Abstract
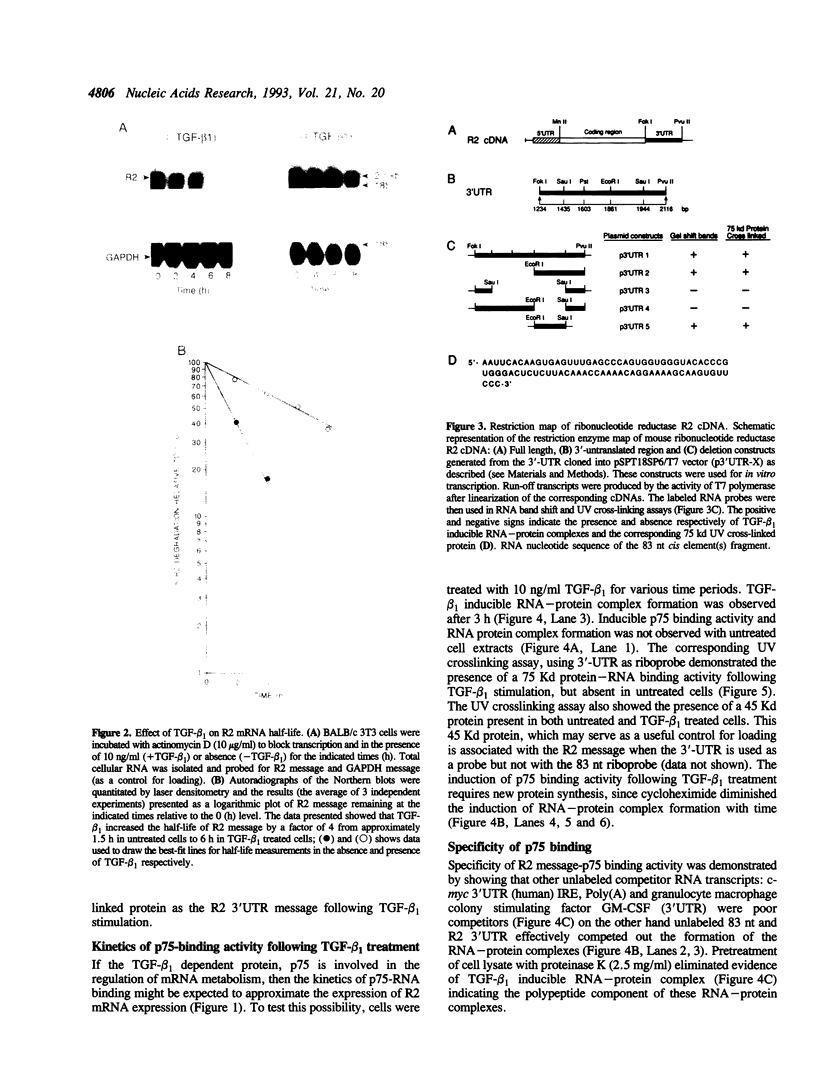
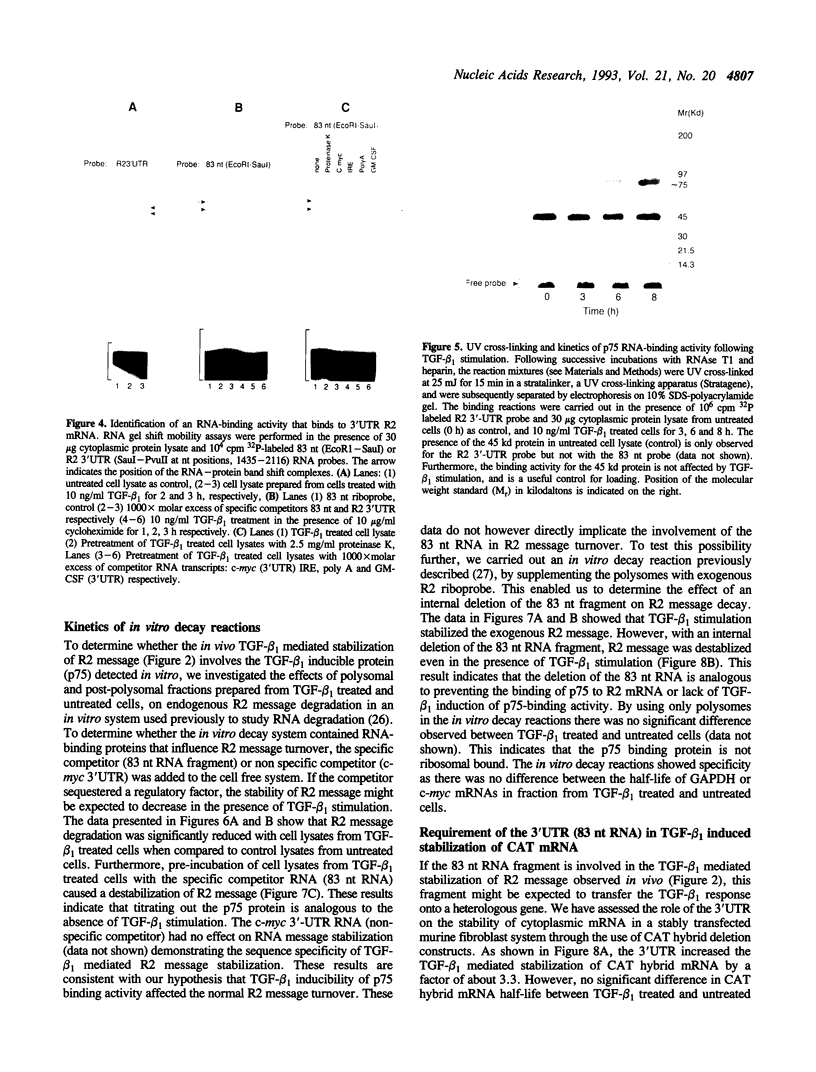
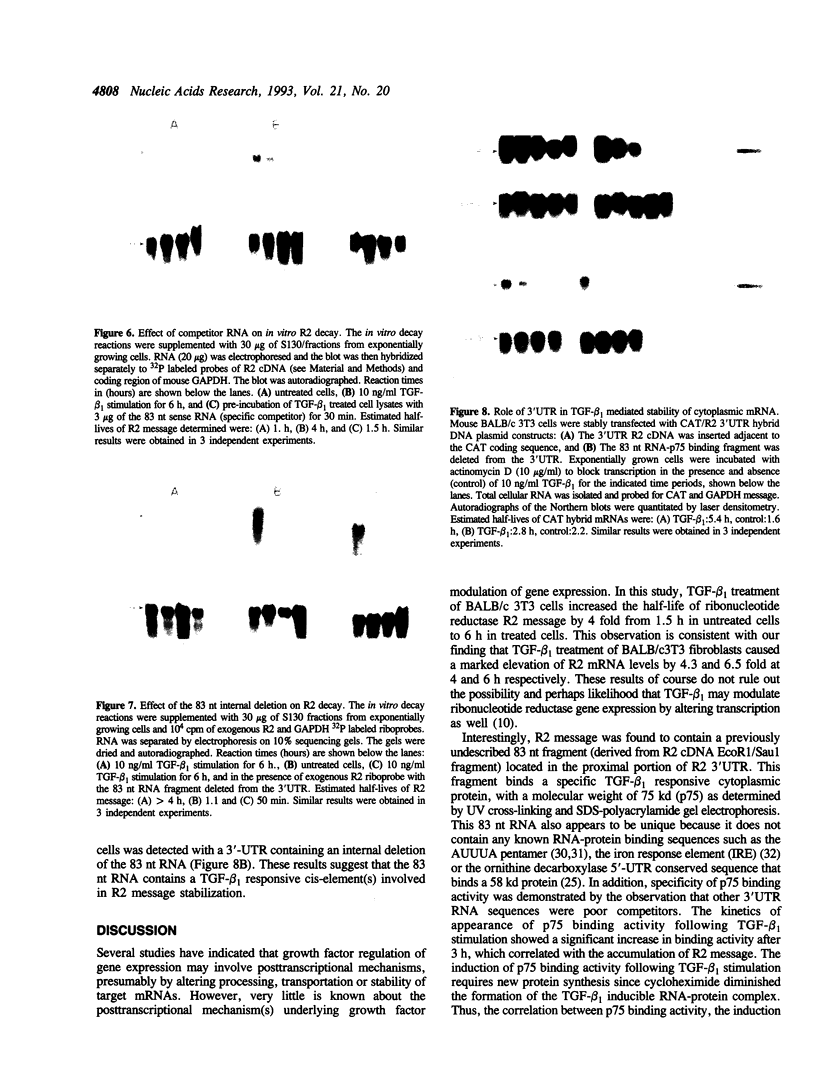
Ribonucleotide reductase is a highly regulated enzyme that provides the four deoxyribonucleotides required for DNA synthesis. Our studies showed that TGF-beta 1 treatment of BALB/c 3T3 mouse fibroblasts markedly elevated ribonucleotide reductase R2 mRNA levels, and also increased the half-life of R2 message by 4-fold from 1.5 h in untreated cells to 6 h in treated cells. We describe a novel 75 Kd sequence-specific cytoplasmic factor (p75) that binds selectively to a 83-nucleotide 3'-untranslated region of R2 mRNA and did not bind to the 5'UTR, the coding region of the R2 message or to the 3'UTRs of other mRNAs (from c-myc, GM-CSF and the iron responsive element from the transferrin receptor mRNA), or to the homopolymer poly(A) sequence. p75-RNA binding activity, which requires new protein synthesis, is not present in untreated cells, but is induced following TGF-beta 1 stimulation. The in vivo kinetics of appearance of p75 binding activity paralleled the accumulation of R2 mRNA. Insertion of the 3'-untranslated region into the chloramphenicol acetyltransferase (CAT) message confers TGF-beta 1 induced stability of RNA in stably transfected cells, while the same insert carrying a deletion of the 83-nucleotide fragment had little affect on RNA levels. Furthermore, in vitro decay reactions that contained the 83-nucleotide RNA or deletion of this fragment caused a significant decrease in TGF-beta 1 stabilization of R2 message. A model is presented of R2 message regulation in which TGF-beta 1 mediated stabilization of R2 message involves a specific interaction of a p75-trans-acting factor with a cis-element(s) stability determinant within the 83-nucleotide sequence which is linked to a reduction in the rate of R2 mRNA degradation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Björklund S., Skog S., Tribukait B., Thelander L. S-phase-specific expression of mammalian ribonucleotide reductase R1 and R2 subunit mRNAs. Biochemistry. 1990 Jun 12;29(23):5452–5458. doi: 10.1021/bi00475a007. [DOI] [PubMed] [Google Scholar]
- Blosmanis R., Wright J. A., Goldenberg G. J. Sensitivity to melphalan as a function of transport activity and proliferative rate in BALB/c 3T3 fibroblasts. Cancer Res. 1987 Mar 1;47(5):1273–1277. [PubMed] [Google Scholar]
- Bohjanen P. R., Petryniak B., June C. H., Thompson C. B., Lindsten T. An inducible cytoplasmic factor (AU-B) binds selectively to AUUUA multimers in the 3' untranslated region of lymphokine mRNA. Mol Cell Biol. 1991 Jun;11(6):3288–3295. doi: 10.1128/mcb.11.6.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer G. An A + U-rich element RNA-binding factor regulates c-myc mRNA stability in vitro. Mol Cell Biol. 1991 May;11(5):2460–2466. doi: 10.1128/mcb.11.5.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer G., Ross J. Regulation of c-myc mRNA stability in vitro by a labile destabilizer with an essential nucleic acid component. Mol Cell Biol. 1989 May;9(5):1996–2006. doi: 10.1128/mcb.9.5.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter B. Z., Malter J. S. Regulation of mRNA stability and its relevance to disease. Lab Invest. 1991 Dec;65(6):610–621. [PubMed] [Google Scholar]
- Choy B. K., McClarty G. A., Wright J. A. Transient elevation of ribonucleotide reductase activity, M2 mRNA and M2 protein in BALB/c 3T3 fibroblasts in the presence of 12-O-tetradecanoylphorbol-13-acetate. Biochem Biophys Res Commun. 1989 Aug 15;162(3):1417–1424. doi: 10.1016/0006-291x(89)90832-2. [DOI] [PubMed] [Google Scholar]
- Edwards D. R., Parfett C. L., Denhardt D. T. Transcriptional regulation of two serum-induced RNAs in mouse fibroblasts: equivalence of one species to B2 repetitive elements. Mol Cell Biol. 1985 Nov;5(11):3280–3288. doi: 10.1128/mcb.5.11.3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis L., Clauser E., Morgan D. O., Edery M., Roth R. A., Rutter W. J. Replacement of insulin receptor tyrosine residues 1162 and 1163 compromises insulin-stimulated kinase activity and uptake of 2-deoxyglucose. Cell. 1986 Jun 6;45(5):721–732. doi: 10.1016/0092-8674(86)90786-5. [DOI] [PubMed] [Google Scholar]
- Gough N. M. Rapid and quantitative preparation of cytoplasmic RNA from small numbers of cells. Anal Biochem. 1988 Aug 15;173(1):93–95. doi: 10.1016/0003-2697(88)90164-9. [DOI] [PubMed] [Google Scholar]
- Hurta R. A., Greenberg A. H., Wright J. A. Transforming growth factor-beta 1 mediated alterations in ribonucleotide reductase gene expression in BALB/c 3T3 fibroblasts. J Cell Physiol. 1992 Sep;152(3):529–535. doi: 10.1002/jcp.1041520312. [DOI] [PubMed] [Google Scholar]
- Hurta R. A., Samuel S. K., Greenberg A. H., Wright J. A. Early induction of ribonucleotide reductase gene expression by transforming growth factor beta 1 in malignant H-ras transformed cell lines. J Biol Chem. 1991 Dec 15;266(35):24097–24100. [PubMed] [Google Scholar]
- Hurta R. A., Wright J. A. Alterations in the activity and regulation of mammalian ribonucleotide reductase by chlorambucil, a DNA damaging agent. J Biol Chem. 1992 Apr 5;267(10):7066–7071. [PubMed] [Google Scholar]
- Hurta R. A., Wright J. A. Regulation of mammalian ribonucleotide reductase by the tumor promoters and protein phosphatase inhibitors okadaic acid and calyculin A. Biochem Cell Biol. 1992 Oct-Nov;70(10-11):1081–1087. doi: 10.1139/o92-153. [DOI] [PubMed] [Google Scholar]
- Iwai Y., Bickel M., Pluznik D. H., Cohen R. B. Identification of sequences within the murine granulocyte-macrophage colony-stimulating factor mRNA 3'-untranslated region that mediate mRNA stabilization induced by mitogen treatment of EL-4 thymoma cells. J Biol Chem. 1991 Sep 25;266(27):17959–17965. [PubMed] [Google Scholar]
- Kerr L. D., Miller D. B., Matrisian L. M. TGF-beta 1 inhibition of transin/stromelysin gene expression is mediated through a Fos binding sequence. Cell. 1990 Apr 20;61(2):267–278. doi: 10.1016/0092-8674(90)90807-q. [DOI] [PubMed] [Google Scholar]
- Leibold E. A., Munro H. N. Cytoplasmic protein binds in vitro to a highly conserved sequence in the 5' untranslated region of ferritin heavy- and light-subunit mRNAs. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2171–2175. doi: 10.1073/pnas.85.7.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leof E. B., Proper J. A., Goustin A. S., Shipley G. D., DiCorleto P. E., Moses H. L. Induction of c-sis mRNA and activity similar to platelet-derived growth factor by transforming growth factor beta: a proposed model for indirect mitogenesis involving autocrine activity. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2453–2457. doi: 10.1073/pnas.83.8.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis W. H., Kuzik B. A., Wright J. A. Assay of ribonucleotide reduction in nucleotide-permeable hamster cells. J Cell Physiol. 1978 Mar;94(3):287–298. doi: 10.1002/jcp.1040940306. [DOI] [PubMed] [Google Scholar]
- Liang H. M., Jost J. P. An estrogen-dependent polysomal protein binds to the 5' untranslated region of the chicken vitellogenin mRNA. Nucleic Acids Res. 1991 May 11;19(9):2289–2294. doi: 10.1093/nar/19.9.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzella J. M., Blackshear P. J. Specific protein binding to a conserved region of the ornithine decarboxylase mRNA 5'-untranslated region. J Biol Chem. 1992 Apr 5;267(10):7077–7082. [PubMed] [Google Scholar]
- Matrisian L. M., Leroy P., Ruhlmann C., Gesnel M. C., Breathnach R. Isolation of the oncogene and epidermal growth factor-induced transin gene: complex control in rat fibroblasts. Mol Cell Biol. 1986 May;6(5):1679–1686. doi: 10.1128/mcb.6.5.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClarty G. A., Chan A. K., Choy B. K., Thelander L., Wright J. A. Molecular mechanisms responsible for the drug-induced posttranscriptional modulation of ribonucleotide reductase levels in a hydroxyurea-resistant mouse L cell line. Biochemistry. 1988 Sep 20;27(19):7524–7531. doi: 10.1021/bi00419a052. [DOI] [PubMed] [Google Scholar]
- Penttinen R. P., Kobayashi S., Bornstein P. Transforming growth factor beta increases mRNA for matrix proteins both in the presence and in the absence of changes in mRNA stability. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1105–1108. doi: 10.1073/pnas.85.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichard P. Interactions between deoxyribonucleotide and DNA synthesis. Annu Rev Biochem. 1988;57:349–374. doi: 10.1146/annurev.bi.57.070188.002025. [DOI] [PubMed] [Google Scholar]
- Rondon I. J., MacMillan L. A., Beckman B. S., Goldberg M. A., Schneider T., Bunn H. F., Malter J. S. Hypoxia up-regulates the activity of a novel erythropoietin mRNA binding protein. J Biol Chem. 1991 Sep 5;266(25):16594–16598. [PubMed] [Google Scholar]
- Rossi P., Karsenty G., Roberts A. B., Roche N. S., Sporn M. B., de Crombrugghe B. A nuclear factor 1 binding site mediates the transcriptional activation of a type I collagen promoter by transforming growth factor-beta. Cell. 1988 Feb 12;52(3):405–414. doi: 10.1016/s0092-8674(88)80033-3. [DOI] [PubMed] [Google Scholar]
- Takehara K., LeRoy E. C., Grotendorst G. R. TGF-beta inhibition of endothelial cell proliferation: alteration of EGF binding and EGF-induced growth-regulatory (competence) gene expression. Cell. 1987 May 8;49(3):415–422. doi: 10.1016/0092-8674(87)90294-7. [DOI] [PubMed] [Google Scholar]
- Weber G. Biochemical strategy of cancer cells and the design of chemotherapy: G. H. A. Clowes Memorial Lecture. Cancer Res. 1983 Aug;43(8):3466–3492. [PubMed] [Google Scholar]
- Wright J. A., Chan A. K., Choy B. K., Hurta R. A., McClarty G. A., Tagger A. Y. Regulation and drug resistance mechanisms of mammalian ribonucleotide reductase, and the significance to DNA synthesis. Biochem Cell Biol. 1990 Dec;68(12):1364–1371. doi: 10.1139/o90-199. [DOI] [PubMed] [Google Scholar]