Abstract
Despite a robust inverse association between high-density lipoprotein (HDL) cholesterol levels and atherosclerotic cardiovascular disease, the development of new therapies based on pharmacologic enhancement of HDL metabolism has proven challenging. Emerging evidence suggests that static measurement of HDL levels has inherent limitations as a surrogate for overall HDL functionality, particularly with regard to the rate of flux through the macrophage reverse cholesterol transport (RCT) pathway. Recent research has provided important insight into the molecular underpinnings of RCT, the process by which excess cellular cholesterol is effluxed from peripheral tissues and returned to the liver for ultimate intestinal excretion. This review discusses the critical importance and current strategies for quantifying RCT flux. It also highlights therapeutic strategies for augmenting macrophage RCT via three conceptual approaches: 1) improved efflux of cellular cholesterol via targeting the macrophage; 2) enhanced cholesterol efflux acceptor functionality of circulating HDL; and 3) increased hepatic uptake and biliary/intestinal excretion.
Keywords: Reverse cholesterol transport, High-density lipoprotein, Lipid metabolism
Introduction
High-density lipoprotein cholesterol (HDL-C) levels have been recognized as a strong inverse predictor of cardiovascular risk since the 1970s [1]. Population-based studies suggest that an increment of even 1 mg/dL in HDL-C is associated with a 3–4% reduction in mortality from cardiovascular disease [2]. However, despite intense efforts in both the private sector and academia, the development of new therapies based on pharmacologic enhancement of HDL metabolism has proven challenging. A number of interventions are linked to increases in HDL-C levels, but robust associations with improved clinical outcomes remain sparse [3, 4•]. Recent research findings that provide novel insight into lipid metabolism, particularly with regard to reverse cholesterol transport (RCT), are likely to prove critical to ongoing drug development efforts.
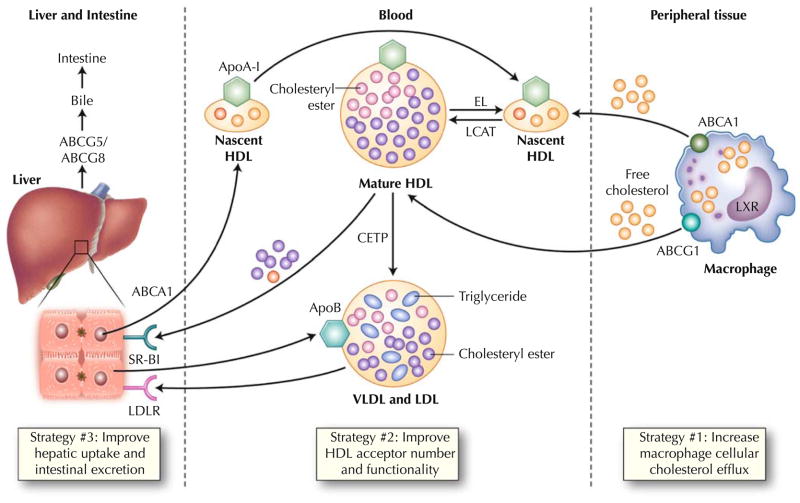
The mechanistic and causal basis of HDL’s apparent atheroprotective effects has not been definitively proven and is of central importance to the field, particularly with regard to drug development. Although likely pleiotropic in nature, HDL’s promotion of RCT is thought to play a key role in the attenuation, and potential regression, of atherosclerosis (Fig. 1) [5]. RCT involves the return of excess cholesterol from peripheral tissues to the liver and ultimately biliary excretion into the intestines [6]. Although macrophage RCT represents only a small fraction of total flux through the pathway, it may warrant particular focus given the importance of cholesterol-laden macrophage foam cells in the development of atheromatous lesions [7, 8].
Fig. 1.
Physiology of macrophage reverse cholesterol transport. Both the liver and intestine synthesize apolipoprotein A-I (ApoA-I), which is secreted in lipid-poor form. These particles are lipidated with both phospholipids and free cholesterol via the hepatocyte ATP-binding cassette A1 (ABCA1) transporter to form nascent high-density lipoprotein (HDL). In peripheral tissues, these HDL particles obtain additional free cholesterol via the macrophage ABCA1 transporter. Lecithin cholesterol acyltransferase (LCAT) esterifies free cholesterol to cholesteryl esters, generating mature HDL. These larger HDL particles serve as additional acceptors of cholesterol efflux via the macrophage ATP-binding cassette G1 (ABCG1) pathway. Liver X receptor (LXR) regulates the expression of both ABCA1 and ABCG1 in the macrophage. HDL can be remodeled by lipases such as hepatic lipase and endothelial lipase (EL), which hydrolyze HDL triglyceride and phospholipid, respectively. Mature HDL can transport its cholesterol directly to the liver via the hepatic scavenger receptor class B type 1 (SR-B1) receptor. Alternatively, cholesteryl ester transfer protein (CETP) can mediate transfer of cholesteryl esters from HDL particles to apoB-containing lipoproteins with subsequent uptake in the liver via the low-density lipoprotein receptor (LDLR). This “indirect” pathway is thought to predominate in humans. Hepatic cholesterol is secreted into the bile via the ABCG5 and ABCG8 transporters. Some reenters the circulation via intestinal reabsorption, and the remainder is excreted into the feces. Recent evidence suggests that the intestine may be able to directly excrete cholesterol, bypassing the liver entirely. VLDL—very low density lipoprotein
Static, mass-based measurement of HDL-C has inherent limitations as a metric of overall functionality, particularly with regard to flux through the RCT pathway. Increased HDL levels should thus be viewed as neither necessary nor sufficient as a surrogate for therapeutic efficacy. Indeed, a validated murine assay that quantifies macrophage RCT has proven a better predictor of atherosclerosis measures than HDL concentration [9]. Therefore, careful attention to the subtleties of HDL particle heterogeneity and the RCT pathway offers a way forward in the development of HDL-centric therapeutics.
This article reviews methodologies of quantifying RCT and strategies for augmenting macrophage RCT via three conceptual approaches: 1) increased efflux of cholesterol from macrophage cells; 2) enhanced acceptor functionality of circulating HDL; and 3) improved hepatic uptake and biliary/intestinal excretion (Table 1).
Table 1.
Summary of selected strategies to increase macrophage reverse cholesterol transport
| Therapeutic strategy | Effect on HDL Levels | Effect on murine macrophage RCT | Effect on atherosclerosis | Rationale | Limitations | Stage of clinical development |
|---|---|---|---|---|---|---|
| Strategy #1: Increase macrophage cholesterol efflux | ||||||
| LXR agonism | Variable | Increased | Decreased | Upregulate ABCA1- and ABCG1-mediated efflux; may also increase intestinal secretion | Induction of hepatic steatosis, particularly with nonselective agonists | Early clinical |
| Strategy #2: Increase HDL acceptor number and functionality | ||||||
| PPARα agonism | Variable | Increased | Unknown | Stimulate apoA-I production/turnover; also increases cellular efflux | Nonspecific effects, safety concerns in humans | Early clinical |
| ApoA-I/reconstituted HDL infusions | Increased | Unknown | Decreased | Promote RCT and enhance plaque stability via short-term infusion | Limited human efficacy data; need for intravenous administration | Early clinical |
| ApoA-I mimetic peptides | None | Increased | Decreased | Recapitulate beneficial effects of full-length apoA-1 via oral administration | Modest oral bioavailability, no human efficacy data | Early clinical |
| CETP inhibition | Increased | Variable | Unknown | Prevent transfer of cholesteryl esters from HDL to apoB-containing lipoproteins, increasing HDL levels | Unknown effects on RCT, failure in one large phase 3 clinical trial | Phase 3 clinical trials |
| Endothelial lipase inhibition | Increased | Unknown | Unknown | Decrease hydrolysis of HDL phospholipids, slowing HDL catabolism | Unknown effects on RCT, paucity of small molecule inhibitors | Preclinical |
| Strategy #3: Increase hepatic uptake and intestinal excretion | ||||||
| Ezetemibe | None | Increased | Decreased | Limit intestinal cholesterol reabsorption | Limited data demonstrating improved clinical outcomes in humans | In clinical use |
| PPARδ agonism | Increased | Increased | Unknown | Limit intestinal cholesterol reabsorption, may improve clearance of postprandial triglycerides | Unknown mechanism of increasing HDL levels, minimal data in humans | Early clinical |
| Fish oil | Increased | Increased | Decreased | Enhance hepatic cholesterol excretion via ABCG5/ABCG8 transporters, decrease intestinal cholesterol absorption | Unclear if clinical benefit is related to increased RCT | In clinical use |
ABC ATP-binding cassette, apo apolipoprotein, CETP cholesteryl ester transfer protein, HDL high-density lipoprotein, LXR liver X receptor, PPAR peroxisome proliferator-activated receptor, RCT reverse cholesterol transport
Metrics of Reverse Cholesterol Transport in Mice and Men
Quantification of dynamic flux through the macrophage RCT pathway, although methodologically challenging, would be immensely valuable to the assessment of HDL metabolism in the setting of pharmacotherapy. One approach has been to study efflux of cellular cholesterol ex vivo [10]. These assays allow investigation of both cell-based and acceptor-based interventions. For example, stimulation of liver X receptor (LXR) activity has been shown to promote macrophage cholesterol efflux ex vivo, primarily via the ATP-binding cassette A1 (ABCA1) pathway [11]. Infusion of reconstituted HDL particles, by contrast, was shown to improve the acceptor function (efflux capacity) of plasma from human patients with type 2 diabetes [12•]. Major current limitations of ex vivo cholesterol efflux assays include limited data linking efflux capacity to coronary disease, methodologic variability, and an inability to assess the terminal components of the RCT pathway. However, if validated and standardized, cellular efflux assays may prove highly useful in screening novel therapeutics.
A robust quantitative in vivo assay has been developed in mice that permits detailed assessment of the tissue-specific macrophage RCT pathway. In brief, the technique involves injecting radiolabeled and cholesterol-loaded macrophage “foam cells” into the intraperitoneal space [13]. The radioactive tracer can then be followed as it moves from the implanted cells into the bloodstream, is taken up by the liver, and is excreted into the feces. This assay has provided critical insight into experimental situations where perturbations of HDL levels have seemingly paradoxical effects on atherosclerosis. For example, overexpression of hepatic scavenger receptor class B type 1 (SR-B1) is associated with markedly decreased HDL levels but decreased atherosclerotic burden [14, 15], an effect likely mediated by enhanced hepatic uptake of HDL and increased RCT flux [16]. Recent efforts have demonstrated effects of several therapeutic interventions on murine macrophage RCT, providing important insight in to their potential utility.
However, species-specific differences in lipid metabolism preclude direct extension of animal findings to efficacy in humans. A direct human analog of the macrophage RCT pathway would thus be highly useful to ongoing translational research efforts. Measurement of fecal cholesterol excretion, the terminal event of RCT, has been proposed as a potential proxy for total centripetal flux [17]. One small human study demonstrated increased fecal steroid excretion after infusion of apolipoprotein (apo) A-I precursor liposome complexes [18]. Another strategy applies principles of steady-state isotope dilution in tracking efflux of cholesterol from tissues into plasma, as detailed in a recent review [19•]. These metrics, although conceptually intriguing, remain limited in overall sensitivity and particularly in their ability to assess macrophage-specific RCT. Ongoing research efforts may yield robust assays, ideally macrophage-specific, of human RCT in vivo.
Increasing Macrophage Cholesterol Efflux
Cholesterol efflux, the first step of the RCT pathway, plays a critical role in maintaining intracellular cholesterol homeostasis. Peripheral tissues gain cholesterol via synthetic pathways and direct uptake of circulating lipoproteins but are largely unable to catabolize it. The toxic buildup of cholesterol in arterial foam cells is thought to play a key role in the initiation and progression of atherosclerotic plaque development, and potentially plaque rupture as well. Once assumed to be a largely passive process, recent research has demonstrated that cholesterol efflux occurs largely via ABCA1 and ATP-binding cassette G1 (ABCG1). The two transporters differ in their acceptor specificities, with ABCA1 responsible for efflux to lipid-poor apoA-I and ABCG1 promoting efflux to mature HDL particles.
Animal studies have confirmed that both ABCA1 and ABCG1 facilitate macrophage RCT [20]. Mice deficient in both of these proteins exhibit dramatic increases in foam cell accumulation and atherosclerosis, reinforcing the concept that the macrophage cholesterol efflux pathway is antiatherogenic in vivo [21••, 22]. Similarly, loss of function ABCA1 mutations in humans, which underlies Tangier disease, is associated with inability to lipidate apoA-I particles and cholesterol build-up in peripheral tissues [23–25]. Among patients with rare ABCA1 mutations, an inverse relationship was noted between ability to efflux cellular cholesterol and carotid intimal-media thickness [26]. These findings have fostered interest in upregulating cholesterol efflux activity as a means of increasing RCT.
LXR Agonism
Liver X receptors (LXR), including LXRα and LXRβ, serve as the major regulators of cellular ABCA1 and ABCG1 expression via binding with the heterodimer retinoid X receptor [27]. Accordingly, pharmacologic LXR agonism has been shown to promote macrophage RCT and decrease atherosclerosis in mouse models [28–30]. However, development of first-generation LXR ligands has been hampered by their induction of hepatic steatosis. Stimulation of hepatic LXRα induces increased fatty acid biosynthesis via increased expression of sterol response element binding protein-1c (SREBP-1c), resulting in fat accumulation [31]. Furthermore, the drugs have been shown to increase low-density lipoprotein cholesterol (LDL-C) levels in some species [32].
Recent studies have highlighted the primary role of the macrophage in mediating LXR agonists’ antiatherogenic activity [33, 34]. Because LXRα is the predominant subtype in the liver, selective agonism of LXRβ may help overcome the undesirable hepatic effects seen with nonselective stimulation. Indeed, LXRβ-selective agonists have been developed that retain the ability to promote macrophage cholesterol efflux [35]. As such, LXR agonism remains a highly plausible and conceptually attractive therapeutic target, particularly if it can be accomplished with selective targeting of the macrophage or intestine.
A proof-of-concept study was performed in humans using LXR-623 (Wyeth, Madison, NJ), a nonselective small molecule agonist of LXR [36]. The compound was associated with increased expression of ABCA1 and ABCG1 in blood. However, adverse central nervous system-related effects were noted in more than half of patients, leading to termination of the study. Although these were likely off-target effects of the specific compound, additional study will be needed to confirm that these events were not due to modulation of central nervous system LXR activity.
Improving HDL Acceptor Number and Function
HDL particles are highly heterogenous with regard to size, lipid composition, and protein cargo. Multiple approaches seek to enhance the efflux capacity of circulating plasma in vivo via oral or infusion therapies.
Increased apoA-I Expression
A large body of evidence suggests that apoA-I, which makes up 70% of HDL protein, is antiatherogenic. Increased apoA-I expression increases plasma HDL levels, promotes RCT, and leads to decreased atherosclerosis in several animal models [13]. One study documented atherosclerotic plaque regression, an elusive “holy grail” of modern cardiology, with hepatic apoA-I overexpression [37]. Small molecules that promote hepatic apoA-I expression in humans may recapitulate these strikingly beneficial effects. One such compound, RVX-208 (Resverlogix, Calgary, AB, Canada), recently entered early-phase clinical trials [38]. Serum from human patients treated with RVX-208 exhibited increased cholesterol efflux capacity despite an only modest increase in HDL-C levels [39]. It will be of interest to follow the progress of this and other approaches to upregulation of apoA-I expression that may develop.
A second mechanism of increasing apoA-I production is via peroxisome proliferator-activated receptor α (PPARα) agonism. Although PPARα stimulation has multiple effects on lipid metabolism, including increasing ABCA1 expression, most promising is its stimulation of apoA-I production [40]. Although currently prescribed fibrates are week PPARα agonists, several agents with substantially increased potency and selectivity have been developed. LY518764 (Eli Lilly and Company, Indianapolis, IN) has been shown to increase apoA-I production rate by 31% in patients with metabolic syndrome [41]. Intriguingly, these patients exhibited an equivalent increase in apoA-I catabolic rate, resulting in no change in steady state apoA-I or HDL-C levels. A second study noted potential safety concerns and modest increases in HDL and apoA-I levels [42]. However, the increased apoA-I turnover may in fact promote macrophage RCT, as has been demonstrated in mice treated with a different potent PPARα agonist [43]. Although PPARα is currently not considered an optimal target for new therapies, available data suggest that PPARα agonists more potent than fibrates have the potential to substantially promote macrophage RCT through both hepatic apoA-I and macrophage ABCA1 upregulation.
ApoA-I/Reconstituted HDL Infusions
Given the substantial challenges of increasing endogenous apoA-I expression, an alternate strategy has been to directly infuse apoA-I or reconstituted HDL (rHDL) into the circulation. These infusions were associated with attenuation, and even regression, of atherosclerosis in rabbit models [44, 45]. Subsequent preclinical studies in humans have noted transient increases in apoA-I levels, total and lipid-poor pre-β HDL concentrations, ex vivo cholesterol efflux capacity, and fecal sterol excretion [12•, 18, 46]. A small human trial studied the effects of rHDL/apoA-I disks on coronary atherosclerosis as visualized using angiography and intravascular ultrasound. The intervention was associated with reduction in atheroma volume, consistent with plaque regression, when compared with baseline [47]. However, although intriguing, the study was underpowered to detect differences from the placebo group and thus awaits replication in a larger cohort.
Beyond infusions of wild-type apoA-I, several groups have explored the use of apoA-I engineered to harbor the rare, naturally occurring Milano variant. The association of this point mutation with low HDL and apoA-I levels but no dramatic increase in vascular disease has led to speculation that apoA-I Milano may have improved atheroprotective properties [48]. However, this remains controversial, and mice expressing wild-type and apoA-I Milano exhibit no difference in macrophage RCT in vivo [49]. Nevertheless, weekly infusions of apoA-I Milano/phosphatidylcholine disks were associated with reduction from baseline coronary atheroma volume noted on intravascular ultrasound [50]. Although the optimal formulation remains unclear, results in both humans and animal models suggest that infusions of apoA-I/rHDL may increase RCT and improve atherosclerotic burden. A major limitation is the requirement for intravenous infusion, likely limiting its potential use to a peri-acute coronary syndrome timeframe. Similar infusional approaches are currently in clinical use for antithrombotic and antiplatelet agents, including heparin and glycoprotein IIb/IIIa inhibitors, indicating the feasibility of this approach if proven to be effective.
ApoA-I Mimetic Peptides
Substantial research has focused on the development of a small mimetic peptide, ideally with oral administration, that can mimic the effects of infusing full-length apoA-I [51]. One such peptide, D-4F, was engineered to contain only 18 amino acids but retain its lipid-binding properties. Furthermore, the use of only D-amino acids allows the molecule to escape gastrointestinal peptidase breakdown. Oral D-4F was associated with enhanced RCT and decreased atherosclerosis in mouse models [52, 53]. Early-phase clinical investigation of single-dose D-4F demonstrated proof of concept with regard to safety, modest oral bioavailability, and a dose-dependent improvement in measures of isolated HDL anti-inflammatory activity [54]. The future of D-4F and other apoA-I mimetic peptides will likely depend on improving bioavailability and larger-scale evidence of enhanced HDL functionality.
Cholesteryl Ester Transfer Protein Inhibition
Patients with genetic cholesteryl ester transfer protein (CETP) deficiency have markedly increased HDL-C levels, a discovery that stimulated efforts to achieve similar effects with pharmacologic therapy [55]. CETP inhibition therefore represents a strategy of increasing acceptor concentrations for macrophage cholesterol efflux. This concept was reinforced when both genetic deficiency and pharmacologic CETP inhibition were associated with increased cholesterol efflux capacity, primarily via the ABCG1 transporter [56, 57]. However, a major limitation of in vitro efflux assays is the inability to assess terminal components of the RCT pathway. CETP has a well-established role in mediating transfer of cholesteryl esters from HDL to apoB-containing lipoproteins (LDL or very low density lipoprotein) in exchange for triglycerides. Human kinetic studies suggest that this transfer, with subsequent uptake of LDL particles by hepatic LDL receptors, represents the primary pathway for RCT in humans [58]. The impact of CETP on RCT, and thus potentially cardiovascular disease, may therefore vary according to hepatic LDL clearance efficiency. Animal studies have supported this notion, with CETP facilitating RCT in wild-type mice but decreasing RCT flux in LDL-receptor deficient mice [59].
Despite being an important determinant of plasma HDL levels, the influence of CETP on RCT and cardiovascular disease remains highly controversial [60]. No increase in fecal sterol excretion was noted in a human study of pharmacologic CETP inhibition, although the limitations of the methodology preclude definitive conclusions regarding RCT [61]. The field was dealt a major blow when a phase 3 clinical trial of one potent CETP inhibitor, torcetrapib (Pfizer, New York, NY), was terminated after an increase in mortality and cardiovascular events was noted in the treatment group. This occurred despite the predicted effects on patients’ lipid profiles, with a 72% increase in HDL-C and 25% reduction in LDL-C [62•]. Unfortunately, torcetrapib was linked to off-target elevations in aldosterone levels and blood pressure, potentially explaining a portion of the adverse effects. Two other CETP inhibitors currently in development, anacetrapib (Merck, Whitehouse Station, NJ) and dalcetrapib (Roche Pharmaceuticals, Basel, Switzerland), show no effects on blood pressure and are likely to provide much-needed insight into the mechanism’s utility [63, 64].
Endothelial Lipase Inhibition
Endothelial lipase (EL) plays a major role in modulating both HDL particle composition and catabolism [65]. Like other members of the lipoprotein lipase family, EL is a secreted protein that binds to the endothelial surface. It primarily hydrolyzes phospholipids on circulating HDL particles, resulting in accelerated apoA-I catabolism [66]. Animal studies have noted substantially increased HDL-C levels and decreased atherosclerosis in EL-deficient mice [67]. Decreasing EL activity has thus been proposed as an effective way of increasing plasma levels of HDL acceptors and potentially enhancing RCT.
Human genetic studies have confirmed that variation near the EL gene is an important determinant of plasma HDL-C levels [68, 69]. Furthermore, EL mass is associated with a number of cardiovascular risk factors and increased atherosclerotic burden as assessed by coronary artery calcification [70]. These findings stimulated initial screening efforts to identify small molecule inhibitors of EL [71]. Ongoing efforts in animal models that seek to determine the effects of EL inhibition on HDL phospholipid levels, HDL catabolism, and RCT will inform whether the strategy may ultimately have utility in humans.
Improving Hepatic Uptake and Biliary/Intestinal Cholesterol Excretion
The terminal hepatic and intestinal components of macrophage RCT, although often overlooked, are likely to receive increased attention in coming years. As discussed earlier, diminished hepatic uptake in mice deficient of SRB1 was linked to markedly decreased RCT [16]. Although the physiologic importance of this pathway in humans remains unclear, the finding reinforces the need for additional study of how cholesterol effluxed to HDL particles is ultimately transported into the intestines.
Recent work suggests that cholesterol taken up by hepatocytes is secreted into the bile by the apical membrane sterol transporters ABCG5 and ABCG8. Accordingly, ABCG5/ABCG8 knockout and overexpression studies in mice have noted the predicted effects on biliary cholesterol excretion [72, 73]. Furthermore, humans with mutations in ABCG5 and ABCG8 suffer from sitosterolemia, a genetic disorder involving decreased biliary cholesterol excretion and the accumulation of dietary cholesterol [74]. An additional line of provocative research has called attention to variable rates of intestinal cholesterol absorption as determinants of dynamic RCT flux [75]. Finally, documentation of direct intestinal secretion of cholesterol, bypassing the liver entirely, offers a potentially important revision of the classic RCT model [76].
As with other components of RCT, the in vivo rodent model has facilitated investigations into the importance of terminal RCT components and the effects of various interventions. A recent report noted that acutely induced inflammation impairs macrophage RCT in mice, largely due to decreased flux from liver to feces [77]. Conversely, fish oil, a dietary supplement with an emerging role in cardioprotection [78], increased RCT by ABCG5/ABCG8-mediated hepatic excretion and decreasing intestinal absorption [79]. Statins and fibrates have been shown to similarly increase ABCG5/ABCG8 expression, although enhanced RCT has not been documented [80, 81]. Ezetimibe, a therapeutic used clinically for its LDL-reducing effects, augments RCT by decreasing intestinal cholesterol absorption via inhibition of the Niemann-Pick C1-like 1 (NPC1L1) transporter [75]. Similar effects with regard to decreased NPC1L1 expression and increased RCT were noted after treatment with a PPARδ agonist [82]. Treatment with a PPARδ agonist was associated with modest increases in HDL levels in humans, although the underlying mechanism remains unclear [83]. Finally, LXR activation, discussed previously with a focus on enhancing cellular efflux, stimulated direct intestinal cholesterol excretion in vivo [84]. Given the adverse hepatic effects of nonselective LXR agonism, intestinal-specific activation has emerged as an additional area of interest.
Additional studies are needed to rigorously assess the molecular and genetic underpinnings of hepatic uptake and excretion, as well as the quantitative role of direct intestinal excretion as a proportion of overall macrophage RCT flux. However, the hepato-intestinal components of RCT represent a promising additional target for drug development.
Conclusions
Recent data have reinforced long-standing hypotheses suggesting that HDL’s critical role in the RCT pathway may explain its atheroprotective effects. Although this causal relationship has not been definitively proven, the promotion of macrophage RCT remains a promising target for future drug development. Ongoing efforts to systematically assess the impact of therapeutics on robust assays of RCT flux will be of major importance to the field. Ultimately, we look forward to a future in which the complexities of HDL metabolism are overcome by an RCT-focused therapy that is safe and efficacious in the treatment of human disease.
Acknowledgments
This work was supported by P01-HL22633 from the National Heart, Lung, and Blood Institute; an Alternative Drug Discovery Initiative award to the University of Pennsylvania from GlaxoSmithKline, and a Doris Duke Charitable Foundation Distinguished Clinical Scientist Award (to DJR).
Footnotes
Disclosure Dr. Rader serves as a consultant to several companies that market or are developing therapies targeting HDL or RCT, including Abbott, AstraZeneca, Bristol-Myers-Squibb, Eli Lilly, Johnson & Johnson, Merck & Co, Novartis, and Resverlogix. No other potential conflicts of interest relevant to this article were reported.
Contributor Information
Amit V. Khera, Institute for Translational Medicine and Therapeutics and Cardiovascular Institute, University of Pennsylvania, Philadelphia, PA, USA
Daniel J. Rader, Email: rader@mail.med.upenn.edu, University of Pennsylvania School of Medicine, 654 BRBII/III, 421 Curie Blvd Philadelphia, PA 19104, USA
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Miller GJ, Miller NE. Plasma-high-density-lipoprotein concentration and development of ischaemic heart-disease. Lancet. 1975;1:16–19. doi: 10.1016/s0140-6736(75)92376-4. [DOI] [PubMed] [Google Scholar]
- 2.Gordon DJ. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79:8–15. doi: 10.1161/01.cir.79.1.8. [DOI] [PubMed] [Google Scholar]
- 3.Singh IM, Shishehbor MH, Ansell BJ. High-density lipoprotein as a therapeutic target: a systematic review. JAMA. 2007;298:786–798. doi: 10.1001/jama.298.7.786. [DOI] [PubMed] [Google Scholar]
- 4•.Briel M, Ferreira-Gonzalez I, You JJ, et al. Association between change in high density lipoprotein cholesterol and cardiovascular disease morbidity and mortality: systematic review and meta-regression analysis. BMJ. 2009;338:b92. doi: 10.1136/bmj.b92. This provocative meta-analysis suggested that increased HDL-C levels explain minimal variability with regard to clinical outcomes, reinforcing the need to move beyond simple measures of HDL-C quantity as surrogates of clinical efficacy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tall AR. Cholesterol efflux pathways and other potential mechanisms involved in the atheroprotective effect of high density lipoproteins. J Intern Med. 2008;263:256–273. doi: 10.1111/j.1365-2796.2007.01898.x. [DOI] [PubMed] [Google Scholar]
- 6.Glomset JA. The plasma lecithins:cholesterol acyltransferase reaction. J Lipid Res. 1968;9:155–167. [PubMed] [Google Scholar]
- 7.Tall AR, Wang N, Mucksavage P. Is it time to modify the reverse cholesterol transport model? J Clin Invest. 2001;108:1273–1275. doi: 10.1172/JCI14342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuchel M, Rader DJ. Macrophage reverse cholesterol transport: key to the regression of atherosclerosis? Circulation. 2006;113:2548–2555. doi: 10.1161/CIRCULATIONAHA.104.475715. [DOI] [PubMed] [Google Scholar]
- 9.Rader DJ, Alexander ET, Weibel GL, et al. The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J Lipid Res. 2009;50:S189–S194. doi: 10.1194/jlr.R800088-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de la Llera Moya M, Atger V, Paul JL, et al. A cell culture system for screening human serum for ability to promote cellular cholesterol efflux. Relations between serum components and efflux, esterification, and transfer. Arterioscler Thromb. 1994;14:1056–1065. doi: 10.1161/01.atv.14.7.1056. [DOI] [PubMed] [Google Scholar]
- 11.Venkateswaran A, Laffitte BA, Joseph SB, et al. Control of cellular cholesterol efflux by the nuclear oxysterol receptor LXR alpha. Proc Natl Acad Sci U S A. 2000;97:12097–12102. doi: 10.1073/pnas.200367697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12•.Patel S, Drew BG, Nakhla S, et al. Reconstituted high-density lipoprotein increases plasma high-density lipoprotein anti-inflammatory properties and cholesterol efflux capacity in patients with type 2 diabetes. J Am Coll Cardiol. 2009;53:962–971. doi: 10.1016/j.jacc.2008.12.008. This is a recent study that applied an HDL panel to study multiple aspects of HDL functionality before and after a therapeutic intervention. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Zanotti I, Reilly MP, et al. Overexpression of apolipoprotein A-I promotes reverse transport of cholesterol from macrophages to feces in vivo. Circulation. 2003;108:661–663. doi: 10.1161/01.CIR.0000086981.09834.E0. [DOI] [PubMed] [Google Scholar]
- 14.Kozarsky KF, Donahee MH, Glick JM, et al. Gene transfer and hepatic overexpression of the HDL receptor SR-BI reduces atherosclerosis in the cholesterol-fed LDL receptor-deficient mouse. Arterioscler Thromb Vasc Biol. 2000;20:721–727. doi: 10.1161/01.atv.20.3.721. [DOI] [PubMed] [Google Scholar]
- 15.Arai T, Wang N, Bezouevski M, et al. Decreased atherosclerosis in heterozygous low density lipoprotein receptor-deficient mice expressing the scavenger receptor BI transgene. J Biol Chem. 1999;274:2366–2371. doi: 10.1074/jbc.274.4.2366. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Da Silva JR, Reilly M, et al. Hepatic expression of scavenger receptor class B type I (SR-BI) is a positive regulator of macrophage reverse cholesterol transport in vivo. J Clin Invest. 2005;115:2870–2874. doi: 10.1172/JCI25327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Czubayko F, Beumers B, Lammsfuss S, et al. A simplified micro-method for quantification of fecal excretion of neutral and acidic sterols for outpatient studies in humans. J Lipid Res. 1991;32:1861–1867. [PubMed] [Google Scholar]
- 18.Eriksson M, Carlson LA, Miettinen TA, et al. Stimulation of fecal steroid excretion after infusion of recombinant proapolipoprotein A-I. Potential reverse cholesterol transport in humans. Circulation. 1999;100:594–598. doi: 10.1161/01.cir.100.6.594. [DOI] [PubMed] [Google Scholar]
- 19•.deGoma EM, deGoma RL, Rader DJ. Beyond high-density lipoprotein cholesterol levels evaluating high-density lipoprotein function as influenced by novel therapeutic approaches. J Am Coll Cardiol. 2008;51:2199–2211. doi: 10.1016/j.jacc.2008.03.016. This review outlines current thinking on HDL functionality, particularly with regard to measurement of HDL’s pleiotropic beneficial effects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Collins HL, Ranalletta M, et al. Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J Clin Invest. 2007;117:2216–2224. doi: 10.1172/JCI32057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21••.Yvan-Charvet L, Ranalletta M, Wang N, et al. Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J Clin Invest. 2007;117:3900–3908. doi: 10.1172/JCI33372. This study in mice provided compelling evidence that ABCA1 and ABCG1 activity decreases atherosclerosis via enhanced cellular cholesterol efflux. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Out R, Hoekstra M, Habets K, et al. Combined deletion of macrophage ABCA1 and ABCG1 leads to massive lipid accumulation in tissue macrophages and distinct atherosclerosis at relatively low plasma cholesterol levels. Arterioscler Thromb Vasc Biol. 2008;28:258–264. doi: 10.1161/ATVBAHA.107.156935. [DOI] [PubMed] [Google Scholar]
- 23.Rust S, Rosier M, Funke H, et al. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat Genet. 1999;22:352–355. doi: 10.1038/11921. [DOI] [PubMed] [Google Scholar]
- 24.Bodzioch M, Orsó E, Klucken J, et al. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat Genet. 1999;22:347–351. doi: 10.1038/11914. [DOI] [PubMed] [Google Scholar]
- 25.Brooks-Wilson A, Marcil M, Clee SM, et al. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat Genet. 1999;22:336–345. doi: 10.1038/11905. [DOI] [PubMed] [Google Scholar]
- 26.van Dam MJ, de Groot E, Clee SM, et al. Association between increased arterial-wall thickness and impairment in ABCA1-driven cholesterol efflux: an observational study. Lancet. 2002;359:37–42. doi: 10.1016/S0140-6736(02)07277-X. [DOI] [PubMed] [Google Scholar]
- 27.Repa JJ, Turley SD, Lobaccaro JA, et al. Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science. 2000;289:1524–1529. doi: 10.1126/science.289.5484.1524. [DOI] [PubMed] [Google Scholar]
- 28.Naik SU, Wang X, Da Silva JS, et al. Pharmacological activation of liver X receptors promotes reverse cholesterol transport in vivo. Circulation. 2006;113:90–97. doi: 10.1161/CIRCULATIONAHA.105.560177. [DOI] [PubMed] [Google Scholar]
- 29.Terasaka N, Hiroshima A, Koieyama T, et al. T-0901317, a synthetic liver X receptor ligand, inhibits development of atherosclerosis in LDL receptor-deficient mice. FEBS Lett. 2003;536:6–11. doi: 10.1016/s0014-5793(02)03578-0. [DOI] [PubMed] [Google Scholar]
- 30.Joseph SB, McKilligin E, Pei L, et al. Synthetic LXR ligand inhibits the development of atherosclerosis in mice. Proc Natl Acad Sci U S A. 2002;99:7604–7609. doi: 10.1073/pnas.112059299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li AC, Glass CK. PPAR- and LXR-dependent pathways controlling lipid metabolism and the development of atherosclerosis. J Lipid Res. 2004;45:2161–2173. doi: 10.1194/jlr.R400010-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Groot PH, Pearce NJ, Yates JW, et al. Synthetic LXR agonists increase LDL in CETP species. J Lipid Res. 2005;46:2182–2191. doi: 10.1194/jlr.M500116-JLR200. [DOI] [PubMed] [Google Scholar]
- 33.Levin N, Bischoff ED, Daige CL, et al. Macrophage liver X receptor is required for antiatherogenic activity of LXR agonists. Arterioscler Thromb Vasc Biol. 2005;25:135–142. doi: 10.1161/01.ATV.0000150044.84012.68. [DOI] [PubMed] [Google Scholar]
- 34.Teupser D, Kretzschmar D, Tennert C, et al. Effect of macrophage overexpression of murine liver X receptor-alpha (LXR-alpha) on atherosclerosis in LDL-receptor deficient mice. Arterioscler Thromb Vasc Biol. 2008;28:2009–2015. doi: 10.1161/ATVBAHA.108.175257. [DOI] [PubMed] [Google Scholar]
- 35.Molteni V, Li X, Nabakka J, et al. N-Acylthiadiazolines, a new class of liver X receptor agonists with selectivity for LXRbeta. J Med Chem. 2007;50:4255–4259. doi: 10.1021/jm070453f. [DOI] [PubMed] [Google Scholar]
- 36.Katz A, Udata C, Ott E, et al. Safety, pharmacokinetics, and pharmacodynamics of single doses of LXR-623, a novel liver X-receptor agonist, in healthy participants. J Clin Pharmacol. 2009;49:643–649. doi: 10.1177/0091270009335768. [DOI] [PubMed] [Google Scholar]
- 37.Tangirala RK, Tsukamoto K, Chun SH, et al. Regression of atherosclerosis induced by liver-directed gene transfer of apolipoprotein A-I in mice. Circulation. 1999;100:1816–1822. doi: 10.1161/01.cir.100.17.1816. [DOI] [PubMed] [Google Scholar]
- 38.ClincialTrials.gov. [Accessed October 8, 2009.];A Safety, Pharmacokinetic, and Pharmacodynamic Assessment of 28-Dy Oral Dosing of rvX000222 in Healthy subjects and subjects with Low High Density Lipoprotein (HDL) Available at http://clinicaltrials.gov/ct2/show/NCT00768274.
- 39.Gordon A, Jahagirdar R, Johannson J, et al. RVX-208 a Small Molecule That Induces Apolipoprotein A-I Production Progresses to Phase Ib/IIa Clinical Trials. Presented at the American College of Cardiology Scientific Sessions; Orlando, FL. March 28–30, 2009. [Google Scholar]
- 40.Duffy D, Rader DJ. Update on strategies to increase HDL quantity and function. Nat Rev Cardiol. 2009;6:455–463. doi: 10.1038/nrcardio.2009.94. [DOI] [PubMed] [Google Scholar]
- 41.Millar JS, Duffy D, Gadi R, et al. Potent and selective PPAR-alpha agonist LY518674 upregulates both ApoA-I production and catabolism in human subjects with the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2009;29:140–146. doi: 10.1161/ATVBAHA.108.171223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nissen SE, Nicholls SJ, Wolski K, et al. Effects of a potent and selective PPAR-alpha agonist in patients with atherogenic dyslipidemia or hypercholesterolemia: two randomized controlled trials. JAMA. 2007;297:1362–1373. doi: 10.1001/jama.297.12.1362. [DOI] [PubMed] [Google Scholar]
- 43.Junichiro T, Tanigawa H, Snehal N, et al. PPARα agonism promotes reverse cholesterol transport in a macrophage PPAR α and Liver X Receptor dependent manner. Presented at American Heart Association Scientific Sessions; Orlando, FL. November 15–18, 2009. [Google Scholar]
- 44.Badimon JJ, Badimon L, Fuster V. Regression of atherosclerotic lesions by high density lipoprotein plasma fraction in the cholesterol-fed rabbit. J Clin Invest. 1990;85:1234–1241. doi: 10.1172/JCI114558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miyazaki A, Sakuma S, Morikawa W, et al. Intravenous injection of rabbit apolipoprotein A-I inhibits the progression of atherosclerosis in cholesterol-fed rabbits. Arterioscler Thromb Vasc Biol. 1995;15:1882–1888. doi: 10.1161/01.atv.15.11.1882. [DOI] [PubMed] [Google Scholar]
- 46.Nanjee MN, Cooke CJ, Garvin R, et al. Intravenous apoA-I/lecithin discs increase pre-beta-HDL concentration in tissue fluid and stimulate reverse cholesterol transport in humans. J Lipid Res. 2001;42:1586–1593. [PubMed] [Google Scholar]
- 47.Tardif JC, Grégoire J, L’Allier PL, et al. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: a randomized controlled trial. JAMA. 2007;297:1675–1682. doi: 10.1001/jama.297.15.jpc70004. [DOI] [PubMed] [Google Scholar]
- 48.Chiesa G, Sirtori CR. Apolipoprotein A-I(Milano): current perspectives. Curr Opin Lipidol. 2003;14:159–163. doi: 10.1097/00041433-200304000-00007. [DOI] [PubMed] [Google Scholar]
- 49.Alexander ET, Weibel GL, Joshi MR, et al. Macrophage reverse cholesterol transport in mice expressing ApoA-I Milano. Arterioscler Thromb Vasc Biol. 2009;29:1496–1501. doi: 10.1161/ATVBAHA.109.191379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nissen SE, Tsunoda T, Tuzcu EM, et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 2003;290:2292–2300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- 51.Van Lenten BJ, Wagner AC, Anantharamaiah GM, et al. Apolipoprotein A-I mimetic peptides. Curr Atheroscler Rep. 2009;11:52–57. doi: 10.1007/s11883-009-0008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Navab M, Anantharamaiah GM, Reddy ST, et al. Oral D-4F causes formation of pre-beta high-density lipoprotein and improves high-density lipoprotein-mediated cholesterol efflux and reverse cholesterol transport from macrophages in apolipoprotein E-null mice. Circulation. 2004;109:3215–3220. doi: 10.1161/01.CIR.0000134275.90823.87. [DOI] [PubMed] [Google Scholar]
- 53.Navab M, Anantharamaiah GM, Hama S, et al. Oral administration of an Apo A-I mimetic Peptide synthesized from D-amino acids dramatically reduces atherosclerosis in mice independent of plasma cholesterol. Circulation. 2002;105:290–292. doi: 10.1161/hc0302.103711. [DOI] [PubMed] [Google Scholar]
- 54.Bloedon LT, Dunbar R, Duffy D, et al. Safety, pharmacokinetics, and pharmacodynamics of oral apoA-I mimetic peptide D-4F in high-risk cardiovascular patients. J Lipid Res. 2008;49:1344–1352. doi: 10.1194/jlr.P800003-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brown ML, Inazu A, Hesler CB, et al. Molecular basis of lipid transfer protein deficiency in a family with increased high-density lipoproteins. Nature. 1989;342:448–451. doi: 10.1038/342448a0. [DOI] [PubMed] [Google Scholar]
- 56.Matsuura F, Wang N, Chen W, et al. HDL from CETP-deficient subjects shows enhanced ability to promote cholesterol efflux from macrophages in an apoE- and ABCG1-dependent pathway. J Clin Invest. 2006;116:1435–1442. doi: 10.1172/JCI27602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yvan-Charvet L, Matsuura F, Wang N, et al. Inhibition of cholesteryl ester transfer protein by torcetrapib modestly increases macrophage cholesterol efflux to HDL. Arterioscler Thromb Vasc Biol. 2007;27:1132–1138. doi: 10.1161/ATVBAHA.106.138347. [DOI] [PubMed] [Google Scholar]
- 58.Schwartz CC, VandenBroek JM, Cooper PS. Lipoprotein cholesteryl ester production, transfer, and output in vivo in humans. J Lipid Res. 2004;45:1594–1607. doi: 10.1194/jlr.M300511-JLR200. [DOI] [PubMed] [Google Scholar]
- 59.Tanigawa H, Billheimer JT, Tohyama J, et al. Expression of cholesteryl ester transfer protein in mice promotes macrophage reverse cholesterol transport. Circulation. 2007;116:1267–1273. doi: 10.1161/CIRCULATIONAHA.107.704254. [DOI] [PubMed] [Google Scholar]
- 60.Tall AR, Yvan-Charvet L, Wang N. The failure of torcetrapib: was it the molecule or the mechanism? Arterioscler Thromb Vasc Biol. 2007;27:257–260. doi: 10.1161/01.ATV.0000256728.60226.77. [DOI] [PubMed] [Google Scholar]
- 61.Brousseau ME, Diffenderfer MR, Millar JS, et al. Effects of cholesteryl ester transfer protein inhibition on high-density lipoprotein subspecies, apolipoprotein A-I metabolism, and fecal sterol excretion. Arterioscler Thromb Vasc Biol. 2005;25:1057–1064. doi: 10.1161/01.ATV.0000161928.16334.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62•.Barter PJ, Caulfield M, Eriksson M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. This is a large phase 3 clinical trial that noted a substantial increase in coronary events among those treated with a CETP inhibitor despite substantially increased HDL levels. [DOI] [PubMed] [Google Scholar]
- 63.Krishna R, Anderson MS, Bergman AJ, et al. Effect of the cholesteryl estertransfer protein inhibitor, anacetrapib, on lipoproteins in patients with dyslipidaemia and on 24-h ambulatory blood pressure in healthy individuals: two double-blind, randomised placebo-controlled phase I studies. Lancet. 2007;370:1907–1914. doi: 10.1016/S0140-6736(07)61813-3. [DOI] [PubMed] [Google Scholar]
- 64.Stein EA, Stroes ES, Steiner G, et al. Safety and tolerability of dalcetrapib. Am J Cardiol. 2009;104:82–91. doi: 10.1016/j.amjcard.2009.02.061. [DOI] [PubMed] [Google Scholar]
- 65.Jaye M, Lynch KJ, Krawiec J, et al. A novel endothelial-derived lipase that modulates HDL metabolism. Nat Genet. 1999;21:424–428. doi: 10.1038/7766. [DOI] [PubMed] [Google Scholar]
- 66.Maugeais C, Tietge UJ, Broedl UC, et al. Dose-dependent acceleration of high-density lipoprotein catabolism by endothelial lipase. Circulation. 2003;108:2121–2126. doi: 10.1161/01.CIR.0000092889.24713.DC. [DOI] [PubMed] [Google Scholar]
- 67.Jin W, Millar JS, Broedl U, et al. Inhibition of endothelial lipase causes increased HDL cholesterol levels in vivo. J Clin Invest. 2003;111:357–362. doi: 10.1172/JCI16146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Edmondson AC, Brown RJ, Kathiresan S, et al. Loss-of-function variants in endothelial lipase are a cause of elevated HDL cholesterol in humans. J Clin Invest. 2009;119:1042–1050. doi: 10.1172/JCI37176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kathiresan S, Melander O, Guiducci C, et al. Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat Genet. 2008;40:189–197. doi: 10.1038/ng.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Badellino KO, Wolfe ML, Reilly MP, et al. Endothelial lipase concentrations are increased in metabolic syndrome and associated with coronary atherosclerosis. PLoS Med. 2006;3:e22. doi: 10.1371/journal.pmed.0030022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goodman KB, Bury MJ, Cheung M, et al. Discovery of potent, selective sulfonylfuran urea endothelial lipase inhibitors. Bioorg Med Chem Lett. 2009;19:27–30. doi: 10.1016/j.bmcl.2008.11.033. [DOI] [PubMed] [Google Scholar]
- 72.Yu L, Hammer RE, Li-Hawkins J, et al. Disruption of Abcg5 and Abcg8 in mice reveals their crucial role in biliary cholesterol secretion. Proc Natl Acad Sci U S A. 2002;99:16237–16242. doi: 10.1073/pnas.252582399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yu L, Li-Hawkins J, Hammer RE, et al. Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. J Clin Invest. 2002;110:671–680. doi: 10.1172/JCI16001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Berge KE, Tian H, Graf GA, et al. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science. 2000;290:1771–1775. doi: 10.1126/science.290.5497.1771. [DOI] [PubMed] [Google Scholar]
- 75.Sehayek E, Hazen SL. Cholesterol absorption from the intestine is a major determinant of reverse cholesterol transport from peripheral tissue macrophages. Arterioscler Thromb Vasc Biol. 2008;28:1296–1297. doi: 10.1161/ATVBAHA.108.165803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Groen AK, Oude Elferink RP, et al. The ins and outs of reverse cholesterol transport. Ann Med. 2004;36:135–145. doi: 10.1080/07853890310020635. [DOI] [PubMed] [Google Scholar]
- 77.McGillicuddy FC, de la Llera Moya M, Hinkle CC, et al. Inflammation impairs reverse cholesterol transport in vivo. Circulation. 2009;119:1135–1145. doi: 10.1161/CIRCULATIONAHA.108.810721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lavie CJ, Milani RV, Mehra MR, et al. Omega-3 polyunsaturated fatty acids and cardiovascular diseases. J Am Coll Cardiol. 2009;54:585–594. doi: 10.1016/j.jacc.2009.02.084. [DOI] [PubMed] [Google Scholar]
- 79.Nishimoto T, Pellizzon MA, Aihara M, et al. Fish oil promotes macrophage reverse cholesterol transport in mice. Arterioscler Thromb Vasc Biol. 2009;29:1502–1508. doi: 10.1161/ATVBAHA.109.187252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kamisako T, Ogawa H. Effects of pravastatin and bezafibrate on biliary lipid excretion and hepatic expression of Abcg5 and Abcg8 in the rat. J Gastroenterol Hepatol. 2004;19:879–883. doi: 10.1111/j.1440-1746.2004.03377.x. [DOI] [PubMed] [Google Scholar]
- 81.Roglans N, Vázquez-Carrera M, Alegret M, et al. Fibrates modify the expression of key factors involved in bile-acid synthesis and biliary-lipid secretion in gallstone patients. Eur J Clin Pharmacol. 2004;59:855–861. doi: 10.1007/s00228-003-0704-1. [DOI] [PubMed] [Google Scholar]
- 82.Briand F, Naik SU, Fuki I, et al. Both the peroxisome proliferator-activated receptor δ agonist, GW0742, and ezetimibe promote reverse cholesterol transport in mice by reducing intestinal reabsorption of HDL-derived cholesterol. Clin Transl Sci. 2009;2:127–133. doi: 10.1111/j.1752-8062.2009.00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sprecher DL, Massien C, Pearce G, et al. Triglyceride: high-density lipoprotein cholesterol effects in healthy subjects administered a peroxisome proliferator activated receptor delta agonist. Arterioscler Thromb Vasc Biol. 2007;27:359–365. doi: 10.1161/01.ATV.0000252790.70572.0c. [DOI] [PubMed] [Google Scholar]
- 84.van der Veen JN, van Dijk TH, Vrins CL, et al. Activation of the liver X receptor stimulates trans-intestinal excretion of plasma cholesterol. J Biol Chem. 2009;284:19211–19219. doi: 10.1074/jbc.M109.014860. [DOI] [PMC free article] [PubMed] [Google Scholar]