Abstract
Tetraethylammonium (TEA) is a potassium (K+) channel inhibitor that has been extensively used as a molecular probe to explore the structure of channels’ ion pathway. In this study, we identified that Leu70 of the virus-encoded potassium channel Kcv is a key amino acid that plays an important role in regulating the channel’s TEA sensitivity. Site-directed mutagenesis of Leu70 can change the TEA sensitivity by 1,000-fold from ∼100 µM to ∼100 mM. Because no compelling trends exist to explain this amino acid’s specific interaction with TEA, the role of Leu70 at the binding site is likely to ensure an optimal conformation of the extracellular mouth that confers high TEA affinity. We further assembled the subunits of mutant and wt-Kcv into a series of heterotetramers. The differences in these heterochannels suggest that all of the four subunits in a Kcv channel additively participate in the TEA binding, and each of the four residues at the binding site independently contributes an equal binding energy. We therefore can present a series of mutant/wild-type tetramer combinations that can probe TEA over three orders of magnitude in concentration. This study may give insight into the mechanism for the interaction between the potassium channel and its inhibitor.
INTRODUCTION
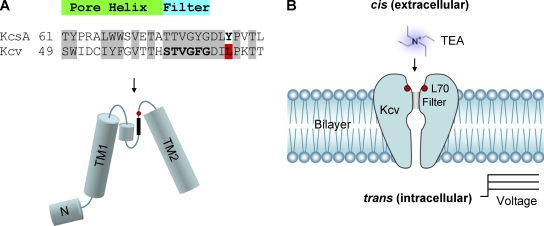
The virus-encoded potassium channel Kcv contains 94 amino acids. It is among the smallest potassium channels discovered to date (Plugge et al., 2000; Kang et al., 2004; Wang et al., 2011), yet possesses conserved selectivity filter domains and transmembrane domains that provide similar channel properties to other potassium channels such as KcsA (>60% homology in P-loop; Fig. 1 A; Plugge et al., 2000). Kcv has been shown to possess analogous selectivity (Plugge et al., 2000), voltage dependence (Gazzarrini et al., 2002, 2003; Shim et al., 2007; Tan et al., 2010), gating (Pagliuca et al., 2007; Shim et al., 2007; Abenavoli et al., 2009; Tan et al., 2010), and ligand blocking (Plugge et al., 2000; Gazzarrini et al., 2003; Syeda et al., 2008). As a result, Kcv is an attractive model protein to use in the study of potassium channel mechanics and biophysics (Balss et al., 2008; Abenavoli et al., 2009; Tayefeh et al., 2009; Gebhardt et al., 2011; Thiel et al., 2011).
Figure 1.
Structure of Kcv and its orientation in the lipid bilayer. (A) Comparison of the KcsA and Kcv protein sequences between two transmembrane domains, including the pore helix and the selectivity filter (top). The arrangement of different domains in Kcv was predicted based on the KcsA structure (bottom). Leu70 is marked in red. (B) Orientation of the Kcv channel in the lipid bilayer, as demonstrated by a series of experiments.
Essential in the determination of biophysical mechanisms is the use of inhibitors to modulate the function of the channel, where the modification of ionic current through the channel can help elucidate and explain interactions with the channel as well as help define the local chemical environment. The quaternary ammonium ion TEA is an important potassium channel inhibitor that has been extensively used as the probe to detect the structure of the potassium channels’ ion permeation pathway, and its blocking properties have been well established for several potassium pores (MacKinnon and Yellen, 1990; Heginbotham and MacKinnon, 1992; Choi et al., 1993; Bretschneider et al., 1999; Heginbotham et al., 1999; Meuser et al., 1999, 2001). Although the effect of TEA on wt-Kcv at the whole-cell and single-channel levels had been tested (Gazzarrini et al., 2003; Syeda et al., 2008), the binding location and the specific nature of the interaction including the per-subunit contribution to the interaction remained unknown. In the absence of an available crystal structure for Kcv, these characterizations are vital for accurate cross-comparison of this model channel with other potassium channels.
In this study, we first used site-directed mutagenesis to identify that Leu70 of Kcv is a key amino acid that determines the Kcv channel’s TEA sensitivity. This position is a homologous residue of KcsA’s external TEA binding site Tyr82 (Meuser et al., 2001; Gazzarrini et al., 2003), and substitution at this position can dramatically alter the TEA sensitivity from 0.1 to 100 mM. We then used the in vitro heterochannel approach (Shim et al., 2007; Tan et al., 2010) to assemble the subunits of mutants and wt-Kcv into a series of heterotetramers. The observed differences between different subunit combinations showed that all four subunits additively participate in the TEA binding, and each of the four residues on the binding site independently contributes an equal energy. Through the judicious choice of certain tetramer mutants and their combinations, we are able to report on a series of mutants that can provide TEA sensitivity over three orders of magnitude, providing a tool for studying the inhibition of potassium channels by the inhibitor over a wide range of concentrations.
MATERIALS AND METHODS
Construction of the Kcv mutants
The Kcv genes were assembled into the pGS-21a expression vector. The Kcv genes containing point mutations at residue Leu70 were constructed using the QuikChange Site-Directed Mutagenesis kit (Agilent Technologies). The gene of the tagged-Kcv (N8) that encodes wt-Kcv with an N-terminal eight-asparagine extension has been constructed in a previous study (Tan et al., 2010).
Synthesis and purification of Kcv proteins
S35-labeled Kcv proteins were synthesized from coupled in vitro transcription and translation using the kit Escherichia coli T7 S30 Extract System for Circular DNA (L1130; Promega). To produce heterotetramers, 4 µl of mixed plasmid of tagged Kcv DNA and untagged Kcv DNA at various ratios was used. The radiolabeled Kcv tetramers were resolved on 12.5% SDS–polyacrylamide gel. Each tetramer was purified from the dried gel, aliquoted, and stored at −20°C. The Kcv protein was synthesized using coupled in vitro transcription and translation, followed by tetramerization and separation on the SDS–polyacrylamide gel (Shim et al., 2007; Tan et al., 2010).
Single-channel recording on lipid bilayer and data analysis
The apparatus and method for single-channel recording has been described previously (Shim et al., 2007). In brief, the lipid bilayer was formed with 1,2-diphytanoylsn-glycerophosphatidylcholine (Avanti Polar Lipids, Inc.) by merging two lipid monolayers over an ∼100-µm-wide aperture in a 25-µm-thick Teflon film that partitions the cis and trans chambers. The Kcv protein purified from the gel was added to the cis chamber, which was electrically grounded (Fig. 1 B). The recording solutions in both trans and cis sides contained 150 mM KCl, 10 mM Tris-Cl, pH 7.2. Single-channel currents were recorded with an Axopatch 200B patch clamp amplifier (Molecular Devices). The current was filtered at 1 kHz with a low-pass Bessel filter and acquired at a sampling rate of 20 kHz. The current amplitude was determined by fitting the all-points histogram with the Gaussian distribution. The inhibition constant IC50 was defined as the concentration of TEA that provides a 50% inhibition to the single-channel open current and was obtained by fitting the dose–response data with the single-component Langmuir equation
| (1) |
where I0 and I are current amplitudes in the absence and presence of TEA. Each current trace under a specific condition was independently collected at least three times based on different channels. The results were shown as mean ± SD. Experiments were conducted at ∼22°C.
Online supplemental material
Fig. S1 illustrates the current-voltage relationship (I-V curves) for wt-Kcv and all Leu70 substitutions. Fig. S2 analyzes the correlation between cis TEA sensitivity and current amplitude at ±60 mV. Fig. S3 analyzes the correlation between cis TEA sensitivity and amino acid hydrophobicity. Fig. S4 shows the weak voltage dependence of cis TEA sensitivity for wt-Kcv and selected Leu70 substitutions. Fig. S5 characterizes the effect of TEA in increasing the open probability of Kcv. Fig. S6 analyzes the relative error for the wide dynamic range composite biosensor formed by tunable ion channels. The supplemental text interprets the discrepancy in TEA sensitivities measured by different methods, describes the principle of ion channel–based composite biosensors for detection of targets in a wide dynamic concentration range, and deducts the expressions for calculating the sensitivity zone boundary in an ion channel sensor. Online supplemental material is available at http://www.jgp.org/cgi/content/full/jgp.201110725/DC1.
RESULTS
Regulation of Kcv’s TEA sensitivity by Leu70 substitutions
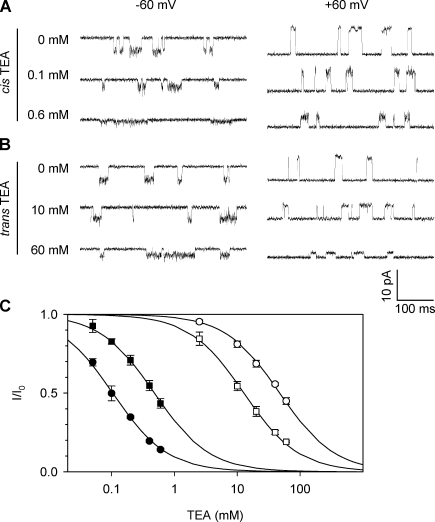
In our Kcv experiments, the protein of Kcv tetramers was added to the cis solution, from which it was inserted into the membrane (Fig. 1 B). Fig. 2 (A and B) shows single-channel current traces of Kcv at −60 mV and 60 mV with various concentrations of TEA added to the cis or trans solution, whereas Fig. 2 C shows the binding curves, i.e., TEA concentration–dependent current reduction (I/I0). The binding curves indicate that both cis and trans TEA can inhibit the Kcv channel, but their sensitivities are completely different. IC50 for the cis TEA was 0.098 ± 0.02 mM at −60 mV and 0.41 ± 0.03 mM at 60 mV, whereas IC50 for the trans TEA was 47 ± 11 mM at −60 mV and 13 ± 2 mM at 60 mV. Thus, the inhibition of Kcv by cis TEA was two orders of magnitude higher than by trans TEA.
Figure 2.
Inhibition of the wt-Kcv K+ channel by TEA. Single-channel currents were recorded in symmetric solutions containing 150 mM KCl buffered with 10 mM Tris, pH 7.2. (A and B) Current traces in the presence of different TEA concentrations added from the cis solution (A) and trans solution (B) at −60 mV (left) and 60 mV (right). (C) Inhibition percentage (I/I0) in various TEA concentrations measured from traces shown in A and B. Closed circles indicate cis TEA, −60 mV; closed boxes indicate cis TEA, 60 mV; open circles indicate trans TEA, −60 mV; open boxes indicate trans TEA, 60 mV. Data are presented as mean ± SD.
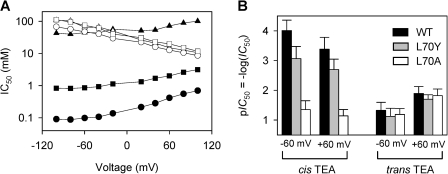
It is known that Tyr82 in KcsA or Phe449 in Shaker are key amino acids required for high sensitivity to external TEA, and substitutions on these sites can significantly alter TEA’s ability to inhibit the channel (Heginbotham and MacKinnon, 1992; Meuser et al., 2001). Structurally, these sites are equivalent to Leu70 of Kcv within the entrance to the selectivity filter (Fig. 1 A), and therefore this amino acid was modified using site-directed mutagenesis. As shown in Fig. 3, the substitution of Leu70 with tyrosine (L70Y) decreased the cis TEA sensitivity by sevenfold to 0.74 ± 0.09 mM (−60 mV), and the substitution by alanine (L70A) sharply decreased the sensitivity by 450-fold to 45 ± 5 mM (−60 mV). In contrast, the trans TEA sensitivity of L70Y (20 ± 4 mM, 60 mV) and L70A (15 ± 2 mM, 60 mV) remained unchanged compared with wt-Kcv (13 ± 2 mM). Thus, the substitution in this Kcv domain can significantly alter the cis TEA sensitivity but has little effect on the trans TEA sensitivity, verifying the cis exposure of Kcv’s substituted domain at Leu70 (Fig. 1 B). This directionality of Kcv is similar to KcsA, which upon being reconstituted from the cis side of the lipid bilayer (Heginbotham et al., 1999), has its extracellular domain face the cis solution.
Figure 3.
Comparison of TEA inhibition between Kcv and substitutions L70Y and L70A. 150 mM KCl buffered with 10 mM Tris, pH 7.2. (A) IC50 at various voltages for wt-Kcv, cis TEA (closed circles); L70Y, cis TEA (closed boxes); L70A, cis TEA (closed triangles); wt-Kcv, trans TEA (open circles); L70Y, trans TEA (open boxes); and L70A, trans TEA (open triangles). (B) Vertical bar comparison of TEA sensitivity from the cis and trans sides at ±60 mV for wt-Kcv, L70Y, and L70A. Data points are presented as mean ± SD.
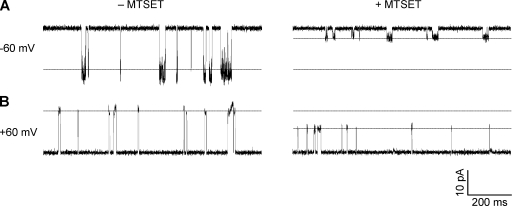
The orientation of Kcv was also confirmed using a chemical modification method. Methanethiosulfonates are common water-soluble sulfhydryl-modifying reagents that can react with cysteine residues. We therefore constructed substitution L70C and introduced the cationic (2-(trimethylammonium)ethyl) methanethiosulfonate (MTSET; bromide salt) to either cis or trans solution. After addition of cis MTSET for 5 min, the conductance of L70C was reduced by 78% at −60 mV (Fig. 4 A) and by 30% at 60 mV (Fig. 4 B). By comparison, MTSET added in the trans solution was not found to reduce the conductance of Kcv (not depicted). Therefore, MTSET lowers the conductance to a greater degree in the cis to trans direction. This rectification is similar to KcsA, in which MTSET reduced 60% of the cis to trans current of Y82C while retaining the current in the reverse direction unchanged (Heginbotham et al., 1999). In the control experiment, we did not observe the conductance change of the wt-Kcv channel in the presence of 0.2 mM cis MTSET, suggesting that MTSET cannot react with wt-Kcv. wt-Kcv also possesses a cysteine at Cys52, but compared with the homologous location within the structure of KcsA, this amino acid is likely buried in the pore helix outside Kcv’s selectivity filter and should be inaccessible to MTSET. Overall, the result of cysteine modification confirms the location of Leu70 near the cis entrances of wt-Kcv.
Figure 4.
Single-channel current changes by MTSET for the cysteine substitution L70C. 0.2 mM MESET was added from the cis side. (A and B) −60 mV (A) and +60 mV (B) in the absence (left) and in the presence of MTSET (right).
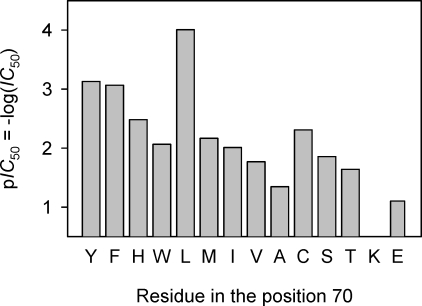
The aforementioned findings suggest the importance of the residue at position 70 in regulating TEA sensitivity. To investigate how the amino acid in this position regulates TEA binding, we constructed a variety of Kcv substitutions and tested homotetramers for their TEA sensitivity. As shown in Table 1 and compared in Fig. 5, the TEA sensitivity of all channels varied broadly over three orders of magnitude from 0.09 to 90 mM, with wt-Kcv possessing the highest TEA sensitivity. Relative to wt-Kcv, the sensitivity decreased by seven- to 86-fold for the aromatic substitutions (L70Y, F, H, and W), 68- to 450-fold for the nonpolar substitutions (L70M, I, V, and A), 45- to 230-fold for polar substitutions (L70C, S, and T), and 900-fold for the charged substitution (L70E). The IC50 values have no correlation with the current amplitude among all channels (Fig. S1 for I-V curves and Fig. S2 for I-IC50 plot), suggesting that the intensity of K+ flow does not influence the TEA sensitivity. Also, the very low TEA sensitivity of L70E suggests that the electrostatic interaction may not be a major contribution to the binding of TEA. We further calculated relative electric distance δ based on the voltage dependence of TEA sensitivity (Fig. S4). Similar to the small δ between 0.16 and 0.21 observed for KcsA (Meuser et al., 2001), the TEA sensitivities for both wt-Kcv and its substitutions were weakly voltage dependent with a δ between 0 and 0.1 for negative voltage data and 0.16 and 0.29 for positive voltage data (Table 1), suggesting that there is a TEA-binding site near the cis surface of the Kcv channel where Leu70 is located (Fig. 1 B).
TABLE 1.
Inhibition constant IC50 and transmembrane electric distance for wt-Kcv and various Leu70 mutants
| Residue type and mutant | I (−60 mV) | IC50 (−60 mV) | δ (−V) | I (60 mV) | IC50 (60 mV) | δ (+V) |
| pA | mM | pA | mM | |||
| Aromatic | ||||||
| L70Y | −15.7 ± 1.2 | 0.74 ± 0.09 | 0.06 | 16.3 ± 1.1 | 1.9 ± 0.2 | 0.25 |
| L70F | −17.6 ± 0.8 | 0.86 ± 0.07 | 0.09 | 15.9 ± 1.2 | 2.0 ± 0.2 | 0.26 |
| L70H | −6.51 ± 0.42 | 3.3 ± 0.5 | 0.00 | 12.6 ± 1.5 | 3.6 ± 0.7 | 0.16 |
| L70W | −15.0 ± 0.9 | 8.6 ± 0.7 | 0.09 | 14.1 ± 0.7 | 22 ± 3 | 0.27 |
| Nonpolar | ||||||
| wt (L70) | −5.43 ± 1.3 | 0.098 ± 0.02 | 0.12 | 12.0 ± 1.1 | 0.41 ± 0.03 | 0.29 |
| L70M | −10.4 ± 0.6 | 6.8 ± 1.1 | 0.15 | 11.4 ± 0.6 | 19 ± 2 | 0.22 |
| L70I | −9.81 ± 1.10 | 9.8 ± 0.7 | 0.06 | 13.7 ± 0.4 | 18 ± 2 | 0.21 |
| L70V | −12.5 ± 0.4 | 17 ± 3 | 0.09 | 15.1 ± 0.9 | 31 ± 4 | 0.23 |
| L70A | −11.3 ± 1.2 | 45 ± 5 | 0.08 | 13.9 ± 1.1 | 72 ± 18 | 0.22 |
| Polar | ||||||
| L70C | −16.4 ± 0.6 | 4.9 ± 0.2 | 0.06 | 14.8 ± 0.2 | 12 ± 2 | 0.23 |
| L70S | −13.4 ± 1.2 | 14 ± 2 | 0.00 | 15.8 ± 1.0 | 21 ± 4 | 0.21 |
| L70T | −13.3 ± 0.8 | 23 ± 4 | 0.12 | 17.6 ± 0.7 | 41 ± 7 | 0.25 |
| Charged | ||||||
| L70Ka | ND | ND | ND | 4.48 ± 0.28 | NS | ND |
| L70E | −11.5 ± 0.6 | 79 ± 11 | ND | 12.8 ± 1.1 | NS | ND |
The relative electrical distance δ was calculated from the voltage dependence of IC50 according to the Woodhull equation I/I0(V) = I/I0(0)exp(−zδFV/RT), where z = 1 is the valence of TEA, F is the Faraday constant, R is the gas constant, and T is the temperature (in K).
The negative current of L70K was not observed; thus, the TEA sensitivity cannot be determined.
Figure 5.
Comparison of inhibition of wt-Kcv and various Leu70 substitutions by the cis (external) TEA. Data were plotted as pIC50 = −log(IC50).
Tuning the TEA sensitivity by manipulating subunit composition
Up until this point, we have exclusively discussed homotetramers of Kcv. To further elucidate the mechanism of TEA binding, we next considered the effect of changing a single subunit in the Kcv tetramer. By synthesizing combinations of high sensitivity L70 subunits and low sensitivity L70A subunits, we can determine the per-subunit contribution to TEA sensitivity using the heterotetramerization method (Tan et al., 2010). In brief, Kcv proteins with high and low TEA sensitivities were coassembled into a series of heterotetramers, each with a specific subunit composition. All types of tetramers were purified using electrophoresis, and their channel activities were examined individually (Shim et al., 2007; Tan et al., 2010). L70A had one of the lowest TEA sensitivities among the tested mutants and was thus used as the TEA-insensitive subunit. We have previously synthesized N8, a wt-Kcv with an eight-asparagine extension at the N terminus. This variant retains the properties of wt-Kcv, including conductance, K+ selectivity, and highest TEA affinity (described in the next section), while allowing formation and separation of hetero–Kcv channels with mutant subunits (Tan et al., 2010). Therefore, N8 was selected to serve as the TEA-sensitive subunit.
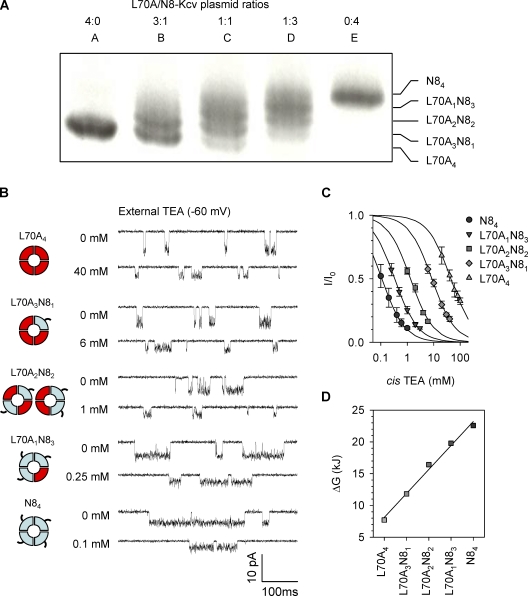
By manipulating the L70A/N8 plasmid ratios, we were able to create a series of heterotetramers that split into five bands on the electrophoresis gel (Fig. 6 A), which correspond to all subunit combinations, L70A4, L70A3N81, L70A2N82, L70A1N83, and N84. Fig. 6 B showed the current traces for the five tetramers in the absence of external TEA (top) and at a TEA concentration around IC50 (bottom). Based on TEA concentration–dependent inhibition (Fig. 6 C), IC50 was calculated (Table 2). The homotetramer L70A4 without Leu70 has the highest IC50 (lowest sensitivity) of 45 ± 7 mM. In contrast, N84 with all four Leu70 residues has the lowest IC50 of 0.11 ± 0.02 mM (identical to wt-Kcv as in Table 1). We identified that IC50 for three heterochannels decreased with the number of N8 subunits in the tetramer: L70A3N81, 8.5 ± 1.2 mM; L70A2N82, 1.3 ± 0.2 mM; and L70A1N83, 0.34 ± 0.2 mM.
Figure 6.
Inhibition of L70A/N8 homo- and heterochannels by external TEA. (A) Electrophoretical separation of five L70A/N8 tetramers. The coexpressing S35-labeled proteins were run on a 12.5% SDS–polyacrylamide gel for 16 h. Lanes A through E were tetramers formed at L70A/N8 plasmid ratios of 4:0, 3:1, 2:2, 1:3, and 0:4. The identified five bands corresponded to all subunit combinations, L70A4-nN8n, where n is the number of N8 subunits in the tetramer, n = 0, 1, 2, 3, and 4. Although there is some overlap in the bands, the lack of any overlap in the data collected using excised tetramers indicates that the correct single tetramers were used for the experiments in B and C, and each type of tetramer has specific conductance, as reported previously (Tan et al., 2010). (B) Current traces for L70A/N8 co-tetramers with five-subunit combinations purified from the gel in A (−60 mV). For each tetramer, the top trace was recorded without TEA and the bottom trace with external TEA at a concentration around IC50. (C) TEA concentration–dependent inhibition fraction for the five L70A/N8 co-tetramers. Data points are presented as mean ± SD. (D) TEA binding energy ΔG for each type of L70A/N8 co-tetramer, as calculated from IC50.
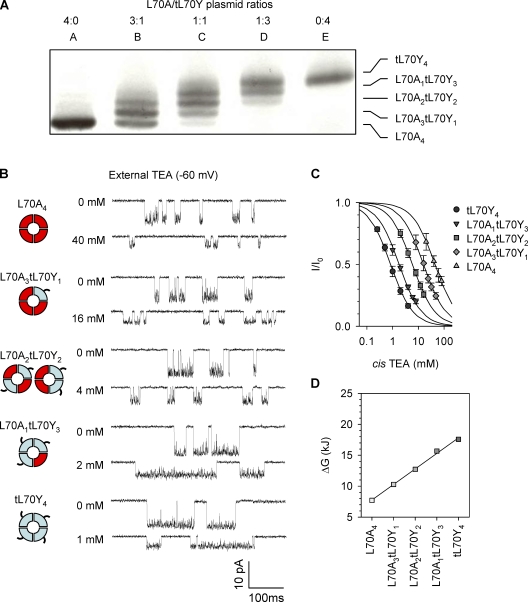
TABLE 2.
IC50, ΔG, and ΔΔG for binding of external TEA to L70A/N8 and L70A/tL70Y tetramers at −60 mV
| Homo- or heterotetramers | IC50 | ΔG | ΔΔGa |
| mM | kJ·mol−1 | kJ·mol−1 | |
| L70A/N8 | |||
| L70A4 | 45 ± 7 | 7.60 | NA |
| L70A3N81 | 8.5 ± 1.2 | 11.7 | 4.1 |
| L70A2N82 | 1.3 ± 0.2 | 16.3 | 4.6 |
| L70A1N83 | 0.34 ± 0.02 | 19.6 | 3.3 |
| N84 | 0.11 ± 0.02 | 22.4 | 2.8 |
| L70A/tL70Y | |||
| L70A4 | 44 ± 6 | 7.66 | NA |
| L70A3tL70Y1 | 16 ± 3 | 10.1 | 2.5 |
| L70A2tL70Y2 | 5.9 ± 0.4 | 12.6 | 2.4 |
| L70A1tL70Y3 | 1.8 ± 0.2 | 15.5 | 2.9 |
| tL70Y4 | 0.83 ± 0.17 | 17.4 | 1.9 |
The mean value of ΔΔG was 3.7 ± 0.8 kJ·mol−1 for the L70A/N8 tetramers and 2.4 ± 0.4 kJ·mol−1 for the L70A/tL70Y tetramers.
To understand whether different subunits cooperatively or independently interact with TEA, we calculated the free energy ΔG from IC50 for each tetramer by ΔG = −RTlnIC50 (IC50 was used as the apparent dissociation constant Kd). As shown in Fig. 6 D, ΔG was found to stepwise increase with the number of N8 (Leu70 containing) subunits. This correlation can be fitted linearly by Eq. 2:
| (2) |
In this expression, ΔG is the energy for a tetramer with n N8 and 4 − n L70A subunits. ΔGA and ΔGL are energies contributed by one L70A or N8 subunit. Their difference, ΔΔG, is the energy increase for the addition of each Leu70-containing subunit in the tetramer; the best fit gave ΔΔG = 3.7 ± 0.8 kJ·mol−1. This result suggests that each subunit with a Leu70 residue contributes equal energy to the interactions in TEA binding and additively modulates the TEA sensitivity without significant intersubunit cooperation.
Finally, to examine whether the additive mechanism applies to other types of residue at position 70 involved in TEA binding, we formed tagged L70Y (tL70Y). As a mark of consistency between experiments, the homotetramer of tL70Y retained the same TEA sensitivity (Table 2) as L70Y without a tag that has been studied above (Table 1). The coexpressed L70A/tL70Y tetramers with five-subunit combinations were purified from the electrophoresis gel (Fig. 7 A), and their inhibition by TEA was examined individually (Fig. 7, B and C) so that their IC50 could be calculated (Table 2). Similar to the L70A/N8 system, we also observed a monotonic increase in TEA sensitivity with the number of Tyr70 subunits. The energy ΔG was also shown to stepwise increase and was linearly correlated with the number of Tyr70 subunits (Fig. 7 D). The addition of each Tyr70 to the channel increases the binding energy at an incremental ΔΔG of 2.5 ± 0.4 kJ·mol−1. Therefore, TEA binding once again follows the additivity model: each subunit with Tyr70 contributes equally and independently to the TEA sensitivity.
Figure 7.
Inhibition of L70A/tL70Y homo- and heterochannels by external TEA. (A) Electrophoretical separation of five homo- and heterotetramers formed by coexpressing L70A and tL70Y proteins on the 12.5% SDS–polyacrylamide gel. (B) Current traces for L70A/tL70Y co-tetramers with five-subunit combinations (−60 mV). For each tetramer, the top trace was recorded without TEA and the bottom one with the external TEA around IC50. (C) TEA concentration–dependent inhibition fraction for the five L70A/tL70Y co-tetramers. Data points are presented as mean ± SD. (D) TEA binding energy ΔG for each type of L70A/tL70Y co-tetramer, as calculated from IC50.
DISCUSSION
TEA sensitivity and Kcv directionality in the lipid bilayer
Kcv demonstrates asymmetrical TEA sensitivity, with cis TEA being >100-fold more potent than trans TEA. To determine the directionality of Kcv in the lipid bilayer, we have measured a series of Leu70 substitutions. This position was chosen because it corresponds to the TEA-binding site Y82 in KcsA according to sequence homology analysis (Fig. 1 A; Gazzarrini et al., 2003). Indeed, the amino acids at position 70 were found to be able to regulate the binding of TEA to Kcv, but the effect depends on the sideness: substitutions of Leu70 decreased the cis TEA sensitivity by hundreds of folds, whereas their trans TEA sensitivities were almost unchanged (Fig. 3). Therefore, Leu70 is expected to face the cis side of the bilayer, which was further confirmed through the MTSET chemical modification method (Fig. 4).
By establishing the directionality of the embedded channel, we are now able to compare with previous studies. A low TEA sensitivity has been reported for Kcv reconstituted into a droplet-interface bilayer (Syeda et al., 2008). In that work, Kcv was in the suspended droplet, whereas TEA was presented in the microwell on the opposite side of the bilayer. This corresponds to trans TEA or intracellular TEA in our configuration (Fig. 1 B). Indeed, the sensitivity reported in that work (70% blockage at 15 mM) is very similar to that for trans TEA (70% blockage at 19 mM) in our study (Figs. 2 and 3). Another low TEA sensitivity was reported for Kcv expressed in Xenopus laevis oocytes and studied using whole-cell recording (Gazzarrini et al., 2003). In that work, 10 mM external TEA only reduced the whole-cell current by 30–40%. This discrepancy between single-channel and whole-cell results could involve divergent mechanisms because of their different channel reconstitution systems and recording methods. However, one reason for the difference could be that TEA can increase the open probability of Kcv (Fig. S5). This effect may influence the apparent whole-cell current reduction, causing a large difference between single-channel and whole-cell measurements (Supplemental text, Interpretation of discrepancy in TEA sensitivities measured by different methods). TEA can also increase the open probability of other K+ channels such as Shaker and Kv2.1 (Choi et al., 1991; Molina et al., 1997; Andalib et al., 2004). This effect is caused by TEA slowing the C-type inactivation. It has been suggested that the C-type inactivation involves a conformational change and structural constriction at the channel’s external mouth (Liu et al., 1996). The binding of external TEA may prevent this constriction via a “foot in the door” model (Molina et al., 1997; Andalib et al., 2004) and slow the inactivation. The selectivity filter of K+ channels is also implicated in the C-type inactivation (Kiss and Korn, 1998, Kiss et al., 1999). Simulation experiments have also suggested that the filter itself acts as a gate (Bernèche and Roux, 2005) because of the rearrangement of certain residues in the filter, which strengthens the coordination with the K+ ion at a binding site. This rearrangement prevents the transition from one K+ occupying configuration to another, thereby inducing the nonconducting state (Bernèche and Roux, 2005). If such a filter gating modulates the C-type inactivation, the binding of TEA to the external mouth may affect the K+ occupancy in the selectivity filter by antagonizing the transition to the nonconducting state, thus slowing the C-type inactivation (Crouzy et al., 2001; Thompson and Begenisich, 2003). In the Kcv channel, which has a very short N terminus (Plugge et al., 2000), this C-type inactivation may play an important role in channel gating. Therefore, it would be very interesting to investigate whether Kcv undergoes the C-type inactivation through filter gating and how the binding of external TEA to L70 affects Kcv gating.
Role of Leu70 in TEA sensitivity
The large variability of TEA sensitivity among Leu70 mutants suggests that this amino acid plays a crucial role in TEA binding. However, this variability alone still lacks a solid trend that is sufficient to shed light on the binding mechanism. It has been suggested that cation-π interaction is involved in the binding of TEA to K+ channels (Heginbotham and MacKinnon, 1992; Ahern et al., 2006). However, the TEA sensitivities of our aromatic L70Y (0.74 mM) and L70F (0.86 mM) are about sevenfold lower than that of wt-Kcv with Leu70 (0.1 mM). Simulation analyses on KcsA have showed that the high affinity could be attributed to the stabilizing hydrophobic interaction, rather than the cation-π interaction, between TEA and the K+ channel (Crouzy et al., 2001; Luzhkov and Åqvist, 2001; Guidoni and Carloni, 2002). However, except wt-Kcv with Leu70, no nonpolar substitutions we made (L70M, I, V, and A) showed high TEA sensitivity. Also, there was no correlation between the residue hydrophobicity and IC50 (r2 = 0.083; Fig. S3). Many other factors such as hydration force (Crouzy et al., 2001) and van der Waals contact (Lenaeus et al., 2005) were also shown to play important roles in extracellular TEA binding. These multifactor effects on TEA binding to Kcv may be precisely dissected in the future relying on crystal structures and molecular dynamics simulation, but our comprehensive mutagenesis study will provide abundant biological data for these investigations. In the absence of compelling trends for cation-π interactions or for hydrophobic interactions, we consider the outlier Leu70 (Fig. S3) as an evolutionarily selected amino acid presented in the wt-Kcv to help the extracellular entrance of channel to adopt an optimal conformation that happens to be energetically favorable for TEA binding.
Additive contribution of subunits to TEA sensitivity
We have demonstrated a set of five heterotetramers of Kcv. Based on their quantitative analysis, each subunit in a channel contributes equal energy to the TEA binding affinity (Figs. 6 and 7). The subunit additivity mechanism has also been identified in other K+ channels such as RBK1 (Kavanaugh et al., 1992) and Shaker (Heginbotham and MacKinnon, 1992). These channels have been studied using more complicated heterochannel methods including coexpression and tandem constructs. ΔΔG for each tyrosine-containing RBK1 subunit (3.0 ± 2.0 kJ·mol−1; Kavanaugh et al., 1992) is consistent with our result for each Leu70-containing (3.7 ± 0.8 kJ·mol−1) and Tyr70-containing (2.4 ± 0.4 kJ·mol−1) Kcv subunits. However, this RBK1 result was associated with a much larger variability (1.7–5.8 kJ·mol−1), which indicates that there is some component of simultaneous interaction of TEA with multiple subunits (Kavanaugh et al., 1992). Overall, the additive regulation of TEA affinity uncovered in several K+ channels suggests a common mechanism for channel inhibition. Such additivity may also be related to the structural symmetry of inhibitors. For instance, the symmetric TEA can simultaneously bind to each subunit of a K+ channel with fourfold symmetry, whereas the asymmetric charybdotoxin can strongly bind only one subunit of Shaker channel (MacKinnon, 1991).
In summary, Kcv can be used as a model potassium channel because of its small structure and ease of channel manipulation, purification, and formation. In this work, we have identified the key amino acid involved in binding of TEA to Kcv. The defined set of five heterotetramers allows the change of the local chemical environment around the binding site in a reproducible and predictable way and therefore characterizing the additive nature of each subunit in TEA binding. Future work with this set can more precisely model this environment: fluorinated amino acids can be used to more precisely probe inhibitor binding (Ahern et al., 2006), and ab initio calculations can demonstrate more precisely the mechanical interactions in these mosaic channels. The heterochannel method can be adapted to elucidate binding mechanisms for various molecular blockers with K+ channels (Syeda et al., 2008), which has implications for both physiology research and pharmacology development. Beyond the field of physiology, the ability to continuously tune the target sensitivity by manipulating subunit composition could be useful in ion channel–based biosensors. In general, ion channels and protein pores can be engineered as biosensors for a variety of important targets (Bayley and Cremer, 2001; Gu and Shim, 2010), but they may have a limited sensitivity range. If each heterochannel has a specific sensitivity range, the group of these heterochannels as a set could be used in tandem to expand the accurate sensitivity range. As a result, this set of channels could be developed as a biosensor capable of detecting targets over a much wider concentration range than is possible with a single pore (Fig. S6 and Supplemental text, Principle of ion channel–based composite biosensors for detection of targets in a wide dynamic concentration range). In future studies, the biosensor model that has been established based on the Kcv/TEA system could be adapted for different channels and for use in the detection of various targets of interest.
Acknowledgments
We thank Drs. Tzyh-Chang Hwang, Kevin Gillis, and Mark Milanick for invaluable discussions on the experimental design and data analysis.
This investigation was partially supported by grants from the National Science Foundation (CAREER 0546165) and the National Institutes of Health (NIH; GM079613) and was conducted in a facility constructed with support from Research Facilities Improvement Program Grant Number C06-RR-016489-01 from the National Center for Research Resources, NIH.
Christopher Miller served as editor.
References
- Abenavoli A., DiFrancesco M.L., Schroeder I., Epimashko S., Gazzarrini S., Hansen U.P., Thiel G., Moroni A. 2009. Fast and slow gating are inherent properties of the pore module of the K+ channel Kcv. J. Gen. Physiol. 134:219–229 10.1085/jgp.200910266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahern C.A., Eastwood A.L., Lester H.A., Dougherty D.A., Horn R. 2006. A cation-π interaction between extracellular TEA and an aromatic residue in potassium channels. J. Gen. Physiol. 128:649–657 10.1085/jgp.200609654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andalib P., Consiglio J.F., Trapani J.G., Korn S.J. 2004. The external TEA binding site and C-type inactivation in voltage-gated potassium channels. Biophys. J. 87:3148–3161 10.1529/biophysj.104.046664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balss J., Papatheodorou P., Mehmel M., Baumeister D., Hertel B., Delaroque N., Chatelain F.C., Minor D.L., Jr, Van Etten J.L., Rassow J., et al. 2008. Transmembrane domain length of viral K+ channels is a signal for mitochondria targeting. Proc. Natl. Acad. Sci. USA. 105:12313–12318 10.1073/pnas.0805709105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley H., Cremer P.S. 2001. Stochastic sensors inspired by biology. Nature. 413:226–230 10.1038/35093038 [DOI] [PubMed] [Google Scholar]
- Bernèche S., Roux B. 2005. A gate in the selectivity filter of potassium channels. Structure. 13:591–600 10.1016/j.str.2004.12.019 [DOI] [PubMed] [Google Scholar]
- Bretschneider F., Wrisch A., Lehmann-Horn F., Grissmer S. 1999. External tetraethylammonium as a molecular caliper for sensing the shape of the outer vestibule of potassium channels. Biophys. J. 76:2351–2360 10.1016/S0006-3495(99)77392-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K.L., Aldrich R.W., Yellen G. 1991. Tetraethylammonium blockade distinguishes two inactivation mechanisms in voltage-activated K+ channels. Proc. Natl. Acad. Sci. USA. 88:5092–5095 10.1073/pnas.88.12.5092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K.L., Mossman C., Aubé J., Yellen G. 1993. The internal quaternary ammonium receptor site of Shaker potassium channels. Neuron. 10:533–541 10.1016/0896-6273(93)90340-W [DOI] [PubMed] [Google Scholar]
- Crouzy S., Bernèche S., Roux B. 2001. Extracellular blockade of K+ channels by TEA: results from molecular dynamics simulations of the KcsA channel. J. Gen. Physiol. 118:207–218 10.1085/jgp.118.2.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzarrini S., Etten J.L., DiFrancesco D., Thiel G., Moroni A. 2002. Voltage-dependence of virus-encoded miniature K+ channel Kcv. J. Membr. Biol. 187:15–25 10.1007/s00232-001-0147-5 [DOI] [PubMed] [Google Scholar]
- Gazzarrini S., Severino M., Lombardi M., Morandi M., DiFrancesco D., Van Etten J.L., Thiel G., Moroni A. 2003. The viral potassium channel Kcv: structural and functional features. FEBS Lett. 552:12–16 10.1016/S0014-5793(03)00777-4 [DOI] [PubMed] [Google Scholar]
- Gebhardt M., Hoffgaard F., Hamacher K., Kast S.M., Moroni A., Thiel G. 2011. Membrane anchoring and interaction between transmembrane domains are crucial for K+ channel function. J. Biol. Chem. 286:11299–11306 10.1074/jbc.M110.211672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu L.Q., Shim J.W. 2010. Single molecule sensing by nanopores and nanopore devices. Analyst (Lond.). 135:441–451 10.1039/b907735a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidoni L., Carloni P. 2002. Tetraethylammonium binding to the outer mouth of the KcsA potassium channel: implications for ion permeation. J. Recept. Signal Transduct. Res. 22:315–331 10.1081/RRS-120014604 [DOI] [PubMed] [Google Scholar]
- Heginbotham L., MacKinnon R. 1992. The aromatic binding site for tetraethylammonium ion on potassium channels. Neuron. 8:483–491 10.1016/0896-6273(92)90276-J [DOI] [PubMed] [Google Scholar]
- Heginbotham L., LeMasurier M., Kolmakova-Partensky L., Miller C. 1999. Single streptomyces lividans K(+) channels: functional asymmetries and sidedness of proton activation. J. Gen. Physiol. 114:551–560 10.1085/jgp.114.4.551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M., Moroni A., Gazzarrini S., DiFrancesco D., Thiel G., Severino M., Van Etten J.L. 2004. Small potassium ion channel proteins encoded by chlorella viruses. Proc. Natl. Acad. Sci. USA. 101:5318–5324 10.1073/pnas.0307824100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanaugh M.P., Hurst R.S., Yakel J., Varnum M.D., Adelman J.P., North R.A. 1992. Multiple subunits of a voltage-dependent potassium channel contribute to the binding site for tetraethylammonium. Neuron. 8:493–497 10.1016/0896-6273(92)90277-K [DOI] [PubMed] [Google Scholar]
- Kiss L., Korn S.J. 1998. Modulation of C-type inactivation by K+ at the potassium channel selectivity filter. Biophys. J. 74:1840–1849 10.1016/S0006-3495(98)77894-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss L., LoTurco J., Korn S.J. 1999. Contribution of the selectivity filter to inactivation in potassium channels. Biophys. J. 76:253–263 10.1016/S0006-3495(99)77194-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenaeus M.J., Vamvouka M., Focia P.J., Gross A. 2005. Structural basis of TEA blockade in a model potassium channel. Nat. Struct. Mol. Biol. 12:454–459 10.1038/nsmb929 [DOI] [PubMed] [Google Scholar]
- Liu Y., Jurman M.E., Yellen G. 1996. Dynamic rearrangement of the outer mouth of a K+ channel during gating. Neuron. 16:859–867 10.1016/S0896-6273(00)80106-3 [DOI] [PubMed] [Google Scholar]
- Luzhkov V.B., Åqvist J. 2001. Mechanisms of tetraethylammonium ion block in the KcsA potassium channel. FEBS Lett. 495:191–196 10.1016/S0014-5793(01)02381-X [DOI] [PubMed] [Google Scholar]
- MacKinnon R. 1991. Determination of the subunit stoichiometry of a voltage-activated potassium channel. Nature. 350:232–235 10.1038/350232a0 [DOI] [PubMed] [Google Scholar]
- MacKinnon R., Yellen G. 1990. Mutations affecting TEA blockade and ion permeation in voltage-activated K+ channels. Science. 250:276–279 10.1126/science.2218530 [DOI] [PubMed] [Google Scholar]
- Meuser D., Splitt H., Wagner R., Schrempf H. 1999. Exploring the open pore of the potassium channel from Streptomyces lividans. FEBS Lett. 462:447–452 10.1016/S0014-5793(99)01579-3 [DOI] [PubMed] [Google Scholar]
- Meuser D., Splitt H., Wagner R., Schrempf H. 2001. Mutations stabilizing an open conformation within the external region of the permeation pathway of the potassium channel KcsA. Eur. Biophys. J. 30:385–391 10.1007/s002490100147 [DOI] [PubMed] [Google Scholar]
- Molina A., Castellano A.G., López-Barneo J. 1997. Pore mutations in Shaker K+ channels distinguish between the sites of tetraethylammonium blockade and C-type inactivation. J. Physiol. 499:361–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliuca C., Goetze T.A., Wagner R., Thiel G., Moroni A., Parcej D. 2007. Molecular properties of Kcv, a virus encoded K+ channel. Biochemistry. 46:1079–1090 10.1021/bi061530w [DOI] [PubMed] [Google Scholar]
- Plugge B., Gazzarrini S., Nelson M., Cerana R., Van Etten J.L., Derst C., DiFrancesco D., Moroni A., Thiel G. 2000. A potassium channel protein encoded by chlorella virus PBCV-1. Science. 287:1641–1644 10.1126/science.287.5458.1641 [DOI] [PubMed] [Google Scholar]
- Shim J.W., Yang M., Gu L.Q. 2007. In vitro synthesis, tetramerization and single channel characterization of virus-encoded potassium channel Kcv. FEBS Lett. 581:1027–1034 10.1016/j.febslet.2007.02.005 [DOI] [PubMed] [Google Scholar]
- Syeda R., Holden M.A., Hwang W.L., Bayley H. 2008. Screening blockers against a potassium channel with a droplet interface bilayer array. J. Am. Chem. Soc. 130:15543–15548 10.1021/ja804968g [DOI] [PubMed] [Google Scholar]
- Tan Q., Shim J.W., Gu L.Q. 2010. Separation of heteromeric potassium channel Kcv towards probing subunit composition-regulated ion permeation and gating. FEBS Lett. 584:1602–1608 10.1016/j.febslet.2010.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayefeh S., Kloss T., Kreim M., Gebhardt M., Baumeister D., Hertel B., Richter C., Schwalbe H., Moroni A., Thiel G., Kast S.M. 2009. Model development for the viral Kcv potassium channel. Biophys. J. 96:485–498 10.1016/j.bpj.2008.09.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel G., Baumeister D., Schroeder I., Kast S.M., Van Etten J.L., Moroni A. 2011. Minimal art: or why small viral K(+) channels are good tools for understanding basic structure and function relations. Biochim. Biophys. Acta. 1808:580–588 10.1016/j.bbamem.2010.04.008 [DOI] [PubMed] [Google Scholar]
- Thompson J., Begenisich T. 2003. External TEA block of shaker K+ channels is coupled to the movement of K+ ions within the selectivity filter. J. Gen. Physiol. 122:239–246 10.1085/jgp.200308848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Xie S., Sun B. 2011. Viral proteins function as ion channels. Biochim. Biophys. Acta. 1808:510–515 10.1016/j.bbamem.2010.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]