Abstract
Objective
Fish oil (FO), and specifically omega 3 fatty acids, has favorable effects on cardiovascular outcomes. The aim of this study was to investigate the effects of FO on the process of macrophage reverse cholesterol transport (RCT) in an in vivo mouse model.
Methods and Results
C57BL/6J mice were fed a FO diet, whereas control mice were fed diets containing alternative sources of fats, soybean oil (SO), and coconut oil (CO) for 4 weeks. Macrophage RCT was assessed by injecting [3H]cholesterol-labeled J774 macrophages intraperitoneally into mice. After 48 hours, tissues were harvested and feces were collected. An increase in the excretion of macrophage-derived [3H]-tracer recovered in fecal neutral sterols for FO-fed mice was observed (273% versus SO and 182% versus CO). FO also decreased [3H]-tracer in hepatic cholesteryl ester compared to SO and CO by 76% and 56%, respectively. To specifically determine the effect of FO on the fate of HDL-derived cholesterol, mice fed FO or SO diets were injected with HDL labeled with [3H]cholesteryl oleate, and the disappearance of [3H]-tracer from blood and its excretion in feces was measured. There was no significant difference in the fractional catabolic rate of [3H]cholesteryl oleate-HDL between the 2 groups. However, there was a 242% increase in the excretion of HDL-derived [3H]-tracer recovered in fecal neutral sterols in FO-fed mice, concordant with significantly increased expression of hepatic Abcg5 and Abcg8 mRNA.
Conclusion
As measured by this tracer-based assay, FO promoted reverse cholesterol transport, primarily by enhancement of the hepatic excretion of macrophage-derived and HDL-derived cholesterol.
Keywords: HDL, atherosclerosis, nutrition
It is well-known that nutritional factors influence the incidence of cardiovascular disease. Dietary fat is considered one of the most important factors associated with the development of atherosclerosis. Saturated fatty acids increase plasma LDL-cholesterol (LDL-C) and promote the progression of atherosclerosis.1 Alternatively, unsaturated fatty acids are thought to protect against the progression of atherosclerosis.2 For example, linoleic acid reduces atherosclerosis by lowering plasma LDL-C.1 Omega-3 fatty acids such as docosahexaenoic acid (DHA) or eicosapentaenoic acid (EPA), abundant in fish oil (FO), reduce clinical cardiovascular complications of atherosclerotic disease.3,4 Several mechanisms have been proposed by which FO reduces cardiovascular events, including triglyceride-lowering effects, antiinflammatory effects, antithrombotic effects, and antiarrhythmic effects.5,6
Reverse cholesterol transport (RCT), by which cholesterol is transported from peripheral macrophages to HDL-based acceptors in the liver for excretion in the bile, is thought to be a protective mechanism against atherosclerosis.7 FO may promote this process by increasing cholesterol efflux from macrophages8 and increasing hepatic uptake of HDL-cholesterol (HDL-C).9 In this study, we evaluated the effect of FO on in vivo macrophage RCT using previously established methods for RCT in mice. We found that FO significantly increased macrophage-to-feces RCT. Additional studies suggest that at least part of the mechanism is via reduction in esterification and promotion in the biliary excretion of HDL-derived cholesterol by the liver.
Methods
Materials
RPMI 1640 were purchased from Invitrogen. [1,2-3H]cholesterol and [1,2-3H]cholesterol oleate were purchased from Perkin-Elmer Life Science. Other reagents without citation in this article were purchased from Fisher Scientific.
Animals and Diets
Female C57BL/6J mice (Jackson Laboratories, Bar Harbor, Me), 13 to 15 weeks old, were housed in polycarbonate cages (4 to 6 animals in each cage) with a 12-hour light/dark cycle. The room temperature was maintained at 22±1°C and humidity at 30% to 50%. All procedures were approved by the University of Pennsylvania Institutional Animal Care and Use Committee.
All diets were based on the AIN-93 mol/L formula and were purchased from Research Diets Inc. The low soybean diet (LSO) contained 9% energy from soybean oil and matched the AIN-93 mol/L formula except that it contained 1.25 g cholesterol/3850 kcals. Three high-fat diets, HSO, CO, and FO diets, contained 1.25 g cholesterol/3851 kcal and 42% energy from fat with 5% energy from soybean oil as a source of essential fatty acids and the remaining fat as soybean oil, hydrogenated coconut oil, and menhaden oil, respectively. Unlike other oils, menhaden oil contains 0.4% (w/w) cholesterol; consequently less cholesterol was added to prepare the FO diet. The fatty acid profile for each diet is provided in supplemental Table I (available online at http://atvb.ahajournals.org).
Diet Treatment
After a 2-week introductory feeding period with the HSO diet, mice were fasted for 4 hours and blood was obtained to determine plasma lipid levels. Mice were divided into 3 groups (n=5 to 6/group) with equal mean plasma total cholesterol (TC) levels and fed either, HSO, CO, or FO diets. In addition, 1 group of mice was fed LSO diet throughout the experiment.
In Vivo RCT With J774 Macrophages
RCT studies were performed as previously described.10 A more detailed description of the methods used can be found in the supplemental materials.
Ex Vivo Cholesterol Efflux From J774 Macrophages
J774 macrophages were used to study the ability of serum, from diet-fed mice, to promote cholesterol efflux ex vivo as previously described.11 A more detailed description of the methods used can be found in the supplemental materials.
Ex Vivo Cholesterol Efflux From Mouse Peritoneal Macrophages
Mouse peritoneal macrophages (MPMs) from diet-fed mice were used to test their ability to efflux cholesterol to defined acceptors ex vivo. A more detailed description of the methods used can be found in the supplemental materials.
HDL Labeling and Turnover
Mouse HDL was isolated from plasma of chow-fed C57BL/6J mice by differential ultracentrifugation, and HDL (1.063<d<1.21) was labeled with [3H]cholesterol oleate according to the methods described previously with slight modifications.12 A more detailed description of the methods used can be found in the supplemental materials.
mRNA Gene Expression Analysis
For RNA expression analysis, livers from the macrophage RCT study were removed and pieces were soaked in RNAlater reagent (Qiagen). The excised small intestines, from HDL turnover study, were washed by ice-cold PBS, and then divided into 6 parts with equal length (duodenum, upper jejunum, mid jejunum, lower jejunum, upper ileum, and lower ileum). Total RNA was isolated from liver or small intestine using EZ1 RNA Tissue Mini Kit (Qiagen). cDNA was produced from total RNA via reverse transcription using Superscript First strand synthesis system Kit (Invitrogen). Quantification of mRNA expression using TaqMan or SYBR green assay systems was performed using an ABI 7300 Real Time thermocycler (Applied Biosystems). Abundance of genes were determined after correcting for loading to Gapdh expression (DCt), and relative expression attributable to diet conditions is compared to the HSO group.
Statistical Analyses
Results are presented as the mean±SD. One-way ANOVA (P<0.05) followed by Tukey HSD-test (a=0.05) was used for detecting the statistical differences among high-fat diet groups (HSO, CO and FO). Student t test was used for detecting differences between LSO and HSO diets to understand the influence of high-fat diet. All analyses were performed using JMP v7.0 (SAS Institute).
Results
Plasma Lipids
Body weight and food intake of mice fed different diets were similar throughout the study (data not shown). Plasma lipids on the different diets are shown in the Table. Compared with the control LSO, the higher level of soybean oil, HSO diet, did not change the plasma total cholesterol (TC) level but it increased HDL-cholesterol (HDL-C) levels and decreased non–HDL-C, triglyceride (TG), and nonesterified fatty acid (NEFA) levels. The CO diet significantly increased TC, HDL-C, non–HDL-C, TG, and NEFA levels compared with the HSO diet. Finally, compared with the other high-fat diets, the FO diet significantly decreased plasma HDL-C levels (13% and 36% compared to HSO and CO, respectively) and NEFA levels (20% and 61% compared to HSO and CO, respectively), and compared with the CO diet the FO diet significantly reduced non–HDL-C (23%) and TG (62%) levels.
Macrophage RCT
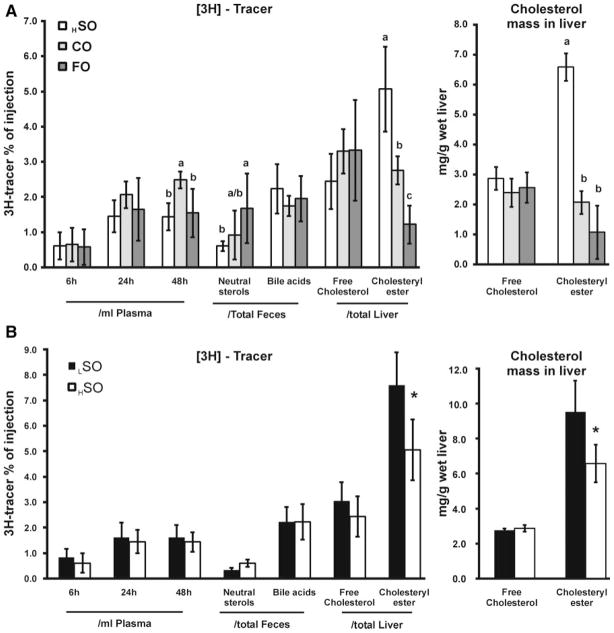
Comparisons among the high-fat diet groups with regard to indices of RCT (Figure 1A) indicate that the plasma [3H]-tracer in FO diet–fed mice was similar to that in HSO diet–fed mice at all time points and significantly less than that in CO diet–fed mice at 48 hours. With regard to fecal neutral sterols, FO substantially increased (by 273% versus HSO, and by 182% versus CO) the excretion of macrophage-derived [3H]-tracer. FO decreased [3H]-tracer recovered in hepatic cholesteryl ester by 76% and 56% compared to HSO and CO, respectively, although there were no differences in [3H]-tracer recovered in hepatic free cholesterol. The reduction in [3H]-tracer in recovered hepatic cholesteryl ester was reflected by a similar reduction in hepatic cholesteryl ester mass in FO diet–fed mice (Figure 1A).
Figure 1. Macrophage RCT in mice.
Mice were fed LSO, HSO, CO, and FO diets (n=6 per each group). A, Comparison of high-fat diet groups. ANOVA was performed for each lipid measurement, and if significance was observed (P<0.01) then Tukey HSD was applied to test for significance between all groups. Different letters indicate significant differences between diets (Tukey HSD, alpha=0.05), absence of letters indicates no significant difference between groups. B, Comparison of low-fat versus high-fat diet (soybean oil). *Significant difference between LSO and HSO by Student t test (P<0.001).
A comparison between the LSO with the HSO diet (Figure 1B) showed no effect on the macrophage-derived [3H]-tracer in plasma. However, the HSO diet had significantly decreased [3H]-tracer in hepatic cholesteryl ester after 48 hours.
Macrophage Cholesterol Efflux
Macrophage cholesterol efflux is the first, and one of the most important, steps in the RCT pathway. The effects of the FO diet on cholesterol efflux from macrophages ex vivo were investigated in 2 different ways. First, we examined the ability of serum obtained from mice on the FO and other diets to promote cholesterol efflux from J774 macrophages (Figure 2). Comparisons among the high-fat diet groups indicate that, in the absence of cAMP (which upregulates ABCA1), the FO diet did not increase the cholesterol efflux capacity of serum, whereas the CO diet significantly increased it compared to the HSO diet. Alternatively, in the presence of cAMP, both the FO and CO diets significantly increased the cholesterol efflux capacity of serum compared to the HSO diet. Comparing the LSO and HSO diets indicates that a higher level of soybean oil increased the cholesterol efflux capacity of serum either with or without cAMP.
Figure 2. Cholesterol efflux.
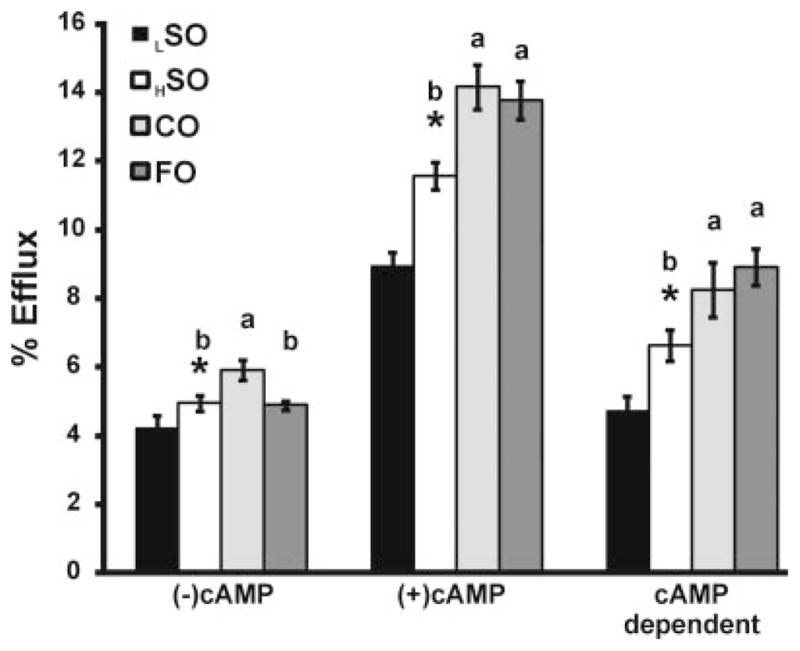
cAMP-dependent cholesterol efflux from J774 cells to serum from mice fed LSO, HSO, CO, and FO diets (n=6 per each group). *Significant difference between LSO and HSO by Student t test (P<0.01). Comparison of high-fat diet groups was performed by ANOVA, and if significance was observed (P<0.01) then Tukey HSD was applied to test for significance between all groups. Different letters indicate significant differences between diets (Tukey HSD alpha=0.05), whereas absence of letters indicates no significant difference between groups.
Secondly, we examined the ability of macrophages isolated from mice fed the experimental diets to efflux cholesterol to defined acceptors; pooled serum (from control chow-fed C57BL/6J mice; 2%) or human ApoA-I (100 μg/mL; Figure 3). Among the high-fat diet groups, there were no differences with regard to cholesterol efflux from MPMs using mouse serum as the acceptor (Figure 3A). Similarly, using human ApoA-I as the acceptor, macrophages from FO-fed mice did not significantly increase cholesterol efflux relative to HSO. Interestingly CO-fed mice had decreased cholesterol efflux compared with macrophages from FO-fed mice (Figure 3B). No differences were observed between the LSO and HSO groups in cholesterol efflux from peritoneal macrophages.
Figure 3. Cholesterol efflux in MPMs.
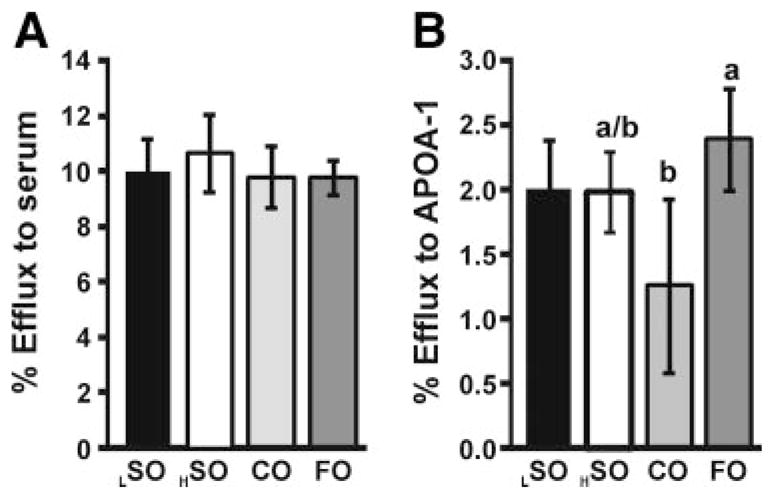
Mice were fed LSO, HSO, CO, and FO diets for 6 weeks then injected with thioglycollate broth. After 4 days, MPMs were collected and individually seeded (n=4 per each group). Cells were then incubated with or without pooled mouse serum (A) or human APOA-1 (B). There were no significant differences between LSO and HSO by t test. Comparisons between high-fat groups was performed by ANOVA, if significance was observed (P<0.01) then Tukey HSD was applied to test for significance between all groups. Different letters indicate significant differences between diets (Tukey HSD, alpha=0.05).
Specific activities of the [3H]-cholesterol in MPM were determined by summing cellular and media [3H]-counts. The specific activities were 48 471±10 865 (HSO), 57 612±8000 (CO), 47 003±6809 (FO), and 49 519±11 948 (LSO), cpm/1×106 cells. The CO group was significantly higher than the HSO (P<0.05) and FO (P<0.01) groups.
Fate of HDL-Derived Cholesteryl Ester
Another key step in RCT is the hepatic uptake and excretion of HDL-derived cholesterol. To determine whether the FO diet may have promoted these steps, a HDL turnover study using [3H]-cholesteryl ester labeled HDL in mice fed the LSO, HSO, and FO diets was performed including tracing the excretion of the labeled cholesterol in the feces (Figure 4). There was no difference between HSO and FO in the plasma FCR of HDL-CE. However, there was a 242% increase in the excretion of HDL-derived [3H]-tracer recovered in fecal neutral sterols in FO diet–fed mice compared to HSO-fed mice (P<0.0001). As observed in the macrophage RCT study, FO did not affect the [3H]-tracer recovered in fecal bile acids. Similarly, as in the RCT study, FO significantly decreased [3H]-tracer in hepatic cholesteryl ester by 79% compared to HSO (P<0.0001). Comparing LSO and HSO indicates that a higher level of soybean oil decreased the FCR of HDL-CE (P=0.004).
Figure 4. HDL kinetics in mice.
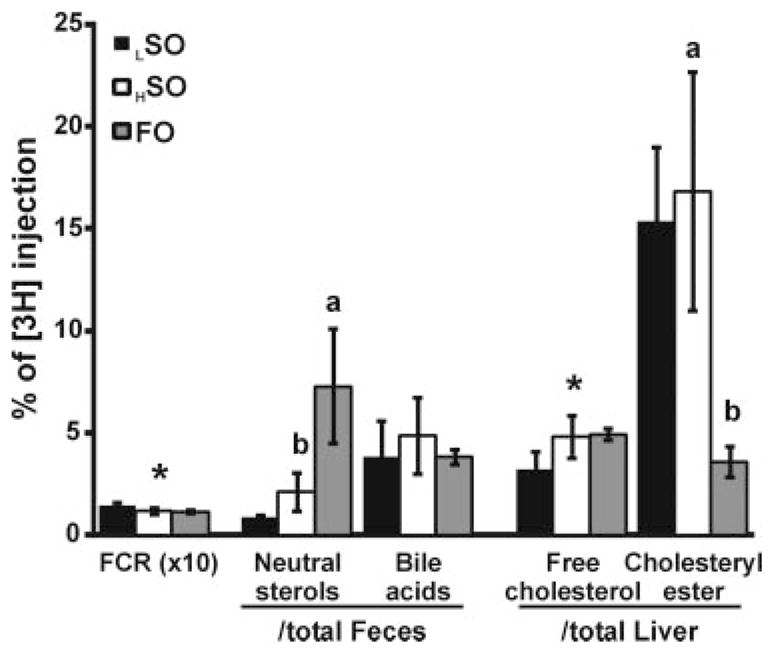
Mice were fed LSO, HSO, or FO diets (n=5 per each group) for 4 weeks and received intravenously injections of [3H]-cholesterol oleate labeled HDL. Fractional catabolic rate (FCR) for plasma HDL-CE was calculated based on the disappearance of [3H]-tracer from plasma of each group. The FCR was transformed by a multiplying by a factor of 10 for scale purposes. *Significant difference between LSO and HSO by Student t test (P<0.01). Different letters between the high-fat groups HSO and FO also indicates a significant difference by Student t test (P<0.01).
Hepatic mRNA Expression Analysis
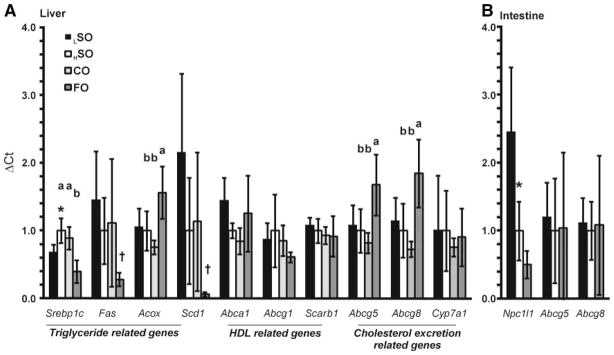
The fish oil diet significantly increased the [3H]-tracer recovered in fecal neutral sterols but not in fecal bile acid in both the RCT and HDL turnover studies. FO markedly decreased [3H]-tracer recovered in hepatic cholesteryl ester in both experiments. These results suggest that FO inhibits the esterification of macrophage and HDL-derived cholesterol and increases the excretion of neutral sterols from liver to bile and possibly from bile to feces. Several liver genes that influence cholesterol esterification and biliary cholesterol excretion were therefore analyzed by RT-QPCR (Figure 5).
Figure 5. mRNA abundance in (A) liver and (B) small intestine from mice kept on each of the 4 diets.
Data are presented relative to HSO, which is set at 1.0 for each gene. *Significant difference between LSO and HSO by Student t test (P<0.01). Statistical comparison of the high-fat diet groups was performed by ANOVA, and if a significant difference was observed (P<0.01) then Tukey HSD was applied. Different letters indicate significant differences between diets (Tukey HSD alpha=0.05), whereas absence of letters indicates no significant difference between groups. A posthoc nonparametric analysis (Wilcoxon/Kruskal–Wallis test) was performed for the genes Fas, Scd1, Abca1, and Abcg1 (Barlett test P<0.001) because their variation was not normally distributed. Under this analysis Fas and Scd1 were significantly lower in FO than in the other 3 high-fat diets (P<0.005) as denoted by the symbol †.
Consistent with a previous report,13 FO significantly decreased mRNA of hepatic triglyceride synthesis related genes (Srebp-1c, Fas, and Scd1) and increased hepatic expression of Acox which catalyzes β-oxidation of fatty acids. The reduction in Scd1 expression, in particular, could result in reduced esterification of HDL-derived cholesterol, consistent with our observations and potentially leading to enhanced excretion into the bile.
In addition, FO did not affect mRNA levels of Scarb1, which is consistent with the HDL-CE turnover data. Western blot analysis also did not show a change (data not shown). The FO diet showed a small, but not significant increase in Abca1 relative to the other high-fat diets, whereas Abcg1 expression was unchanged among the different diet groups.
ABCG5 and ABCG8 play a critical role in biliary excretion of neutral sterols. Comparisons among the high-fat diet groups show FO significantly increased hepatic expression of both Abcg5 and Abcg8 but not Cyp7a1. Compared to the control LSO diet, the HSO diet had little effect on hepatic expression of Abcg5 and Abcg8.
Finally, NPC1L1 plays a crucial role in intestinal absorption of neutral sterols. In the small intestine, a higher level of soybean oil (HSO diet) significantly decreased Npc1l1 mRNA levels compared with the LSO diet (P=0.0029) (Figure 5B). FO further decreased Npc1l1 expression in the small intestine compared to HSO, although the additional decrease was not significant (P=0.23). The significant increase in hepatic Abcg5/g8 and potential decrease in intestinal Npc1l1 mRNA may account for the increased fecal excretion of macrophage and HDL-derived cholesterol.
Discussion
This study shows that a diet high in omega-3 fatty acids substantially increased the fecal excretion of macrophage-derived and HDL-derived [3H]cholesterol, compared with a diet high in soybean oil as well as a low-fat diet. The fish oil diet reduced hepatic esterification of HDL-derived cholesterol, increased the fecal excretion of HDL-derived cholesterol, upregulated hepatic Abcg5 and Abcg8, and downregulated intestinal Npc1l1, all of which may have contributed to the increased rate of RCT.
Marmillot et al reported that FO feeding in rats enhanced the ability of their HDL to promote cholesterol efflux from J774 cells.8 In the present study, the serum from FO-fed mice promoted cAMP-dependent cholesterol efflux (but not basal efflux) from J774 cells, compared with that from HSO fed mice. Because the addition of cAMP to the cells causes an increase in ABCA1 at the cell surface, our results suggest that serum from FO-fed mice has a greater capacity to promote efflux of cellular cholesterol through the ABCA1-dependent pathway. Serum from CO-fed mice also increased efflux compared to HSO both in the absence and presence of cAMP. Because 2% serum is used as an acceptor, the increase is probably attributable to the 38% increase in HDL in the CO-fed mice. We also tested the relative ability of MPMs isolated from mice fed different diets to efflux cholesterol. There was no difference in efflux to pooled mouse serum from MPM derived from the different diet groups.
In the absence of compelling data to suggest that the FO diet promoted cholesterol efflux from macrophages, we hypothesized that the effect of the FO diet on RCT was attributable to effects downstream from the efflux step. To evaluate later steps involved in RCT which may be affected by FO, we performed an HDL turnover study using [3H]cholesteryl oleate-labeled HDL. There was no change in FCR of plasma HDL-CE between FO and HSO diet–fed mice and no change in hepatic Scarb1 expression. Le Morvan et al reported that FO-fed mice had an increased FCR and hepatic uptake of HDL-CE, accompanied by an increase in Scarb1 expression in FO-fed mice,9 in contrast with data presented here. The differences in these results may be attributable to the difference in the diet composition: Le Morvan et al used a grain-based basal diet, whereas here, a purified AIN-93 mol/L basal diet was used with added cholesterol. Vasandani et al reported that hepatic Scarb1 was unchanged by a semisynthetic diet containing EPA and DHA in LDL receptor– deficient mice,14 which is consistent with observations made here. However, despite the unchanged turnover of HDL-CE in FO-fed mice, FO substantially increased the fecal excretion of HDL-derived cholesterol to a similar extent as was observed in the macrophage RCT study. These results suggest that FO promotes in vivo macrophage RCT at least in part via an enhancement of the excretion of HDL-derived cholesterol from liver to feces.
Our results suggest several complementary mechanisms of this effect of FO on the biliary and fecal excretion of HDL-derived cholesterol. First, we demonstrate a striking reduction in macrophage- and HDL-derived [3H]-tracer in hepatic cholesteryl ester of FO-fed mice relative to both CO and HSO diets, which increases the pool of unesterified cholesterol available for biliary excretion. This observation with the tracer studies is consistent with a significant decrease in hepatic cholesteryl ester mass in FO-fed mice. Omega-3 fatty acids such as EPA and DHA decreased hepatic cholesteryl ester compared with omega-6 fatty acids in LDL receptor– deficient mice.14 EPA and DHA are poor substrates for the ACAT reaction.15 In addition, FO feeding inhibited Fas expression, as previously shown,14 which can cause a depletion of endogenous substrates for acyl-CoA cholesterol acyl transferase (ACAT). Furthermore, SCD1 depletion causes a reduction in not only hepatic triglyceride but also hepatic cholesteryl ester levels.16 In this study FO significantly decreased Scd1 mRNA expression in liver, which may contribute to the depletion of cholesteryl ester in the liver. Thus, in FO-fed mice, macrophage- and HDL-derived free cholesterol is less effectively esterified, making it more available for biliary excretion.
A second mechanism to explain our finding is that the FO diet significantly increased hepatic mRNA levels of Abcg5/g8, key proteins which regulate hepatic cholesterol secretion into bile. LXR agonists induce Abcg5/g8 expression.17 Recently, Uehara et al reported that EPA decreased LXR regulated genes, Abcg1 and Abca1, in an in vitro study.18 However, other LXR regulated genes, Abcg5/g8 and Cyp7a1, were not affected by a diet with 10% FO (w/w) in an in vivo rat model compared to those fed the same diet with olive oil.19 The FO diet used here contains 0.15% cholesterol which in the presence of the FO diet could have resulted in increased levels of unesterified cholesterol in the liver and increased generation of oxysterols, which could serve to activate LXR. Furthermore, PPARα agonists modestly increased Abcg5/g8 expression.20 EPA and DHA are more potent PPARα agonists compared with monounsaturated or saturated fatty acid.21 Indeed, in our study the FO diet increased hepatic mRNA expression of Acox, a classic target gene of PPARα. Furthermore, we observed a significant correlation between Abcg5/g8 and Acox mRNA expression. Although further investigation is needed to clarify the mechanism by which FO increases Abcg5/g8 expression, activation of LXR or PPARα are among possible mechanisms.
A third potential mechanism is reduced intestinal reabsorption of biliary HDL-derived cholesterol attributable to downregulation of intestinal Npc1l1. We have shown that ezetimibe, which inhibits Npc1l1, promotes the fecal excretion of macrophage and HDL-derived cholesterol.22 Our data here suggest that the FO diet reduced the expression of Npc1l1. Athough the mechanism of this observation is unknown, PPARδ agonists have been shown to downregulate Npc1l122, 23 leading to increased macrophage RCT,22 and it is possible that FO could influence Npc1l1 expression through this mechanism.
In conclusion, our data indicate that a FO diet promoted the excretion of macrophage- and HDL-derived cholesterol into the feces in mice, primarily because of increased hepatic fecal excretion of HDL-derived cholesterol as a result of several complementary mechanisms. We suggest that this effect of fish oils could contribute to their antiatherosclerotic and cardioprotective properties.
Supplementary Material
Table.
Plasma Lipids of Diet-Fed Mice
| Lipid | Diet | n | Mean | Std DEV | Sig |
|---|---|---|---|---|---|
| TC | LSO | 6 | 94.5 | 9.5 | |
|
| |||||
| HSO | 6 | 95.3 | 3.3 | b | |
| CO | 6 | 133.0 | 6.2 | a | |
| FO | 6 | 89.5 | 6.4 | b | |
|
| |||||
| HDL-C | LSO | 6 | 58.0 | 11.0 | * |
|
| |||||
| HSO | 6 | 71.2 | 3.6 | b | |
| CO | 6 | 97.5 | 7.1 | a | |
| FO | 6 | 62.0 | 4.0 | c | |
|
| |||||
| Non–HDL-C | LSO | 6 | 36.5 | 3.5 | ** |
|
| |||||
| HSO | 6 | 24.2 | 2.4 | b | |
| CO | 6 | 35.5 | 2.3 | a | |
| FO | 6 | 27.5 | 3.6 | b | |
|
| |||||
| TG | LSO | 6 | 14.5 | 2.3 | ** |
|
| |||||
| HSO | 6 | 7.5 | 2.3 | b | |
| CO | 6 | 29.7 | 6.3 | a | |
| FO | 6 | 11.3 | 3.8 | b | |
|
| |||||
| NEFA | LSO | 6 | 1.2 | 0.2 | * |
|
| |||||
| HSO | 6 | 1.0 | 0.1 | b | |
| CO | 6 | 1.7 | 0.2 | a | |
| FO | 6 | 0.8 | 0.1 | c | |
Values are given as mean mg/dL ±SD; n=6 mice per group. ANOVA indicates a significant difference between groups for all lipids (P<0.01). Sig indicates the statistical significance where different letters indicate significantly differences among the high-fat diet groups determined by Tukey HSD (alpha=0.05). The same letter indicates no significant difference between groups. Two-tailed tests were performed to examine differences between low-fat LSO and high-fat HSO diets (*P<0.05, **P<0.01).
Acknowledgments
We are indebted to Dawn Marchadier, Debra Cromley, Aisha Wilson, Edwige Edouard, Mao-Sen Sun, Michelle Joshi, Anna DiFlorio, and Linda Morrell for their excellent technical assistance; and to Drs John Millar and Ginny Weibel for helpful discussions.
Sources of Funding
This project was supported by Takeda Pharmaceutical Company Limited and by P01 HL22633.
Footnotes
Disclosures
None.
References
- 1.Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77:1146–1155. doi: 10.1093/ajcn/77.5.1146. [DOI] [PubMed] [Google Scholar]
- 2.Wijendran V, Hayes KC. Dietary n-6 and n-3 fatty acid balance and cardiovascular health. Annu Rev Nutr. 2004;24:597–615. doi: 10.1146/annurev.nutr.24.012003.132106. [DOI] [PubMed] [Google Scholar]
- 3.Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA. 2006;296:1885–1899. doi: 10.1001/jama.296.15.1885. [DOI] [PubMed] [Google Scholar]
- 4.Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–1098. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 5.Harrison N, Abhyankar B. The mechanism of action of omega-3 fatty acids in secondary prevention post-myocardial infarction. Curr Med Res Opin. 2005;21:95–100. doi: 10.1185/030079904x17956. [DOI] [PubMed] [Google Scholar]
- 6.Lee KW, Lip GY. The role of omega-3 fatty acids in the secondary prevention of cardiovascular disease. QJM. 2003;96:465–480. doi: 10.1093/qjmed/hcg092. [DOI] [PubMed] [Google Scholar]
- 7.Rader DJ, Alexander ET, Weibel GL, Billheimer J, Rothblat GH. Role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J Lipid Res. 2009;50:S189–S194. doi: 10.1194/jlr.R800088-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marmillot P, Rao MN, Liu QH, Chirtel SJ, Lakshman MR. Effect of dietary omega-3 fatty acids and chronic ethanol consumption on reverse cholesterol transport in rats. Metabolism. 2000;49:508–512. doi: 10.1016/s0026-0495(00)80017-7. [DOI] [PubMed] [Google Scholar]
- 9.le Morvan V, Dumon MF, Palos-Pinto A, Berard AM. n-3 FA increase liver uptake of HDL-cholesterol in mice. Lipids. 2002;37:767–772. doi: 10.1007/s11745-002-0959-2. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Zanotti I, Reilly MP, Glick JM, Rothblat GH, Rader DJ. Overexpression of apolipoprotein A-I promotes reverse transport of cholesterol from macrophages to feces in vivo. Circulation. 2003;108:661–663. doi: 10.1161/01.CIR.0000086981.09834.E0. [DOI] [PubMed] [Google Scholar]
- 11.Adorni MP, Zimetti F, Billheimer JT, Wang N, Rader DJ, Phillips MC, Rothblat GH. The roles of different pathways in the release of cholesterol from macrophages. J Lipid Res. 2007;48:2453–2462. doi: 10.1194/jlr.M700274-JLR200. [DOI] [PubMed] [Google Scholar]
- 12.Tietge UJ, Maugeais C, Cain W, Grass D, Glick JM, de beer FC, Rader DJ. Overexpression of secretory phospholipase A(2) causes rapid catabolism and altered tissue uptake of high density lipoprotein cholesteryl ester and apolipoprotein A-I. J Biol Chem. 2000;275:10077–10084. doi: 10.1074/jbc.275.14.10077. [DOI] [PubMed] [Google Scholar]
- 13.Kim HJ, Takahashi M, Ezaki O. Fish oil feeding decreases mature sterol regulatory element-binding protein 1 (SREBP-1) by down-regulation of SREBP-1c mRNA in mouse liver. A possible mechanism for down-regulation of lipogenic enzyme mRNAs. J Biol Chem. 1999;274:25892–25898. doi: 10.1074/jbc.274.36.25892. [DOI] [PubMed] [Google Scholar]
- 14.Vasandani C, Kafrouni AI, Caronna A, Bashmakov Y, Gotthardt M, Horton JD, Spady DK. Upregulation of hepatic LDL transport by n-3 fatty acids in LDL receptor knockout mice. J Lipid Res. 2002;43:772–784. [PubMed] [Google Scholar]
- 15.Rustan AC, Nossen JO, Osmundsen H, Drevon CA. Eicosapentaenoic acid inhibits cholesterol esterification in cultured parenchymal cells and isolated microsomes from rat liver. J Biol Chem. 1988;263:8126–8132. [PubMed] [Google Scholar]
- 16.Miyazaki M, Kim YC, Gray-Keller MP, Attie AD, Ntambi JM. The biosynthesis of hepatic cholesterol esters and triglycerides is impaired in mice with a disruption of the gene for stearoyl-CoA desaturase 1. J Biol Chem. 2000;275:30132–30138. doi: 10.1074/jbc.M005488200. [DOI] [PubMed] [Google Scholar]
- 17.Repa JJ, Berge KE, Pomajzl C, Richardson JA, Hobbs H, Mangelsdorf DJ. Regulation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 by the liver X receptors alpha and beta. J BiolChem. 2002;277:18793–18800. doi: 10.1074/jbc.M109927200. [DOI] [PubMed] [Google Scholar]
- 18.Uehara Y, Miura S, von Eckardstein A, Abe S, Fujii A, Matsuo Y, Rust S, Lorkowski S, Assmann G, Yamada T, Saku K. Unsaturated fatty acids suppress the expression of the ATP-binding cassette transporter G1 (ABCG1) and ABCA1 genes via an LXR/RXR responsive element. Atherosclerosis. 2007;191:11–21. doi: 10.1016/j.atherosclerosis.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 19.Pawar A, Botolin D, Mangelsdorf DJ, Jump DB. The role of liver X receptor-alpha in the fatty acid regulation of hepatic gene expression. J Biol Chem. 2003;278:40736–40743. doi: 10.1074/jbc.M307973200. [DOI] [PubMed] [Google Scholar]
- 20.Roglans N, Vazquez-Carrera M, Alegret M, Novell F, Zambon D, Ros E, Laguna JC, Sanchez RM. Fibrates modify the expression of key factors involved in bile-acid synthesis and biliary-lipid secretion in gallstone patients. Eur J Clin Pharmacol. 2004;59:855–861. doi: 10.1007/s00228-003-0704-1. [DOI] [PubMed] [Google Scholar]
- 21.Pawar A, Jump DB. Unsaturated fatty acid regulation of peroxisome proliferator-activated receptor alpha activity in rat primary hepatocytes. J Biol Chem. 2003;278:35931–35939. doi: 10.1074/jbc.M306238200. [DOI] [PubMed] [Google Scholar]
- 22.Briand F, Naik SU, Fuki I, Millar JS, MacPhee C, Walker M, Billheimer J, Rothblat G, Rader DJ. Both the peroxisome proliferator-activated receptor delta agonist, GW0742, and ezetimbe promote reverse cholesterol transport in mice by reducing intestinal reabsorption of HDL-derived cholesterol. Clin Trans Sci. 2009;2:127–133. doi: 10.1111/j.1752-8062.2009.00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Veen JN, Kruit JK, Havinga R, Baller JF, Chimini G, Lestavel S, Staels B, Groot PH, Groen AK, Kuipers F. Reduced cholesterol absorption upon PPARdelta activation coincides with decreased intestinal expression of NPC1L1. J Lipid Res. 2005;46:526–534. doi: 10.1194/jlr.M400400-JLR200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.