Abstract
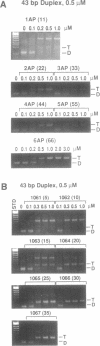
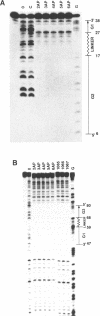
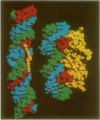
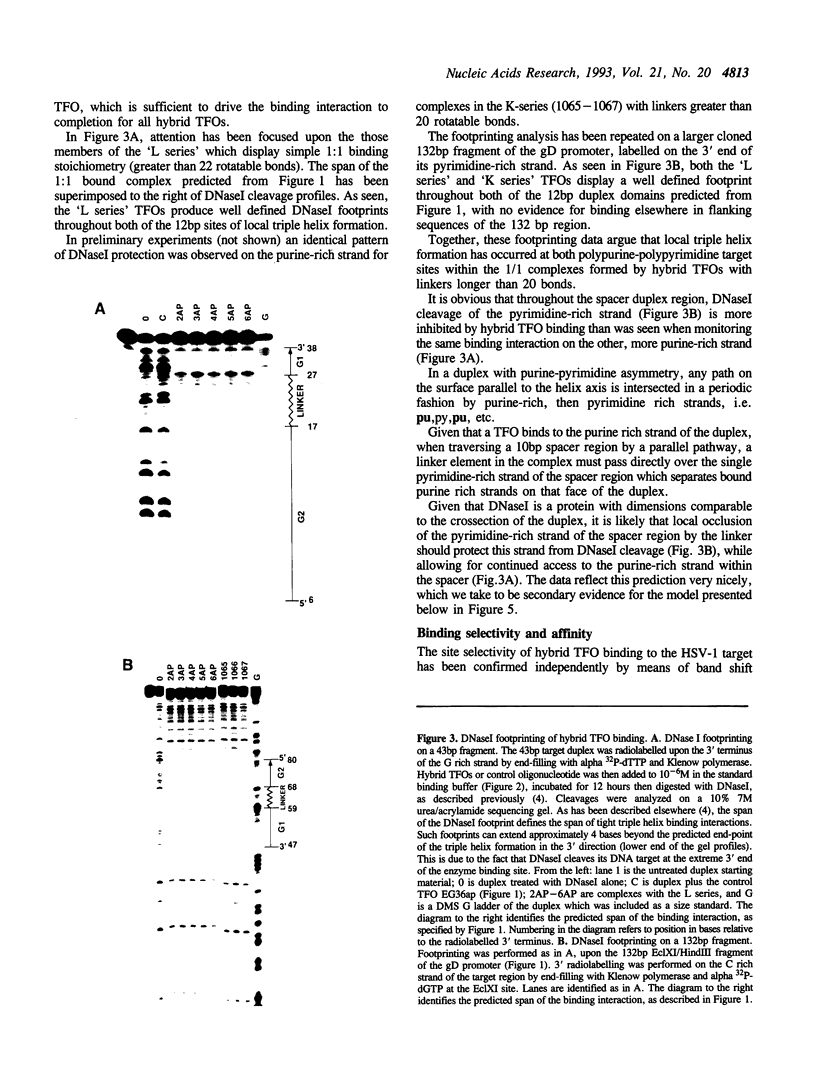
An oligonucleotide hybrid is described which possesses two triple helix forming oligonucleotides which have been connected by a flexible polymeric linker chain. As a prototype, binding of this class of oligonucleotide to duplex DNA has been studied using a segment of the HSV-1 D-glycoprotein promoter, which possesses a pair of 12bp target sites for stable triple helix formation, separated by a duplex spacer region which is one helical turn long. Band shift and footprinting analysis show that such hybrids can bind to both 12bp elements simultaneously, if flexible linkers are included which are longer than 20-25 rotatable bonds. Molecular modeling confirms that a flexible polymeric linker as short as 22 rotatable bonds is enough to link the two distant segments of triple helix, providing that the linker element travels a path which is external to the helix grooves and parallel to the long helix axis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beal P. A., Dervan P. B. Second structural motif for recognition of DNA by oligonucleotide-directed triple-helix formation. Science. 1991 Mar 15;251(4999):1360–1363. doi: 10.1126/science.2003222. [DOI] [PubMed] [Google Scholar]
- Durland R. H., Kessler D. J., Gunnell S., Duvic M., Pettitt B. M., Hogan M. E. Binding of triple helix forming oligonucleotides to sites in gene promoters. Biochemistry. 1991 Sep 24;30(38):9246–9255. doi: 10.1021/bi00102a017. [DOI] [PubMed] [Google Scholar]
- Jayasena S. D., Johnston B. H. Oligonucleotide-directed triple helix formation at adjacent oligopurine and oligopyrimidine DNA tracts by alternate strand recognition. Nucleic Acids Res. 1992 Oct 25;20(20):5279–5288. doi: 10.1093/nar/20.20.5279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohwi Y., Kohwi-Shigematsu T. Magnesium ion-dependent triple-helix structure formed by homopurine-homopyrimidine sequences in supercoiled plasmid DNA. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3781–3785. doi: 10.1073/pnas.85.11.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misiura K., Durrant I., Evans M. R., Gait M. J. Biotinyl and phosphotyrosinyl phosphoramidite derivatives useful in the incorporation of multiple reporter groups on synthetic oligonucleotides. Nucleic Acids Res. 1990 Aug 11;18(15):4345–4354. doi: 10.1093/nar/18.15.4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondragón A., Harrison S. C. The phage 434 Cro/OR1 complex at 2.5 A resolution. J Mol Biol. 1991 May 20;219(2):321–334. doi: 10.1016/0022-2836(91)90568-q. [DOI] [PubMed] [Google Scholar]
- Moser H. E., Dervan P. B. Sequence-specific cleavage of double helical DNA by triple helix formation. Science. 1987 Oct 30;238(4827):645–650. doi: 10.1126/science.3118463. [DOI] [PubMed] [Google Scholar]
- Tedder D. G., Everett R. D., Wilcox K. W., Beard P., Pizer L. I. ICP4-binding sites in the promoter and coding regions of the herpes simplex virus gD gene contribute to activation of in vitro transcription by ICP4. J Virol. 1989 Jun;63(6):2510–2520. doi: 10.1128/jvi.63.6.2510-2520.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trung Le Doan, Perrouault L., Chassignol M., Nguyen T. T., Hélène C. Sequence-targeted chemical modifications of nucleic acids by complementary oligonucleotides covalently linked to porphyrins. Nucleic Acids Res. 1987 Nov 11;15(21):8643–8659. doi: 10.1093/nar/15.21.8643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de los Santos C., Rosen M., Patel D. NMR studies of DNA (R+)n.(Y-)n.(Y+)n triple helices in solution: imino and amino proton markers of T.A.T and C.G.C+ base-triple formation. Biochemistry. 1989 Sep 5;28(18):7282–7289. doi: 10.1021/bi00444a021. [DOI] [PubMed] [Google Scholar]