Abstract
Background
Familial nonmedullary thyroid carcinoma (FNMTC) is frequently detected, but the prevalence or the aggressiveness of FNMTC is still unclear. We aimed to investigate the prevalence, clinical characteristics, and prognosis of FNMTC.
Methods
This study included 3056 nonmedullary thyroid carcinoma (NMTC) patients who were pathologically confirmed to exhibit differentiated thyroid carcinoma from January 1962 through March 2010. The duration of follow-up was 6.2±6.2 years.
Results
The prevalence of FNMTC was 9.6%; 37.9% of the FNMTC patients exhibited a parent-offspring relationship, and 62.1% exhibited a sibling relationship. FNMTC was smaller in tumor size (1.2±0.9 vs. 1.4±1.1 cm) and more multifocal (33.6% vs. 27.0%) than sporadic cases. FNMTC presented higher recurrence rates (29.5% vs. 19.8%) and shorter recurrence-free survival than sporadic NMTC (p=0.046). When we compared sporadic NMTC with parent-offspring or sibling FNMTC separately, parent-offspring FNMTC was more multifocal (39.3% vs. 27.0%), while sibling FNMTC was more prevalent in female patients (89.6% vs. 82.5%) and presented smaller tumors (1.2±0.8 vs. 1.4±1.1 cm) than sporadic NMTC. The recurrence rate was higher than that of sporadic NMTC in parent-offspring FNMTC (35.6% vs. 19.8%) but not in sibling FNMTC. Among the 123 parent-offspring FNMTC cases, the second generation exhibited an earlier age at the diagnosis (38±11 vs. 57±11 years), more extrathyroidal invasion (57.8% vs. 29.4%), a higher recurrence rate (50.0% vs. 19.0%), and shorter recurrence-free survival (p=0.015) than the first generation.
Conclusion
FNMTC was found to have a very high prevalence in our population. Parent-offspring FNMTC demonstrated higher recurrence than sporadic NMTC; specifically, the second generation of parent-offspring FNMTC cases exhibited more aggressive clinical characteristics than the first generation.
Introduction
Thyroid carcinoma is the most common endocrine cancer, and its incidence has been rapidly increasing. In particular, the incidence of thyroid carcinoma in Korea increased more than 5-fold from 1999 (7.2 per 100,000) to 2007 (38.1 per 100,000) (1). Therefore, it is important to identify patients at high risk for thyroid cancer. It is known that patients who have previously been exposed to radiation in their neck area (2) or who have familial histories of certain hereditary cancers have an increased risk of thyroid cancer (3), but the number of such patients is low.
Some previous studies have shown that first-degree relatives of patients with thyroid cancer have an increased risk of thyroid cancer (4,5) and that a family history of thyroid cancer is considered one of the risk factors for thyroid carcinoma. Some susceptibility genes have been suggested, although they have not been clearly identified yet (6). Familial nonmedullary thyroid carcinoma (FNMTC) is defined as occurring when two or more first-degree relatives have nonmedullary thyroid carcinoma (NMTC) in the absence of other known associated cancers (7,8). The reported prevalence of FNMTC is ∼5% (9–14), but the number of subjects in most studies has been less than 1000, except in Japanese studies, so the exact prevalence of FNMTC is still unclear. There also exist controversies regarding the prognosis of FNMTC; some researchers have reported that FNMTC is associated with bad prognoses (9,13,15,16), while others have found no difference between the prognoses of FNMTC and NMTC (10–12,14,17). If FNMTC is related to a poor prognosis, then it is important to adequately treat these patients. However, the prognostic value of FNMTC is not yet clear.
In this study, we took detailed familial histories of thyroid cancer from 3056 patients who visited our clinic, and we investigated the prevalence and the clinical characteristics, including the long-term outcomes of FNMTC, among this relatively large group of NMTC patients.
Materials and Methods
Patients and methods
This study included patients who were pathologically confirmed to have differentiated thyroid carcinoma from January 1962 through March 2010 and who were followed up at the Seoul National University Hospital. We retrospectively surveyed the family histories of differentiated thyroid carcinoma in all patients who visited our clinic from February 2009 to June 2010. We directly asked the patients whether they had any first-degree relatives who had been diagnosed with thyroid cancer. First-degree relatives included parents, offspring, and siblings (5). The FNMTC group included the patients whose first-degree relative(s) was (were) also followed in our hospital or who were confident that their first-degree relative(s) had been diagnosed with thyroid cancer after operations. Patients with prior exposure to radiation and with coexisting anaplastic thyroid carcinoma, medullary thyroid carcinoma, malignant lymphoma, or other inherited familial cancer syndromes (e.g., familial adenomatous polyposis, Gardner's syndrome, Cowden's disease, Carney's complex, and Werner's syndrome) were excluded from this study. Among the 3056 total patients, 2738 had sporadic NMTC, and 318 had FNMTC. Among the 318 FNMTC cases, 28 related pairs (56 patients) were followed together at the Seoul National University Hospital, and the other 262 patients' family members were followed up at other hospitals. To more precisely quantify the prevalence of FNMTC, we calculated the prevalence after excluding 28 of the related patients from the FNMTC group and from the total patient number. However, when we compared the clinicopathologic characteristics, we included all 318 FNMTC patients. The mean follow-up period from the diagnosis of thyroid cancer to the most recent hospital visit was 6.2±6.2 years (range 0.3–48.5 years), and there was no difference between FNMTC and sporadic NMTC in the follow-up period (Table 1).
Table 1.
Number of Patients According to the Diagnostic Periods and Relationships of Affected First-Degree Family Members of Familial Nonmedullary Thyroid Carcinoma Families
| Year of diagnosis | Total | Before 2000 | 2000–2004 | 2005–2010 |
|---|---|---|---|---|
| Number (na) | 3056/3028a | 542/510a | 598/596a | 1946/1922a |
| Sporadic NMTC n | 2738 (89.6%) | 509 (90.2%) | 539 (90.1%) | 1740 (89.2%) |
| Mean F/U duration (years) | 6.2±6.2 | 17.6±5.7 | 7.7±1.4 | 2.6±1.4 |
| Familial NMTC | 318/290a (9.6%) | 53/51a (10.0%) | 59/57a (9.6%) | 206/182a (9.4%) |
| Mean F/U duration (years) | 6.2±6.6 | 18.9±5.7 | 7.3±1.2 | 2.7±1.5 |
| Parent-offspring (total) | 123/110a (3.6%) | 21/20a (3.9%) | 28/28a (4.7%) | 75/62a (3.2%) |
| Parent-one-offspring | 117/104a (3.4%) | 18/17a (3.3%) | 25/25a (4.2%) | 75/62a (3.2%) |
| Parent-two or more-offspring | 6/6a (0.2%) | 3/3a (0.6%) | 3/3a (0.5%) | 0/0a (0%) |
| Siblings (total) | 195/180a (6.0%) | 32/31a (6.1%) | 31/29a (4.9%) | 131/120a (6.2%) |
| Two siblings | 185/171a (5.7%) | 31/30a (5.9%) | 30/28a (4.7%) | 123/113a (5.9%) |
| Three-or-more siblings | 10/9a (0.3%) | 1/1a (0.2%) | 1/1a (0.2%) | 8/7a (0.3%) |
The number was calculated by counting the patient whose other family member(s) was (were) also followed up in our clinics as “one” patient (total 28 families, 56 patients), and used for the calculation of prevalence (%).
NMTC, nonmedullary thyroid carcinoma; F/U, follow-up.
To compare the clinicopathologic characteristics of FNMTC and sporadic NMTC, the following parameters were examined and analyzed: age at the diagnosis of NMTC, sex, histopathology, tumor size, lymph node metastasis, multifocality, extrathyroidal invasion, combined chronic thyroiditis, treatment with radioactive iodine, staging, recurrence risk stratification, and recurrence. Recurrence was defined as locoregional or distant, which was confirmed by histology or a whole-body scan and serum thyroglobulin following radioactive iodine therapy. We subdivided FNMTC into sibling FNMTC and parent-offspring FNMTC. Sibling FNMTC was further divided into two groups, one group consisting of patients with only one sibling and the other group consisting of patients with two or more siblings. Parent-offspring FNMTC was also divided into two groups, one group containing parents of a single offspring and the other group including parents with two offspring. The study's protocol and survey were approved by the Seoul National University Hospital Institutional Review Board.
Statistical analysis
All continuous variables were expressed as means±SDs. Statistical analyses were performed using Pearson's chi-square test or Student's t-test. Recurrence-free survival curves were drawn using the Kaplan-Meier method and were statistically analyzed with the log-rank test. A p-value <0.05 was considered statistically significant. These analyses were performed using SPSS for Windows (SPSS 15.0; SPSS, Inc., Chicago, IL).
Results
Prevalence of FNMTC
Among the total of 3056 NMTC patients, 318 had family histories of NMTC, and the prevalence of FNMTC was 9.6% (290/3028). As shown in Table 1, to exclude the confounding effects of the duration of the follow-up, we calculated the prevalence of FNMTC in different diagnostic periods (before 2000, 2000–2004, and 2005–2010), and the prevalence of FNMTC was greater than 9% during all periods of time, regardless of the duration of follow-up. In the 318 FNMTC patients, 35.9% exhibited a one-parent and one-offspring relationship, 2.0% a one-parent and two-sibling relationship, 59.0% a two-sibling relationship, and 3.1% a three-or-more sibling relationship (Table 1). The prevalence of offspring or sibling FNMTC was also similar among the different periods.
Comparison of clinicopathological characteristics of sporadic and familial NMTC
The clinicopathological characteristics of sporadic NMTC and FNMTC are shown in Table 2. Tumor size was smaller in FNMTC than in sporadic NMTC (1.2±0.9 vs. 1.4±1.1 cm, p=0.005). Multifocality was more frequent in FNMTC than in sporadic NMTC (33.6% vs. 27.0%, p=0.014). There were no significant differences between the two groups with regard to the other parameters. When we compared the clinicopathologic parameters between sporadic NMTC and parent-offspring or sibling FNMTC separately, we found that parent-offspring FNMTC was more multifocal (39.3% vs. 27.0%, p=0.003) than sporadic NMTC, and we also found that sibling FNMTC was more prevalent in female patients (89.6% vs. 82.5%, p=0.037) and presented a smaller tumor size (1.2±0.8 vs. 1.4±1.1 cm, p=0.021) than sporadic NMTC.
Table 2.
Demographic and Disease Characteristics of Sporadic and Familial Nonmedullary Thyroid Carcinoma Patients
| |
|
Familial NMTC (n=318) |
||
|---|---|---|---|---|
| Sporadic NMTC (n=2738) | Total (n=318) | Sibling (n=194) | Parent-offspring (n=118) | |
| Age at diagnosis (years) | 47±11 | 47±12 | 48±10 | 45±15 |
| Female sex rate (%) | 82.5 | 85.8 | 89.7a,b | 79.7 |
| Histopathology (% of papillary carcinoma) | 94.8 | 96.2 | 97.4 | 94.0 |
| Tumor size (cm) | 1.4±1.1 | 1.2±0.9a | 1.2±0.8a | 1.3±1.1 |
| LN metastasis (%) | 33.9 | 39.9 | 41.2 | 36.4 |
| Multifocality (%) | 27.0 | 33.6a | 30.9 | 39.3a |
| Extrathyroidal invasion (%) | 45.3 | 46.4 | 45.9 | 48.5 |
| Combined chronic thyroiditis (%) | 23.0 | 27.9 | 30.6a | 23.5 |
| TNM staging (%)c | 52.4/8.2/38.4/1.0 | 53.4/7.6/38.6/0.4 | 50.6/8.6/40.2/0.6 | 59.0/6.0/35.0/0.0 |
| T (%)d | 36.3/4.4/58.8/0.5 | 33.6/4.5/61.9/0.0 | 35.1/3.0/61.9/0.0 | 32.0/7.2/60.8/0.0 |
| N (%)e | 58.0/42.0 | 53.1/46.9 | 51.2/48.8 | 57.3/42.7 |
| M (%)f | 98.4/1.6 | 98.7/1.3 | 99.0/1.0 | 99.2/0.8 |
| ATA risk (%)g | 56.8/35.9/7.3 | 55.6/36.7/7.7 | 55.6/34.4/10.0 | 54.9/42.2/2.9 |
| Recurrence rate (%) | 19.8 | 29.5a | 26.8 | 35.6a |
p<0.05 versus sporadic NMTC, bp<0.05 versus parent-offspring FNMTC, cstage 1/2/3/4 rate, dT 1/2/3/4, eN 0/1, fM 0/1, gATA recurrence risk stratification, low/intermediate/high risk rate.
FNMTC, familial nonmedullary thyroid carcinoma; LN, lymph node; ATA, American Thyroid Association.
We also evaluated whether there were any differences in the clinicopathologic parameters between parent-offspring and sibling FNMTC (Table 2). Women more commonly exhibited sibling FNMTC (89.7% vs. 79.7%, p=0.013), and the age at diagnosis was younger in parent-offspring FNMTC; however, this difference was not significant (45±15 vs. 48±10 years, p=0.071). No significant differences were found in the other parameters.
To minimize the influence of the follow-up periods, we compared the clinicopathologic characteristics of FNMTC and sporadic NMTC patients who were diagnosed before 2005 because they had relatively long follow-up periods (12.3±6.5 years). However, the results were not different from those of the total group of patients above (data not shown).
Recurrence rate and recurrence-free survival of FNMTC and sporadic NMTC
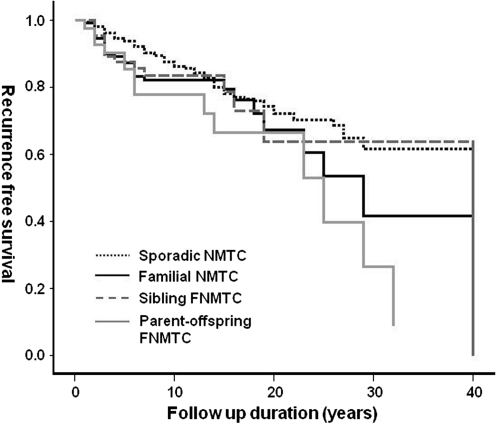
In addition to the recurrence rate we analyzed recurrence-free survival between FNMTC (n=122) and sporadic NMTC (n=1179) in patients who were followed up for more than 5 years to minimize the influence of follow-up periods. The recurrence rate was higher in FNMTC (29.5% vs. 19.8%, p=0.018) and in parent-offspring FNMTC (35.6% vs. 19.8%, p=0.011) than in sporadic NMTC (Table 2). As shown in Figure 1, the recurrence-free survival of FNMTC patients was significantly shorter than that of sporadic NMTC patients (p=0.046 by the log-rank test). This difference can be attributed to parent-offspring FNMTC (Fig. 1, p=0.019 by log-rank test), as no differences were found in the recurrence-free survival rates of sibling FNMTC and sporadic NMTC.
FIG. 1.
Recurrence-free survival curves of sporadic nonmedullary thyroid carcinoma (NMTC), familial NMTC (FNMTC), and sibling-related and parent-offspring–related FNMTC. Total FNMTC (solid black line) and parent-offspring–related FNMTC patients (solid gray line) had a shorter recurrence-free survival than sporadic NMTC patients (short-dotted black line) (p<0.05 by log-rank test). Sibling FNMTC (dotted gray line) patients showed no significant difference than sporadic FNMTC patients.
Comparing familial NMTC with two versus three or more affected members
It has been suggested that the probability of developing FNMTC is greater than 99% if three or more family members are affected (18). Thus, we compared the clincopathological characteristics and recurrence-free survival rates of patients with two affected family members (n=302) and those with three or more affected family members (n=16). However, with the limited number of subjects, we could not find any differences between the two groups (data not shown).
Comparing the clinicopathological differences between the first (parent) and second (offspring) generation
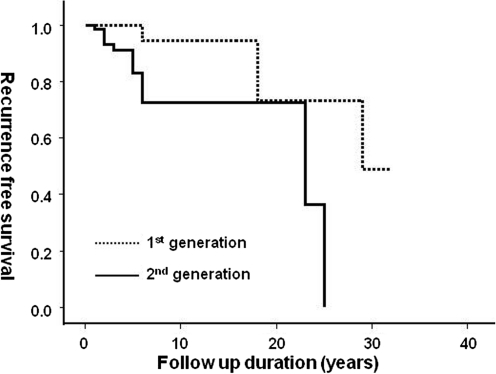
We examined the differences between the first and second generations in parent-offspring FNMTC. As shown in Table 3, the age at diagnosis was younger in the second generation (38±11 vs. 57±11 years, p<0.0001). Additionally, the second generation presented more extrathyroidal invasion (57.8% vs. 29.4%, p=0.011), a higher recurrence rate (50.0% vs. 19.0%, p=0.030), and a shorter recurrence-free survival period (Fig. 2, p=0.015 by log-rank test) than the first generation. Using the American Thyroid Association's recurrence risk stratification, the second generation had a higher risk rate than the first generation (53.0% vs. 22.2%, p=0.009).
Table 3.
Demographic and Disease Characteristics of First- and Second-Generation Patients Among Parent-Offspring Familial Nonmedullary Thyroid Carcinoma
| Parameters | First generation (n=43) | Second generation (n=75) | p |
|---|---|---|---|
| Age at diagnosis (years) | 57±11 | 38±12 | <0.0001 |
| Female sex rate (%) | 83.7% | 77.3% | 0.407 |
| Histopathology (% of papillary carcinoma) | 95.3 | 93.2 | 0.631 |
| Tumor size (cm) | 1.4±1.4 | 1.2±0.8 | 0.499 |
| LN metastasis (%) | 27.9 | 41.3 | 0.270 |
| Multifocality (%) | 37.2 | 40.0 | 0.765 |
| Extrathyroidal invasion (%) | 29.4 | 58.5 | 0.006 |
| Combined chronic thyroiditis (%) | 25.0 | 23.9 | 0.889 |
| TNM staging (%)a | 35.1/8.1/56.8/0.0 | 73.0/4.8/22.2/0.0 | 0.014 |
| T staging (%)b | 34.3/5.7/60.0/0.0 | 30.6/8.1/61.3/0.0 | 0.874 |
| N staging (%)c | 63.3/36.4 | 53.6/46.4 | 0.383 |
| M staging (%)d | 100.0/0.0 | 98.7/1.3 | 0.447 |
| ATA riske | 75.0/22.2/2.8 | 43.9/53.0/3.0 | 0.009 |
| Recurrence rate (%) | 19.0 | 50.0 | 0.030 |
Stage 1/2/3/4 rate.
T 1/2/3/4.
N 0/1.
M 0/1.
ATA recurrence risk stratification, low/intermediate/high risk rate.
FIG. 2.
Recurrence-free survival curves of the first- and second-generation FNMTC patients. The second-generation patients (solid line) showed shorter recurrence-free survival than the first-generation patients (dotted line) (p<0.05 by log-rank test).
Discussion
In this study, we found that the prevalence of FNMTC in Korea was very high, up to 9.6%, and the second generation of FNMTC patients exhibited poorer long-term prognoses than the sporadic NMTC patients.
The reported prevalence of FNMTC was ∼5% of cases, varying from 2.5% to 11.3% (9–14). Our study showed that the prevalence of FNMTC in the Korean population was 9.6%; 37.9% of these cases were parent-offspring relationships, and 62.1% were sibling relationships. This rate is quite high and is comparable with the prevalence in Italy (9) and in the United States (14). However, due to the high prevalence of thyroid cancer, clustering of sporadic thyroid cancer in one family may not be rare. It has been estimated that 62–69% of families with two affected relatives are sporadic occurrences (19). Approximately 95% of our FNMTC families had two affected family members; thus, if we applied the estimated percentages to our study, the true prevalence of FNMTC could have been roughly 3–4%, which is still a high prevalence.
The reason for the higher prevalence of FNMTC in this study is unclear. Recently, the incidence of thyroid cancer has increased surprisingly, probably due to the increased detection of earlier, smaller thyroid cancers (20–22). Since 2007, the age-standardized incidence rate of thyroid cancer ranked highest among Korean women (64.8% per year) and was 38.1% per year in the entire Korean population (1). To exclude the possible confounding effects on the high prevalence of FNMTC of increased screening among family members of recently diagnosed patients, we calculated the prevalence of FNMTC according to the period of diagnosis and the duration of follow-up. The prevalence of FNMTC did not depend on the duration of follow-up, and the percentages of patient-offspring and sibling relationships were also similar during each period (Table 1). Interestingly, the prevalence of FNMTC before 2000 was also 10% over 17.6±5.7 or 18.9±5.7 years of follow-up for sporadic NMTC or FNMTC, respectively. This result implies that the prevalence of FNMTC is high in the Korean population regardless of frequent diagnosis using ultrasound screening. Considering the duration of the follow-up periods, the prevalence of FNMTC in recently diagnosed patients (2005–2010) could increase because of more screening within families.
At the same time, we should consider the possible confounding effects of coexisting chronic autoimmune thyroiditis. It has been suggested that chronic autoimmune thyroiditis is commonly associated with papillary thyroid carcinoma (23,24). Chronic thyroiditis is the autoimmune disease that most commonly exhibits familial aggregation, and the reported prevalence of positive thyroid peroxidase antibodies is 9.2–11.3% (25–28). In our study, the prevalence of combined chronic autoimmune thyroiditis in FNMTC was 27.9%; in particular, coexisting chronic autoimmune thyroiditis was more common in sibling FNMTC cases than in sporadic NMTC cases (30.6% vs. 23.0%, p<0.05). Thus, the high coexistence of chronic autoimmune thyroiditis may have contributed to the high prevalence of FNMTC in this study. However, the prevalence of coexisting chronic autoimmune thyroiditis was not different between parent-offspring FNMTC cases and sporadic NMTC cases, and the difference between sibling FNMTC and sporadic NMTC cases was only 7%; thus, the confounding effects of coexisting chronic thyroiditis were not significant.
Genetic abnormalities could lead to FNMTC, and although the genes responsible for FNMTC have not been characterized to date, six potential regions have been identified: MNG1 (14q32), TCO (19p13.2), fPTC/PRN (1q21), NMTC1 (2q21), FTEN (8p23.1–p22), and the telomere–telomerase complex (17). Our population had a relatively homogenous genetic background (all of the patients were Korean); therefore, genetic analysis including these six candidate genes could provide useful information and might explain the reason for the higher prevalence of FNMTC in our population.
Whether FNMTC is more aggressive than sporadic NMTC remains controversial. Most studies have reported that patients with FNMTC had increased risks of multifocality (9,13), local invasion, and lymph node metastases (15), leading to higher recurrence rates and decreased disease-free survival (9,13,16,29). However, some other studies have not found FNMTC to be more aggressive than sporadic disease (10–12,14,17). In addition, some studies have reported that patients with FNMTC presented earlier cancer onset (16) or smaller primary tumors (30,31). In our study, FNMTC cases exhibited greater multifocality, a higher recurrence rate, and shorter recurrence-free survival than sporadic NMTC cases.
When we subdivided FNMTC into parent-offspring and sibling FNMTC and compared each of them with sporadic NMTC, only parent-offspring FNMTC, specifically the offspring (second generation), exhibited higher extrathyroidal invasion, higher ATA risk (32), and a higher recurrence rate than sporadic NMTC, while sibling FNMTC exhibited no difference in prognosis. However, in sibling FNMTC, the tumor size was smaller, coexisting chronic autoimmune thyroiditis was more prevalent, and the prevalence in female patients was higher than sporadic NMTC. Until now, there have been no studies comparing parent-offspring FNMTC or sibling FNMTC with sporadic NMTC. Our results suggest that parent-offspring FNMTC might have a more aggressive prognosis than sibling FNMTC. However, it is possible that the prognosis of sibling FNMTC was attenuated by chronic autoimmune thyroiditis. Sibling FNMTC included more frequent coexisting chronic autoimmune thyroiditis than parent-offspring FNMTC, and papillary thyroid carcinoma combined with chronic autoimmune thyroiditis exhibited a smaller tumor size, a lower recurrence rate, and a more favorable prognosis (33–35). Therefore, the smaller tumor size and the greater percentage of female patients in sibling FNMTC were also explained by more frequent coexisting chronic autoimmune thyroiditis.
FNMTC is usually indistinguishable from sporadic NMTC by family history, histology, or genetics (8). However, it has been suggested that the probability of a sporadic case in families with three or more affected members is less than 6% (19), and Triponez et al. reported that patients with three or more affected family members had significantly lower survival rates than controls (36). Thus, we evaluated the prognosis of FNMTC in those three or more affected members, but we failed to demonstrate poor prognoses in these cases because there were only 16 individuals (Table 1).
In recent studies, the second generation in parent-offspring FNMTC was associated with an earlier age at diagnosis, greater multifocality (9,37), and a higher metastasis rate (9). In accordance with previous results, we found that the second generation was diagnosed with thyroid cancer at a younger age (Table 3), supporting the presence of “genetic anticipation,” a phenomenon defined as the occurrence of a genetic disorder at progressively earlier ages and with increased severity in successive generations (9,37), in FNMTC. The mean ages at diagnosis of the second generation were 27 and 31 in the previous two studies and 38 years in our study. In addition, the second generation had more extrathyroidal invasion and a higher recurrence rate than the first generation, which suggested that FNMTC diagnosed in the second generation requires more aggressive treatment than sporadic NMTC. If we consider only the high prevalence of FNMTC and the poorer prognosis in second-generation cancer, then screening for thyroid cancer should start at least in the early 30s in the offspring of patients with thyroid cancer. However, thyroid cancer usually shows good prognostic behavior, and there is no diagnostic method to detect true FNMTC at the present time. Thus, routine ultrasound screening in the offspring should not be recommended, but a careful evaluation should be performed if the offspring has a thyroid nodule, especially when there are any sonographically suspicious findings (38).
We were able to confirm the diagnoses of 56 family members in the FNMTC group who were also followed at our hospital, while we could not ascertain whether the relatives of the other 290 FNMTC patients had been diagnosed with thyroid cancer. However, we included the patients as FNMTC only when the patient was confident that they had family member who was diagnosed with thyroid cancer after operations. Therefore, we expect that the error rate was low. However, the lack of confirmation of the index cases is a limitation of our study. It is regrettable that we could not compare the index cases with their family members, because those results would have supplied us with valuable information.
In summary, the prevalence of FNMTC in our study was 9.6%, which is higher than that reported in other studies. FNMTC with a parent-offspring relationship exhibited a poorer prognosis than sporadic NMTC. The second generation in parent-offspring FNMTC was diagnosed at an earlier age and had a higher recurrence rate. These findings suggest that we should take careful familial histories of thyroid cancer patients and make decisions about treatment or diagnostic modalities after considering family incidence in NMTC patients with thyroid cancer or nodules.
Acknowledgments
This work was supported by Chung-Ang University research grants in 2011 and by research grant number CB-2011-03-01 of the Korean Foundation for Cancer Research.
Disclosure Statement
The authors declare that no competing financial interests exist.
References
- 1.Korean Cancer Statistics of National Cancer Information Center. www.cancer.go.kr/cms/statics/ [Dec 30;2010 ]. www.cancer.go.kr/cms/statics/
- 2.Shibata Y. Yamashita S. Masyakin VB. Panasyuk GD. Nagataki S. 15 years after Chernobyl: new evidence of thyroid cancer. Lancet. 2001;358:1965–1966. doi: 10.1016/S0140-6736(01)06971-9. [DOI] [PubMed] [Google Scholar]
- 3.Malchoff CD. Malchoff DM. Familial nonmedullary thyroid carcinoma. Cancer Control. 2006;13:106–110. doi: 10.1177/107327480601300204. [DOI] [PubMed] [Google Scholar]
- 4.Hemminki K. Eng C. Chen B. Familial risks for nonmedullary thyroid cancer. J Clin Endocrinol Metab. 2005;90:5747–5753. doi: 10.1210/jc.2005-0935. [DOI] [PubMed] [Google Scholar]
- 5.Pal T. Vogl FD. Chappuis PO. Tsang R. Brierley J. Renard H. Sanders K. Kantemiroff T. Bagha S. Goldgar DE. Narod SA. Foulkes WD. Increased risk for nonmedullary thyroid cancer in the first degree relatives of prevalent cases of nonmedullary thyroid cancer: a hospital-based study. J Clin Endocrinol Metab. 2001;86:5307–5312. doi: 10.1210/jcem.86.11.8010. [DOI] [PubMed] [Google Scholar]
- 6.Khan A. Smellie J. Nutting C. Harrington K. Newbold K. Familial nonmedullary thyroid cancer: a review of the genetics. Thyroid. 2010;20:795–801. doi: 10.1089/thy.2009.0216. [DOI] [PubMed] [Google Scholar]
- 7.Sippel RS. Caron NR. Clark OH. An evidence-based approach to familial nonmedullary thyroid cancer: screening, clinical management, and follow-up. World J Surg. 2007;31:924–933. doi: 10.1007/s00268-006-0847-1. [DOI] [PubMed] [Google Scholar]
- 8.Sturgeon C. Clark OH. Familial nonmedullary thyroid cancer. Thyroid. 2005;15:588–593. doi: 10.1089/thy.2005.15.588. [DOI] [PubMed] [Google Scholar]
- 9.Capezzone M. Marchisotta S. Cantara S. Busonero G. Brilli L. Pazaitou-Panayiotou K. Carli AF. Caruso G. Toti P. Capitani S. Pammolli A. Pacini F. Familial non-medullary thyroid carcinoma displays the features of clinical anticipation suggestive of a distinct biological entity. Endocr Relat Cancer. 2008;15:1075–1081. doi: 10.1677/ERC-08-0080. [DOI] [PubMed] [Google Scholar]
- 10.Ito Y. Kakudo K. Hirokawa M. Fukushima M. Yabuta T. Tomoda C. Inoue H. Kihara M. Higashiyama T. Uruno T. Takamura Y. Miya A. Kobayashi K. Matsuzuka F. Miyauchi A. Biological behavior and prognosis of familial papillary thyroid carcinoma. Surgery. 2009;145:100–105. doi: 10.1016/j.surg.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Loh KC. Familial nonmedullary thyroid carcinoma: a meta-review of case series. Thyroid. 1997;7:107–113. doi: 10.1089/thy.1997.7.107. [DOI] [PubMed] [Google Scholar]
- 12.Maxwell EL. Hall FT. Freeman JL. Familial non-medullary thyroid cancer: a matched-case control study. Laryngoscope. 2004;114:2182–2186. doi: 10.1097/01.mlg.0000149454.91005.65. [DOI] [PubMed] [Google Scholar]
- 13.Uchino S. Noguchi S. Kawamoto H. Yamashita H. Watanabe S. Shuto S. Familial nonmedullary thyroid carcinoma characterized by multifocality and a high recurrence rate in a large study population. World J Surg. 2002;26:897–902. doi: 10.1007/s00268-002-6615-y. [DOI] [PubMed] [Google Scholar]
- 14.Moses W. Weng J. Kebebew E. Prevalence, clinicopathologic features, and somatic genetic mutation profile in familial versus sporadic nonmedullary thyroid cancer. Thyroid. 2011;21:367–371. doi: 10.1089/thy.2010.0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grossman RF. Tu SH. Duh QY. Siperstein AE. Novosolov F. Clark OH. Familial nonmedullary thyroid cancer. An emerging entity that warrants aggressive treatment. Arch Surg. 1995;130:892–897. doi: 10.1001/archsurg.1995.01430080094015. discussion 898–899. [DOI] [PubMed] [Google Scholar]
- 16.Alsanea O. Wada N. Ain K. Wong M. Taylor K. Ituarte PH. Treseler PA. Weier HU. Freimer N. Siperstein AE. Duh QY. Takami H. Clark OH. Is familial non-medullary thyroid carcinoma more aggressive than sporadic thyroid cancer? A multicenter series. Surgery. 2000;128:1043–1050. doi: 10.1067/msy.2000.110848. discussion 1050–1041. [DOI] [PubMed] [Google Scholar]
- 17.Robenshtok E. Tzvetov G. Grozinsky-Glasberg S. Shraga-Slutzky I. Weinstein R. Lazar L. Serov S. Singer J. Hirsch D. Shimon I. Benbassat C. Clinical characteristics and outcome of familial nonmedullary thyroid cancer: a retrospective controlled study. Thyroid. 2011;21:43–48. doi: 10.1089/thy.2009.0406. [DOI] [PubMed] [Google Scholar]
- 18.Charkes ND. On the prevalence of familial nonmedullary thyroid cancer. Thyroid. 1998;8:857–858. doi: 10.1089/thy.1998.8.857. [DOI] [PubMed] [Google Scholar]
- 19.Charkes ND. On the prevalence of familial nonmedullary thyroid cancer in multiply affected kindreds. Thyroid. 2006;16:181–186. doi: 10.1089/thy.2006.16.181. [DOI] [PubMed] [Google Scholar]
- 20.Liu S. Semenciw R. Mao Y. Cervical cancer: the increasing incidence of adenocarcinoma and adenosquamous carcinoma in younger women. CMAJ. 2001;164:1151–1152. [PMC free article] [PubMed] [Google Scholar]
- 21.Davies L. Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 22.Leenhardt L. Grosclaude P. Cherie-Challine L. Increased incidence of thyroid carcinoma in france: a true epidemic or thyroid nodule management effects? Report from the French Thyroid Cancer Committee. Thyroid. 2004;14:1056–1060. doi: 10.1089/thy.2004.14.1056. [DOI] [PubMed] [Google Scholar]
- 23.Loh KC. Greenspan FS. Dong F. Miller TR. Yeo PP. Influence of lymphocytic thyroiditis on the prognostic outcome of patients with papillary thyroid carcinoma. J Clin Endocrinol Metab. 1999;84:458–463. doi: 10.1210/jcem.84.2.5443. [DOI] [PubMed] [Google Scholar]
- 24.Repplinger D. Bargren A. Zhang YW. Adler JT. Haymart M. Chen H. Is Hashimoto's thyroiditis a risk factor for papillary thyroid cancer? J Surg Res. 2008;150:49–52. doi: 10.1016/j.jss.2007.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hollowell JG. Staehling NW. Flanders WD. Hannon WH. Gunter EW. Spencer CA. Braverman LE. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87:489–499. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 26.Bjoro T. Holmen J. Kruger O. Midthjell K. Hunstad K. Schreiner T. Sandnes L. Brochmann H. Prevalence of thyroid disease, thyroid dysfunction and thyroid peroxidase antibodies in a large, unselected population. The Health Study of Nord-Trondelag (HUNT) Eur J Endocrinol. 2000;143:639–647. doi: 10.1530/eje.0.1430639. [DOI] [PubMed] [Google Scholar]
- 27.Teng W. Shan Z. Teng X. Guan H. Li Y. Teng D. Jin Y. Yu X. Fan C. Chong W. Yang F. Dai H. Yu Y. Li J. Chen Y. Zhao D. Shi X. Hu F. Mao J. Gu X. Yang R. Tong Y. Wang W. Gao T. Li C. Effect of iodine intake on thyroid diseases in China. N Engl J Med. 2006;354:2783–2793. doi: 10.1056/NEJMoa054022. [DOI] [PubMed] [Google Scholar]
- 28.O'Leary PC. Feddema PH. Michelangeli VP. Leedman PJ. Chew GT. Knuiman M. Kaye J. Walsh JP. Investigations of thyroid hormones and antibodies based on a community health survey: the Busselton thyroid study. Clin Endocrinol (Oxf) 2006;64:97–104. doi: 10.1111/j.1365-2265.2005.02424.x. [DOI] [PubMed] [Google Scholar]
- 29.Lupoli G. Vitale G. Caraglia M. Fittipaldi MR. Abbruzzese A. Tagliaferri P. Bianco AR. Familial papillary thyroid microcarcinoma: a new clinical entity. Lancet. 1999;353:637–639. doi: 10.1016/S0140-6736(98)08004-0. [DOI] [PubMed] [Google Scholar]
- 30.Lynch HT. Lynch PM. Albano WA. Edney J. Organ CH. Lynch JF. Hereditary cancer: ascertainment and management. CA Cancer J Clin. 1979;29:216–232. doi: 10.3322/canjclin.29.4.216. [DOI] [PubMed] [Google Scholar]
- 31.Ozaki O. Ito K. Kobayashi K. Suzuki A. Manabe Y. Hosoda Y. Familial occurrence of differentiated, nonmedullary thyroid carcinoma. World J Surg. 1988;12:565–571. doi: 10.1007/BF01655453. [DOI] [PubMed] [Google Scholar]
- 32.Cooper DS. Doherty GM. Haugen BR. Kloos RT. Lee SL. Mandel SJ. Mazzaferri EL. McIver B. Pacini F. Schlumberger M. Sherman SI. Steward DL. Tuttle RM. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 33.Kashima K. Yokoyama S. Noguchi S. Murakami N. Yamashita H. Watanabe S. Uchino S. Toda M. Sasaki A. Daa T. Nakayama I. Chronic thyroiditis as a favorable prognostic factor in papillary thyroid carcinoma. Thyroid. 1998;8:197–202. doi: 10.1089/thy.1998.8.197. [DOI] [PubMed] [Google Scholar]
- 34.Kim EY. Kim WG. Kim WB. Kim TY. Kim JM. Ryu JS. Hong SJ. Gong G. Shong YK. Coexistence of chronic lymphocytic thyroiditis is associated with lower recurrence rates in patients with papillary thyroid carcinoma. Clin Endocrinol (Oxf) 2009;71:581–586. doi: 10.1111/j.1365-2265.2009.03537.x. [DOI] [PubMed] [Google Scholar]
- 35.Matsubayashi H. Tomaru M. Sawa M. Nonaka M. Oguma Y. Epistatic interactions among the P element-induced high interspecific crossability strains in Drosophila melanogaster. Jpn J Genet. 1995;70:211–221. doi: 10.1266/jjg.70.211. [DOI] [PubMed] [Google Scholar]
- 36.Triponez F. Wong M. Sturgeon C. Caron N. Ginzinger DG. Segal MR. Kebebew E. Duh QY. Clark OH. Does familial non-medullary thyroid cancer adversely affect survival? World J Surg. 2006;30:787–793. doi: 10.1007/s00268-005-0398-x. [DOI] [PubMed] [Google Scholar]
- 37.Hillenbrand A. Varhaug JE. Brauckhoff M. Pandev R. Haufe S. Dotzenrath C. Koberle R. Hoffmann R. Klein G. Kadmon M. Negele T. Hagieva T. Henne-Bruns D. Luster M. Weber T. Familial nonmedullary thyroid carcinoma-clinical relevance and prognosis. A European multicenter study. ESES Vienna presentation. Langenbecks Arch Surg. 2010;395:851–858. doi: 10.1007/s00423-010-0696-0. [DOI] [PubMed] [Google Scholar]
- 38.Moon WJ. Jung SL. Lee JH. Na DG. Baek JH. Lee YH. Kim J. Kim HS. Byun JS. Lee DH. Benign and malignant thyroid nodules: US differentiation—multicenter retrospective study. Radiology. 2008;247:762–770. doi: 10.1148/radiol.2473070944. [DOI] [PubMed] [Google Scholar]