Abstract
Malignant glioma tumors are the most common primary central nervous system tumors. Despite the multidisciplinary approach to treatment, prognosis remains poor. In this study, we demonstrated that the Salmonella typhimurium A1-R tumor-targeting strain can inhibit and eradicate human glioma in an orthotopic nude-mouse model. S. typhimurium A1-R was administered by injection through a craniotomy open-window or intravenously in nude mice. To establish the model, 2 × 105 U87-RFP human glioma cells were injected stereotactically into the mouse brain through the craniotomy open window. Two weeks after glioma-cell implantation, mice were treated with S. typhimurium A1-R [2 × 107 CFU/200 µl intravenous injection (i.v.) or 1 × 106 CFU/1 µl intracranial injection (i.c.)] once a week for 3 weeks. Brain tumors were observed by fluorescence imaging through the craniotomy open window over time. S. typhimurium A1-R, administered i.c., inhibited brain tumor growth 7.6-fold compared with untreated mice (p = 0.009) and improved survival 73% (p = 0.001). Two of ten mice appeared to have their tumors eradicated. Intravenous administration of S. typhimurium A1-R was not effective. The craniotomy open window enabled observation of tumor growth in the brain in real time in both treated and untreated mice. The results of the present study demonstrate that bacterial therapy of brain cancer is a novel, effective and safe treatment strategy in a highly treatment-resistance cancer.
Key words: Salmonella typhimurium A1-R, fluorescent proteins, brain cancer, mouse model, in vivo imaging
Introduction
Malignant glioma tumors are the most common primary central nervous system (CNS) tumors and account for 78% of overall CNS tumors in adults.1 The current standard of care for the treatment of malignant glioma are surgery, radiotherapy and chemotherapy.2 Despite the multidisciplinary approach to treatment, prognosis remains poor and progression of disease is relatively common.3–6
There have been reports since the early 19th century that cancer patients who have been infected with bacteria have shown regression of their tumors.7 At the end of the 19th century, Coley treated cancer patients with bacteria and later used extracts of the bacteria, called Coley's toxins, as a therapeutic. Coley's toxins showed significant efficacy in a large group of cancer patients.8 Coley's toxins went out of favor after his death in the 1930s. Starting in the mid-20th century, there were preclinical studies on bacterial therapy of cancer and recently studies have been performed with obligate anaerobes such as Bifidobacterium and Clostridium in mouse models of cancer.9–14 However, obligate anaerobes can only grow in the necrotic areas of tumors, which limits their efficacy. The facultative anaerobe Salmonella typhimurium (S. typhimurium) has also been attenuated for use as an anticancer agent and tested in a clinical trial.15 Although the Phase I clinical trial showed safety of this approach, tumor colonization by the bacteria was limited, perhaps due to over-attenuation.
We have previously developed S. typhimurium A1, which is auxotrophic (leu/arg-dependent) but receives sufficient nutritional support from tumor tissue. However, S. typhimurium A1-R does not continuously infect normal tissues.
To increase the tumor-targeting capability of S. typhimurium A1, the strain was re-isolated after infection into a human colon tumor growing in nude mice. The tumor-isolated strain, termed A1-R, demonstrated efficacy in treatment of mouse models of human breast cancer,16 human prostate cancer,17 fibrosarcoma,18,19 pancreatic cancer,20,21 spinal cord glioma22 and lung cancer.23,24
In the present study, we demonstrate that S. typhimurium A1-R, administered by intracranial injection through a craniotomy open window, can effectively treat human glioma in an orthotopic nude-mouse model.
Results and Discussion
Real-time in vivo imaging of U87-red fluorescent protein (RFP) glioma growing orthotopically in nude mice.
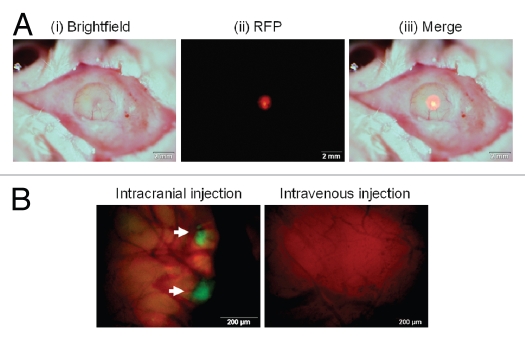
RFP-expressing U87 glioma tumors in the brain were observed through the craniotomy open window (Fig. 1A). The craniotomy open window enables imaging tumor growth in the brain over time while keeping animals alive and without further invasive procedures. Colonies of GFP-labeled S. typhimurium A1-R were observed in the U87-RFP brain tumor after cranial injection, but no bacterial colonies were found in the tumor treated with intravenous injection of S. typhimurium A1-R (Fig. 1B).
Figure 1.
RFP-expressing U87 glioma tumor and GFP-labeled S. typhimurium A1-R in the brain observed through the craniotomy open window in live mice. (A) (i) Using a skin biopsy punch, a 4 mm diameter craniotomy was made over the right parietal bone. (ii) RFP-expressing U87 tumor growing in the brain was observed with florescence imaging through the craniotomy open window. (iii) Merged image of bright field and florescence image. (B) Colonies of GFP-labeled S. typhimurium A1-R (arrows) were observed in the U87-RFP brain tumor treated by intracranial injection of S. typhimurium A1-R (left column). No S. typhimurium colonies were found in the tumor treated with intravenous injection (right column). Scale bars: (A) 2 mm; (B) 200 µm.
Dose-limiting toxicity of S. typhimurium A1-R administered by intracranial injection.
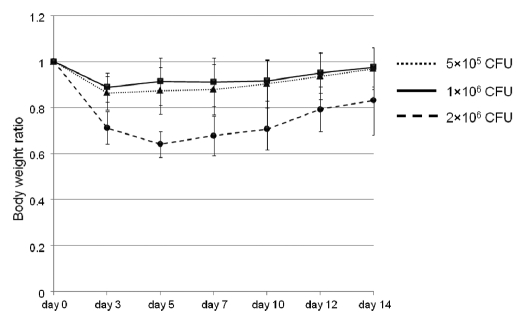
Mice without tumors were tested for determination of S. typhimurium A1-R dose-limiting toxicity after intracranial administration of the bacteria. The mice were injected intracranially weekly with different doses of the bacteria (5 × 105 CFU/µl, 1 × 106 CFU/µl and 2 × 106 CFU/µl). The body weight ratio (body weight at each day/body weight at day 0) was compared in the three groups to determine toxicity (Fig. 2). In the 5 × 105 CFU/µl and 1 × 106 CFU/µl groups, the maximum weight loss was 13.7% and 11.2%, respectively, and weight recovered by day 14 in both groups. In the 2 × 106 CFU/µl group, however, the maximum weight loss was 35.9% on day 5, exceeding 20% weight loss, and by day 14, body weight did not recover. The treatment dose of S. typhimurium A1-R for brain cancer was therefore chosen as 1 × 106 CFU/µl.
Figure 2.
Dose-limiting toxicity determination of S. typhimurium A1-R in the brain. Mice without tumors were tested for determination of S. typhimurium A1-R dose-limiting toxicity using intracranial injection. The mice were injected intracranially weekly with different doses of the bacteria (5 × 105 CFU/µl, 1 × 106 CFU/µl and 2 × 106 CFU/µl). The body weight ratio was compared within three groups to decide the optional dosage of the bacteria. In the 5 × 105 CFU/µl and 1 × 106 CFU/µl groups, the maximum weight loss was 13.7% and 11.2%, respectively, and weight recovered by day 14 in both groups. In the 2 × 106 CFU/µl group, however, the maximum weight loss was 35.9% on day 5, and on day 14, the weight loss was still 12.9%. The optimal dose of S. typhimurium A1-R in the brain was therefore determined to be 1 × 106 CFU/µl.
Evaluation of S. typhimurium A1-R therapeutic brain cancer efficacy over time.
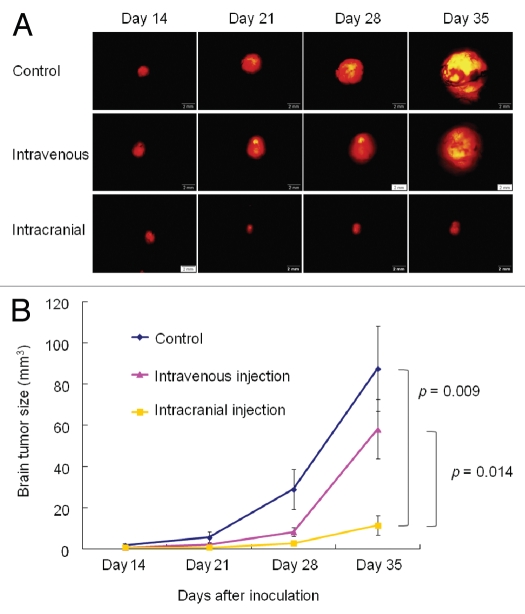
Mice in the treatment group were given S. typhimurium A1-R weekly by intravenous injection (i.v.) or intracranial injection (i.c.) for 3 weeks, beginning 14 d after tumor transplantation. Mice were administered the same volume of PBS as the untreated group (control). Tumor size was measured using RFP imaging at days 14, 21, 28 and 35 (Fig. 3A). The bacteria therapy was evaluated by tumor volume, which was measured using fluorescence imaging. Efficacy of the bacteria treatment is shown in Figure 3B. Average tumor size on day 14 (the day S. typhimurium A1-R was administered) was 1.81 mm3 in the control group, 0.74 mm3 in the intravenous group, and 0.68 mm3 in the intracranial group. There was no significant difference among three groups. Tumor volume in the intracranialtreatment group was 2.87 mm3 and 11.5 mm3 on days 28 and 35, respectively. These volumes were significantly smaller than in the other two groups (on day 28, vs. control: 29.0 mm3, p = 0.034; vs. intravenous: 8.22 mm3, p = 0.048; on day 35, vs. control: 87.6 mm3, p = 0.009; vs. intravenous: 58.1 mm3, p = 0.014). However, there was no significant difference in tumor volume between the control group and the intravenous group (at day 21, p = 0.233; at day 28, p = 0.075; and at day 35, p = 0.265).
Figure 3.
Real-time, in vivo imaging of U87-RFP glioma growing orthotopically in nude mice and evaluation of S. typhimurium A1-R therapeutic efficacy over time. (A) Representative images of tumor growth in untreated group (control); treated with intravenous injection (i.v. injection); and treated by cranial injection of S. typhimurium A1-R (intracranial injection). Mice in the treatment groups were given S. typhimurium A1-R weekly by i.v. injection or intracranial injection for 3 weeks, beginning 14 d after tumor transplantation. Tumor size was measured using RFP imaging at days 14, 21, 28 and 35. Scale bars: 2 mm. (B) Tumor volumes in each group were compared. Tumor volume in the intracranial-injection group was significantly smaller than in the other two groups (at day 35, vs. control: p = 0.009; vs. intravenous: p = 0.014). However, there was no significant difference in tumor volume between the control group and intravenous-injection group. Seven mice were used in each group. The experimental data are expressed as the mean ± SD. Statistical analysis was performed using the Student t-test.
Survival efficacy of S. typhimurium A1-R therapy on U87-RFP glioma.
Survival efficacy of S. typhimurium A1-R therapy in the orthotopic model is shown in Figure 4. Death from disease was rapid in the control group and intravenous group. Their median survival times were 37 and 43 d, respectively. Treatment with intracranial injection significantly prolonged median survival to 64 d (p = 0.001). Two out of seven mice treated by intracranial injection survived at day 80.
Figure 4.
Survival efficacy of S. typhimurium A1-R therapy on U87-RFP glioma growing orthotopically in nude mice. Treatment by intracranial injection significantly prolonged survival. Median survival increased from 37 and 43 d in the control group and intravenous injection group, respectively, to 64 d in the intracranial injection group (p = 0.001). Two out of seven mice treated intracranial injection with the bacteria survived at day 80. Seven mice were used in each group. Statistical analysis was performed using the Kaplan-Meier analysis with log-rank test.
Intracranial injection of S. typhimurium was significantly more effective and resulted in prolonged survival in mice compared with intravenous injection (Figs. 3 and 4). It appears that S. typhimurium poorly penetrates the blood-brain barrier. Unlike systemic therapy, intracranial injection of S. typhimurium A1-R can bypass the blood-brain barrier, which is the major obstacle in treatment of brain cancer. Intracranial administration delivers S. typhimurium A1-R directly to the brain tumor.
S. typhimurium A1-R is auxotrophic for both leucine and arginine, which appears to preclude these bacteria from mounting a sustained infection in normal tissue. Tumors, however, appear to be able to supply these amino acids sufficiently and enable S. typhimurium A1-R to grow in and kill tumors.25 S. typhimurium A1-R was selected for increased virulence by passage through a growing tumor in a mouse.16
Weekly administration of bacteria at the appropriate dose by intracranial delivery had no apparent adverse effects (such as meningitis, encephalitis). The mice tolerated the treatment well and appeared healthy during the experiment. These safety results suggest that direct cranial injection of bacteria in patients with brain cancer could be a clinically effective method for treatment of this highly drug-resistance disease.
In conclusion, we have demonstrated that S. typhimurium A1-R monotherapy rapidly inhibits human U87 glioma in the brain of mice in an orthotopic brain tumor model. U87 cancer cells were highly sensitive to S. typhimurium A1-R therapy, which prolonged survival of the mice. The craniotomy open window also enables imaging of tumor growth in the brain over time, keeping animals alive, and enables evaluation of the efficacy of a treatment in real time. Bacterial therapy of brain cancer is a novel and effective treatment strategy for this disease.
Our results also suggest potential use of S. typhimurium A1-R to target26 glioma properties such as delivery of glioma-suppressing microRNAs;27 targeting enzymes involved with aerobic glycolysis;28 targeting the hypoxic glioma tumor microenvironment29 and targeting the Tie2/TEK tyrosine kinase receptor.30
Materials and Methods
Establishment of RFP labeled cancer cell line.
For RFP gene transduction of cancer cells, 70% confluent human glioma (U87) cells were used. In brief, cells were incubated with a 1:1 precipitated mixture of retroviral supernatants of PT67-RFP cells and RPMI 1640 (Irvine Scientific) containing 10% fetal bovine serum (FBS) (Hyclone Laboratories) for 72 h. Fresh medium was replenished at this time. Cells were harvested with trypsin/EDTA 72 h post-transduction and subcultured at a ratio of 1:15 into selective medium, which contained 200 µg/ml of G418. The level of G418 was increased stepwise up to 800 µg/ ml.
Cell culture.
U87 cells were maintained in DMEM medium (Hyclone Laboratories) supplemented with 10% FBS. The cells were incubated at 37°C in a humidified atmosphere of 5% CO2 in air. The cells were collected after trypsinization and stained with trypan blue (Sigma-Aldrich). Only viable cells were counted with a hemocytometer (Hausser Scientific).
Green fluorescent protein (GFP) gene transfection of S. typhimurium.
S. typhimurium (ATCC 14028) was grown at 37°C to mid-logarithmic phase in liquid Luria Bertani (LB) and harvested at 4°C. Bacteria (2.0 × 108), in 40 µl 10% glycerol, were mixed with 2 µl pEGFP vector (Clontech). The preparation was placed on ice for 5 min before electroporation, using the Gene Pulser apparatus (Bio-Rad) according to the manufacturer's instructions. Electroporation was performed at 1.8 kV, with the pulse controller at 1,000 Ω parallel resistance.31 Bacteria engineered to express light-emitting proteins have been shown to be readily visualized in tumors.32,33
Induction of bacterial mutations with nitrosoguanidine and selection for auxotrophs of S. typhimurium-GFP.
Freshly prepared nitrosoguanidine (NTG; 1 mg/ml in sterile water) was added to the washed culture to a final concentration of 100 µg/ml in Tris-maleic acid buffer (pH 6.0). S. typhimurium-GFP were incubated with NTG for 30 min. NTG-treated cells were grown in nutrient broth to express any mutations that had been induced. Bacterial colonies were replica-plated in supplemented minimal agar plates, containing specific amino acids in order to identify auxotrophic requirements. Auxotroph A1, which required leucine and arginine, was initially isolated.25
Selection of the high-tumor-targeting variant S. typhimurium A1-R.
Selection of a high-tumor-targeting variant of S. typhimurium A1-GFP was performed as follows: A1 bacteria were injected into the tail vein of an HT-29 human colon tumorbearing nude mouse. Three days after infection, tumor tissue was removed from the infected animal. Tumor tissue was then homogenized and diluted with phosphate-buffered saline (PBS). The resulting supernatant of the tumor tissue was cultured in LB agar plates at 37°C overnight. The bacterial colony with the brightest green fluorescence was selected and cultured in 5 ml of LB medium. This strain was termed A1-R.16
Growth of S. typhimurium A1-R for treatment.
S. typhimurium A1-R were grown overnight in LB medium and then diluted 1:10 in LB medium. Bacteria were harvested at latelog phase, washed with PBS and then diluted in PBS. Bacteria were then ready for injection in mice.16
Animals.
Athymic NCR nude mice (nu/nu) at 4–6 weeks of age were used in this study. The breeding pairs were obtained from Charles River Laboratories. Mice were kept in a barrier facility under HEPA filtration. Mice were fed with autoclaved laboratory rodent diet. All animal studies were conducted in accordance with the principals and procedures outlined in the NIH Guide for the Care and Use of Laboratory Animals under PHS Assurance Number A3873-1.
Craniotomy open window.
Mice were anesthetized with a ketamine mixture (10 µl ketamine HCL, 7.6 µl xylazine, 2.4 µl acepromazine maleate and 10 µl H2O) via s.c. injection. After fixing the mice in a prone position, a 1.5 cm incision was made directly down the midline of the scalp. The scalp was retracted and the skull was exposed. Using a skin biopsy punch (Acuderm Inc.), a 4 mm diameter craniotomy was made over the right parietal bone (Fig. 1A). The bone fragment was removed carefully in order not to injure the meninges and brain tissue. The craniotomy open window was covered only by the scalp. Thus, only scalp retraction was needed in order to image tumor growth or inject S. typhimurium in the brain. The incision was then closed with 6-0 surgical suture (ETHICON, Inc.). All mice were kept in an oxygenated warmed chamber until they recovered from anesthesia.
Stereotactic injection.
The mice were anesthetized with a ketamine mixture via s.c. injection. After the craniotomy open window was made, 1 µl of a suspension containing 2 × 105 U87-RFP cells was injected stereotactically into the mouse brain using a 10 µl Hamilton syringe. Cells were injected in the middle of the craniotomy open window to a depth of 1 mm.
Bacterial therapy in the brain tumor model.
Two weeks after inoculation, mice were treated with S. typhimurium A1-R (2 × 107 CFU/200 µl PBS intravenous injection from the tail vein or 1 × 106 CFU/1 µl PBS intracranial injection through the craniotomy open window) once a week for 3 weeks. Mice were administered the same volume of PBS as the untreated control group. After administration of S. typhimurium A1-R, fluorescence imaging (please see below) was performed (Fig. 1A) and changes in the diameters of the RFP-expressing tumors were recorded each week for 3 weeks. Tumor diameters were measured each week after S. typhimurium A1-R administration. Tumor volume was calculated by the formula (width2 × length × 0.5). Seven mice were used in each group.
Fluorescence imaging.
The Olympus OV100 Small Animal Imaging System (Olympus), containing an MT-20 light source (Olympus Biosystems) and DP70 CCD camera (Olympus), was used for imaging live mice.34 High-resolution images were captured directly on a personal computer (Fujitsu Siemens Computers). Images were analyzed with the use of CellR software (Olympus Biosystems).
Survival analysis.
The experimental data are expressed as the mean ± SD. Statistical analysis was performed using the Student t-test. Kaplan-Meier analysis with a log-rank test was used to determine survival and difference between treatment groups. A p-value less than 0.05 was used to indicate a significant difference.
Acknowledgements
This study was supported in part by National Cancer Institute grant CA126023.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.CBTRUS, author. Statistical report: Primary brain tumors in the United States, 1998–2002. Chicago IL: CBTRUS; 2006. [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Fine HA, Dear KBG, Loeffler JS, Black PM, Canellos GP. Meta-analysis of radiation therapy with and without adjuvant chemotherapy for malignant gliomas in adults. Cancer. 1993;71:2585–2597. doi: 10.1002/1097-0142(19930415)71:8<2585::AIDCNCR2820710825>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 4.Grossman SA, Batara JF. Current management of glioblastoma multiforme. Semin Oncol. 2004;31:635–644. doi: 10.1053/j.seminoncol.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Carpentier AF. Neuro-oncology: the growing role of chemotherapy in glioma. Lancet Neurol. 2005;4:4–5. doi: 10.1016/S1474-4422(04)00944-5. [DOI] [PubMed] [Google Scholar]
- 6.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 7.Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas: with a report of ten original cases. Am J Med Sci. 1893;105:487–511. doi: 10.1097/00000441-189305000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Coley WB. Late results of the treatment of inoperable sarcoma by the mixed toxins of erysipelas and Bacillus prodigiosus. Am J Med Sci. 1906;131:375–430. [Google Scholar]
- 9.Malmgren RA, Flanigan CC. Localization of the vegetative form of Clostridium tetani in mouse tumors following intravenous spore administration. Cancer Res. 1955;15:473–478. [PubMed] [Google Scholar]
- 10.Gericke D, Engelbart K. Oncolysis by clostridia. II Experiments on a tumor spectrum with a variety of clostridia in combination with heavy metal. Cancer Res. 1964;24:217–221. [PubMed] [Google Scholar]
- 11.Moese JR, Moese G. Oncolysis by clostridia. I. Activity of clostridium butyricum (M-55) and other nonpathogenic clostridia against the Ehrlich carcinoma. Cancer Res. 1964;24:212–216. [PubMed] [Google Scholar]
- 12.Thiele EH, Arison RN, Boxer GE. Oncolysis by clostridia. III Effects of clostridia and chemotherapeutic agents on rodent tumors. Cancer Res. 1964;24:222–233. [PubMed] [Google Scholar]
- 13.Kohwi Y, Imai K, Tamura Z, Hashimoto Y. Antitumor effect of Bifidobacterium infantis in mice. Gann. 1978;69:613–618. [PubMed] [Google Scholar]
- 14.Kimura NT, Taniguchi S, Aoki K, Baba T. Selective localization and growth of Bifidobacterium bifidum in mouse tumors following intravenous administration. Cancer Res. 1980;40:2061–2068. [PubMed] [Google Scholar]
- 15.Toso JF, Gill VJ, Hwu P, Marincola FM, Restifo NP, Schwartzentruber DJ, et al. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J Clin Oncol. 2002;20:142–152. doi: 10.1200/JCO.20.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao M, Yang M, Ma H, Li X, Tan X, Li S, et al. Targeted therapy with a Salmonella typhimurium leucine-arginine auxotroph cures orthotopic human breast tumors in nude mice. Cancer Res. 2006;66:7647–7652. doi: 10.1158/0008-5472.CAN-06-0716. [DOI] [PubMed] [Google Scholar]
- 17.Zhao M, Geller J, Ma H, Yang M, Penman S, Hoffman RM. Monotherapy with a tumor-targeting mutant of Salmonella typhimurium cures orthotopic metastatic mouse models of human prostate cancer. Proc Natl Acad Sci USA. 2007;104:10170–10174. doi: 10.1073/pnas.0703867104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayashi K, Zhao M, Yamauchi K, Yamamoto N, Tsuchiya H, Tomita K, et al. Systemic targeting of primary bone tumor and lung metastasis of high-grade osteosarcoma in nude mice with a tumor-selective strain of Salmonella typhimurium. Cell Cycle. 2009;8:870–875. doi: 10.4161/cc.8.6.7891. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi K, Zhao M, Yamauchi K, Yamamoto N, Tsuchiya H, Tomita K, et al. Cancer metastasis directly eradicated by targeted therapy with a modified Salmonella typhimurium. J Cell Biochem. 2009;106:992–998. doi: 10.1002/jcb.22078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagakura C, Hayashi K, Zhao M, Yamauchi K, Yamamoto N, Tsuchiya H, et al. Efficacy of a genetically-modified Salmonella typhimurium in an orthotopic human pancreatic cancer in nude mice. Anticancer Res. 2009;29:1873–1878. [PubMed] [Google Scholar]
- 21.Yam C, Zhao M, Hayashi K, Ma H, Kishimoto H, McElroy M, et al. Monotherapy with a tumor-targeting mutant of S. typhimurium inhibits liver metastasis in a mouse model of pancreatic cancer. J Surg Res. 2010;164:248–255. doi: 10.1016/j.jss.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kimura H, Zhang L, Zhao M, Hayashi K, Tsuchiya H, Tomita K, et al. Targeted therapy of spinal cord glioma with a genetically modified Salmonella typhimurium. Cell Prolif. 2010;43:41–48. doi: 10.1111/j.1365-2184.2009.00652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu F, Zhang L, Hoffman RM, Zhao M. Vessel destruction by tumor-targeting Salmonella typhimurium A1-R is enhanced by high tumor vascularity. Cell Cycle. 2010;9:4518–4524. doi: 10.4161/cc.9.22.13744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao M, Suetsugu A, Ma H, Zhang L, Liu F, Zhang Y, et al. Efficacy against lung metastasis with a tumortargeting mutant of Salmonella typhimurium in immunocompetent mice. Cell Cycle. 2012:11. doi: 10.4161/cc.11.1.18667. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao M, Yang M, Li XM, Jiang P, Baranov E, Li S, et al. Tumor-targeting bacterial therapy with amino acid auxotrophs of GFP-expressing Salmonella typhimurium. Proc Natl Acad Sci USA. 2005;102:755–760. doi: 10.1073/pnas.0408422102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wall DM, Srikanth CV, McCormick BA. Targeting tumors with Salmonella typhimurium-potential for therapy. Oncotarget. 2010;1:721–728. doi: 10.18632/oncotarget.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guessous F, Zhang Y, Kofman A, Catania A, Li Y, Schiff D, et al. microRNA-34a is tumor suppressive in brain tumors and glioma stem cells. Cell Cycle. 2010;9:1031–1036. doi: 10.4161/cc.9.6.10987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolf A, Agnihotri S, Guha A. Targeting metabolic remodeling in glioblastoma multiforme. Oncotarget. 2010;1:552–562. doi: 10.18632/oncotarget.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heddleston JM, Li Z, McLendon RE, Hjelmeland AB, Rich JN. The hypoxic microenvironment maintains glioblastoma stem cells and promotes reprogramming towards a cancer stem cell phenotype. Cell Cycle. 2009;8:3274–3284. doi: 10.4161/cc.8.20.9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu D, Martin V, Fueyo J, Lee OH, Xu J, Cortes-Santiago N, et al. Tie2/TEK modulates the interaction of glioma and brain tumor stem cells with endothelial cells and promotes an invasive phenotype. Oncotarget. 2010;1:700–709. doi: 10.18632/oncotarget.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoffman RM, Zhao M. Whole-body imaging of bacterial infection and antibiotic response. Nat Protoc. 2006;1:2988–2994. doi: 10.1038/nprot.2006.376. [DOI] [PubMed] [Google Scholar]
- 32.Yu YA, Timiryasova T, Zhang Q, Beltz R, Szalay AA. Optical imaging: bacteria, viruses and mammalian cells encoding light-emitting proteins reveal the locations of primary tumors and metastases in animals. Anal Bioanal Chem. 2003;377:964–972. doi: 10.1007/s00216-003-2065-0. [DOI] [PubMed] [Google Scholar]
- 33.Yu YA, Shabahang S, Timiryasova TM, Zhang Q, Beltz R, Gentschev I, et al. Visualization of tumors and metastases in live animals with bacteria and vaccinia virus encoding light-emitting proteins. Nat Biotechnol. 2004;22:313–320. doi: 10.1038/nbt937. [DOI] [PubMed] [Google Scholar]
- 34.Yamauchi K, Yang M, Jiang P, Xu M, Yamamoto N, Tsuchiya H, et al. Development of real-time subcellular dynamic multicolor imaging of cancer-cell trafficking in live mice with a variable-magnification whole-mouse imaging system. Cancer Res. 2006;66:4208–4214. doi: 10.1158/0008-5472.CAN-05-3927. [DOI] [PubMed] [Google Scholar]