Abstract
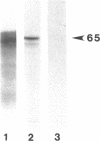
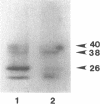
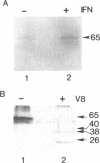
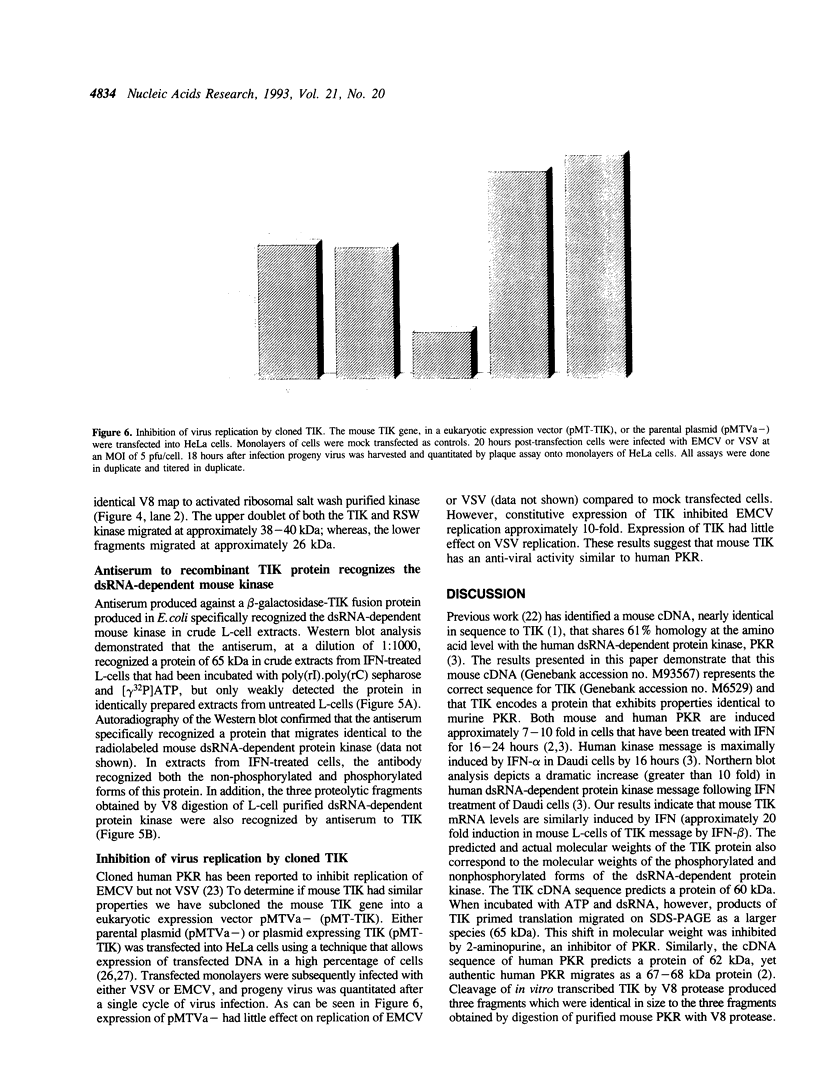
The mouse TIK protein, a serine/threonine kinase, was originally isolated from a murine pre-B cell expression library by its ability to bind anti-phosphotyrosine antibodies (Icely et al., J. Biol. Chem. 266, 16073-16077, 1991). The 67 kDa protein was found to have an associated autophosphorylation activity when incubated with ATP. Our results show that TIK is actually the mouse interferon-induced, dsRNA-dependent protein kinase, PKR. We demonstrate that the TIK message is interferon-inducible in mouse L-cells and in vitro transcription and translation of the TIK cDNA produces a protein that is capable of binding double-stranded RNA. The in vitro synthesized TIK protein migrated as a 65 kDa protein on SDS-PAGE when incubated with ATP, but migrated as a 60 kDa protein when incubated with an inhibitor of PKR, 2-aminopurine. We further show that proteolytic digestion of TIK with Staphylococcus aureus V8 protease results in a cleavage pattern identical to that obtained by V8 digestion of authentic PKR. Antiserum to TIK specifically recognized PKR. Cloned TIK had inhibitory activity for replication of EMCV but not VSV. From these observations we conclude that TIK kinase is the mouse interferon-induced, double-stranded RNA-dependent kinase, PKR.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baeuerle P. A. The inducible transcription activator NF-kappa B: regulation by distinct protein subunits. Biochim Biophys Acta. 1991 Apr 16;1072(1):63–80. doi: 10.1016/0304-419x(91)90007-8. [DOI] [PubMed] [Google Scholar]
- Chang H. W., Jacobs B. L. Identification of a conserved motif that is necessary for binding of the vaccinia virus E3L gene products to double-stranded RNA. Virology. 1993 Jun;194(2):537–547. doi: 10.1006/viro.1993.1292. [DOI] [PubMed] [Google Scholar]
- Chong K. L., Feng L., Schappert K., Meurs E., Donahue T. F., Friesen J. D., Hovanessian A. G., Williams B. R. Human p68 kinase exhibits growth suppression in yeast and homology to the translational regulator GCN2. EMBO J. 1992 Apr;11(4):1553–1562. doi: 10.1002/j.1460-2075.1992.tb05200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Felber B. K., Paskalis H., Kleinman-Ewing C., Wong-Staal F., Pavlakis G. N. The pX protein of HTLV-I is a transcriptional activator of its long terminal repeats. Science. 1985 Aug 16;229(4714):675–679. doi: 10.1126/science.2992082. [DOI] [PubMed] [Google Scholar]
- Feng G. S., Chong K., Kumar A., Williams B. R. Identification of double-stranded RNA-binding domains in the interferon-induced double-stranded RNA-activated p68 kinase. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5447–5451. doi: 10.1073/pnas.89.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatignol A., Buckler-White A., Berkhout B., Jeang K. T. Characterization of a human TAR RNA-binding protein that activates the HIV-1 LTR. Science. 1991 Mar 29;251(5001):1597–1600. doi: 10.1126/science.2011739. [DOI] [PubMed] [Google Scholar]
- Gilmore T. D. NF-kappa B, KBF1, dorsal, and related matters. Cell. 1990 Sep 7;62(5):841–843. doi: 10.1016/0092-8674(90)90257-f. [DOI] [PubMed] [Google Scholar]
- Goebel S. J., Johnson G. P., Perkus M. E., Davis S. W., Winslow J. P., Paoletti E. The complete DNA sequence of vaccinia virus. Virology. 1990 Nov;179(1):247-66, 517-63. doi: 10.1016/0042-6822(90)90294-2. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hammacher A., Mellström K., Heldin C. H., Westermark B. Isoform-specific induction of actin reorganization by platelet-derived growth factor suggests that the functionally active receptor is a dimer. EMBO J. 1989 Sep;8(9):2489–2495. doi: 10.1002/j.1460-2075.1989.tb08385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Icely P. L., Gros P., Bergeron J. J., Devault A., Afar D. E., Bell J. C. TIK, a novel serine/threonine kinase, is recognized by antibodies directed against phosphotyrosine. J Biol Chem. 1991 Aug 25;266(24):16073–16077. [PubMed] [Google Scholar]
- Koromilas A. E., Roy S., Barber G. N., Katze M. G., Sonenberg N. Malignant transformation by a mutant of the IFN-inducible dsRNA-dependent protein kinase. Science. 1992 Sep 18;257(5077):1685–1689. doi: 10.1126/science.1382315. [DOI] [PubMed] [Google Scholar]
- Langland J. O., Jacobs B. L. Cytosolic double-stranded RNA-dependent protein kinase is likely a dimer of partially phosphorylated Mr = 66,000 subunits. J Biol Chem. 1992 May 25;267(15):10729–10736. [PubMed] [Google Scholar]
- Lengyel P. Biochemistry of interferons and their actions. Annu Rev Biochem. 1982;51:251–282. doi: 10.1146/annurev.bi.51.070182.001343. [DOI] [PubMed] [Google Scholar]
- Marcus P. I., Sekellick M. J. Interferon induction by viruses. XVI. 2-Aminopurine blocks selectively and reversibly an early stage in interferon induction. J Gen Virol. 1988 Jul;69(Pt 7):1637–1645. doi: 10.1099/0022-1317-69-7-1637. [DOI] [PubMed] [Google Scholar]
- McClain D. A., Maegawa H., Lee J., Dull T. J., Ulrich A., Olefsky J. M. A mutant insulin receptor with defective tyrosine kinase displays no biologic activity and does not undergo endocytosis. J Biol Chem. 1987 Oct 25;262(30):14663–14671. [PubMed] [Google Scholar]
- Meurs E. F., Watanabe Y., Kadereit S., Barber G. N., Katze M. G., Chong K., Williams B. R., Hovanessian A. G. Constitutive expression of human double-stranded RNA-activated p68 kinase in murine cells mediates phosphorylation of eukaryotic initiation factor 2 and partial resistance to encephalomyocarditis virus growth. J Virol. 1992 Oct;66(10):5805–5814. doi: 10.1128/jvi.66.10.5805-5814.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meurs E., Chong K., Galabru J., Thomas N. S., Kerr I. M., Williams B. R., Hovanessian A. G. Molecular cloning and characterization of the human double-stranded RNA-activated protein kinase induced by interferon. Cell. 1990 Jul 27;62(2):379–390. doi: 10.1016/0092-8674(90)90374-n. [DOI] [PubMed] [Google Scholar]
- Miyamoto N. G., Jacobs B. L., Samuel C. E. Mechanism of interferon action. Effect of double-stranded RNA and the 5'-O-monophosphate form of 2',5'-oligoadenylate on the inhibition of reovirus mRNA translation in vitro. J Biol Chem. 1983 Dec 25;258(24):15232–15237. [PubMed] [Google Scholar]
- Moreno S., Nurse P. Substrates for p34cdc2: in vivo veritas? Cell. 1990 May 18;61(4):549–551. doi: 10.1016/0092-8674(90)90463-o. [DOI] [PubMed] [Google Scholar]
- Ochoa S. Regulation of protein synthesis initiation in eucaryotes. Arch Biochem Biophys. 1983 Jun;223(2):325–349. doi: 10.1016/0003-9861(83)90598-2. [DOI] [PubMed] [Google Scholar]
- Pain V. M. Initiation of protein synthesis in mammalian cells. Biochem J. 1986 May 1;235(3):625–637. doi: 10.1042/bj2350625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R. C., Sen G. C. Construction and expression of an enzymatically active human-mouse chimeric double-stranded RNA-dependent protein kinase. J Interferon Res. 1992 Oct;12(5):389–393. doi: 10.1089/jir.1992.12.389. [DOI] [PubMed] [Google Scholar]
- Patel R. C., Sen G. C. Identification of the double-stranded RNA-binding domain of the human interferon-inducible protein kinase. J Biol Chem. 1992 Apr 15;267(11):7671–7676. [PubMed] [Google Scholar]
- Pathak V. K., Schindler D., Hershey J. W. Generation of a mutant form of protein synthesis initiation factor eIF-2 lacking the site of phosphorylation by eIF-2 kinases. Mol Cell Biol. 1988 Feb;8(2):993–995. doi: 10.1128/mcb.8.2.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka S., Langer J. A., Zoon K. C., Samuel C. E. Interferons and their actions. Annu Rev Biochem. 1987;56:727–777. doi: 10.1146/annurev.bi.56.070187.003455. [DOI] [PubMed] [Google Scholar]
- Qian Y. A., Jiang B. M., Saif L. J., Kang S. Y., Ojeh C. K., Green K. Y. Molecular analysis of the gene 6 from a porcine group C rotavirus that encodes the NS34 equivalent of group A rotaviruses. Virology. 1991 Oct;184(2):752–757. doi: 10.1016/0042-6822(91)90446-i. [DOI] [PubMed] [Google Scholar]
- Samuel C. E. Antiviral actions of interferon. Interferon-regulated cellular proteins and their surprisingly selective antiviral activities. Virology. 1991 Jul;183(1):1–11. doi: 10.1016/0042-6822(91)90112-o. [DOI] [PubMed] [Google Scholar]
- Samuel C. E., Knutson G. S., Berry M. J., Atwater J. A., Lasky S. R. Purification of double-stranded RNA-dependent protein kinase from mouse fibroblasts. Methods Enzymol. 1986;119:499–516. doi: 10.1016/0076-6879(86)19070-7. [DOI] [PubMed] [Google Scholar]
- Samuel C. E. Mechanism of interferon action: phosphorylation of protein synthesis initiation factor eIF-2 in interferon-treated human cells by a ribosome-associated kinase processing site specificity similar to hemin-regulated rabbit reticulocyte kinase. Proc Natl Acad Sci U S A. 1979 Feb;76(2):600–604. doi: 10.1073/pnas.76.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel C. E. Mechanisms of the antiviral action of interferons. Prog Nucleic Acid Res Mol Biol. 1988;35:27–72. doi: 10.1016/s0079-6603(08)60609-1. [DOI] [PubMed] [Google Scholar]
- Thomis D. C., Doohan J. P., Samuel C. E. Mechanism of interferon action: cDNA structure, expression, and regulation of the interferon-induced, RNA-dependent P1/eIF-2 alpha protein kinase from human cells. Virology. 1992 May;188(1):33–46. doi: 10.1016/0042-6822(92)90732-5. [DOI] [PubMed] [Google Scholar]
- Tiwari R. K., Kusari J., Kumar R., Sen G. C. Gene induction by interferons and double-stranded RNA: selective inhibition by 2-aminopurine. Mol Cell Biol. 1988 Oct;8(10):4289–4294. doi: 10.1128/mcb.8.10.4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvanathan K. V., Goodbourn S. Double-stranded RNA activates binding of NF-kappa B to an inducible element in the human beta-interferon promoter. EMBO J. 1989 Apr;8(4):1129–1138. doi: 10.1002/j.1460-2075.1989.tb03483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg R. A. Tumor suppressor genes. Science. 1991 Nov 22;254(5035):1138–1146. doi: 10.1126/science.1659741. [DOI] [PubMed] [Google Scholar]
- Zinn K., Keller A., Whittemore L. A., Maniatis T. 2-Aminopurine selectively inhibits the induction of beta-interferon, c-fos, and c-myc gene expression. Science. 1988 Apr 8;240(4849):210–213. doi: 10.1126/science.3281258. [DOI] [PubMed] [Google Scholar]