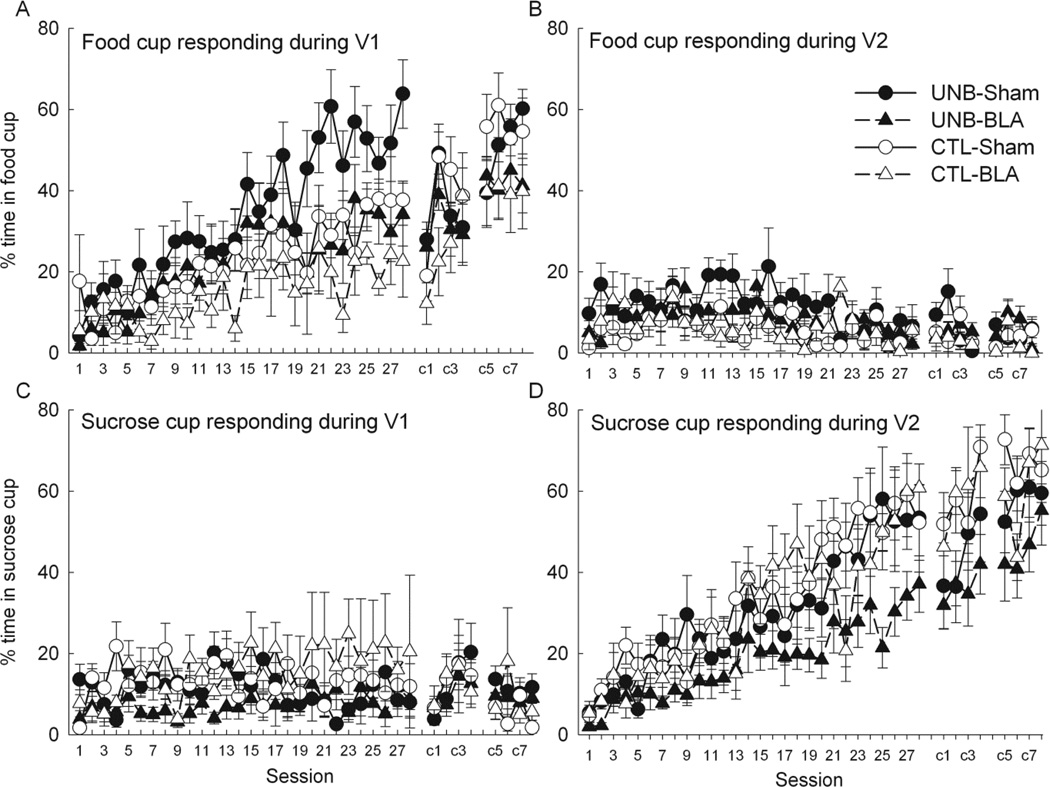
Figure 4A shows the acquisition of food cup behavior during V1 in Phase 1 and during the V1N compound during Phase 2. By the end of Phase 1 training especially, rats in the UNB condition showed greater food cup responding than rats in the CTL condition. A repeated-measures ANOVA of Phase 1 responding revealed significant main effects of treatment (F1,24 = 4.29; p = .049) and session (F27,648 = 14.36, p < .001) and their interaction (F27,648 = 1.75; p = .011). This greater food cup responding in the UNB condition was to be expected, because in the UNB condition, V1 was paired with food only, whereas in the CTL condition, V1 was paired with both food and (5 s later) sucrose. Thus, in the CTL condition, a tendency to enter the sucrose cup may have interfered with the display of food cup responding (see Fig. 4C, below). In addition, by the end of Phase 1 training there was greater responding in sham than BLA-lesioned rats, as indicated by a marginally significant main effect of lesion (F1,24 = 4.03, p = .056) and a significant lesion X session interaction (F27,648 = 1.55, p = .039). There were no treatment X lesion (F1,24 = 0.17, p = .683) or lesion X treatment X session (F27,648 = 1.14; p =.285) interactions.
Figure 4.
Mean (± sem) food- and sucrose-cup responding in phases 1 and 2 of Experiment 2. Panels A and C show food cup and sucrose cup responding, respectively, during the V1 visual cue in phase 1 and the V1 + noise compound in phase 2 of Experiment 2. Panels B and D show food and sucrose cup behavior, respectively, during the V2 visual cue in phases 1 and 2. Test sessions were administered after compound sessions c4 and c8.
When the noise was introduced in Phase 2, food cup responding dropped substantially, recovering gradually over the course of Phase 2; the main effect of session was significant (F7,168 = 13.52; p <.001). However, the differences in food cup responding observed between groups during Phase 1 appeared diminished, with no effect of treatment (F1,24 = 0.01; p = .933), consistent with the fact that the two treatments were identical in Phase 2. The effect of lesion was marginally significant (F1,24 = 3.80; p =.063), but the treatment X lesion (F1,24 = 0.50; p =.486) and treatment X lesion X sessions (F7,168 = 0.69; p = .680) interactions were not.
Sucrose cup responding during V1
Figure 4C shows sucrose cup responding during V1. Although there was a tendency in Phase 1 for sucrose cup responding to be greater in Group CTL, in which V1 was followed eventually by sucrose, than in Group UNB, in which V1 was never paired with sucrose, that difference was not significant, (F1,24 = 2.51, p = .126). The lesion X session interaction was significant, F(27,648) = 1.76, p = .010); however, no systematic contrasts of the lesion effect across sessions (e.g., first- vs second half of training) were significant, ps > .100. In Phase 2, sucrose cup responding was initially disrupted, recovered briefly and then declined (effect of sessions, F(7, 168 = 3.76, p < .001; quadratic trend over sessions, F(1,24) = 8.69, p = .007). No other effects or interactions were significant, ps > .277.
Sucrose cup responding during V2
Figure 4D shows the acquisition of sucrose cup behavior during V2 in Phases 1 and 2. Rats in the CTL condition showed numerically greater sucrose cup behavior than rats in the UNB condition. This nonsignificant (F1,24 = 2.96, p =.098) difference is consistent with generalization between V1 and V2; rats in the CTL condition received sucrose on both V2 and V1 trials, whereas rats in the UNB condition received sucrose only on V2 trials. ANOVA showed a significant effect of session (F27,648 = 22.97, p <.001), but neither the effect of lesion (F1,24 = 2.44; p = .131), the treatment X lesion interaction (F1,24 = 1.10, p = .304), nor any other interaction (Fs27,648 ≤ 1.37; p ≥ .104) was significant.
These trends continued during Phase 2. ANOVA of sucrose cup behavior during V2 showed significant main effects of session (F7,168 = 7.22; p < .001) and treatment (F1,24 = 5.18; p = .032), but no main effect of lesion (F1,24 = 1.07; p = .310), or treatment X lesion interaction (F1,25 = 0.25; p = .621). No other interactions were significant (Fs7,168 <1.59; ps > .141).
Food cup responding during V2
Although the rats displayed relatively little food cup responding during V2 (Figure 4B), rats in the CTL condition showed less food cup behavior than those in the in the UNB condition, consistent with their displaying more sucrose behavior (described previously, Figure 4D). ANOVA of Phase 1 food cup responding showed significant effects of treatment (F1,24 = 5.22, p = .031) and sessions (F27,648 = 2.16, p < .001), but no other effects or interactions were significant, ps > .208. The lower level of food cup responding during V2 in rats in the CTL condition was maintained in Phase 2, although not significantly (F1,24 = 3.36, p = .079). No other main effects or interactions were significant, ps > .129.
Test Behavior
Rats showed little evidence of conditioned responding in the first test session, which was administered after only half as many compound conditioning trials as in Experiment 1. Thus, we present only the data from the second test session. Figure 5A shows food cup responding during each 5-s interval on noise-alone trials in Test 2. Figure 5B shows responding collapsed across the measurement intervals (second 5-s of the CS and both 5-s US intervals). Sham-lesioned rats showed significant unblocking, but BLA-lesioned rats did not. A treatment X lesion ANOVA of the food cup responding displayed in Figure 5B revealed no significant effect of treatment (F1,24 = 2.00; p = .170), but a significant main effect of lesion (F1,24 = 10.29, p = .004) and, most important, a significant treatment X lesion interaction (F1,24 = 5.95; p = .022). Planned comparisons showed that there was significantly more food cup behavior in the UNB than CTL conditions in sham-lesioned rats (F1,24 = 8.22, p = .009) but not in BLA-lesioned rats (F1,25 = 0.48, p = .495). Furthermore, rats in the UNB-Sham condition showed more food cup responding than rats in the UNB-BLA condition (F1,24 = 17.65, p < .001), but responding in the CTL condition did not differ between the lesion conditions (F1,24 = 0.27, p = .609).
Figure 5.
Mean (±sem) food cup responding during the second test session of Experiment 2. Panel A shows responding during each of the 5-s test intervals, including the pre-CS interval, the first (N1) and second (N2) 5-s period of the noise cue, the 5-s period when food (fd) would have been delivered in phase 2, the 5-s period when sucrose (suc) would have been delivered in phase 2, and the 5-s post-reinforcer period. Panel B shows average responding collapsed over the N2, fd, and suc periods. No food or sucrose was presented in the test phase.
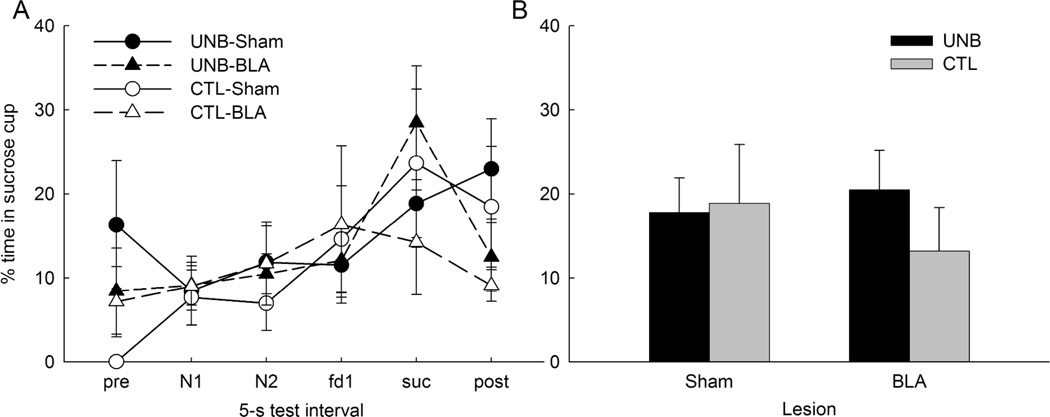
Figure 6 shows sucrose cup responding during the test. Rats gradually spent more time in the sucrose cup during the later stages of each trial, peaking in the 5-s interval when sucrose would have been delivered or the subsequent (post) interval. These results are supported by a repeated-measures ANOVA that revealed a main effect of period (F5,120 = 9.91, p <.001). However, we did not find a main effect of lesion (F1,24 = 0.05; p =.825), treatment (F1,24 = 0.23; p =.637), or a treatment X lesion interaction (F1,24 = 0.12; p =.727). Similar ANOVAs of responding in the former food, sucrose, or post-sucrose periods alone or combined also yielded no significant effects of treatment, lesion or treatment X lesion interaction, ps > .211, although for the post-sucrose period alone, the effect of lesion was marginally significant (p = .099). Thus, we found no evidence for unblocking of learning about the added sucrose reinforcer in either sham or lesioned rats.
Figure 6.
Mean (±sem) sucrose cup responding during the second test session of Experiment 2. Panel A shows responding during each of the 5-s test intervals, including the pre-CS interval, the first (N1) and second (N2) 5-s period of the noise cue, the 5-s period when food (fd) would have been delivered in phase 2, the 5-s period when sucrose (suc) would have been delivered in phase 2, and the 5-s post-reinforcer period. Panel B shows average responding collapsed over the fd, suc and post-sucrose periods. No food or sucrose was presented in the test phase.
Discussion
In Experiment 2, rats with BLA lesions showed no evidence for unblocking in a procedure designed to distinguish unblocking that arises from additional conditioning between the added cue and the added (sucrose) reinforcer from unblocking that reflects enhanced learning of associations between the added cue and the initial (food) reinforcer. Although theories of either the Rescorla-Wagner (1972) or Pearce-Hall (1980) type could account for the former outcome, the enhanced noise-food learning after the surprising delivery of sucrose shown by sham-lesioned rats in Experiment 2 is inconsistent with the Rescorla-Wagner (1972) model, and more in accord with models such as that of Pearce and Hall (1980). Thus, our observation that this new learning was absent in rats with BLA lesions supports Roesch et al.’s (2010) suggestion that BLA neurons that code unsigned prediction error after reinforcer shifts may be important for learning enhancements specified by the Pearce-Hall (1980) model.
It might be argued that the results of Experiment 2 are compromised somewhat by evidence for another learning deficit in rats in the UNB condition. In Phase 1, food cup responding to V1 was lower in lesioned rats than in sham-lesioned rats, and sucrose cup responding to V2 showed an insignificant trend in the same direction. This deficit may be related to the known involvement of the BLA in the differential-outcome-expectancy (DOE) effect. Although our event delivery procedures were Pavlovian (i.e., independent of the rats’ behavior), the actual receipt of the reinforcers was contingent on the rats’ entering the correct reinforcer cup. Thus, rats were reinforced for performing food cup responses in the presence of V1 (but not V2) and for performing sucrose cup responses in the presence of V2 (but not V1). In intact rats, arranging a consistent relation between explicit instrumental responses (e.g., left- and right-lever presses) and qualitatively different reinforcers often accelerates discrimination learning, compared to control conditions in which both responses are reinforced with the same outcome, or each correct response is reinforced randomly with either of the two reinforcers (McDannald, Saddoris, Gallagher, & Holland, 2005; Trapold & Overmier, 1972). This DOE effect is thought to reflect the fact that outcome expectancy information provided by the discriminative cues is uniquely associated with particular responses in the DOE case, but is equally associated with both responses in the control cases. Thus, any reward expectancies conditioned to the discriminative cues would enhance the discriminability of those cues in the DOE case by providing additional relevant cues, but reduce their discriminabilty in the control conditions by providing additional irrelevant cues. Importantly, BLA lesions are known to affect rats’ ability to form or use cue-outcome expectancies (Hatfield et al., 1996; Holland & Gallagher, 2004; Johnson, Gallagher, & Holland, 2009). More specifically, rats with BLAs lesions fail to display a DOE effect (Blundell, Hall, & Killcross, 2001; McDannald et al., 2005). Consequently, it is possible that our sham-lesioned rats showed better V1 learning than BLA-lesioned rats in Phase 1 because of implicit instrumental DOE contingencies embedded in Pavlovian training.
However, for several reasons, we do not think this deficit contributed to our observation of reduced unblocking in BLA-lesioned rats. First, if learning about the visual cues in Phase 1 was impaired in BLA-lesioned rats, then V1 should be a relatively poor blocking stimulus in Phase 2. Hence, one would expect more learning to the noise in BLA-lesioned rats than in sham-lesioned rats. Instead, we observed similar levels of blocking, and less unblocking in BLA-lesioned rats. Second, although implicit DOE contingencies may have been present for the visual cues, these conditions were not applicable for the noise target cue in Phase 2, which was trained in a nondiscriminative fashion (both food cup and sucrose cup responses would have been reinforced after noise presentations in that phase). Third, if BLA-lesioned rats were unable to differentially represent the qualitative properties of the food and sucrose reinforcers, then the food→sucrose reinforcer of Experiment 2 would be functionally identical to the food→food reinforcer of Experiment 1, in which the BLA lesion had no effects on unblocking.
Thus, we conclude that BLA lesions prevented unblocking with upshifts in reward number under conditions in which that phenomenon is not attributable to additional learning about the added sucrose reinforcer, as specified by the Rescorla-Wagner (1972) model, but rather to increased learning of the association between the added cue and the original food reinforcer. Indeed, we found no evidence that the surprising addition of the sucrose reinforcer produced any unblocking of noise-sucrose learning, compared to noise-sucrose learning in control rats for which the sucrose was already well-predicted on the basis of prior learning to V1 alone.
General discussion
In Experiment 1, we found no effect of BLA lesions in unblocking with upshifts in reinforcer number, using a simple design that did not distinguish among accounts for the phenomenon. For example, according to the Rescorla-Wagner (1972) model, the shift to the more valuable food→food reinforcer in the compound phase in the UNB condition would have permitted additional conditioning to accrue to the noise that was introduced at that time. By contrast, in the CTL condition, because the food→food reinforcer was already anticipated on the basis of the visual cue alone, that reinforcer would have been ineffective in establishing associations with the added noise. Alternately, according to Pearce and Hall (1980), the surprise (prediction error) produced by delivery of the second set of food pellets may have enhanced the associability of that noise, permitting greater learning than in the CTL condition, in which no such enhancement would have occurred.
In Experiment 2, we found that rats with BLA lesions failed to show unblocking under conditions in which that phenomenon was not attributable to mechanisms specified by the Rescorla-Wagner (1972) model. In sham-lesioned rats, surprising presentation of a sucrose reinforcer in the UNB condition enhanced the formation of associations between the added noise and the original, expected food reinforcer, an outcome consistent with Pearce and Hall’s (1980) theory but not that of Rescorla and Wagner (1972).
From these results it is tempting to conclude, informed by Roesch et al. (2010), that BLA plays a critical role in surprise-induced enhancements of cue associability, as described by Pearce and Hall (1980). However, previously we (Holland, Hatfield, & Gallagher, 2001) found that BLA lesions had no effect on another example of surprise-induced enhancement in cue processing. In this ‘serial prediction’ (Wilson, Boumphrey, & Pearce, 1992) task, rats first received extensive training with a serial light→tone compound, reinforced with food on half its presentations and nonreinforced on the other half. In a second phase, the tone was omitted on the nonreinforced trials for rats in a “surprise” condition, whereas training continued as before in a “consistent” condition. Enhancements in the light’s associability produced by the surprising omission of the tone were revealed as more rapid learning of new associations to the light in a subsequent test phase in which the light was directly paired with food. Although lesions of a number of brain regions, including the CeA (Holland & Gallagher, 1993a), the substantia innominata (Chiba, Bucci, Holland, & Gallagher, 1995; Han, Gallagher, & Holland, 1999), the posterior parietal cortex (Bucci, Holland, & Gallagher, 1998) and the substantia nigra pars compacta (Lee et al., 2006, 2008) prevent these enhancements, Holland et al. (2001) found rats with BLA lesions to be unimpaired in this task, despite displaying substantial deficits in another task known to be BLA-dependent. To the extent that BLA is the source of unsigned prediction errors critical to the adjustment of cue associability, one would expect BLA lesions to disrupt all phenomena thought to be mediated by such adjustments.
Notably, the serial prediction task just described differs from the unblocking task examined here in several ways. First, it involves the omission of an expected event (downshift), rather than the presentation of an unexpected event (upshift) as in the present study. Perhaps the BLA is specialized for enhanced cue processing after upshifts (positive prediction errors) and CeA is specialized for enhanced cue processing after downshifts (negative prediction error). Although this particular specialization is novel, many authors have suggested contrasting functions of BLA and CeA in a variety of experimental contexts (e.g., Corbit & Balleine, 2005; Holland & Gallagher, 1999, 2003). Previously, we have found that rats with CeA lesions fail to display unblocking after downshifts, that is, they do not show excitatory learning about a cue that accompanied the surprising omission of the second of two expected reinforcers, but show normal unblocking with upshifts (Holland & Gallagher, 1993b), even with the experimental design used in Experiment 2 (Holland, 2006). If this specialization for processing of positive and negative prediction error were the case, then one would expect that BLA lesions would fail to affect unblocking with downshifts.
A second way in which the unblocking and serial prediction tasks differ is that whereas unblocking involves disconfirmations of reward expectancies, the latter task involves disconfirmation of expectancies about a relatively neutral stimulus, such as a tone. If the BLA were specialized to process reward expectancy information, then one would expect lesions of the BLA to affect unblocking with either upshifts or downshifts, but to have no effect in tasks in which reward expectancies were not manipulated, such as the serial prediction task. From this perspective, Holland et al.’s (2001) failure to find effects of BLA lesions on surprise-induced cue associability enhancements in the serial prediction task is not inconsistent with Roesch et al.’s (2010) suggestion that BLA provides an unsigned prediction error signal after episodes of either positive or negative (reward) prediction error. Although data implicate BLA more generally in performances associated with reward downshift, for example, in the successive negative contrast effect (e.g., Pecoraro & Dallman, 2005), further research is needed to determine whether the BLA is critical for surprise-induced enhancements in learning produced by surprising reward omission.
A recent study suggests another important difference between the nature of learning enhancements in the unblocking and serial prediction tasks. Holland and Kenmuir (2005) found evidence supporting the assertion that the surprising omission or presentation of the second of two expected reinforcers in unblocking tasks may enhance processing of the first reinforcer, rather than of the cues that preceded that reinforcer. Thus, Holland and Kenmuir (2005) suggested that the effectiveness of the first reward was altered indirectly, as a consequence of the unsigned prediction error related to the second reward, in the same manner as such a signal is thought to alter cue associability in the Pearce-Hall (1980) model. It should be recognized that although Roesch et al. (2010) suggested that the unsigned error signal they observed in BLA could be identified with the Pearce-Hall error term used to alter cue associability, that signal might just as easily be used to alter the efficacy of the reward itself. Notably, in Roesch et al.’s (2010) study, the unsigned error signal was observed in response to the reinforcers, not to the cues. Indeed, there was little evidence of alterations in neuronal responses to the cues themselves as the prediction error signal varied. Thus, another potential description of the role of BLA in surprise-induce enhancements of learning is that it is involved in ‘indirect’ (Schultz & Dickinson, 2000) changes in reward processing, as described by Holland and Kenmuir (2005).
Finally, another potential account for the different effects of BLA lesions in different procedures that reveal surprise-induced enhancements of learning rate is that BLA is only required for such enhancements when multiple, qualitatively different reinforcers are involved. A similar assertion has been made in other areas of investigation. For example, many investigators have found that BLA is critical to selective Pavlovian-instrumental transfer (PIT) but not to single-reinforcer PIT (Corbit & Balleine, 2005; Holland & Gallagher, 2003). Similarly, Johnson et al. (2009) found that post-training lesions of BLA disrupted both Pavlovian and instrumental devaluation effects after training with multiple reinforcers but not after training with a single reinforcer. Within this view, an effect of BLA lesions would be expected in Experiment 2, but not in previous serial prediction studies, or more important, in Experiment 1, in which only a single reinforcer type (pellets) was used. This explanation would also help resolve a puzzling aspect of the results of the present experiments. In Experiment 2, although the surprising presentation of sucrose reward produced subtstantial enhancement of noise-food learning in sham-lesioned rats, we saw no evidence for direct noise-sucrose learning. This failure to observe unblocking of the type anticipated by Rescorla and Wagner (1972) in Experiment 2 makes less plausible the suggestion that BLA lesions did not affect unblocking in Experiment 1 because the unblocking in that experiment reflected direct noise-food2 conditioning. If adding a second (sucrose) reinforcer in Experiment 2 failed to produce evidence for unblocking due to a Rescorla-Wagner (1972) mechanism, why should we expect that adding food2 would do so in Experiment 1?
Accounts for other observations of task performance being BLA dependent only when multiple, qualitatively different reinforcers are involved, have involved assertions that BLA function is necessary to properly represent sensory aspects of expected rewards, or to associate those sensory features with affective properties of the rewards. Although, as noted in our discussion of the results of Experiment 2, one might expect that nondifferential coding of the food and sucrose reinforcers of Experiment 2 would make them more like the two food deliveries of Experiment 1 (in which unblocking was unaffected by the BLA lesions), the lesion might disturb processing of the reinforcers in some other way. Within this more generic view, BLA lesions affected unblocking in the present Experiment 2 but did not affect unblocking in Experiment 1 (or learning enhancements in the serial prediction task), because only in the first case were two qualitatively different reinforcers involved. From this perspective, BLA lesions would also not affect unblocking with downshifts with a single reinforcer, but would impair unblocking with either upshifts or downshifts (and performance in the serial prediction task) if multiple reinforcers were made critical to those tasks.
Electrophysiological data (Roesch et al., 2009) show that BLA neurons encode not only reward value, but also upshifts and downshifts in that value. Importantly, in that study, the same neurons appeared to code both positive and negative prediction errors in terms of their absolute value, that is, as unsigned prediction errors. Such an error signal is represented in models such as that of Pearce and Hall (1980) but not the Rescorla-Wagner (1972) model. Here, using an unblocking task in which the occurrence of that phenomenon is not consistent with the Rescorla-Wagner (1972) model, we found that BLA was critical to enhanced learning after an upshift in reward, at least when multiple reinforcers were used. Although there is evidence that BLA is also important to some performances associated with reward downshifts (e.g., Pecoraro & Dallman, 2005), it is not a critical contributor to enhanced learning after omission of expected cues in a serial prediction task (Holland et al., 2001). Further investigation of the role of BLA in enhanced learning produced after the omission of expected rewards, as in downshift unblocking, when multiple versus single reinforcers are used, and when surprise induces enhancement in processing of reinforcers (Holland & Kenmuir, 2005) rather than cues, could help resolve these discrepancies in the literature and provide more complete information about mechanisms by which reward prediction error signals guide learning.
Acknowledgments
This research was supported by grant MH53367.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne
References
- Blundell P, Hall G, Killcross S. Lesions of the basolateral amygdala disrupt selective aspects of reinforcer representation in rats. Journal of Neuroscience. 2001;21:9018–9026. doi: 10.1523/JNEUROSCI.21-22-09018.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci DJ, Holland PC, Gallagher PC. Removal of cholinergic input to rat posterior parietal cortex disrupts incremental processing of conditioned stimuli. Journal of Neuroscience. 1998;18:8038–8046. doi: 10.1523/JNEUROSCI.18-19-08038.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calu DJ, Roesch MR, Haney RZ, Holland PC, Schoenbaum G. Neural correlates of variations in event processing during learning in central nucleus of amygdala. Neuron. 2010;68:991–1001. doi: 10.1016/j.neuron.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba AA, Bucci DJ, Holland PC, Gallagher M. Basal forebrain cholinergic lesions disrupt increments but not decrements in conditioned stimulus processing. Journal of Neuroscience. 1995;15:7315–7322. doi: 10.1523/JNEUROSCI.15-11-07315.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW. Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of Pavlovian-instrumental transfer. Journal of Neuroscience. 2005;25:962–970. doi: 10.1523/JNEUROSCI.4507-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson A, Hall G, Mackintosh NJ. Surprise and the attenuation of blocking. Journal of Experimental Psychology: Animal Behavior Processes. 1976;2:313–322. [Google Scholar]
- Esber GR, Haselgrove M. Reconciling the influence of predictiveness and uncertainty on stimulus salience: A model of attention in associative learning. Proceedings of the Royal Society: B. 2011 doi: 10.1098/rspb.2011.0836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J-S, Holland PC, Gallagher M. Disconnection of the amygdala central nucleus and substantia innominata/nucleus basalis disrupts increments in conditioned stimulus processing in rats. Behavioral Neuroscience. 1999;113:143–151. doi: 10.1037//0735-7044.113.1.143. [DOI] [PubMed] [Google Scholar]
- Hatfield T, Han JS, Conley M, Gallagher M, Holland PC. Neurotoxic lesions of basolateral, but not central, amygdala interfere with Pavlovian second-order conditioning and reinforcer devaluation effects. Journal of Neuroscience. 1996;16:5256–5265. doi: 10.1523/JNEUROSCI.16-16-05256.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC. Conditioned stimulus as a determinant of the form of the Pavlovian conditioned response. Journal of Experimental Psychology: Animal Behavior Processes. 1977;3:77–104. doi: 10.1037//0097-7403.3.1.77. [DOI] [PubMed] [Google Scholar]
- Holland PC. Unblocking in Pavlovian appetitive conditioning. Journal of Experimental Psychology: Animal Behavior Processes. 1984;10:476–497. [PubMed] [Google Scholar]
- Holland PC. Excitation and inhibition in unblocking. Journal of Experimental Psychology: Animal Behavior Processes. 1988;14:261–279. [PubMed] [Google Scholar]
- Holland PC. Enhanced conditioning produced by surprising increases in reinforcer value are unaffected by lesions of the amygdala central nucleus. Neurobiology of Learning and Memory. 2006;85:30–35. doi: 10.1016/j.nlm.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Amygdala central nucleus lesions disrupt increments, but not decrements, in conditioned stimulus processing. Behavioral Neuroscience. 1993;107:246–253. doi: 10.1037//0735-7044.107.2.246. (a) [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Effects of amygdala central nucleus lesions on blocking and unblocking. Behavioral Neuroscience. 1993;107:235–245. doi: 10.1037//0735-7044.107.2.235. (b) [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Amygdala circuitry in attentional and representational processes. Trends in Cognitive Sciences. 1999;3:65–73. doi: 10.1016/s1364-6613(98)01271-6. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Double dissociation of the effects of lesions of basolateral and central amygdala on CS-potentiated feeding and Pavlovian-instrumental transfer. European Journal of Neuroscience. 2003;17:1680–1694. doi: 10.1046/j.1460-9568.2003.02585.x. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Amygdala-frontal interactions and reward expectancy. Current Opinion in Neurobiology. 2004;14:148–155. doi: 10.1016/j.conb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Holland PC, Hatfield T, Gallagher M. Rats with lesions of basolateral amygdala show normal increases in conditioned stimulus processing but reduced conditioned potentiation of eating. Behavioral Neuroscience. 2001;115:945–950. [PubMed] [Google Scholar]
- Holland PC, Kenmuir C. Variations in unconditioned stimulus processing in unblocking. Journal of Experimental Psychology: Animal Behavior Processes. 2005;31:155–171. doi: 10.1037/0097-7403.31.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC, Maddux J-M. Brain systems of attention in associative learning. In: Mitchell CJ, LePelley ME, editors. Attention and learning. Oxford: Oxford University Press; 2010. pp. 305–349. [Google Scholar]
- Johnson AW, Gallagher M, Holland PC. The basolateral amygdala is critical to the expression of Pavlovian and instrumental outcome-specific reinforcer devaluation effects. Journal of Neuroscience. 2009;29:696–704. doi: 10.1523/JNEUROSCI.3758-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamin LJ. Attention-like processes in classical conditioning. In: Jones MR, editor. Miami symposium on the prediction of behavior: Aversive stimulation. Coral Gables, FL: University of Miami Press; 1968. pp. 9–32. [Google Scholar]
- Le Pelley ME. The role of associative history in models of associative learning: A selective review and a hybrid model. Quarterly Journal of Experimental Psychology: Comparative and Physiological Psychology. 2004;57(B):193–243. doi: 10.1080/02724990344000141. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Youn JM, O MJ, Gallagher M, Holland PC. Role of substantia nigra-amygdala connections in surprise-induced enhancement of attention. Journal of Neuroscience. 2006;26:6077–6081. doi: 10.1523/JNEUROSCI.1316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Youn JM, Gallagher M, Holland PC. Temporally-limited role of substantia nigra-central amygdala connections in surprise-induced enhancement of learning. European Journal of Neuroscience. 2008;27:3043–3049. doi: 10.1111/j.1460-9568.2008.06272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackintosh NJ. A theory of attention: Variations in the associability of stimuli with reinforcement. Psychological Review. 1975;82:276–298. [Google Scholar]
- McDannald MA, Saddoris MP, Gallagher M, Holland PC. Lesions of orbitofrontal cortex impair rats’ differential outcome expectancy learning but not conditioned stimulus-potentiated feeding. Journal of Neuroscience. 2005;25:4626–4632. doi: 10.1523/JNEUROSCI.5301-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Pearce JM, Hall G. A model for Pavlovian learning: Variations in the effectiveness of conditioned but not of unconditioned stimuli. Psychological Review. 1980;87:532–552. [PubMed] [Google Scholar]
- Pearce JM, Mackintosh NJ. Two theoreis of attention: a review and a possible integration. In: Mitchell CJ, LePelley ME, editors. Attention and learning. Oxford: Oxford University Press; 2010. pp. 11–39. [Google Scholar]
- Pecoraro N, Dallman MF. c-Fos after incentive shifts: expectancy, incredulity, and recovery. Behavioral Neuroscience. 2005;119:366–387. doi: 10.1037/0735-7044.119.2.366. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF, editors. Classical conditioning II. New York: Appleton-Century-Crofts; 1972. pp. 64–99. [Google Scholar]
- Roesch MR, Calu DJ, Esber GR, Schoenbaurm G. Neural correlates of variations in event processing during learning in basolateral amygdala. Journal of Neuroscience. 2010;30:2464–2471. doi: 10.1523/JNEUROSCI.5781-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dickinson A. Neuronal coding of prediction errors. Annual Review of Neuroscience. 2000;23:473–500. doi: 10.1146/annurev.neuro.23.1.473. [DOI] [PubMed] [Google Scholar]
- Sutton RS, Barto AG. Toward a modern theory of adaptive networks: expectations and prediction. Psychological Review. 1981;88:135–170. [PubMed] [Google Scholar]
- Trapold MA, Overmier JB. The second learning process in instrumental training. In: Black A, Prokasy WF, editors. Classical Conditioning II. New York: Appleton-Century-Crofts; 1972. pp. 427–452. [Google Scholar]
- Wilson PN, Boumphrey P, Pearce JM. Restoration of the orienting response to a light by a change in its predictive accuracy. Quarterly Journal of Experimental Psychology. 1992;44B:17–36. [Google Scholar]