Abstract
Recent work has identified Behavioral Approach System (BAS) sensitivity as a risk factor for the first onset and recurrence of mood episodes in bipolar disorder, but little work has evaluated risk factors for the prospective development of hypomanic symptoms in individuals at risk for, but without a history of, bipolar disorder. The present study used a prospective behavioral high-risk design to evaluate the impact of positive overgeneralization, a cognitive correlate of risk for hypomania, on hypomanic symptoms in individuals with high vs. moderate BAS sensitivity, but without a history of mood elevation. Hierarchical linear regressions indicated that upwards positive overgeneralization and BAS sensitivity interacted to predict increased levels of hypomanic symptoms at follow-up, controlling for initial hypomanic symptoms. The pattern of this interaction was such that positive overgeneralization predicted higher levels of hypomanic symptoms among high-BAS, but not moderate-BAS, individuals. Thus, the self-reported tendency to experience grandiose increases in confidence following success may confer additional risk for mood elevation among individuals already at risk for developing bipolar disorder. Potential implications for prevention and treatment are discussed.
Keywords: Generalization, Cognitive Style, Goal-Striving, Behavioral Approach System, Bipolar Disorder, Hypomania, Vulnerability
Bipolar disorder (BD) can be a severe and chronic mood disorder that is characterized by alternating periods of depression and mood elevation or irritability. Bipolar disorders (BDs) encompass a spectrum of severity, with milder forms sometimes progressing to more severe BDs (e.g., Akiskal, Djenderedjian, Rosenthal, & Khani, 1977; Alloy et al., in press-a; Birmaher et al., 2009). The milder cyclothymic disorder and bipolar II disorder may be characterized by episodes of hypomania, an elevated mood state characterized by persistent elevated or irritable mood, but without functional impairment, whereas full-blown bipolar I disorder is characterized by significantly impairing manic episodes involving more pervasive expansive or irritable mood. BDs occur in approximately 4.4% of the population (Merikangas et al., 2007) and are associated with impairment in many areas of functioning, including poorer academic and work achievement, divorce, substance abuse, and suicide (Angst, Stassen, Clayton, & Angst, 2002; Dilsaver, 2009; Goodwin & Jamison, 2007; Grant et al., 2004; Kessler et al., 2006; Nusslock, Alloy, Abramson, Harmon-Jones, & Hogan, 2008). Onset of BD is generally seen between the ages of 15 and 19 (Alloy, Abramson, Walshaw, Keyser, & Gerstein, 2006a; Burke, Burke, Regier, & Rae, 1990; Kennedy et al., 2005; Kessler, Rubinow, Holmes, Abelson, & Zhao, 1997), with earlier onset predicting a greater severity of illness and a more unrelenting course (Alloy et al., in press-a; Perlis et al., 2004). Thus, it is important to identify those at risk for developing BDs, so that interventions may be designed and implemented to prevent onset or improve prognosis.
One recent theory that helps to account for risk for BDs is the Behavioral Approach System (BAS) hypersensitivity model (Alloy & Abramson, 2010; Alloy, Abramson, Urosevic, Bender & Wagner, 2009; Depue, Krauss & Spoont, 1987; Depue & Iacono, 1989; Johnson, 2005; Urosevic, Abramson, Harmon-Jones, & Alloy, 2008). The BAS is a motivational system theorized to regulate approach behavior towards goals and rewards (Gray, 1991). The BAS hypersensitivity model proposes that individuals with BDs have an overly sensitive BAS that leads to the development of hypomanic or manic symptoms when the BAS is activated, as well as depressive symptoms when the BAS is deactivated (Alloy & Abramson, 2010; Alloy et al., 2009; Urosevic et al., 2008). Activation of the BAS is theorized to occur in response to events involving goal-striving or attainment, and extreme or prolonged activation may lead to symptoms of mood elevation and potentially to hypomanic or manic episodes (Depue & Iacono, 1989; Fowles, 1993; Urosevic et al., 2008). Deactivation of the BAS is theorized to occur in response to cues of irreconcilable failure to achieve goals, leading to depressive symptoms such as low mood, anhedonia, lack of energy, and hopelessness (Depue et al., 1987; Fowles, 1988, 1993; Urosevic et al., 2008). People without a hypersensitive BAS are expected to report normal, transient responses to stimuli involving achievement or failure (Urosevic et al., 2008). However, individuals with BD who have this BAS vulnerability are overly responsive to these cues, resulting in the approach system becoming excessively activated or deactivated in such situations. For example, individuals with high BAS sensitivity are highly responsive to rewards (e.g., Depue & Collins, 1999; Gray, 1994), which results in increased motor behavior, positive goal-striving emotions, and incentive-reward motivation, characteristics that parallel hypomania and mania. In sum, individuals with BAS hypersensitivity are prone to experiencing mood elevation in response to BAS-activating events, and to experiencing depressed mood in response to BAS-deactivating events, fluctuations in mood that are characteristic of BD.
Recent findings have been supportive of the BAS model of BDs. Alloy et al. (2006b) found that individuals high in BAS sensitivity were six times more likely to have a lifetime bipolar spectrum disorder diagnosis, and were more prone to experiencing hypomanic symptoms, than were individuals with moderate BAS sensitivity. Similarly, Salavert et al. (2007) found that individuals with bipolar I disorder had higher levels of BAS sensitivity than did healthy control participants, even after controlling for concurrent mood symptoms. BAS hypersensitivity has also been found to prospectively predict a shorter time to onset of hypomanic and manic episodes across an average of 33 months of follow-up (Alloy et al., 2008) and to prospectively predict a greater likelihood of progression to bipolar I disorder among individuals with less severe disorders in the bipolar spectrum (Alloy et al., in press-a). In another prospective study, individuals with high BAS sensitivity were more likely to experience a first onset of a BD compared to those with moderate BAS sensitivity (Alloy et al., in press-b). Altogether, much evidence supports the BAS hypersensitivity model of BD.
Although the BAS hypersensitivity theory has been useful in understanding BD, identifying additional factors that contribute to the development of hypomanic symptoms would add to the ability to predict which individuals may be at risk for developing BD. Hypomania is characteristic of the less severe BDs and distinguishes BDs from unipolar depressive disorders. Furthermore, understanding what factors confer risk to hypomania may help us better understand the roots of manic symptoms, as the milder forms of BDs often progress to the more severe (e.g., Alloy et al., in press-a). Several cognitive correlates of hypomanic symptoms that are relevant to the BAS have been noted in individuals with BDs and among those at risk for BDs, fitting with the goal-directed nature of hypomania. In particular, several studies have shown that BD tends to be characterized by setting overly ambitious goals and striving for success (Alloy et al., 2009b; Carver & Johnson, 2009; Gruber & Johnson, 2009; Johnson & Carver, 2006; Johnson & Jones, 2009; Johnson, Ruggero, & Carver, 2005; Meyer & Krumm-Merabet, 2003; for a review, see Johnson, 2005). When individuals with BD experience success, they may also experience positive mood and robust increases in confidence. In combination with high trait goal-striving, this likely contributes to excessive pursuit of goals and may precipitate an escalation into mania (Johnson, 2005). Studies have found that those with bipolar I disorder, even while euthymic, have higher trait levels of goal setting, and place higher values on achieving goals than do healthy controls (Lam, Wright, & Smith, 2004; Meyer, Johnson, & Winters, 2001; Scott, Stanton, Garland, & Ferrier, 2000; Spielberger, Parker, & Becker, 1963), and these findings have been replicated among individuals at risk for BD (Johnson & Carver, 2006).
Individuals with BD also may maintain positive emotions by reflecting on their positive thoughts and moods, an effect often called basking (Segerstrom et al., 2003). More specifically, researchers have found that individuals with BDs (Johnson et al., 2008) as well as those at risk for developing BDs (Feldman, Joormann, & Johnson, 2008) are significantly more likely to respond to positive affect with additional thoughts about their own positive qualities, previous positive experiences, and positive circumstances (termed rumination on positive affect). Furthermore, trait-like levels of positive emotionality and rumination on positive affect have been shown to be correlated with lifetime mania frequency (Gruber, Eidelman, Johnson, Smith, & Harvey, 2011). Similarly, whereas it has been theorized that most individuals tend to reduce effort after progress towards a goal exceeds expectations (Carver & Scheier, 1998; Carver, 2003), individuals with BDs have been shown to be less likely to “coast,” and instead actually increase their efforts towards said goal (Fulford, Johnson, Llabre, & Carver, 2010). It is theorized that by increasing actions directed towards goals, these individuals are able to experience increasing amounts of confidence; hence, their lack of goal coasting may be considered another form of basking because strategies are employed in order to maintain positive emotions. Although the lack of goal coasting in BDs needs further investigation, the authors suggested that increases in efforts towards a goal may underlie the goal-directed activity seen in hypomania (Fulford et al., 2010; see Johnson, 2005 for a review). In sum, these forms of basking are thought to be emotion regulation strategies designed to maintain or enhance positive mood states. Positive overgeneralization may be conceived as a cognitive characteristic that results in positive affect maintenance and also leads to increased goal-striving across domains of life following initial perceptions of success.
In addition to engaging in ambitious goal-striving, individuals at risk for BD, as well as those with a BD history, are also thought to have a tendency to respond to success with excessive confidence, and to generalize from the causes of positive events to broader aspects of life (Eisner, Johnson, & Carver, 2008). This positive overgeneralization (POG) is thought to be the positive complement to the negative overgeneralization that is often seen in depressive disorders (Eisner et al., 2008), and may lead to higher levels of goal pursuit or higher aspirations, as is often seen in BD (Johnson, 2005; Johnson & Carver, 2006; Johnson, Eisner, & Carver, 2009). To date, all published reports of POG have been cross-sectional (Eisner et al., 2008; Johnson & Jones, 2009; Carver, Sinclair, & Johnson, 2010; Fulford, Johnson, & Carver, 2008). Although these studies have shown that POG reliably correlates with hypomanic personality, it cannot be concluded from such designs whether elevations on POG confer risk for the development of hypomanic symptoms prospectively.
One of the best study designs to evaluate whether hypothesized risk factors confer vulnerability for the development of mental disorders is the prospective behavioral high-risk design (Riskind & Alloy, 2006). In this design, because behavioral vulnerabilities are thought to create liabilities to mental health problems, participants are recruited who do not meet criteria for the disorder in question but do exhibit the related vulnerabilities (Alloy, Abramson, Raniere, & Dyller, 1999; Just, Abramson, & Alloy, 2001). These high-risk participants, as well as a comparison group of individuals who do not show such vulnerabilities, are then followed prospectively (e.g., Alloy et al., 2006b). In this way, researchers are able to determine whether the vulnerability does confer higher risk to the disorder in question (e.g., BD), without contamination by the condition if it were to exist already. Indeed, research has suggested that mood episodes may confer cognitive “scars” and change individuals’ cognition and responses to future changes in affect (Lewinsohn, Steinmetz, Larson, & Franklin, 1981). In studies of risk factors for mania in participants who are in remission from BD, for example, it is impossible to determine whether the risk factors were present prior to the onset of the disorder, or are consequences of the disorder (Just et al., 2001). It is also important to determine what risk factors are involved in the initial development of BD so that early intervention or prevention approaches can be formed.
The Present Study
The present study employed a prospective behavioral high-risk design, using BAS sensitivity to select groups hypothesized to be at high versus low risk for BD. We evaluated the effect of POG on prospective levels of hypomanic symptoms, controlling for initial hypomanic symptoms. We hypothesized that higher levels of POG would predict higher levels of hypomanic symptoms in the group at high risk, but not low risk, for BD. Thus, the present study integrated the BAS and POG models of risk for BD, and evaluated whether higher levels of both characteristics conferred greatest risk for prospective symptoms of hypomania.
Method
Participants and Procedures
Participants were selected based on a two-phase screening procedure from the greater Philadelphia area. In the first phase of screening, 9,991 students (14-19 year olds) from Philadelphia School District public high schools and Philadelphia universities completed the Behavioral Inhibition System/Behavioral Activation System (BIS/BAS) Scales (Carver & White, 1994) and the Sensitivity to Punishment and Sensitivity to Reward Questionnaire (SPSRQ; Torrubia, Avila, Molto, & Caseras, 2001). Students scoring in the top 15th percentile on both the BAS-Total (BAS-T) of the BIS/BAS Scales and the Sensitivity to Reward Scale (SR) of the SPSRQ composed the high BAS (HBAS) group and those who scored between the 40th and 60th percentiles on both of these measures composed the moderate BAS (MBAS) group. The final sample was representative of adolescents ages 14-19 in the Philadelphia area on a number of demographic characteristics, including race, sex, and age.
At the second phase of screening, parents provided written consent and adolescents provided written assent for those under the age of 18 and participants 18 or older completed their own written consent. A random subset of HBAS and MBAS participants (n = 390) were invited to participate in the Phase II screening and were administered the mood and psychosis sections of an expanded Schedule for Affective Disorders and Schizophrenia—Lifetime (exp-SADS–L; Endicott & Spitzer, 1978) diagnostic interview by interviewers blind to participants’ BAS risk group status. Participants also completed the Beck Depression Inventory (BDI; Beck, Rush, Shaw, & Emery, 1979) to assess depressive symptoms and the Altman Self-Rating Mania Scale (ASRM; Altman, Hedeker, Peterson, & Davis, 1997) to assess hypomanic symptoms. Participants were excluded from the final sample if they met Diagnostic and Statistical Manual of Mental Disorders 4th ed. (DSM–IV-TR; American Psychiatric Association, 2000) and/or Research Diagnostic Criteria (RDC; Spitzer, Endicott, & Robins, 1978) criteria for any bipolar spectrum diagnosis (bipolar I, II, or cyclothymia) or a hypomanic or manic episode prior to the date of participation in Phase I of the study, but were not excluded based on past history of depressive episodes if there was no history of hypomanic or manic episodes. In addition, participants with a lifetime history of any psychotic disorder or who were not fluent in English were also excluded. Of the students who completed Phase II, 22 participants were excluded on the basis of meeting criteria for bipolar spectrum disorder or hypomanic episode(s) prior to completing Phase I, 7 were excluded due to history of psychotic disorder, and 5 were excluded due to poor English ability. Furthermore, 66 eligible participants had not yet completed baseline assessments, and thus, were not included due to incomplete information.
After completing the screening processes, participants came in for an initial session and completed further interviews and questionnaires. At Time 1, participants completed the remainder of the SADS-L, as well as several self-report measures described below. After completing the Time 1 assessment, participants were followed prospectively with an average of 263 days (SD = 93 days; median = 230 days) between assessments. At follow-up, participants completed self-report measures of symptom levels. The final sample for the present analyses consisted of 99 HBAS and 75 MBAS participants who completed the Time 1 assessment and at least one regular prospective assessment of the longitudinal study. The HBAS and MBAS groups did not differ on the basis of age, gender, or ethnicity (see Table 1). The HBAS group scored higher than the MBAS group on BAS and SR scores, which was expected due to their classification. However, the groups did not differ on BIS or Sensitivity to Punishment (SP) scores. This pattern of results was consistent with previous literature suggesting that risk for mood elevation is associated uniquely with BAS and reward sensitivity (e.g., Alloy et al., 2006b; Harmon-Jones & Allen, 1997; Meyer, Johnson, & Carver, 1999). Finally, the two groups did not differ on Time 1 BDI scores, but did differ on Time 1 ASRM scores. Mean BIS/BAS, SPSRQ, BDI and ASRM scores by group are presented in Table 1.
Table 1.
Demographic Characteristics of the Study Sample
| High BAS (N = 99) |
Moderate BAS (N = 75) |
t / χ2 | |
|---|---|---|---|
| Age | 18.15 (1.58) | 17.99 (1.56) | 0.57 |
| Sex | 62.3% Female | 72.0% Female | 1.69 |
| Race | 55.6% Caucasian | 47.2% Caucasian | 0.64 |
| 21.2% African American | 36.1% African American | ||
| 14.1% Asian/Pacific Islander | 13.9% Asian/Pacific Islander | ||
| 2.0% Biracial | 0.0% Biracial | ||
| 4.0% Other | 4.7% Other | ||
| Ethnicity | 8.1% Hispanic/Latino | 5.6% Hispanic/Latino | 0.64 |
| BIS | 20.01 (4.17) | 19.46 (2.90) | 1.02 |
| BAS-Total | 46.00 (2.73) | 38.13 (0.87) | 25.98*** |
| BAS-Drive | 13.36 (1.72) | 10.40 (1.39) | 12.42*** |
| BAS-Fun | 13.90 (1.56) | 11.29 (1.61) | 10.71*** |
| BAS-Reward | 18.70 (1.17) | 16.44 (1.51) | 10.75*** |
| SPSRQ-SR | 8.47 (1.21) | 5.08 (1.47) | 15.98*** |
| ASRM (Time 1) | 6.60 (4.11) | 5.11 (3.35) | 2.63** |
| ASRM (Follow- up) |
5.90 (3.95) | 3.95 (3.43) | 3.48** |
p < .05;
p < .01;
p < .001.
Note. Standard deviations are in parentheses. BIS = Behavioral Inhibition System scores from the BIS/BAS Scales; BAS-T = Behavioral Approach System – Total scores from the BIS/BAS Scales; SPSRQ-SP = Sensitivity to Punishment and Sensitivity to Reward Questionnaire - Sensitivity to Punishment Subscale; SPSRQ-SR = Sensitivity to Punishment and Sensitivity to Reward Questionnaire - Sensitivity to Reward Subscale; BDI = Beck Depression Inventory; ASRM = Altman Self-Rating Mania Scale.
Measures
Behavioral Approach System sensitivity
As described, the BIS/BAS Scale (Carver & White, 1994) was used to select the HBAS and MBAS groups. The BIS/BAS Scale is a widely used self-report questionnaire that assesses individual differences in trait sensitivity to threats and rewards. Participants were asked to respond to questions on a 4-point Likert scale ranging from strongly disagree to strongly agree. The BAS-Total (BAS-T) score, which we used to select the HBAS and MBAS groups, is the sum of all the BAS items. Internal consistencies (α’s = .66-.76) and test-retest reliabilities (r’s = .59-.69) for the subscales have been found to be satisfactory (Carver & White, 1994). In the current study, the BIS/BAS Scale was given at Phase I, and the BAS-T demonstrated good internal consistency (α = .80).
As described, in addition to the BIS/BAS Scales, the SPSRQ (Torrubia et al., 2001) was used to select the HBAS and MBAS groups. This measure was used in conjunction with the BIS/BAS Scale to improve the theoretical consistency with Gray’s BIS/BAS theory, construct validity, and address weaknesses in the BIS/BAS Scale’s item content. The SPSRQ is composed of two subscales, sensitivity to reward (SR) and sensitivity to punishment (SP) with 24 “yes” or “no” questions in each. Both subscales have demonstrated good internal consistency and retest reliability (Torrubia et al., 2001). In addition, research supports the construct validity of both the BIS/BAS and SPRSQ measures (Harmon-Jones & Allen, 1997; Heponiemi, Keltikangas-Jarvinen, Kettunen, Puttonen, & Ravaja, 2004; Kambouropoulos & Staiger, 2004). In the current study, the SR and SP scales demonstrated good internal consistency with α’s = .76 and .84, respectively, and the SR subscale was correlated with the BAS-T (r = .40, p < .01) in our Phase I sample.
Positive overgeneralization
The Positive Overgeneralization scale (POG; Eisner et al., 2008) was used to assess the tendency to overgeneralize from a given successful experience to broader aspects of life. On the 18-item self-report questionnaire, participants are asked to respond to items by indicating if they agree or disagree with the statements, with possible responses ranging from 1 (I disagree with the statement a lot) to 4 (I agree with the statement a lot). The scale is composed of three subscales: Lateral generalization (LG) from a good outcome in one domain to positive outcomes in other areas of life (e.g., “When something good happens to me, it makes me expect good things in other parts of my life too”); Upward generalization (UG) to more lofty goals in the same domain (e.g., “When one thing goes right, it makes me feel my possibilities are limitless”); and Social generalization (SG; e.g., “When someone compliments me about something I’ve said, it makes me think about impressing lots of other people”). Internal consistencies for the subscales in a previous study ranged from α = .51 (SG) to .82 (LG; Eisner et al., 2008). In the present study, the POG was given at Time 1 with internal consistencies of α =.70 (LG), α = .69 (UG), and α = .70 (SG).
Hypomanic symptoms
The Altman Self-Rating Mania Scale (ASRM; Altman et al., 1997) was used to assess symptoms of hypomania. The ASRM is a 5-item self-report questionnaire that assesses symptoms such as inflated self-confidence, talkativeness, elation, reduced need for sleep, and excessive activity. Participants read a group of statements and choose the statement that best describes them in the last month on a 5-point Likert scale. In a factor analysis, the ASRM items loaded onto a single factor (Altman et al., 1997) and total scores demonstrated high correlation with both clinical interviews and other measures of mania (Altman, Hedeker, Peterson, & Davis, 2001). In the current study, the ASRM was given at Phase II and at follow-up and demonstrated good internal consistency at both timepoints, α = .75 and .76, respectively. The ASRM has been used successfully to assess symptoms of mania and hypomania in individuals with bipolar spectrum disorders (e.g., Savitz, van der Merwe, & Ramesar, 2008).
Results
Table 2 displays bivariate correlations between BAS risk group, BAS-T, SR, POG subscales, and ASRM scores at Time 1 and follow-up. BAS risk group was positively correlated with each of the POG subscales and with hypomanic symptoms, indicating that the HBAS group had higher levels of POG and hypomanic symptoms than did the MBAS group. Each POG subscale was also positively correlated with hypomanic symptoms at both timepoints.
Table 2.
Correlations Between All Primary Variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|
| 1 POG LG | - | .46*** | .56*** | .17* | .13 | .16* | .22** | .29*** |
| 2 POG UG | - | .62*** | .17* | .17* | .21** | .28*** | .20** | |
| 3 POG SG | - | .30*** | .26** | .33*** | .19* | .19* | ||
| 4 BAS Total | - | .73*** | .87*** | .16* | .23** | |||
| 5 SPSRQ-SR | - | .78*** | .20** | 0.14 | ||||
| 6 Risk Group | - | .19* | .25** | |||||
| 7 ASRM - Time 1 | - | .33*** | ||||||
| 8 ASRM - Follow-Up | - |
p < .05;
p < .01;
p < .001.
Note. POG LG = Positive Overgeneralization Lateral Generalization subscale; POG UG = Positive Overgeneralization Upward Generalization subscale; POG SG = Positive Overgeneralization Social Generalization subscale; BAS = Behavioral Approach System risk group; SPSRQ-SR = Sensitivity to Punishment and Sensitivity to Reward Questionnaire - Sensitivity to Reward Subscale; Risk Group = BAS risk group based on high (1) versus moderate (0) BAS sensitivity; ASRM = Altman Self-Rating Mania Scale score.
To evaluate whether POG, in interaction with BAS risk group, would predict hypomanic symptoms at follow-up, controlling for initial hypomanic symptoms, we conducted hierarchical linear regressions. Following the procedures outlined by Aiken and West (1991), POG subscales were centered at their means prior to analysis, and BAS risk group was dummy coded, with 1 indicating the HBAS group and 0 indicating the MBAS group. To test our hypotheses, we regressed a number of predictor variables on follow-up ASRM scores (see Table 3). In Step 1, Time 1 ASRM score was entered, thus creating a residual score reflecting change in hypomanic symptoms from Time 1 to follow-up. In Step 2, main effects of a POG subscale and BAS risk group were entered. Finally, in Step 3, the interaction term between the POG subscale and BAS risk group was entered.1
Table 3.
Hierarchical Linear Regressions of BAS Risk Group and POG Subscales Predicting Hypomanic Symptoms at Follow-Up, Controlling for Time 1 Hypomanic Symptoms
| POG LG |
POG UG |
POG SG |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ß | t | ß | t | ß | t | ß | t | ß | t | ß | t | |
| T1 ASRM | 0.26 | 3.63*** | 0.25 | 3.50** | 0.29 | 3.9*** | 0.29 | 3.90*** | 0.29 | 4.02*** | 0.28 | 3.86*** |
| BAS | 1.21 | 2.20* | 1.25 | 2.26* | 1.32 | 2.34* | 1.44 | 2.56* | 1.22 | 2.08* | 1.41 | 2.36* |
| POG LG | 0.22 | 2.95** | 0.14 | 1.11 | ||||||||
| POG LG x BAS | 0.13 | 0.82 | ||||||||||
| POG UG | 0.08 | 1.19 | −0.12 | −0.93 | ||||||||
| POG UG x BAS | 0.30 | 2.70* | ||||||||||
| POG SG | 0.08 | 1.06 | −0.09 | −0.73 | ||||||||
| POG SG x BAS | 0.25 | 1.60 | ||||||||||
| Intercept | 2.80*** | 2.78*** | 2.58*** | 2.41*** | 2.60*** | 2.39*** | ||||||
|
| ||||||||||||
| R2 | .191 | .194 | .163 | .184 | .154 | .167 | ||||||
p< .001,
p< .01,
p< .05.
Note. T1 ASRM = Time 1 Altman Self-Rating Mania Scale; BAS = Behavioral Approach System risk group; POG LG = Positive Overgeneralization Lateral Generalization subscale; POG UG = Positive Overgeneralization Upward Generalization subscale; POG SG = Positive Overgeneralization Social Generalization subscale. In each analysis, T1 ASRM was entered as Step 1, the main effects of BAS and POG Subscale were entered as predictors in Step 2, and the cross-product was entered in Step 3. Each model is presented first with only the main effects entered as predictors in Step 2, and subsequently with the cross-product entered in Step 3.
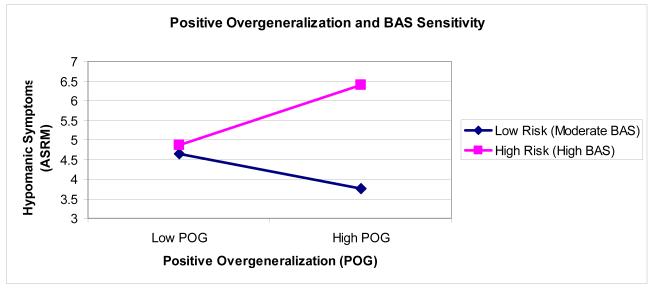
POG Upward Generalization (UG) interacted with BAS risk group significantly to predict ASRM at follow-up, controlling for Time 1 ASRM (see Table 3). To probe the form of the interaction (Figure 1), we tested the simple slopes of POG UG and BAS at one standard deviation above and below the mean of the other variable (Aiken & West, 1991). Consistent with our hypotheses, among HBAS individuals, there was a significant effect of POG UG, t = 2.20, p = .03, such that higher POG UG predicted higher levels of hypomanic symptoms at follow-up. Among MBAS individuals, there was not a significant effect of POG UG, t = −0.93, p = .36. Additionally, at higher levels of POG UG, there was a significant effect of BAS risk group, t = 3.14, p < .005, such that HBAS individuals experienced greater levels of hypomanic symptoms at follow-up than did MBAS individuals. At lower levels of POG UG, there was not a significant effect of BAS risk group, t = 0.30, p = .76. There was also a significant main effect of BAS risk group, such that the HBAS group experienced greater increases in hypomanic symptoms than the MBAS group, t = 2.56, p = .01. Neither POG Lateral Generalization (LG) nor POG Social Generalization interacted significantly with BAS risk group to predict ASRM at follow-up, controlling for Time 1 ASRM. However, there was a significant main effect of POG LG, indicating that individuals with higher levels of lateral generalization experienced greater increases in hypomanic symptoms than did individuals with lower lateral generalization. Additionally, there were significant main effects of BAS risk group in all regressions, such that the HBAS group experienced greater increases in hypomanic symptoms than did the MBAS group (see Table 3).
Figure 1.
Interaction between positive overgeneralization and BAS sensitivity predicting follow-up hypomanic symptoms, controlling for Time 1 hypomanic symptoms.
Discussion
Consistent with our hypotheses, BAS sensitivity significantly interacted with POG to predict increases in hypomanic symptoms at follow-up, controlling for Time 1 hypomanic symptoms. Specifically, high-BAS, but not moderate-BAS, individuals with higher levels of upward overgeneralization experienced increases in hypomanic symptoms across the study. As hypothesized by the BAS hypersensitivity theory of BD, high BAS participants also experienced greater increases in hypomanic symptoms than did moderate BAS participants. Taken together, these findings suggest that among individuals already at risk for BD due to high BAS sensitivity, positive overgeneralization may confer additional risk for hypomania.
The results of the present study are consistent with previous work evaluating positive overgeneralization. The POG Upward Generalization scale measures an individual’s self-reported tendency towards grandiose increases in confidence following small successes. Eisner et al. (2008) found that this subscale was more predictive of risk for hypomania than the two other POG subscales, Lateral Generalization (the tendency to generalize positive outcomes in one domain to positive outcomes in other domains) and Social Generalization (the tendency to expect broad positive social gains following small positive social experiences). Consistent with these findings, our prospective results indicated that the POG Upward scale was the only subscale that interacted with BAS risk group to predict hypomanic symptoms. It may be that having a dispositional tendency to generalize in a grandiose manner (far exceeding what is realistic given the magnitude of success) may be a risk factor for hypomania among individuals already at high risk. Eisner et al. (2008) hypothesized that upward positive overgeneralization may be associated with aspirations to popular fame and increased lofty goal setting, both of which characterize individuals with bipolar disorder. It also may be the case that upward positive overgeneralization is less realistic and less adaptive than other types of overgeneralization and thus may be more highly predictive of clinically significant outcomes. Although POG Social Generalization scores were higher in the high-BAS group, they did not predict increases in hypomanic symptoms in interaction with BAS group. It may be that the generalization captured by the Social subscale is less suggestive of cognitive potential for hypomanic activation than is the Upward Generalization subscale. It is also possible that there was less power to detect an interaction between BAS and POG Social Generalization because there is greater overlap between these two measures than there is between BAS and the other two POG subscales (Table 2). There was also some evidence that POG Lateral Generalization predicted increases in hypomanic symptoms, but this finding was not specific to the high-BAS group. This suggests that the tendency to generalize from a good outcome in one domain to positive outcomes in other areas of life may also contribute to the development of symptoms of mood elevation.
These findings are also consistent with previous research that has demonstrated that individuals at risk for hypomania may have an overly positive cognitive style (Jones, Mansell, & Waller, 2006; Lam et al., 2005) and experience increases in self-confidence and goal-setting in response to success (Eisner et al., 2008; Johnson et al., 2005). Additionally, individuals with BD, as well as those who are at risk for BD, have been shown to have a tendency towards an overly positive appraisal of one’s self and one’s mood states (Jones et al., 2006; Jones & Day, 2008), which is associated with current hypomanic symptoms (Mansell, Rigby, Tai, & Lowe, 2008). Among those at risk for developing hypomania, an initial success or positive mood may induce a positive appraisal of self and increased self-confidence. This, in turn, may increase expectancies for future successes, which may be manifested in positive overgeneralization and in extreme cases, grandiosity. Johnson (2005) suggested that individuals with vulnerability to hypomania may not only expect greater achievement following an initial success, but consequently, may also be more likely to pursue more numerous and difficult goals with decreased attention to potential risks. Increased goal setting and pursuit may translate into hypomanic symptoms such as increased activity and loss of sleep, which, in turn, may amplify other symptoms of mania or hypomania, leading to diagnosable episodes of mood elevation (Johnson, 2005).
Additional research has investigated the interaction of positive cognitive styles and life events in predicting symptoms of BD. Among individuals with cyclothymia or a history of hypomania, positive attributional style and life events have been shown to interact to prospectively predict increases in hypomanic symptoms (Alloy, Reilly-Harrington, Fresco, Whitehouse, & Zechmeister, 1999). These findings suggest that whereas for some individuals a positive cognitive style may be protective against depression, overly positive attributional tendencies in individuals with bipolar spectrum disorders may precipitate mood elevation in the face of high levels of positive life events, leading to hypomania. Further research has suggested that specific types of positive life events may be more likely to interact with positive cognitive styles to lead to hypomanic symptoms. Johnson (2005) suggested that excessive engagement in goal pursuit should lead to symptoms of hypomania following initial life success or goal achievement. Consistent with this proposal, Nusslock, Abramson, Harmon-Jones, Alloy, and Hogan (2007) demonstrated that goal-striving life events prospectively predicted the onset of hypomanic episodes among individuals with BD. Urosevic et al. (2008) proposed that goal-striving life events are a subset of BAS-activating life events which offer the opportunity for attainment of goals and/or rewards, and that BAS-activating life events are most likely to result in hypomanic symptoms among those with BD or vulnerability to BD who have high expectations about their efficacy in achieving goals or attaining rewards. Future research should examine the interaction between positive overgeneralization and life events, particularly life events that activate the BAS (Urosevic et al., 2008). It is possible that BAS-activating life events may be especially predictive of mood elevation in individuals with high POG (and particularly among those also with high BAS sensitivity), who may perceive these positive events as having overly positive implications for themselves.
The present findings suggest that positive overgeneralization may play an important role in the course of mood symptomotology among those with or at risk for BD, which may have important implications for treatment. Currently, cognitive therapy (CT) for BD typically seeks to employ cognitive behavioral skills to prevent mood episode onset by promoting self-monitoring of mood and sleep patterns, and by targeting unrealistic goal-striving cognitions (Lam et al., 2005). BD patients with a sense of “hyper-positive self” have been shown to have a poorer response to traditional short-term CT (Lam et al., 2005), suggesting that overly positive cognitive styles may hinder treatment response among those with BD. Based on these findings, it is possible that CT for BD may also be improved by targeting positive overgeneralization as well as targeting individuals with overly-positive self-beliefs. However, given the widespread occurrence of negative affect, hopelessness and suicidality in individuals with bipolar disorder, tackling positive overgeneralization should be done with caution. Indeed, a certain level of positive beliefs about the future is likely adaptive in instilling hope and may serve as a protective factor against negative mood (e.g., as a self-serving optimistic bias against depression; Abramson & Alloy, 1981; Mezulis, Abramson, Hyde, & Hankin, 2004). However, the results of the present study must be replicated among individuals with BD before these clinical hypotheses can be evaluated.
The current study had several strengths. It is the first study to examine the interaction of BAS sensitivity and POG in prospectively predicting hypomanic symptoms. It is unique in that it utilizes a prospective behavioral high-risk design, evaluating individuals with no history of BD but who are at high risk for the development of BD (as demonstrated by Alloy et al., in press-b), which provides a powerful test of the BAS hypersensitivity theory of BD.
Nevertheless, several limitations of this study must also be noted. One significant limitation of this study is the use of a self-report measure of hypomanic symptoms, as opposed to a structured diagnostic interview assessment of hypomania. Given that the participants in this study had no history of BD, and consequently, are unlikely to have as high levels and severity of hypomanic symptoms as might be found in clinical BD patients, it is likely that the self-report measures used represent an accurate portrayal of symptomatology. However, it is not entirely clear whether these findings would generalize to participants with higher levels of hypomanic symptoms or who have experienced diagnosable mood episodes. Additionally, it is important to note that experiencing increases in hypomanic symptoms does not necessarily indicate that these symptoms were clinically significant. However, because Alloy et al. (in press-b) showed that individuals with high BAS sensitivity are significantly more likely than moderate-BAS individuals to develop a first onset of bipolar spectrum disorders, it will be important to continue to identify predictors of hypomanic symptoms such as POG in these high-risk individuals. Additionally, Alloy et al.’s (in press-a) finding that BAS prospectively predicts progression of severity of bipolar spectrum disorders highlights the importance of understanding predictors of mood elevation in high-BAS individuals before the onset of a potentially clinically-impairing bipolar disorder.
Furthermore, the current study did not examine the role of overgeneralizing cognitive styles in predicting depressive symptoms in individuals at risk for BD. This may be particularly important to address in future research given that depressive episodes have a greater negative impact on quality of life in individuals with bipolar disorder than hypomanic episodes (Merikangas et al., 2011). The interaction of negative appraisals of affect and internal state with BAS sensitivity may also be important in predicting both hypomanic and depressive episodes in individuals with and at risk for bipolar disorder. Recent research suggests that negative appraisals of activated internal states and moods may differentiate individuals with bipolar disorder from those with unipolar depression (Alatiq, Crane, Williams & Goodwin, 2010; Kelly et al., 2011) and no history of mood disorders (Mansell et al., 2011). Negative goal-related appraisals have also been associated with a history of hypomanic episodes, although it is important to note that this relationship may be mediated by the higher prevalence of depressive symptoms among those with a history of hypomania (Meyer, Beevers & Johnson, 2008). Taken together with the current study, this work suggests that positive and negative evaluations of the meaning of success and of internal state may be relevant to mood symptomatology in bipolar disorder. Future research should seek to assess the interaction of these types of positive and negative appraisals (e.g., Kelly et al., 2011) in predicting mood symptoms and episodes in individuals with and at risk for bipolar disorder.
Based on previous literature on the relationship of cognitive styles, BAS, and life events, it is likely that positive or BAS-activating life events may play a role in moderating the interaction of POG and BAS, which we did not evaluate in the current report. Future research should seek to determine the role of positive and BAS relevant life events in the relationship between BAS and POG. Additionally, it is possible that the range of POG scores observed in the present study was restricted because of the correlation between POG and BAS sensitivity. However, because POG and BAS sensitivity are correlated in unselected samples (e.g. Carver et al., 2010), it was expected that there would be differences in POG between the high and moderate BAS risk groups as selected by the high risk design. Consequently, the results observed should still be representative of a full range of POG scores. Finally, because the present study evaluated changes in hypomanic symptoms, we did not report data using the Hypomanic Personality Scale (Eckblad & Chapman, 1986), a well-validated instrument of risk for hypomania. Nevertheless, using a behavioral high-risk design allowed us to select an ultra-high risk group based on BAS sensitivity, resulting in the findings having potentially greater clinical significance than when using a continuous measure of mania risk. Still, the ASRM, the outcome measure used in the present study, assesses symptoms of affect, self-confidence, sleep, rate of speech, and behavioral activity, but does not assess other symptoms often associated with mood elevation such as negative affect, dysphoria, irritability, panic, worry, and aggression. Future studies should evaluate POG as a predictor of these additional common symptoms of hypomania through a variety of methods (e.g., self-report and interviewer ratings of mood symptoms).
Based on the results of this study, it appears that upward positive overgeneralization may confer additional risk for development of hypomanic symptoms, particularly for individuals already at high risk for BD based on high BAS sensitivity. Future work should continue to evaluate excessive confidence and overgeneralization as predictors of episodes of mood elevation in samples at risk for BD and in individuals with an existing diagnosis of BD.
Highlights.
We prospectively evaluated risk factors for the development of hypomanic symptoms
We used a sample at high versus low risk for the first onset of bipolar disorder
Positive overgeneralization (POG) predicted increases in hypomanic symptoms
POG only predicted hypomanic symptoms among high-BAS individuals
POG may confer additional risk for developing mood elevation
Acknowledgements
This research was supported by National Institute of Mental Health Grant MH 77908 to Lauren B. Alloy.
Footnotes
In addition to predicting hypomanic symptoms, we repeated each analysis to predict follow-up depressive symptoms, controlling for Time 1 depressive symptoms. None of the three POG subscales interacted with BAS group to predict depressive symptoms.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jonathan P. Stange, Temple University
Ashleigh R. Molz, Temple University
Chelsea L. Black, Temple University
Benjamin G. Shapero, Temple University
Joanna M. Bacelli, Temple University
Lyn Y. Abramson, University of Wisconsin-Madison
Lauren B. Alloy, Temple University
References
- Abramson LY, Alloy LB. Depression, nondepression, and cognitive illusions: Reply to Schwartz. Journal of Experimental Psychology. 1981;110:436–447. doi: 10.1037//0096-3445.110.3.436. [DOI] [PubMed] [Google Scholar]
- Aiken LS, West SG. Multiple Regression: Testing and interpreting interactions. Sage; Newbury Park, CA: 1991. [Google Scholar]
- Akiskal HS, Djenderedjian AH, Rosenthal RH, Khani MK. Cyclothymic disorder: Validating criteria for inclusion in the bipolar affective group. American Journal of Psychiatry. 1977;134:1227–1233. doi: 10.1176/ajp.134.11.1227. [DOI] [PubMed] [Google Scholar]
- Alatiq C, Crane C, Williams JMG, Goodwin GM. Dysfunctional beliefs in bipolar disorder: Hypomanic vs. depressive attitudes. Journal of Affective Disorders. 2010;122:294–300. doi: 10.1016/j.jad.2009.08.021. doi: 10.1016/j.jad.2009.08.021. [DOI] [PubMed] [Google Scholar]
- Alloy LB, Abramson LY. The role of the Behavioral Approach System (BAS) in bipolar spectrum disorders. Current Directions in Psychological Science. 2010;19:189–194. doi: 10.1177/0963721410370292. doi: 10.1177/0963721410370292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Abramson LY, Raniere D, Dyller IM. Research methods in adult psychopathology. In: Kendall PC, Butcher JN, Holmbeck GN, editors. Handbook of research methods in clinical psychology. 2nd ed Wiley; New York: 1999. pp. 466–498. [Google Scholar]
- Alloy LB, Abramson LY, Urosevic S, Bender RE, Wagner CA. Longitudinal predictors of bipolar spectrum disorders: A behavioral approach system perspective. Clinical Psychology: Science and Practice. 2009;16:206–226. doi: 10.1111/j.1468-2850.2009.01160.x. doi: 10.1111/j.1468-2850.2009.01160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Abramson LY, Walshaw PD, Cogswell A, Smith J, Hughes M, Nusslock R. Behavioral Approach System (BAS) sensitivity and bipolar spectrum disorders: A retrospective and concurrent behavioral high-risk design. Motivation & Emotion. 2006b;30:143–155. doi: 10.1007/s11031-006-9003-3. [Google Scholar]
- Alloy LB, Abramson LY, Walshaw PD, Cogswell A, Grandin LD, Hughes ME, Hogan ME. Behavioral Approach System and Behavioral Inhibition System sensitivities: Prospective prediction of bipolar mood episodes. Bipolar Disorders. 2008;10:310–322. doi: 10.1111/j.1399-5618.2007.00547.x. doi: 10.1111/j.1399-5618.2007.00547.x. [DOI] [PubMed] [Google Scholar]
- Alloy LB, Abramson LY, Walshaw PD, Keyser J, Gerstein RK. A cognitive vulnerability stress perspective on bipolar spectrum disorders in a normative adolescent brain, cognitive, and emotional development context. Development and Psychopathology. 2006a;18:1055–1103. doi: 10.1017/S0954579406060524. doi: 10.1017/S0954579406060524. [DOI] [PubMed] [Google Scholar]
- Alloy LB, Abramson LY, Walshaw PD, Gerstein RK, Keyser JD, Whitehouse WG, Harmon-Jones E. Behavioral Approach System (BAS)-relevant cognitive styles and bipolar spectrum disorders: Concurrent and prospective associations. Journal of Abnormal Psychology. 2009b;118(3):459–471. doi: 10.1037/a0016604. doi: 10.1037/a0016604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Bender RE, Whitehouse WG, Wagner CA, Liu RT, Grant DA, et al. High Behavioral Approach System (BAS) sensitivity and reward responsiveness predict first onset of bipolar spectrum disorders: A prospective behavioral high-risk design. Journal of Abnormal Psychology. doi: 10.1037/a0025877. (in press-b) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Urosevic S, Abramson LY, Jager-Hyman S, Nusslock R, Whitehouse WG, Hogan ME. Progression along the bipolar spectrum: A longitudinal study of predictors of conversion from bipolar spectrum conditions to bipolar I and II disorders. Journal of Abnormal Psychology. 120 doi: 10.1037/a0023973. (in press-a) doi: 10.1037/a0023973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman EG, Hedeker D, Peterson JL, Davis JM. The Altman self-rating mania scale. Biological Psychiatry. 1997;42:948–955. doi: 10.1016/S0006-3223(96)00548-3. doi: 10.1016/S0006-3223(96)00548-3. [DOI] [PubMed] [Google Scholar]
- Altman EG, Hedeker D, Peterson JL, Davis JM. A comparative evaluation of three self-rating scales for acute mania. Biological Psychiatry. 2001;50:468–471. doi: 10.1016/s0006-3223(01)01065-4. doi: 10.1016/S0006-3223(01)01065-4. [DOI] [PubMed] [Google Scholar]
- Angst F, Stassen HH, Clayton PJ, Angst J. Mortality of patients with mood disorders: Follow-up over 34–38 years. Journal of Affective Disorders. 2002;68:167–181. doi: 10.1016/s0165-0327(01)00377-9. doi: 10.1016/S0165-0327(01)00377-9. [DOI] [PubMed] [Google Scholar]
- Beck A, Rush A, Shaw B, Emery G. Cognitive therapy of depression. Guilford Press; New York: 1979. [Google Scholar]
- Birmaher B, Axelson D, Goldstein B, Strober M, Gill MK, Hunt J, et al. Four-year longitudinal course of children and adolescents with bipolar spectrum disorders: The Course and Outcome of Bipolar Youth (COBY) study. American Journal of Psychiatry. 2009;166:795–804. doi: 10.1176/appi.ajp.2009.08101569. doi: 10.1176/appi.ajp.2009.08101569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke KC, Burke JD, Jr., Regier DA, Rae DS. Age at onset of selected mental disorders in five community populations. Archives of General Psychiatry. 1990;47(6):511–518. doi: 10.1001/archpsyc.1990.01810180011002. [DOI] [PubMed] [Google Scholar]
- Carver CS. Pleasure as a sign you can attend to something else: Placing positive feelings within a general model of affect. Cognition & Emotion. 2003;17:241–261. doi: 10.1080/02699930302294. doi: 10.1080/02699930244000291. [DOI] [PubMed] [Google Scholar]
- Carver CS, Johnson SL. Tendencies toward mania and tendencies toward depression have distinct motivational, affective, and cognitive correlates. Cognitive Therapy and Research. 2009;33:552–569. doi: 10.1007/s10608-008-9213-y. doi: 10.1007/s10608-008-9213-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, Scheier MF. On the self-regulation of behavior. Cambridge University Press; New York: 1998. [Google Scholar]
- Carver CS, Sinclair S, Johnson SL. Authentic and hubristic pride: Differential relations to aspects of goal regulation, affect, and self-control. Journal of Research in Personality. 2010;44:698–703. doi: 10.1016/j.jrp.2010.09.004. doi: 10.1016/j.jrp.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. Journal of Personality and Social Psychology. 1994;67:319–333. doi: 10.1037/0022-3514.67.2.319. [Google Scholar]
- Depue RA, Collins PF. Neurobiology of the structure of personality: Dopamine, facilitation of incentive motivation, and extraversion. Behavioral and Brain Sciences. 1999;22:491–517. doi: 10.1017/s0140525x99002046. [DOI] [PubMed] [Google Scholar]
- Depue RA, Iacono WG. Neurobehavioral aspects of affective disorders. Annual Reviews in Psychology. 1989;40:457–492. doi: 10.1146/annurev.ps.40.020189.002325. [DOI] [PubMed] [Google Scholar]
- Depue RA, Krauss S, Spoont MR. A two-dimensional threshold model of seasonal bipolar affective disorder. In: Magnusson D, Ohman A, editors. Psychopathology: An interactional perspective. Academic Press; New York: 1987. pp. 95–123. [Google Scholar]
- Dilsaver SC. An estimate of the minimum economic burden of bipolar I and II disorders in the United States. Journal of Affective Disorders. 2009;129(1-3):79–83. doi: 10.1016/j.jad.2010.08.030. doi: 10.1016/j.jad.2010.08.030. [DOI] [PubMed] [Google Scholar]
- Eckblad M, Chapman LJ. Development and validation of a scale for hypomanic personality. Journal of Abnormal Psychology. 1986;95:214–222. doi: 10.1037//0021-843x.95.3.214. [DOI] [PubMed] [Google Scholar]
- Eisner L, Johnson SL, Carver CS. Cognitive responses to failure and success relate uniquely to bipolar depression versus mania. Journal of Abnormal Psychology. 2008;117:154–163. doi: 10.1037/0021-843X.117.1.154. doi: 10.1037/0021-843X.117.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endicott J, Spitzer RL. A diagnostic interview: The schedule for Affective Disorders and Schizophrenia. Archives of General Psychiatry. 1978;35:837–844. doi: 10.1001/archpsyc.1978.01770310043002. [DOI] [PubMed] [Google Scholar]
- Feldman GC, Joormann J, Johnson SL. Responses to positive affect: A self-report measure of rumination and dampening. Cognitive Therapy and Research. 2008;32:507–525. doi: 10.1007/s10608-006-9083-0. 10.1007/s10608-006-9083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowles DC. Psychophysiology and psychopathology: A motivational approach. Psychophysiology. 1988;25:373–391. doi: 10.1111/j.1469-8986.1988.tb01873.x. doi: 10.1111/j.1469-8986.1988.tb01873.x. [DOI] [PubMed] [Google Scholar]
- Fowles DC. Biological variables in psychopathology: A psychobiological perspective. In: Sutker PB, Adams HE, editors. Comprehensive handbook of psychopathology. 2nd Edition Plenum Press; New York: 1993. pp. 57–82. [Google Scholar]
- Fulford D, Johnson SL, Carver CS. Commonalities and differences in characteristics of persons at risk for narcissism and mania. Journal of Research in Personality. 2008;42:1427–1438. doi: 10.1016/j.jrp.2008.06.002. doi: 10.1016/j.jrp.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulford D, Johnson SL, Llabre MM, Carver CS. Pushing and coasting in dynamic goal pursuit: Coasting is attenuated in bipolar disorder. Psychological Science. 2010;21:1021–1027. doi: 10.1177/0956797610373372. doi: 10.1177/095679761037337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin FK, Jamison KR. Manic-depressive illness. 2nd edition Oxford University Press; New York: 2007. [Google Scholar]
- Grant BF, Stinston FS, Dawson DA, Chou P, Dufour MC, Compton W, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Archives of General Psychiatry. 2004;61:807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- Gray JA. Neural systems, emotion and personality. In: Madden J, editor. Neurobiology of learning, emotion and affect. Raven Press; New York: 1991. pp. 273–306. [Google Scholar]
- Gray JA. Three fundamental emotion systems. In: Eckman P, Davidson RJ, editors. The nature of emotion: Fundamental questions. Oxford University Press; New York: 1994. pp. 243–247. [Google Scholar]
- Gruber J, Eidelman P, Johnson SL, Smith B, Harvey AG. Hooked on a feeling: Rumination about positive and negative emotion in inter-episode bipolar disorder. Journal of Abnormal Psychology. 2011;120(4):956–961. doi: 10.1037/a0023667. doi: 10.1037/a0023667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber J, Johnson SL. Positive emotional traits and ambitious goals among people at risk for bipolar disorder: The need for specificity. International Journal of Cognitive Therapy. 2009;2(2):176–187. doi: 10.1521/ijct.2009.2.2.176. doi: 10.1521/ijct.2009.2.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon-Jones E, Allen JJB. Behavioral activation sensitivity and resting frontal EEG asymmetry: Covariation of putative indicators related to risk for mood disorders. Journal of Abnormal Psychology. 1997;106:159–163. doi: 10.1037//0021-843x.106.1.159. doi: 10.1037/0021-843X.106.1.159. [DOI] [PubMed] [Google Scholar]
- Heponiemi T, Keltikangas-Jarvinen L, Kettunen J, Puttonen S, Ravaja N. BIS-BAS sensitivity and cardiac autonomic stress profiles. Psychophysiology. 2004;41(1):37–45. doi: 10.1111/1469-8986.00118. doi: 10.1111/1469-8986.00118. [DOI] [PubMed] [Google Scholar]
- Johnson SL. Mania and dysregulation in goal pursuit: A review. Clinical Psychology Review. 2005;25:241–262. doi: 10.1016/j.cpr.2004.11.002. doi: 10.1016/j.cpr.2004.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Carver CS. Extreme goal setting and vulnerability to mania among undiagnosed young adults. Cognitive Therapy and Research. 2006;30:377–395. doi: 10.1007/s10608-006-9044-7. doi: 10.1007/s10608-006-9044-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Eisner LR, Carver CS. Elevated expectancies among persons diagnosed with bipolar disorder. British Journal of Clinical Psychology. 2009;48:217–222. doi: 10.1348/014466509X414655. doi: 10.1348/014466509X414655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Jones S. Cognitive correlates of mania risk: Are responses to success, positive moods, and manic symptoms distinct or overlapping? Journal of Clinical Psychology. 2009;65(9):891–905. doi: 10.1002/jclp.20585. doi: 10.1002/jclp.20585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, McKenzie G, McMurrich S. Ruminative responses to negative and positive affect among students diagnosed with bipolar disorder and major depressive disorder. Cognitive Therapy and Research. 2008;32:702–713. doi: 10.1007/s10608-007-9158-6. doi: 10.1007/s10608-007-9158-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Ruggero CJ, Carver CS. Cognitive, behavioral, and affective responses to reward: Links with hypomanic symptoms. Journal of Social and Clinical Psychology. 2005;24:894–906. doi: 10.1521/jscp.2005.24.6.894. [Google Scholar]
- Johnson SL, Sandrow D, Meyer B, Winters R, Miller I, Solomon D, Keitner G. Increases in manic symptoms after life events involving goal attainment. Journal of Abnormal Psychology. 2000;109(4):721–727. doi: 10.1037//0021-843x.109.4.721. doi: 10.1037/0021-843X.109.4.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Day C. Self appraisal and behavioural activation in the prediction of hypomanic personality and depressive symptoms. Personality and Individual Differences. 2008;45:643–648. doi: 10.1016/j.paid.2008.07.008. [Google Scholar]
- Jones S, Mansell W, Waller L. Appraisal of hypomania-relevant experiences: Development of a questionnaire to assess positive self-dispositional appraisals in bipolar and behavioural high risk samples. Journal of Affective Disorders. 2006;93:19–28. doi: 10.1016/j.jad.2006.01.017. doi: 10.1016/j.jad.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Just N, Abramson LY, Alloy LB. Remitted depression studies as tests of the cognitive vulnerability hypotheses of depression onset: A critique and conceptual analysis. Clinical Psychology Review. 2001;21:63–83. doi: 10.1016/s0272-7358(99)00035-5. doi: 10.1016/S0272-7358(99)00035-5. [DOI] [PubMed] [Google Scholar]
- Kambouropoulos N, Staiger PK. Reactivity to alcohol-related cues: Relationship among cue type, motivational processes, and personality. Psychology of Addictive Behaviors. 2004;18:275–283. doi: 10.1037/0893-164X.18.3.275. doi: 10.1037/0893-164X.18.3.275. [DOI] [PubMed] [Google Scholar]
- Kelly RE, Mansell W, Wood AM, Alatiq Y, Dodd A, Searson R. Extreme positive and negative appraisals of activated states interact to discriminate bipolar disorder from unipolar depression and non-clinical controls. Journal of Affective Disorders. 2011;134:484–443. doi: 10.1016/j.jad.2011.05.042. doi:10.1016/j.jad.2011.05.042. [DOI] [PubMed] [Google Scholar]
- Kennedy N, Everitt B, Boydell J, Van Os J, Jones PB, Murray RM. Incidence and distribution of first-episode mania by age: results from a 35-year study. Psychological Medicine. 2005;35(6):855–863. doi: 10.1017/s0033291704003307. doi: 10.1017/S0033291704003307. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Rubinow DR, Holmes C, Abelson JM, Zhao S. The epidemiology of DSM-III-R bipolar I disorder in a general population survey. Psychological Medicine. 1997;27:1079–1089. doi: 10.1017/s0033291797005333. doi: 10.1017/S0033291797005333. [DOI] [PubMed] [Google Scholar]
- Lam D, Wright K, Sham P. Sense of hyper-positive self and response to cognitive therapy in bipolar disorder. Psychological Medicine. 2005;35:69–77. doi: 10.1017/s0033291704002910. doi: 10.1017/S0033291704002910. [DOI] [PubMed] [Google Scholar]
- Lam D, Wright K, Smith N. Dysfunctional assumptions in bipolar disorder. Journal of Affective Disorders. 2004;79:193–199. doi: 10.1016/S0165-0327(02)00462-7. doi: 10.1016/S0165-0327(02)00462-7. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Steinmetz JL, Larson DW, Franklin J. Depression-related cognitions: Antecedent or consequence? Journal of Abnormal Psychology. 1981;90(3):213–219. doi: 10.1037/0021-843X.90.3.213. [Google Scholar]
- Mansell W, Rigby Z, Tai S, Lowe C. Do current beliefs predict hypomanic symptoms beyond personality style? Factor analysis of the hypomanic attitudes and positive predictions inventory (HAPPI) and its association with hypomanic symptoms in a student population. Journal of Clinical Psychology. 2008;64:450–465. doi: 10.1002/jclp.20455. doi: 10.1002/jclp.20455. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Akiskal HS, Angst J, Greenberg PE, Hirschfeld RM, Petukhova M, Kessler RC. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Archives of General Psychiatry. 2007;64:543–552. doi: 10.1001/archpsyc.64.5.543. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, Jin R, He J, Kessler RC, Lee S, Sampson NA, Zarkov Z. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Archives of General Psychiatry. 2011;68(3):241–251. doi: 10.1001/archgenpsychiatry.2011.12. doi: 10.1001/archgenpsychiatry.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer B, Beevers CG, Johnson SL. Goal appraisals and vulnerability to bipolar disorder: A personal projects analysis. Cognitive Therapy and Research. 2004;28(2):173–182. doi: 10.1023/B:COTR.0000021538.34160.52. [Google Scholar]
- Meyer B, Johnson SL, Carver CS. Exploring behavioral activation and inhibition sensitivities among college students at risk for bipolar spectrum symptomatology. Journal of Psychopathology and Behavioral Assessment. 1999;21(4):275–292. doi: 10.1023/A:1022119414440. doi: 10.1023/A:1022119414440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer B, Johnson SL, Winters R. Responsiveness to threat and incentive in bipolar disorder: Relations of the BIS/BAS scales with symptoms. Journal of Psychopathology and Behavioral Assessment. 2001;23:133–143. doi: 10.1023/A:1010929402770. doi: 10.1023/A:1010929402770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer TD, Krumm-Merabet C. Academic performance and expectations for the future in relation to a vulnerability marker for bipolar disorders: The hypomanic temperament. Personality and Individual Differences. 2003;35:785–796. doi: 10.1016/S0191-8869(02)00283-0. [Google Scholar]
- Mezulis AH, Abramson LY, Hyde JS, Hankin BL. Is there a universal positivity bias in attributions? A meta-analytic review of individual, developmental, and cultural differences in the self-serving attributional bias. Psychological Bulletin. 2004;130(5):711–747. doi: 10.1037/0033-2909.130.5.711. [DOI] [PubMed] [Google Scholar]
- Nusslock R, Abramson LY, Harmon-Jones E, Alloy LB, Hogan ME. A goal-striving life event and the onset of bipolar episodes: Perspective from the behavioral approach system (BAS) dysregulation theory. Journal of Abnormal Psychology. 2007;116:105–115. doi: 10.1037/0021-843X.116.1.105. doi: 10.1037/0021-843X.116.1.105. [DOI] [PubMed] [Google Scholar]
- Perlis RH, Miyahara S, Marangell LB, Wisniewski SR, Ostacher M, DelBello MP, et al. Long-term implications of early onset in bipolar disorder: data from the first 1000 participants in the systematic treatment enhancement program for bipolar disorder (STEP-BD) Biological Psychiatry. 2004;55(9):875–881. doi: 10.1016/j.biopsych.2004.01.022. doi: 10.1016/j.biopsych.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Riskind JH, Alloy LB. Cognitive vulnerability to emotional disorders: Theory and research design/methodology. In: Alloy LB, Riskind JH, editors. Cognitive vulnerability to emotional disorders. Erlbaum; New York: 2006. pp. 1–32. [Google Scholar]
- Salavert J, Caseras X, Torrubia R, Furest S, Arranz B, Duenas R, et al. The functioning of the Behavioral Activation and Inhibition Systems in bipolar I euthymic patients and its influence in subsequent episodes over an 18-month period. Personality and Individual Differences. 2007;42:1323–1331. doi: 10.1016/j.paid.2006.10.010. [Google Scholar]
- Savitz J, van der Merwe L, Ramesar R. Hypomanic, cyclothymic and hostile personality traits in bipolar spectrum illness: a family-based study. Journal of Psychiatric Research. 2008;42(11):920–929. doi: 10.1016/j.jpsychires.2007.10.011. doi: 10.1016/j.jpsychires.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Scott J, Stanton B, Garland A, Ferrier IN. Cognitive vulnerability in patients with bipolar disorder. Psychological Medicine. 2000;30(2):467–72. doi: 10.1017/s0033291799008879. doi: 10.1017/S0033291799008879. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, Stanton AL, Alden LE, Shortridge BE. A multidimensional structure for repetitive thought: What’s on your mind, and how, and how much? Journal of Personality and Social Psychology. 2003;85:909–921. doi: 10.1037/0022-3514.85.5.909. doi: 10.1037/0022-3514.85.5.909. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Parker JB, Becker J. Conformity and achievement in remitted manic-depressive patients. Journal of Nervous and Mental Disease. 1963;137:162–172. doi: 10.1097/00005053-196308000-00007. doi: 10.1097/00005053-196308000-00007. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Endicott J, Robins E. Research diagnostic criteria: Rationale and reliability. Archives of General Psychiatry. 1978;35:773–782. doi: 10.1001/archpsyc.1978.01770300115013. [DOI] [PubMed] [Google Scholar]
- Torrubia R, Avila C, Molto J, Caseras X. The Sensitivity to Punishment and Sensitivity to Reward Questionnaire (SPSRQ) as a measure of Gray’s anxiety and impulsivity dimensions. Personality and Individual Differences. 2001;31:837–862. doi: 10.1016/S0191-8869(00)00183-5. [Google Scholar]
- Urosevic S, Abramson LY, Harmon-Jones E, Donovan PM, Van Voorhis LL, Hogan ME, Alloy LB. The behavioral approach system (BAS) and bipolar spectrum disorders: Relationship of BAS and behavioral inhibition system (BIS) sensitivities to bipolar spectrum diagnoses and hypomanic personality. Clinical Psychology Review. 2008;28:1188–1205. doi: 10.1016/j.cpr.2008.04.004. doi: 10.1016/j.cpr.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]