Abstract
Adoptive cell therapy (ACT) of metastatic melanoma with autologous tumor infiltrating lymphocytes (TIL) is clinically effective, but TIL production can be challenging. Here we describe a simplified method for initial TIL culture and rapid expansion in gas-permeable flasks. TIL were initially cultured from tumor digests and fragments in 40 mL capacity flasks with a 10 cm2 gas-permeable silicone bottom, G-Rex10. A TIL rapid expansion protocol (REP) was developed using 500 mL capacity flasks with a 100 cm2 gas-permeable silicone bottom, G-Rex100. TIL growth was successfully initiated in G-Rex10 flasks from tumor digests from 13 of 14 patients and from tumor fragments in all 11 tumor samples tested. TIL could then be expanded to 8–10×109 cells in a two-step REP which began by seeding 5 × 106 TIL into a G-Rex100 flask, followed by expansion at day 7 into 3 G-Rex100 flasks. To obtain the 30 to 60 × 109 cells used for patient treatment we seeded 6 G-Rex100 flasks with 5×106 cells and expanded into 18 G-Rex100 flasks. Large scale TIL REP in gas-permeable flasks requires approximately 9 to 10 liters of media, about 3 to 4 times less than other methods. In conclusion, TIL initiation and REP in gas-permeable G-Rex flasks require fewer total vessels, less media, less incubator space and less labor than initiation and REP in 24-well plates, tissue culture flasks and bags. TIL culture in G-Rex flasks will facilitate the production of TIL at the numbers required for patient treatment at most cell processing laboratories.
Introduction
Adoptive cell therapy (ACT) with tumor infiltrating lymphocytes (TIL) following host lymphodepletion leads to objective response rates of 50% to 70% patients with metastatic melanoma (1–4). TIL therapy involves culturing cytotoxic T cells from dissected tumor fragments or enzymatically digested tumor and expanding the cells by serial passage (3;5). Patients enrolled in initial clinical trials received an average dose of 60 × 109 TIL. Since generating these large quantities of cells over a short period of time is labor intensive and requires a skilled laboratory staff, it is desirable to simplify TIL production.
Traditionally, TIL were grown from melanoma fragments in microcultures with interleukin-2 (IL-2), selected for tumor-specific reactivity and were then rapidly expanded. Recent studies evaluated the means for the growth of TIL that enabled the administration of cells with shorter culture times derived from single cell suspensions of tumors (6;7). These “Young TIL” had properties that were associated with improved in vivo persistence and response rates including long telomeres and increased expression of CD27 and CD28 (8–12). Production of young TIL involves initial TIL culture from dissected tumor fragments or digested tumor in media containing human AB serum and IL-2 in 24-well plates, conditions that favor the growth of TIL cells over tumor and other cells. If after approximately two to four weeks in culture an adequate quantity of TIL ranging from 50 to 200 × 106 can be obtained, a portion of these TIL are then further expanded 500- to 2000-fold over 2 weeks to obtain 30 to 60 × 109 TIL. This rapid expansion protocol (REP) for TIL typically is performed in tissue culture flasks and gas-permeable bags. The first step of REP involves the co-culture of TIL with irradiated autologous or allogeneic peripheral blood mononuclear cells (PBMCs) as feeders in T-175 flasks in media with IL-2 and anti-CD3 for 7 days. The cells are then transferred to gas-permeable bags and are cultured for an additional 7 days. Since the optimal density of cells cultured in bags is approximately 0.5 to 2×106 cells per mL, the final volume of the culture is 30 to 60 L. At the end of the culture period the cells must be concentrated, washed and resuspended in a volume that can be administered intravenously over 1 to 2 hours.
Although the production of young TIL is simpler than previous described methods, initial TIL growth and REP using 24-well plates, tissue culture flasks and gas-permeable bags can be challenging and these procedures are not well suited for cell growth and expansion under the current good manufacturing practice (cGMP) conditions that are required for Phase III clinical trials. Initial TIL culture normally involves the growth of TIL from tumors in 24-well plates which is an “open” system and susceptible to microbial contamination. There are also several limitations to TIL REP in tissue culture flasks and bags. First, the initial step of REP in tissue culture flasks is also an open system which further increases the risk of microbial contamination during culture. Second, numerous containers are used which makes the process labor intensive and which also increases the risk of microbial contamination. Third, the final culture volume is large and requires considerable incubator capacity, specialized instruments for concentrating and washing the cells, and large quantities of culture media and additives. Fourth, the process requires a highly trained and highly skilled staff.
The goal of this study was to develop an initial TIL culture and REP process that can be used by most cell processing laboratories. We found that both initial TIL culture and REP could be performed in gas-permeable flasks using far less media, reagents and total number of containers than previously described methods.
Methods
Initial TIL culture
Patients were entered into clinical protocols and signed informed consents that were approved by the Institutional Review Board of the National Cancer Institute prior to tumor resection. TIL were initially cultured from enzymatic tumor digests and tumor fragments (1–8 mm3) produced by sharp dissection. Tumor digests were generated by incubation in enzyme media (RPMI 1640, 2mM Glutmax, 10 μg/mL gentamicin, 30 units/mL DNase and 1.0 mg/mL collagenase) followed by mechanical dissociation (GentleMACS, Miltenyi Biotec, Auburn, CA). Immediately after placing the tumor in enzyme media, it was mechanically dissociated for approximately 1 minute. The solution was then incubated for 30 minutes at 37°C in 5% CO2 and it was then mechanically disrupted again for approximately 1 minute. After being incubated again for 30 minutes at 37°C in 5% CO2, the tumor was mechanically disrupted a third time for approximately one minute. If after the third mechanical disruption, large pieces of tissue were present, one or two additional mechanical dissociations were applied to the sample, with or without 30 additional minutes of incubation at 37°C in 5% CO2. At the end of the final incubation if the cell suspension contained a large number of red blood cells or dead cells, a density gradient separation using ficoll was performed to remove these cells.
When TIL cultures were initiated in 24-well plates (Costar 24 well cell culture cluster, flat bottom, Corning Incorporated, Corning, NY), each well was seeded with 1×106 tumor digest cells or one tumor fragment approximately 1 to 8 mm3 in size in 2 mL of complete medium (CM) with IL-2 (6000 IU/mL, Chiron Corp., Emeryville, CA). CM consisted of RPMI 1640 with glutamine, supplemented with 10% human AB serum, 25 mM Hepes and 10 μg/mL gentamicin. When cultures were initiated in gas-permeable flasks with a 40 mL capacity and a 10 cm2 gas-permeable silicon bottom (G-Rex10, Wilson Wolf Manufacturing, New Brighton, MN, USA) (Figure 1), each flask was loaded with 10 to 40×106 viable tumor digest cells or 5 to 30 tumor fragments in 10 to 40 mL of CM with IL-2. Both the G-Rex10 and 24-well plates were incubated in a humidified incubator at 37°C in 5% CO2 and five days after culture initiation, half the media was removed and replaced with fresh CM and IL-2 and after day 5, half the media was changed every 2 to 3 days.
Figure 1. G-Rex flasks.

Three types of flasks with silicone gas-permeable bottoms were used in these studies. The G-Rex10 flask has a 10 cm2 silicon bottom and holds approximately 40 mL (left). The G-Rex100 flask has a 100 cm2 silicon bottom and holds approximately 500 mL (center). The G-Rex100L has the same 100 cm2 silicon bottom as the G-Rex100 flask but it holds approximately 2000 mL (right).
TIL Rapid Expansion Protocol
REP of TIL was performed using T-175 flasks and gas permeable bags as previously described (8;13) or gas permeable G-Rex flasks. For TIL REP in T-175 flasks, 1×106 TIL suspended in 150 mL of media was added to each T-175 flask. The TIL were cultured with irradiated (50 Gy) allogeneic peripheral blood mononuclear cells (PBMC) as “feeder” cells at a ratio of 1 to 100 and the cells were cultured in a 1 to 1 mixture of CM and AIM-V medium (50/50 medium), supplemented with 3000 IU per mL of IL-2 and 30 ng per mL of anti-CD3. The T-175 flasks were incubated at 37°C in 5% CO2. Half the media was changed on day 5 using 50/50 medium with 3000 IU per mL of IL-2. On day 7 cells from two T-175 flasks were combined in a 3 liter bag and 300 mL of AIM V with 5% human AB serum and 3000 IU per mL of IL-2 was added to the 300 mL of TIL suspension. The number of cells in each bag was counted every day or two and fresh media was added to keep the cell count between 0.5 and 2.0×106 cells/mL.
For TIL REP in 500 mL capacity flasks with 100 cm2 gas-permeable silicon bottoms (G-Rex100, Wilson Wolf) (Figure 1) 5×106 or 10×106 TIL were cultured with irradiated allogeneic PBMC at a ratio of 1 to 100 in 400 mL of 50/50 medium, supplemented with 5% human AB serum, 3000 IU per mL of IL-2 and 30 ng per ml of anti-CD3. The G-Rex100 flasks were incubated at 37°C in 5% CO2. On day 5, 250 mL of supernatant was removed and placed into centrifuge bottles and centrifuged at 1500 rpm (491 xg) for 10 minutes. The TIL pellets were re-suspended with 150 mL of fresh medium with 5% human AB serum, 3000 IU per mL of IL-2, and added back to the original G-Rex100 flasks. When TIL were expanded serially in G-Rex100 flasks, on day 7 the TIL in each G-Rex100 were suspended in the 300 mL of media present in each flask and the cell suspension was divided into 3 100 mL aliquots that were used to seed 3 G-Rex100 flasks. Then 150 mL of AIM-V with 5% human AB serum and 3000 IU per mL of IL-2 was added to each flask. The G-Rex100 flasks were incubated at 37°C in 5% CO2 and after 4 days 150 mL of AIM-V with 3000 IU per mL of IL-2 was added to each G-Rex100 flask. The cells were harvested on day 14 of culture.
Cell counts, viability, flow cytometery
The expression of CD3, CD4, CD8 and CD56 was measured by flow cytometry with antibodies from BD Biosciences (BD Biosciences, San Jose, CA) using a FACSCanto flow cytometer (BD Biosciences). The cells were counted manually using a disposable c-chip hemacytometer (VWR, Batavia, IL) and viability was assessed using trypan blue staining.
Cytokine Release Assays
TIL were evaluated for interferon-gamma (IFN-γ) secretion in response to stimulation either with OKT3 antibody or co-culture with autologous tumor digest. For OKT3 stimulation, TIL were washed extensively, and duplicate wells were prepared with 1 × 105 cells in 0.2ml CM in 96 well flat-bottom plates pre-coated with 0.1 or 1.0μg /mL of OKT-3 antibody diluted in PBS. After overnight incubation, the supernatants were harvested and IFN-γ in the supernatant was measured by ELISA (Pierce/Endogen, Woburn, MA). For the co-culture assay, TIL cells were placed into a 96-well plate with autologous tumor cells. After a 24 hour incubation, supernatants were harvested and IFN-γ release was quantified by ELISA.
Statistical Analysis
Values in the text are mean one ± standard error of the mean (SEM) unless otherwise indicated. Groups were compared using paired T tests.
Results
Initial TIL Culture
We compared the growth of TIL from tumors using gas-permeable flasks with a 40 mL capacity and 10 cm2 gas permeable silicone bottom (G-Rex10) with growth in 24-well plates. Fourteen melanoma samples were tested: 9 consisting of freshly prepared tumor digests and 5 thawed samples from previously frozen tumor digests. Except for one fresh sample (#3522), the ratio of harvested TIL to initially seeded cells at day 17 to 29 was similar to or better in the G-Rex10 flasks than in the 24-well plates (Table 1). The viability and percentage of cells expressing CD3 and CD8 grown in the G-Rex10 flasks and 24-well plates were similar (Table 1). TIL were obtained from 13 of the 14 samples. We could not culture TIL in either the G-Rex10 or 24-well plates from frozen tumor digest from patient 2653. These results suggest that TIL growth in G-Rex10 flasks is comparable to that in 24-well plates. We compared IFN-γ production by TIL cultured in G-Rex10 flasks with those cultured in 24-well plates. IFN-γ production following stimulation with autologous tumor by TIL from 4 patients cultured in both types of vessels was similar (Table 2).
Table 1.
Comparison of initial TIL culture in G-Rex10 flasks with 24-well plates using either fresh or frozen tumor digests
| Sample Type | Patient # | Ratio of # TIL harvested/# cell seeded | TIL Viability (%) | TIL Phenotype (% expressing CD3+CD8+) | |||
|---|---|---|---|---|---|---|---|
| 24-well plate | G-Rex10 | 24-well plate | G-Rex10 | 24-well plate | G-Rex10 | ||
| Fresh Tumor Digest | 3520 | 1.28 | 1.17 | 92.80 | 93.30 | 26.50 | 31.60 |
| 3522 | 2.14 | 1.46 | 92.10 | 95.10 | 39.80 | 48.70 | |
| 3523 | 2.34 | 2.30 | 94.50 | 93.60 | 15.00 | 37.60 | |
| 3524 | 1.27 | 6.22 | 80.20 | 95.00 | 76.70 | 86.60 | |
| 3546 | 5.86 | 7.60 | 90.40 | 93.80 | 44.60 | 37.30 | |
| 3552 | 3.20 | 5.83 | 94.90 | 97.10 | 43.10 | 72.40 | |
| 3556 | 3.60 | 4.15 | 96.20 | 96.20 | 35.40 | 31.20 | |
| 3560 | 6.06 | 6.25 | 95.80 | 97.20 | 32.30 | 38.60 | |
| 3561 | 4.76 | 6.38 | 94.70 | 93.90 | 65.80 | 83.40 | |
| Average ± SEM | 3.39±0.61 | 4.60±0.80 | 92.40±1.65 | 95.02±0.50 | 42.13±6.33 | 51.93±7.51 | |
| Frozen Tumor Digest | 2653 | 0.16 | 0.19 | 87.70 | 90.60 | N.T. | N.T. |
| 3289 | 2.43 | 5.30 | 95.10 | 98.00 | 70.40 | 73.60 | |
| 2976 | 7.26 | 7.50 | 99.00 | 99.00 | 66.30 | 70.00 | |
| 3071 | 1.79 | 6.05 | 88.40 | 97.70 | 37.50 | 29.10 | |
| 2998 | 3.36 | 5.00 | 96.30 | 97.00 | 64.40 | 75.80 | |
| Average ± SEM | 3.00±1.18 | 4.81±1.23 | 93.30±2.24 | 96.46±1.50 | 59.65±6.70 | 62.12±9.91 | |
N.T. = Not Tested
Table 2.
Interferon-γ release (pg/ml) by unstimulated and tumor-stimulated TIL
| Patient | Growth method | Tumor target1 |
||
|---|---|---|---|---|
| None | Allogeneic | Autologous | ||
| 3552 | plate | 114 | 157 | >1541 |
| G-Rex10 | 81 | 338 | >1577 | |
|
| ||||
| 3556 | plate | 90 | 184 | 139 |
| G-Rex10 | 87 | 363 | 282 | |
|
| ||||
| 3560 | plate | 135 | 175 | 318 |
| G-Rex10 | 104 | 212 | 572 | |
|
| ||||
| 3561 | plate | 70 | 97 | 207 |
| G-Rex10 | 84 | 128 | 253 | |
Cryopreserved enzymatically digested single cell tumor suspension was thawed and 1×105 viable tumor cells were cocultured with TIL (1:1 ratio) overnight before quantifying interferon-γ in the supernatant by ELISA. Values in bold are more than two times background and >200 pg/ml.
We next compared the growth of TIL in G-Rex10 flasks or 24-well plates from tumor fragments. For each tumor sample, fragments approximately 1 to 8 mm3 in size were seeded into 24-well plates at 1 piece per well and into G-Rex10 flasks at 5, 10, 20 or 30 pieces per flask. A total of 11 tumor samples collected from 9 patients were tested; 3 samples were from 1 patient, but they were from different metastatic tumors. TIL could be grown from tumor fragments from all 11 samples in both the G-Rex10 flasks and 24-well plates, but after 7 to 23 days in culture greater quantities of TIL were obtained from the G-Rex10 flasks. The head-to-head comparison of culturing 10 fragments in the two types of vessels showed that TIL yields from G-Rex10 flasks were consistently higher than those from 24-well plate (Figure 2, Panel A). The optimal numbers of fragments seeded into each G-Rex10 flasks was further assessed. The quantities of TIL obtained per tumor fragment decreased as the number of pieces added to each G-Rex10 flask increased (Figure 2, panel B), however, total TIL yield was higher as more fragments were cultured in the G-Rex10 flasks until 20 or more tumor fragments were cultured in each G-Rex10 flask (Figure 2, panel C). The viability of TIL obtained from G-Rex10 flasks was similar to that of TIL obtained from 24-well plates (96.6±0.6% vs 95.3±0.8%) as was the proportion of TIL expressing CD3 and CD8 (67.8±7.2% vs 63.3±7.7%). TIL were also obtained from 3 of the 11 samples by the culture of mechanically dissociated samples in G-Rex10 flasks, but greater yields were obtained using tumor fragments as the starting material (data not shown). It is not certain why the number of TIL produced per fragment decreased when more than 10 fragments were added but this may be related to the quantity of nutrients and oxygen available or release of suppressive factors by the tumor cells.
Figure 2. Initial TIL culture using tumor fragments in G-Rex10 flasks.
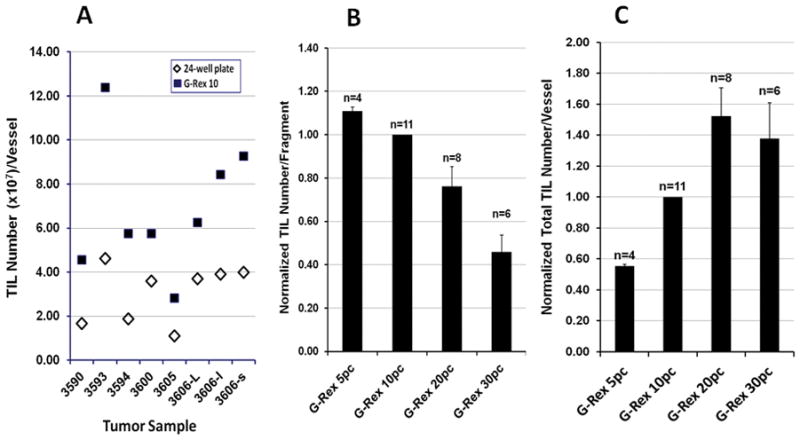
Panel A) Comparison of TIL numbers produced by 10 tumor fragments in 24-well plates and G-Rex10 flasks. For each individual tumor sample, 10 fragments were seeded into a 24-well plate at 1 piece per well and 10 fragments were seeded into a single G-Rex 10 flask. Cells were harvested by 7 to 23 days of culture, pooled if collected from 24-well plate, and counted. A total of 8 samples were tested. Panel B) The number of TIL produced per each tumor fragment by 7 to 23 days of culture of 5, 10, 20 and 30 tumor fragments in G-Rex10 flasks. Since only G-Rex10 flasks with 10 fragments were used in all experiments, the data was normalized using the number of cells produced in G-Rex10 flasks with 10 fragments. The number of TIL produced in each flask was divided by the number of fragments in the flask and this value was divided by the number of TIL produced in G-Rex10 flasks with10 fragments from the same patient divided by 10. The average number of TIL produced by each tumor fragment in G-Rex10 flasks seeded with 10 fragments was 7.51×106 cells per fragment (n=11). Panel C) The total number of TIL produced by 7 to 23 days of culture of 5, 10, 20 and 30 tumor fragments in G-Rex10 flasks. The data was again normalized using the number of cells produced in G-Rex10 flasks with 10 fragments. The total number TIL produced in each G-Rex10 flask was divided by the number of TIL produced by each G-Rex10 flask seeded with 10 tumor fragments from the same patient. The average number of TIL produced by G-Rex10 flasks seeded with 10 tumor fragments was 75.1×106 (n=11). The values displayed in both panels represent the mean ± SEM.
Comparison of T-175 and G-Rex100 flasks for the first step of TIL REP
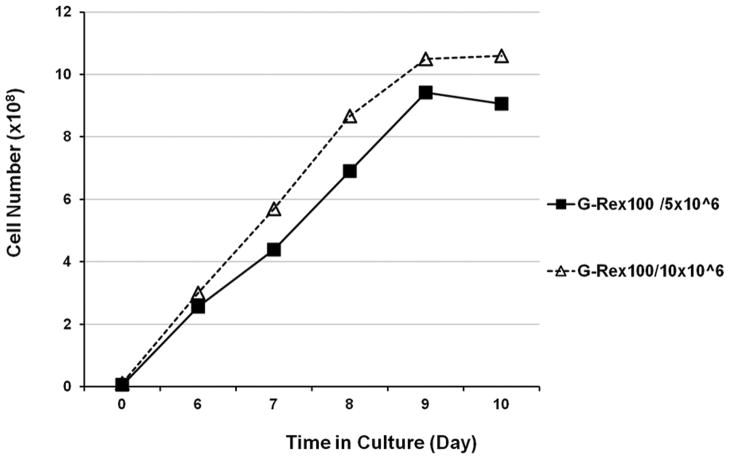
The first step in TIL REP has traditionally been performed in T-175 flasks. We compared the expansion of TIL in T-175 flasks to expansion in G-Rex100 flasks. We first assessed the kinetics of TIL growth in gas-permeable flasks. TIL from one patient were cultured in G-Rex100 flasks seeded at a density of 5 × 106 and 10×106 cells per flask. The cells were counted daily after day 6. On day 6 the number of cells in the G-Rex100 flask seeded at 5×106 cells was 255×106 cells and at 10×106 cells was 300×106 cells. The quantity of TIL in G-Rex100 flasks seeded at each cell density increased steadily until day 9, but there was little increase in cell counts between days 9 and 10. After 10 days 906×106 cells were harvested from the G-Rex100 flask seeded with 5×106 TIL and 1,050×106 cells from G-Rex100 flask seeded with 10×106 TIL (Figure 3). Although TIL expanded well for 9 days, in order to keep the G-Rex100 flask expansion process similar to REP in T-flasks and gas-permeable bags where TIL are transferred from T-flasks to bags on day 7, for further studies we focused on TIL expansion in the G-Rex100 flasks for 7 days.
Figure 3. Kinetics of TIL rapid expansion in a single gas-permeable G-Rex100 flask.
TIL from one patient were expanded in a G-Rex100 flask seeded with 5 x106 cells (solid line and squares) and a G-Rex100 flask seeded with 10×106 cells (dashed line and triangles). For all flasks the TIL were incubated with irradiated PBMC feeder cells at a ratio of 1 to 100 and with IL-2 (3000 IU per mL) and anti-CD3 (30 ng/mL).
Next, we identified the optimal seeding density for REP in G-Rex100 flasks. We compared the expansion of TIL from 4 patients over 7 days in G-Rex100 flasks seeded with 5×106 and 10×106 cells and compared this with TIL expansion in T-175 flasks. After 7 days of culture in T-175 flasks the number of TIL increased to 206±51×106 cells which represented an expansion of 167±37 fold (Table 3). The culture of TIL from the same 4 patients in G-Rex100 flasks seeded with 5×106 cells resulted in the production of 877±182×106 cells which represented an expansion of 176±36 fold. The culture of TIL from 2 of the 4 patients in G-Rex100 flasks seeded with 10×106 cells resulted in the production of 980±290×106 cells which represented an expansion of 98±29 fold. The viability and proportion of cells that expressed CD3 and CD8 were similar among those produced by the three different conditions (Table 3). These results suggested that the performance of G-Rex100 flasks seeded at the lower seeding density was comparable to that of the T-175 flasks. Since the 7-day culture of TIL in G-Rex100 flasks seeded with 10×106 cells did not produce a significantly greater number of cells than the G-Rex100 flasks seeded with 5×106 cells, the lower seeding density was preferable.
Table 3.
Comparison of TIL rapid expansion protocol (REP) over 7 days in T-175 flasks* and G-Rex100 flasks seeded with 5×106 or 10×106 TIL.
| Patient | Fold Increase | Viability (%) | Phenotype (%CD3+CD8+) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| T-175 | G-Rex100 5×106 | G-Rex100 10×106 | T-175 | G- Rex100 5×106 | G-Rex100 10×106 | T-175 | G-Rex100 5×106 | G-Rex100 10×106 | |
| 2812 | 74 | 88 | 57 | 95.8 | 96.6 | 94.7 | 66.4 | 64.6 | 60.5 |
| 3289 | 183 | 218 | 139 | 96.4 | 94.8 | 95.1 | 79.7 | 83.7 | 84.1 |
| 2976 | 254 | 250 | N.T. | 94.1 | 96.1 | N.T. | 55.2 | 59.9 | N.T. |
| 3071 | 156 | 146 | N.T. | 87.4 | 90.3 | N.T. | 44.6 | 41.6 | N.T. |
| Mean | 167±37 | 176±36 | 98±29 | 93.5±1.4 | 94.5±1.4 | 94.9±0.3 | 61.5±7.5 | 62.5±8.6 | 72.3±16.7 |
T-175 flasks were seeded with 1×106 TIL
N.T. = not tested
TIL REP using serial culture in G-Rex100 flasks
Since the maximum number of TIL present in one G-Rex100 flask reached a plateau after approximately 9 days, the production of adequate quantities of TIL for clinical therapy required the splitting of cells during REP and transferring the cells into multiple G-Rex flasks for further culture. We tested TIL REP by serial culture in G-Rex flasks. In addition to the G-Rex100 flask there is another type of gas-permeable flask that is commercially available for the large scale cell expansion: the G-Rex100L. The G-Rex100L has the same gas-permeable surface area on the silicone bottom of the flask as the G-Rex100, but the G-Rex100L is taller (Figure 1). As a result the media capacity of the G-Rex100L flask is approximately 2000 mL compared to approximately 500 mL for the G-Rex100. We compared TIL expansion using these two types of flasks. TIL were initially seeded at a density of 5×106 cells for both the G-Rex100 and G-Rex100L flasks and were cultured for 7 days in both types of flasks. After 7 days the cells from the G-Rex100 flask were split into 3 equal parts, and seeded into 3 G-Rex100 flasks and the cells from the G-Rex100L flask were split into two equal parts, seeded into 2 G-Rex100L flasks. The TIL were cultured for an additional 7 days in the G-Rex100 and G-Rex100L flasks.
The expansion of TIL from two patients was compared in the G-Rex100 and G-Rex100L flasks. Both patients’ TIL growth slowed after 13 or 14 days (Figure 4). The total number of cells produced after 14 days by culture in the 3 G-Rex100 flasks and the 2 G-Rex100L flasks was similar for one patient but was greater in the G-Rex100 flask for the second. Since a similar volume of media is required to produce a similar number of cells in the G-Rex100 and in the G-Rex100L flasks and since the G-Rex100 flasks are easier to handle, we elected to expand TIL initially in one G-Rex100 flask followed by expansion in 3 G-Rex100 flasks.
Figure 4. Comparison of serial gas-permeable flask TIL rapid expansion in G-Rex100 or G-Rex100L flasks.
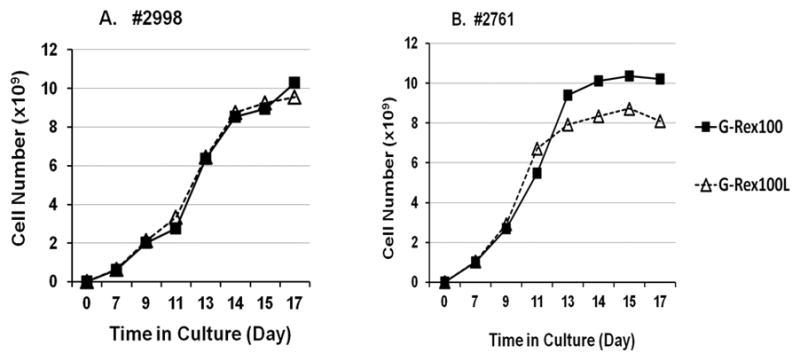
For the first step of REP 5×106 TIL were seeded into a G-Rex100 and a G-Rex100L gas-permeable flask. For expansion in both types of flasks, the TIL were cultured with irradiated PBMCs at a TIL to feeder cell ratio of 1 to 100 and the cells were cultured in a 1 to 1 mixture of CM and AIM-V medium, supplemented with IL-2 (3000 IU per mL) and anti-CD3 (30 ng per mL). After 7 days the TIL in the G-Rex100 flask were split equally into 3 and the culture was continued in the 3 G-Rex100 flasks. The TIL grown in the G-Rex100L flask were also divided after 7 days, but they were split in half and cultured in 2 G-Rex100L flasks. The cells in both types of containers were maintained in culture for 17 days. TIL from two patients were tested, patients 2998 (Panel A) and 2761 (Panel B). Growth of cells in the G-Rex100 flasks are shown by the solid lines and squares, the G-Rex100L flasks by the dashed lines and triangles. TIL for REP was prepared by growth in 24-well plates from fresh tumor digests.
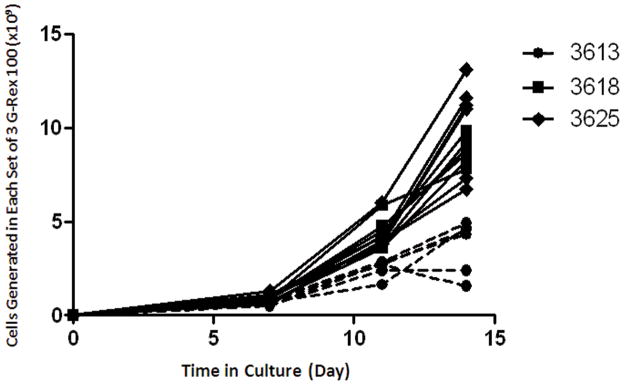
We next tested the consistency of serial TIL expansion in G-Rex100 flasks using cells from 14 patients (Figure 5). Initially, 5×106 TIL were seeded into a G-Rex100 flask and the cells were cultured for 7 days. They were then split into 3 equal parts, seeded into 3 G-Rex100 flasks. After 14 days in culture, 8.60±0.75 x109 TIL with a range of 2.24×109 to 12.8 x109 were produced (Figure 5). The number of TIL produced after 14 days was similar for 12 patients, but lower for two others. When the 2 patients with the lowest overall TIL expansion were excluded, the mean quantity of TIL produced was 9.55×109 cells per original G-Rex100 flask. The mean cell concentration in G-Rex100 flasks at the end of the culture was 7.95×106 cells per mL.
Figure 5. Consistency of serial TIL culture in G-Rex100 gas-permeable flasks for rapid expansion.
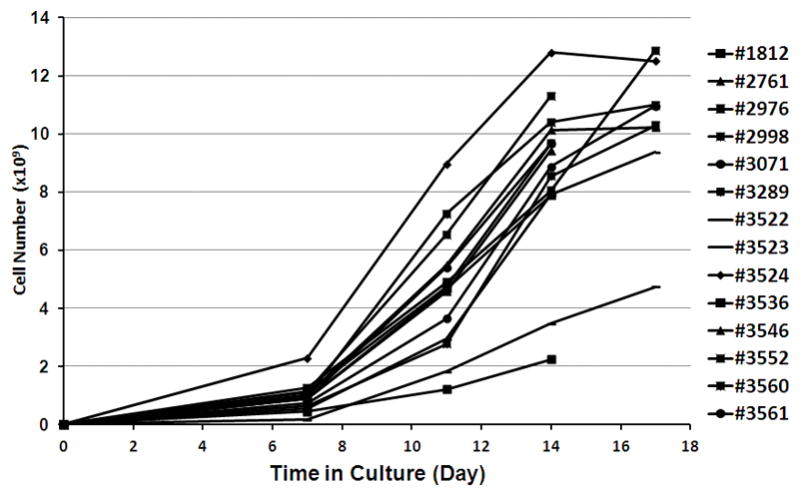
TIL from 14 patients were expanded over 14 days in G-Rex100 flasks using a two-step process. For the first step of TIL REP a G-Rex100 flask was seeded with 5×106 TIL and irradiated PBMCs at a TIL to feeder cell ratio of 1 to 100 and the cells were cultured in a 1 to 1 mixture of CM and AIM-V medium, supplemented with IL-2 (3000 IU/mL) and anti-CD3 (30 ng/mL). After 7 days of culture the TIL were harvested and divided. One third of the cells were cultured 7 more days in a second G-Rex100 flask. The total number of cells that would have been produced using 3 G-Rex100 flasks for the second step of expansion is shown. TIL for REP were prepared by growth in 24-well plates from fresh tumor digests from 8 patients and by growth in 24-well plates from frozen tumor digests from 6 patients (samples 1812, 3282, 2976, 3020, 2998, and 2761).
The IFN-γ release from TIL produced by G-Rex100 REP and T-175 flask/bag REP was compared. TIL samples produced by both REP methods using the same tumor samples from 4 patients were tested. Following stimulation by anti-CD3 IFN-γ production by TIL expanded in G-Rex100 flasks was similar to that of TIL expanded in T-175 flasks and bags (Table 4).
Table 4.
Comparison of interferon-γ production by TIL expanded in T flasks/bags and G-Rex100 flasks.
| Patient1 | Sample | IFN-γ release (pg/ml) 2 |
||
|---|---|---|---|---|
| OKT3 1.0ug/ml | OKT3 0.1ug/ml | None | ||
| 3536 | Flask/Bag | 631, 672 | 457, 390 | 0, 0 |
| G-Rex100 | 579, 553 | 243, 277 | 0, 0 | |
|
| ||||
| 3539 | Flask/Bag | 12272, 14350 | 10553, 11039 | 179, 176 |
| G-Rex100 | 29792, 29550 | 26670, 23835 | 73, 80 | |
|
| ||||
| 3135 | Flask/Bag | 831, 1124 | 704, 643 | 0, 0 |
| G-Rex100 | 581, 635 | 151, 74 | 2, 1 | |
|
| ||||
| 3533 | Flask/Bag | 6870, 6370 | 4280, 500 | 114, 146 |
| G-Rex100 | 5100, 4510 | 1513, 1407 | 136, 150 | |
TIL cells were stimulated by overnight incubation on plate bound OKT3 (anti-CD3 antibody) coated at the concentration indicated.
Values are IFN-γ (pg/ml) detected in duplicate wells measured by ELISA (see Materials and Methods).
These results suggested that 20 to 30×109 TIL could be produced by the initial culture of 15×106 TIL in 3 G-Rex100 flasks for 7 days followed by a second 7 day culture in 9 G-Rex100 flasks (Figure 6). To test this “full scale” G-Rex100 REP, 15×106 TIL from one patient were divided among three G-Rex100 flasks, A, B and C. After 7 days in culture the TIL in each flask were split into 3 equal parts, seeded into 3 G-Rex100 flasks and cultured for an additional 7 days. The mean number of TIL harvested from each of the 3 G-Rex100 flasks used for initial expansion was 875± 30.8×106and ranged from 849×106 to 909×106 TIL and the mean number harvested from each of the 9 G-Rex100 flasks used for the secondary expansion was 2.63± 0.03×109and ranged from 2.55×109 to 2.76×109 TIL (Table 5). The total TIL collection yield was 23.6×109 and 21.0×109 remained after washing the cells. Their viability was 96% and 69% of the cells expressed CD3 and CD8.
Figure 6. Description of large scale TIL REP using G-Rex100 flasks.
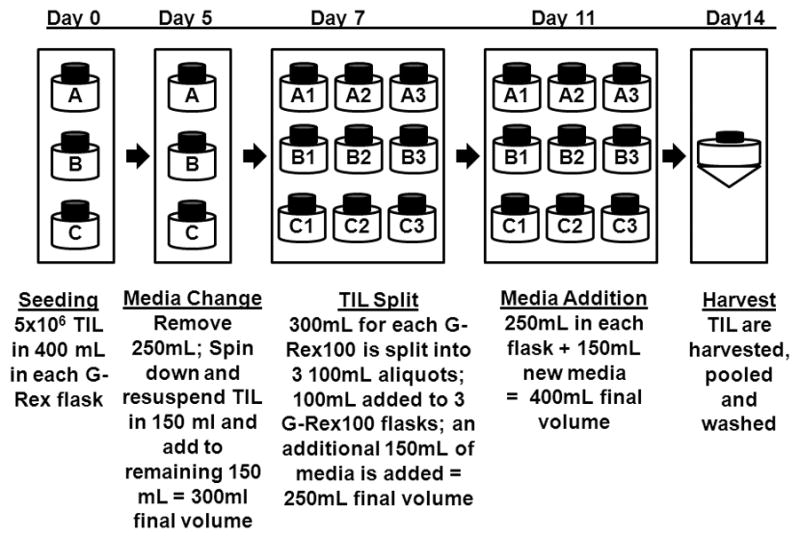
For large scale TIL REP in G-Rex100 gas-permeable flasks 5×106 TIL were cultured in 3 G-Rex100 flasks (A, B and C) with irradiated allogeneic PBMC at a ratio of 1 to 100 in 400 mL of a 1 to 1 mixture of CM and AIM-V medium, supplemented with IL-2 (3000 IU/mL) and anti-CD3 (30 ng/mL) and the flasks were incubated at 37°C in 5% CO2. On day 5, 250 mL of supernatant was removed and centrifuged. The TIL were suspended and 150 mL of a 1 to 1 mixture of CM and AIM-V media with 3000 IU per mL of IL-2 was added to the remaining 150 mL of cells. On day 7 the TIL in each G-Rex100 (A, B and C) were suspended in the 300 mL of media present in each flask and the cell suspension was divided into three 100 mL aliquots that were used to seed 3 G-Rex100 flasks (A1 A2, and A3; B1, B2 and B3; and C1, C2 and C3). After the 100 mL cell suspension was added to each G-Rex100 flask, 150 mL of AIM-V with 5% human AB serum and IL-2 (3000 IU/mL) was added to each of the 9 G-Rex100 flasks. The G-Rex100 flasks were incubated at 37°C in 5% CO2 and on day 11 150 mL of AIM-V with IL-2 (3000 IU/mL) was added to each flask. The cells were harvested, pooled and washed on day 14 of culture.
Table 5.
Number of cells produced by a two-step G-Rex100 TIL rapid expansion protocol (REP); the first step involved growth in 3 Rex100 flasks and the second growth in 9 G-Rex100 flasks.
| First G-Rex100 Flask | Day 0 | Day 7 | Second G-Rex100 Flask | Day 11 | Day 14 |
|---|---|---|---|---|---|
| A | 5×106 | 849×106 | A1 | 1.44×109 | 2.67×109 |
| A2 | N.T. | 2.43×109 | |||
| A3 | N.T. | 2.66×109 | |||
| B | 5×106 | 867×106 | B1 | 1.55×109 | 2.70×109 |
| B2 | N.T. | 2.66×109 | |||
| B3 | N.T. | 2.76×109 | |||
| C | 5×106 | 909×106 | C1 | 1.44×109 | 2.62×109 |
| C2 | N.T. | 2.60×109 | |||
| C3 | N.T. | 2.55×109 | |||
| Total | 15×106 | 2.63×109 | 14×109 | 23.6×109 |
N.T. = Not Tested
Clinical Scale TIL Initiation and REP in G-Rex Flasks
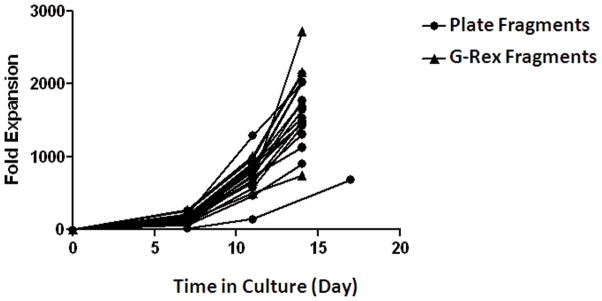
We next tested clinical scale TIL production using tumor fragments from 3 patients by initially culturing TIL in G-Rex10 flasks followed by REP in G-Rex100 flasks. For each patient, 6 G-Rex10 flasks were seeded with 5 tumor fragments and after 14 to 15 days 5×106 TIL from each G-Rex10 flask were seeded into one G-Rex100 flask. After 7 days TIL from each G-Rex100 flask were split into 3 G-Rex100 flasks and after an additional 7 days in culture TIL were harvested from the 18 G-Rex100 flasks. For two patients, 3613 and 3618, enough TIL could be harvested from each of the 6 G-Rex10 flasks for TIL REP in a G-Rex100 flask. The quantity of TIL harvested from each of the G-Rex10 flasks ranged from 47.5 to 97.8 x106 cells for patient 3613 and 24.6 to 64.2×106 for patient 3618 (Table 6). For patient 3625 sufficient quantities of TIL were obtained from 4 of the 6 G-Rex10 flasks. The quantity harvested from these 4 flasks ranged from 59.7 to 140×106 cells (Table 6). For patients 3613 and 3618, 5×106 TIL from each of the 6 G-Rex10 flasks was seeded into a G-Rex100 flask. For patient 3625, 5×106 TIL from 2 G-Rex10 flasks were each seeded into one G-Rex100 flask and 10×106 TIL from the other 2 GP40 flasks were split and used to seed 4 G-Rex100 each with 5×106 TIL. At the completion of REP using patient 3613 cells 22.4×109 TIL were harvested, while REP using patient 3618 cells yielded 52.7×109 TIL and patient 3625 cells yielded 61.0×109 TIL. The number of TIL produced by each of the 6 sets of 3 G-Rex100 flasks was similar for each patient (Figure 7). These results show that G-Rex100 flasks can produce sufficient quantities of TIL for clinical therapy using TIL initially cultured from tumor fragments in G-Rex10 flasks. The same G-Rex100 REP protocol was also successful in expanding TIL that were initially cultured from tumor fragments in 24-well plates. There was no significant differences in fold expansion using either TIL initially cultured from tumor fragments in 24-well plates or G-Rex10 flasks (Figure 8).
Table 6.
Clinical scale initial TIL culture from tumor fragments in G-Rex10 flasks
| Patient # | Culture Vessel | # of Fragments Seeded | Day TIL Harvested | # TIL Harvested | #TIL/Fragment | Viability (%) | TIL Phenotype (% CD3+ CD8+) |
|---|---|---|---|---|---|---|---|
| 3613 | 1 | 5 | 14 | 47.5×106 | 9.50×106 | N.T. | 70 |
| 2 | 5 | 14 | 94.5×106 | 18.9×106 | N.T. | 74 | |
| 3 | 5 | 14 | 57.5×106 | 11.5×106 | N.T. | 74 | |
| 4 | 5 | 14 | 97.8×106 | 19.6×106 | N.T. | 73 | |
| 5 | 5 | 14 | 55.0×106 | 11.0×106 | N.T. | 67 | |
| 6 | 5 | 14 | 67.0×106 | 13.4×106 | N.T. | 67 | |
| 3618 | 1 | 5 | 14 | 61.0×106 | 12.2×106 | 99 | 77 |
| 2 | 5 | 14 | 64.2×106 | 12.8×107 | 97 | 84 | |
| 3 | 5 | 14 | 24.6×106 | 4.92×106 | 99.2 | N.T. | |
| 4 | 5 | 14 | 64.2×106 | 12.8×106 | 96.4 | 86 | |
| 5 | 5 | 14 | 40.2×106 | 8.04×106 | 97.1 | 77 | |
| 6 | 5 | 14 | 57.0×106 | 11.4×106 | 99.3 | 85 | |
| 3625 | 1 | 5 | 15 | 86.0×106 | 17.2×106 | 96.8 | 73 |
| 2 | 5 | 15 | 59.4×106 | 11.9×106 | 98.7 | 91 | |
| 3 | 5 | 15 | 71.8×106 | 14.4×106 | 98.6 | 73 | |
| 4 | 5 | 15 | 1.80×106** | 0.36×106 | 64.3 | N.T. | |
| 5 | 5 | 15 | 2.20×106** | 0.440×106 | 78.6 | N.T. | |
| 6 | 5 | 15 | 140×106 | 28.0×106 | 100 | 74 |
N.T. = Not Tested
Insufficient number of cells for clinical REP
Figure 7. Clinical scale REP of TIL from 3 patients in G-Rex100 flasks using TIL grown from tumor fragments in G-Rex10 flasks.
TIL initially cultured from tumor fragments in G-Rex10 flasks from 3 patients, 3613, 3618, and 3625, were used for clinical scale REP in G-Rex100 flasks. For each patient, 6 G-Rex100 flasks were seeded with 5×106 TIL and irradiated PBMC feeder cells at a TIL to feeder cell ratio of 1 to 100 as described in the methods section. For patients 3613 and 3618 each of the 6 G-Rex100 flasks was seeded with TIL obtained from initial culture in a single G-Rex10 flask. Since for patient 3625 TIL could only be obtained from 4 of 6 G-Rex10 flasks, 2 G-Rex100 flasks were each seeded with TIL obtained from a single G-Rex10 flask and for the other 4 G-Rex100 flasks, cells from each of the two remaining G-Rex10 flasks were used to seed 2 G-Rex100 flasks each. On day 7 of REP, the cells were split 1:3 and seeded into 3 G-Rex100 flasks. The quantity of cells harvested from each of the 6 sets of 3 G-Rex100 flasks is shown for the 3 patients. Patient 3613 TIL are shown by circles, patient 3618 by squares and patient 3225 by diamonds.
Figure 8. Comparison of clinical scale G-Rex REP of TIL grown from tumor fragments in 24-well plates and G-Rex10 flasks.
TIL were expanded over 14 days in G-Rex100 flasks. For each patient 6 G-Rex100 flasks were seeded with 5×106 TIL and irradiated PBMCs at a TIL to feeder cell ratio of 1 to 100. After 7 days of culture the TIL from each G-Rex100 was divided between 3 G-Rex100 flasks and were then cultured for 7 more days. The fold expansion of TIL over 14 days is shown. TIL for REP were prepared by growth in 24-well plates from tumor fragments for 14 patients (circles) and by growth in G-Rex10 flasks from tumor fragments for 5 patients (triangles).
Discussion
We describe a new and simplified method for the growth of human TIL to numbers suitable for cancer treatment using gas-permeable G-Rex flasks. We found that the entire TIL production process which typically involves 4 to 6 weeks of cell culture in 24-well plates, T-175 flasks and gas permeable bags can be completed entirely in gas-permeable G-Rex flasks. This method yields 25 to 60 x109 TIL by culturing 30 to 40 x106 tumor digest cells in one G-Rex10 or 30 to 60 pieces of tumor in 6 G-Rex10 flasks for 1 to 4 weeks to obtain 50 to 400 x106TIL followed by expansion for 7 days in 6 G-Rex100 gas-permeable flasks seeded with 30 x106 TIL and another expansion for 7 days in a total of 18 G-Rex100 flasks. The phenotype and cytokine production of TIL grown in G-Rex flasks were similar to TIL produced with 24-well plates, T-175 flasks, and bags.
The initial growth of TIL from tumor in G-Rex10 flasks has an advantage over culture in 24-well plates in that more cells were obtained in gas-permeable G-Rex flasks and it is a semi-closed system. Enough TIL for REP can be obtained from tumor digests in one G-Rex10 flask compared to two 24-well plates. Working with G-Rex10 flasks is also simpler and less labor intensive than working with two 24-well plates and the G-Rex10 flasks are less subject to microbial contamination than 24-well plates. We also found that TIL can be grown from tumor pieces that have not been treated with proteolytic enzymes or mechanically disrupted. This reduces the manipulation of the tumor and obviates the need for GMP grade proteolytic enzymes and an instrument to disrupt the tissue.
TIL REP in G-Rex flasks has several advantages over other REP methods. First, G-Rex flask REP requires far fewer total vessels and far less media, because these flasks support the growth of TIL at much greater cell densities than tissue culture flasks or gas-permeable bags. In order to produce a clinical TIL dose of 25 to 60 billion cells a total of 18 G-Rex100 flasks are required compared to approximately 30–50 T-175 flasks and 15 to 25 3-liter gas-permeable bags for young TIL REP. G-Rex100 flask TIL REP requires approximately 9–10 liters of media as compared to 30 to 40 liters for the T-175 flask/gas-permeable bag REP. This results in an approximately 3- to 4-fold reduction in the use of media, IL-2, CD3 monoclonal antibodies and AB serum.
The reduced culture volume also reduces the equipment needs of the cell processing laboratory. G-Rex flask REP requires less incubator space. The REP of TIL in 15–25 3-liter bags requires the use of 2 or more incubators, but culture in 18 G-Rex100 flasks requires only one or two shelves of a standard sized incubator. The use of G-Rex flasks for REP also eliminates the need for specialized cell harvesting and concentrating devices. Many cell washing devices can be used to concentrate in a few hours the cells in the approximately 10 liter cell suspension harvested from 18 G-Rex100 flasks, including instruments found in many blood banks and blood centers. One such device is the Cobe 2991 Cell Processor manufactured by Caridian BCT. However, specialized cell washers are required to process 30 to 40 liters of cell suspensions in a few hours. Harvesting these large volumes of cell suspensions has become especially problematic since a cell harvester that has been used for many years to harvest and wash large volume TIL suspensions is no longer commercially available.
One disadvantage of TIL culture in G-Rex flasks is that cells cannot be easily visualized. In addition, G-Rex flasks are not a closed system, but using a reduced number of G-Rex flasks compared to T-175 flasks reduces the chance of contamination. Furthermore, the manufacturer of G-Rex flasks is working to add ports to these flasks to make this a more closed system. The manufacturer of the G-Rex flasks is also working to develop a single gas-permeable flask that could take the place of 9 to 12 G-Rex100 flasks. Preliminary testing of a prototype vessel with a 650 cm2 gas-permeable membrane and a capacity of approximately 5 liters showed that the vessel could produce 20 to 25 x109 TIL using an initial seeding number of 10 x106 TIL.
TIL REP using G-Rex100 flasks also requires fewer media changes. In T-175 flask/bag REP media is generally changed every other day, but for G-Rex100 REP media is changed every 3 to 4 days. The reduced need to change media reduces the risk of contamination since the flasks are entered less often.
Less labor is required for TIL growth in G-Rex flasks for several reasons. Laboratory staff must maintain fewer containers, media changes are less frequent, less media must be prepared and less volume of cell suspension that must be harvested.
Initial TIL growth and culture in G-Rex flasks also has advantages over the other methods described for TIL production. TIL REP can be performed in a single-pass perfusion, closed-system bioreactor, Aastrom (14). However, this system is limited by the fixed design of its cell culture cassette. Since the maximum quantity of TIL that can be produced in each cassette is approximately 5×109 cells, several bioreactors would be required to produce sufficient quantities of TIL to treat one patient. Hollow fiber (15;16) and WAVE bioreactors (17) have also been used for TIL REP. The WAVE bioreactor can be used for the entire REP process (18), but the hollow fiber and Aastrom bioreactors can only be used for the later stages of REP.
Another group has used G-Rex flasks to expand Epstein-Barr virus (EBV)-specific cytotoxic lymphocytes (CTLs), but these studies expanded cells from the peripheral blood rather than tumor derived cells (19). In addition, an irradiated EBV-lymphpblastoid cell line (LCL) was used as feeders to expand the EBV-specific CTLs rather than irradiated PBMCs.
In conclusion, the use of gas-permeable G-Rex flasks for initial TIL culture and REP allows for the reliable and reproducible production of TIL using fewer reagents than other methods. These improvements in TIL initial culture and REP will allow TIL production to move from highly specialized cell therapy laboratories to cell processing laboratories found at many academic healthcare centers that produce a range of cellular therapies. This could lead to the wide spread use of TIL cells to treat patients with metastatic melanoma. This technology may also be useful for expanding autologous or allogeneic cells for adoptive immunotherapy of cancer such as NK cells or lymphocytes with engineered T cell receptors.
Acknowledgments
The authors thank the staff of TIL Laboratory, Surgery Branch, NCI and Cell Processing Laboratory, Department of Transfusion Medicine, CC for their support and help with these studies.
Footnotes
Financial Disclosure: John Wilson is an employee of Wilson Wolf Manufacturing, which manufactures the gas permeable flasks used in this study. All other authors have declared there are no conflicts of interest in regards to this work.
References
- 1.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002 Oct 25;298(5594):850–4. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005 Apr 1;23(10):2346–57. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988 Dec 22;319(25):1676–80. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 4.Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008 Nov 10;26(32):5233–9. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenberg SA, Yannelli JR, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, et al. Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J Natl Cancer Inst. 1994 Aug 3;86(15):1159–66. doi: 10.1093/jnci/86.15.1159. [DOI] [PubMed] [Google Scholar]
- 6.Schwartzentruber DJ, Hom SS, Dadmarz R, White DE, Yannelli JR, Steinberg SM, et al. In vitro predictors of therapeutic response in melanoma patients receiving tumor-infiltrating lymphocytes and interleukin-2. J Clin Oncol. 1994 Jul;12(7):1475–83. doi: 10.1200/JCO.1994.12.7.1475. [DOI] [PubMed] [Google Scholar]
- 7.Besser MJ, Shapira-Frommer R, Treves AJ, Zippel D, Itzhaki O, Hershkovitz L, et al. Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin Cancer Res. 2010 May 1;16(9):2646–55. doi: 10.1158/1078-0432.CCR-10-0041. [DOI] [PubMed] [Google Scholar]
- 8.Tran KQ, Zhou J, Durflinger KH, Langhan MM, Shelton TE, Wunderlich JR, et al. Minimally cultured tumor-infiltrating lymphocytes display optimal characteristics for adoptive cell therapy. J Immunother. 2008 Oct;31(8):742–51. doi: 10.1097/CJI.0b013e31818403d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou J, Shen X, Huang J, Hodes RJ, Rosenberg SA, Robbins PF. Telomere length of transferred lymphocytes correlates with in vivo persistence and tumor regression in melanoma patients receiving cell transfer therapy. J Immunol. 2005 Nov 15;175(10):7046–52. doi: 10.4049/jimmunol.175.10.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang J, Khong HT, Dudley ME, El-Gamil M, Li YF, Rosenberg SA, et al. Survival, persistence, and progressive differentiation of adoptively transferred tumor-reactive T cells associated with tumor regression. J Immunother. 2005 May;28(3):258–67. doi: 10.1097/01.cji.0000158855.92792.7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang J, Kerstann KW, Ahmadzadeh M, Li YF, El-Gamil M, Rosenberg SA, et al. Modulation by IL-2 of CD70 and CD27 expression on CD8+ T cells: importance for the therapeutic effectiveness of cell transfer immunotherapy. J Immunol. 2006 Jun 15;176(12):7726–35. doi: 10.4049/jimmunol.176.12.7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou J, Dudley ME, Rosenberg SA, Robbins PF. Persistence of multiple tumor-specific T-cell clones is associated with complete tumor regression in a melanoma patient receiving adoptive cell transfer therapy. J Immunother. 2005 Jan;28(1):53–62. doi: 10.1097/00002371-200501000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dudley ME, Wunderlich JR, Shelton TE, Even J, Rosenberg SA. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother. 2003 Jul;26(4):332–42. doi: 10.1097/00002371-200307000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klapper JA, Thomasian AA, Smith DM, Gorgas GC, Wunderlich JR, Smith FO, et al. Single-pass, closed-system rapid expansion of lymphocyte cultures for adoptive cell therapy. J Immunol Methods. 2009 Jun 30;345(1–2):90–9. doi: 10.1016/j.jim.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knazek RA, Wu YW, Aebersold PM, Rosenberg SA. Culture of human tumor infiltrating lymphocytes in hollow fiber bioreactors. J Immunol Methods. 1990 Feb 20;127(1):29–37. doi: 10.1016/0022-1759(90)90337-u. [DOI] [PubMed] [Google Scholar]
- 16.Malone CC, Schiltz PM, Mackintosh AD, Beutel LD, Heinemann FS, Dillman RO. Characterization of human tumor-infiltrating lymphocytes expanded in hollow-fiber bioreactors for immunotherapy of cancer. Cancer Biother Radiopharm. 2001 Oct;16(5):381–90. doi: 10.1089/108497801753354285. [DOI] [PubMed] [Google Scholar]
- 17.Tran CA, Burton L, Russom D, Wagner JR, Jensen MC, Forman SJ, et al. Manufacturing of large numbers of patient-specific T cells for adoptive immunotherapy: an approach to improving product safety, composition, and production capacity. J Immunother. 2007 Sep;30(6):644–54. doi: 10.1097/CJI.0b013e318052e1f4. [DOI] [PubMed] [Google Scholar]
- 18.Sadeghi A, Pauler L, Anneren C, Friberg A, Brandhorst D, Korsgren O, et al. Large-scale bioreactor expansion of tumor-infiltrating lymphocytes. J Immunol Methods. 2011 Feb 1;364(1–2):94–100. doi: 10.1016/j.jim.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Vera JF, Brenner LJ, Gerdemann U, Ngo MC, Sili U, Liu H, et al. Accelerated production of antigen-specific T cells for preclinical and clinical applications using gas-permeable rapid expansion cultureware (G-Rex) J Immunother. 2010 Apr;33(3):305–15. doi: 10.1097/CJI.0b013e3181c0c3cb. [DOI] [PMC free article] [PubMed] [Google Scholar]