Abstract
Purpose
The aim of this study was to investigate the sensitization, pharmacokinetics, and absorption of FK506 after corneal transplantation.
Methods
New Zealand albino rabbits were divided into normal and corneal transplantation groups. Each group was divided into 5 subgroups—saline, blank matrix, high-dose, medium-dose, and low-dose, respectively. There were 10 rabbits in each subgroup. One drop (25 μL) of FK506 was administered topically to both eyes of the rabbits 4 times daily for 30 days. Thirty days later, 5 rabbits of each subgroup were sacrificed after the administration of the last dose. Both eyes were enucleated; the left eye was used for pathologic examination and the right eye for the determination of FK506 distribution. The other 5 rabbits in each subgroup were sacrificed 14 days after the former 5 rabbits were sacrificed, and their eyes were enucleated for pathologic examination and tissue distribution determination as the former 5 rabbits in each subgroup (the second batch).
Results
Fluorescein staining and local ocular reaction provided evidence that there were no significant differences between control and FK506-instilled eyes in the rabbit model at any of the tested doses. Histologic examination revealed no ocular abnormality in the rabbits instilled with any doses of FK506 eyedrop. The peak serum concentration (Cmax) of systemic absorption ranged from 4.31±0.79 ng/mL to 14.89±6.85 ng/mL.
Conclusion
Our study suggests that up to 0.1% FK506 administered 4 times a day (q.i.d.) topically is safe for the rabbit eye. However, further safety studies are required in view of systemic adverse effects.
Introduction
FK506 (tacrolimus hydrate)1 is a novel macrolide immunosuppressant discovered in 1984 by the Exploratory Research Laboratories of Fujisawa Pharmaceutical Co., Ltd. (Osaka, Japan) and is now used as an immunosuppressant after organ transplantation worldwide. It has a mechanism of action similar to that of ciclosporine A, but is 50–100 times more potent and less likely to induce systemic hypertension and lipid abnormalities.2–7 Its mechanism involves binding to the cytoplasmic FK506-binding protein (FKBP-12). The complex inhibits the Ca2+- and calmodulin-dependent dephosphatase activity of calcineurin, which inhibits the ability of calcineurin to dephosphorylate the nuclear factor of activated T cells (NFAT), a transcription factor that activates the genes of interleukin (IL)-2 granulocyte macrophage colony-stimulating factor, tumor necrosis factor, interferon, and other ILs that are required for the development of an immune response.8–10 In 1989, Kobayashi11 first reported that FK506 suppressed corneal graft rejection in rabbits. Since then, the use of FK506 is of special interest in ophthalmology because it is indicated to be effective in the treatment of immune-mediated diseases such as corneal graft rejection, ocular inflammation, ocular pemphigoid, and uveitis.
Due to its highly hydrophobic macrolide lactone and relatively high molecular weight (822 daltons), FK506 is almost insoluble in water and has its limitation in corneal penetration. Previously, we reported the investigation of the preliminary stability of laboratory-prepared FK506 suspension pharmacokinetics in rabbit aqueous humor and distribution in eye tissues after single-dose administration.12 In this study, we further investigated its long-term safety, sensitization, pharmacokinetics, and absorption in systemic circulation after 1 month of ocular instillation.
Methods
Materials
FK506 and the preparation of FK506 eye drop was obtained as described previously.12 FK506 was suspended in sterile purified water containing glycol, sodium citrate, and merthiolate. The preliminary study showed that the particles with diameters less than 6 μm constituted up 75% of all the particles in the suspension. The suspension solution showed a constant decline in sedimentation volume up to 40 days, whereas the decline was minimized after 45 days. On the 45th day, the sedimentation rate was 0.93. The flocculation value was 6.3, and it took 3 s for the suspension to redisperse. After 3 months of storage at 4°C, no significant differences were found in terms of content, pH (7.06), character, and redispersal. The blank matrix contained all of the materials except FK506. High-dose FK506 contained 0.1% FK506, whereas medium-dose and low-dose contained 0.05% and 0.025% FK506.
Animals
One hundred New Zealand albino rabbits (Animal Center of The State Key Laboratory of Safety Evaluation on New Drug in Guangzhou) weighing between 2 and 3 kg were raised for at least 1 week under standardized temperature (25–28°C), humidity (50%–60%), and light (12 h light°dark) conditions before the experiment. Animals were allowed free access to standard food and tap water. All care and handling of rabbits adhered to the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research, with the approval of Institutional Authority for Laboratory Animal Care. At 24 h before the drug administration process, bilateral eyes of the animals were inspected with 2% fluorescein sodium to make sure all of them had no irritation, corneal defects, and conjunctival damage.
Study design
The animals were divided into 2 groups—the normal group and the corneal transplantation group. Both groups were divided into 5 subgroups: Saline (groups An and Ac), blank matrix (groups Bn and Bc), high-dose (groups Cn and Cc), medium-dose (groups Dn and Dc), and low-dose (groups En and Ec) in the normal group (n) and corneal transplantation group (c), respectively. According to weight and gender, the rabbits were assigned to each subgroup with stochastic user equilibrium assignment. There were 10 rabbits in each subgroup. A standard corneal transplantation procedure was carried on in both eyes of all the rabbits in the corneal transplantation group 1 day before the eye drop administration started. The eye drop, each time 1 drop (25 μL), was administered topically to both eyes of the rabbits 4 times daily for 30 days. Then 5 rabbits of each subgroup were sacrificed. Both eyes were enucleated; the left eye was for pathologic examination and the right eye for determination of FK506 tissue distribution. The other 5 rabbits in each subgroup were left under observation until 14 days after the last eye drop instillation. Then they were sacrificed, and their eyes were enucleated for pathologic examination and tissue distribution determination the same as the former 5 rabbits in each subgroup.
Observation methods
Fluorescein staining inspection
Before the first FK506 instillation each day and 1, 2, 4, 24, and 48 h after the last instillation, fluorescein staining inspection was carried out. One drop of 2% fluorescein sodium was instilled into the rabbit eye, and 15 s later, the rabbit eye was rinsed thoroughly with sterilized saline. Then the rabbit eyes were inspected with a slit-lamp microscope.
Local ocular reaction
Before the first FK506 instillation each day and 1, 2, 4, and 24 h after the last instillation, a slit-lamp microscope was employed to inspect the local ocular reaction of the rabbit eyes. During the period between 48 h and 14 days after the last instillation, the rabbit eyes were inspected with the slit-lamp microscope every day. The score was marked according to the local ocular reaction. The scoring criteria and evaluation criteria are listed in Tables 1 and 2, respectively.
Table 1.
Scoring Criteria of Eye Irritative Reaction
| Ocular Reaction | Score |
|---|---|
| Cornea | |
| No opacity | 0 |
| Scatter or disseminated opacity, iris clearly seen | 1 |
| Translucent area distinguishable, iris blurred and indistinct | 2 |
| Grey translucent area appeared, iris indistinct, pupil merely seen | 3 |
| Cornea opacity, iris inrecognizable | 4 |
| Iris | |
| Normal | 0 |
| Iridial folds deepen, congestion, slight congestion around cornea, pupil reative to light | 1 |
| Hemorrhage/macroscopic necrosis/ unreactive to light | 2 |
| Conjunctiva | |
| Congestion (including palpebral conjunctiva and in palpebral conjunctiva) | |
| Normal capillary | 0 |
| Capillary congestion with bright red colour | 1 |
| Capillary congestion with bright red colour, vascular inrecognizable | 2 |
| Disseminated congestion with purple red colour | 3 |
| Edema | |
| Absence | 0 |
| Slightly edema (eyelids including) | 1 |
| Significant edema with eyelid ectropion | 2 |
| Significant edema with eyelid half closure | 3 |
| Significant edema with eyelid more than half closure | 4 |
| Exudates | |
| Absence | 0 |
| Minor | 1 |
| Enough to wet or adhere the eyelid and eyelash | 2 |
| Enough to wet or adhere the entire ocular area | 3 |
| Maximum total scores | 16 |
Table 2.
Evaluation Criteria of Eye Irritative Reaction
| Scores | Evaluation |
|---|---|
| 0–3 | Nonirrative |
| 4–8 | Slightly irritative |
| 9–12 | Moderate irritative |
| 13–16 | Severe irritative |
Histologic examination
All rabbits were killed humanely with an overdose of pentobarbital after the last instillation or 14 days after the last instillation. The eyes were immediately enucleated, and the sclera were incised 2 mm posterior to the limbus and fixed in a solution of 10% formalin. The eyes were sectioned, and the tissues were processed, embedded in paraffin, sectioned at a thickness of 5 μm, and stained with Hematoxylin & Eosin. Histologic examination was performed with light microscopy.
Blood sampling
On the first day, 15th day, and 30th day after the first instillation, a series of blood samplings was carried out. The sampling time points of the first and the 15th day were 5 min before instillation, 10 min, 30 min, 1 h, 1.5 h, 2 h, 3 h, 4.5 h, and 7.5 h after the first instillation of the day. The sampling time points of the 30th day were 5 min before instillation, 10 min, 0.5 h, 1 h, 2 h, 3 h, 6 h, and 10 h after the first instillation of the day. Approximately 1 mL of whole-blood samples were collected from the marginal ear vein of the rabbits. The whole-blood samples were infused into the tubes containing heparin and mixed.
Tissue dissection
When the first 5 rabbits were sacrificed, the anterior segment was dissected to obtain samples of conjunctiva, iris, cornea, and sclera. Approximately 0.4–0.6 mL of vitreous humor was collected, with care taken not to aspirate fragments of the ciliary body or retina. When necessary, a microsponge was used to remove excess vitreous humor from the posterior tissues. Chorioretinal samples were also collected. All eyes were dissected with sterile instruments, whole and undamaged if possible, rinsed thoroughly with sterile saline, and blotted dry with sterile filter paper, and stored at −80°C until analysis.
Sample preparation
Whole-blood samples (30 μL each) were spiked with 60 μL of ritonavir (50 ng/mL) as internal standard (IS). Then 60 μL of methanol was added to precipitate protein and 1 mL of ethyl acetate was added for extraction. All of the tissue samples were cut into small particles with scissors and ground to fine powder with a mortar and pestle in liquid nitrogen. The powder was then transferred into a clean polypropylene tube, and 0.5 mL of sterile saline and IS were added. The tube was stirred for 2 min. All of the mixtures were homogenized for 1.5 min and centrifuged at 13,000 rpm for 10 min. The resulting upper organic phase was transferred to a clean polypropylene tube. The organic solvent was evaporated to dryness at 55°C under a stream of nitrogen. The residue was reconstituted in 0.1 mL of mobile phase (0.1% formic acid in water, methanol, and acetonitrile, vol/vol/vol, 5:85:10) by vortexing for 2 min. The resulting sample was transferred to high-performance liquid chromatography (HPLC) autosampler vials, and then 10 μL of the organic phase solution was injected into a liquid chromatography tandem mass spectrometry (LC–MS/MS) system. The LC-MS/MS system, including the Surveyor autosampler and MS pump, was purchased from Thermo Finnigan (Thermo-Finnigan, San Jose, CA). The determination method was the same as we previously described.13
Statistical analysis
Data were expressed in means and standard deviations. Noncompartmental pharmacokinetic parameter calculations were performed using the TopFit 2.0 software package (Thomae GmbH, Germany). Maximum aqueous humor concentration (Cmax) and the time-to-maximum concentration (Tmax) were estimated by visual inspection of semilogarithmic plots of the concentration–time curves. The area under the curve (AUC0–480min) was calculated using the linear-trapezoidal rule, with extrapolation to infinity (AUC0–∞) from the last detectable concentration using the terminal elimination rate constant (ke) calculated by linear regression of the final log-linear part of the drug concentration–time curve. Apparent elimination half-life (t1/2) was calculated as t1/2=0.693/ke.
Comparisons between each group were conducted using an analysis of variance test for numeric variables and Mantel–Haenszel chi-squared test for categorical variables. The confidence level was set to 95% for all tests. Statistical analyses were performed using SAS (version 9.1.2).
Results
General observation
During the experimental period, no premature deaths relating to the treatment occurred throughout the experiments. No seizures, manifest changes in animal behavior, or overall appearance were observed during the study. However, somewhat reduced locomotive activity and tendency to reduced feed intake were noted in the group treated with the high dose in the normal group (En) and high dose group in the corneal transplantation group (Ec). The relative body weight gain was significant in control and vehicle groups during the whole study. Results of the dissection in the main organs revealed no obvious abnormality.
Visual inspection
The scoring criteria were based on Guidance for Irritation, Sensitization and hematolysis Testing of Chemical Drugs issued by the State Food and Drug Administration (SFDA). During the research period and the drug withdrawal period, the results of irritative reaction of cornea, conjunctiva, and iris are listed in Table 3. Within the normal group, the score indicated a dose-responsive increase, whereas no statistical difference between each subgroup was observed. The scores of the corneal transplantation group were significantly higher than the normal group. The scores of the corneal transplantation group were 6.5, 6.7, 7.0, 7.1, and 7.2 for saline group (group Ac), blank matrix group (group Bc), high-dose group (group Cc), medium-dose group (group Dc), and low-dose group (group Ec), respectively. There was no statistical difference between each subgroup. Considering that the scores of saline group (group Ac and group An) and blank matrix group (group Bc and group Bn) were not significantly diverged, the matrix of the suspension eye drop may not be the source of irritation. Due to the FK506 dose-responsive, but not statistically significant, increase of the score, it is indicated that FK506 might be somewhat irritating, yet the signs of irritation in each subgroup in the corneal transplantation group was possibly due to the corneal surgery. Thus, we suggested that little irritation was caused by the FK506 eye drop itself.
Table 3.
Total Scores of Each Subgroup in the Observation Period
| Subgroup | Total scores | Agregate number of observation | Intergral mean value |
|---|---|---|---|
| An | 1,074 | 2550 | 0.42 |
| Ac | 12,576 | 1936 | 6.5 |
| Bn | 1,362 | 2550 | 0.53 |
| Bc | 15,383 | 2280 | 6.7 |
| Cn | 1,428 | 2550 | 0.56 |
| Cc | 14,216 | 2044 | 7 |
| Dn | 1,438 | 2550 | 0.56 |
| DC | 13,355 | 1886 | 7.1 |
| En | 2,544 | 2550 | 1 |
| EC | 15,917 | 2214 | 7.2 |
n, Normal; c, corneal transplantation; An, Ac, saline; Bn, Bc, blank matrix; Cn, Cc, high dose; Dn, Dc, medium dose; En, Ec, low dose.
Histological inspection
The normal group
In both the first and second batches, a small amount of inflammatory cell infiltration was observed near the corneoscleral limbal. No obvious irritative reaction was observed in the whole eyeball.
The corneal transplantation group
In the first batch, infiltration by quite a few inflammatory cells was observed in the cornea in subgroup Ac, with two cases of local vascular proliferation, one case of iris synechia, and one case of corneal stromal edema. These are the morphologic features of a pathological healing process after corneal transplantation. In subgroup Bc, there was one case of stromal edema thickening with infiltration by quite a few inflammatory cells, and one case of a small amount of inflammatory cells infiltration near corneoscleral limbal. The pathological changes in subgroup Cc, Dc, and Ec were similar to that of subgroup Bc. In the second batch, the conditions of pathologic corneal stromal thickening, local inflammatory cell infiltration, and local avascular proliferation in all the subgroups were not significantly different from their counterpart in the first batch.
Systemic pharmacokinetic study
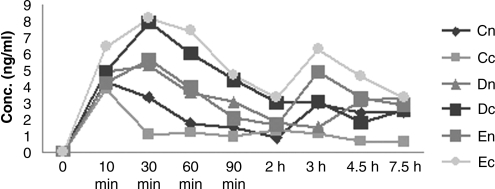
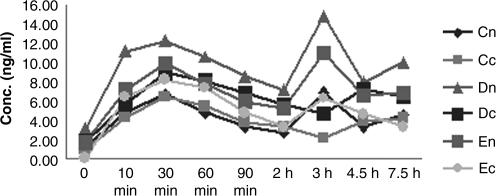
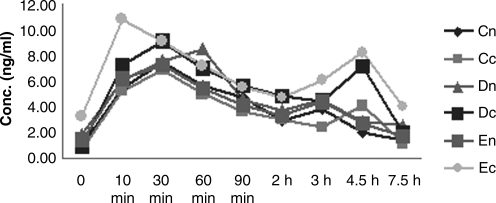
The results of systemic pharmacokinetic study are listed in Table 4. Cmax, t1/2, AUC0-t, and AUC0-∞ of FK506 in whole-blood samples were determined in day 1, day 15, and day 30. Figures 1, 2, and 3 show their curves for the mean concentration versus time, respectively. There were no unscheduled plasmin-related deaths or premature sacrifices during these two studies, and no treatment-related changes in appearance and behavior were observed. Body weights were also unaffected by treatment at any dose level in both groups.
Table 4.
FK506 Pharmacokinetics of Each Subgroup at Different Time Points
| |
|
Sampling time |
||
|---|---|---|---|---|
| Parameters | Subgroup | Day 1 | Day 15 | Day 30 |
| Cmax | Cn | 4.306±0.785 ng·mL−1 | 6.95±2.99 ng·mL−1 | 4.31±0.78 ng·mL−1 |
| Cc | 6.23±1.23 ng·mL−1 | 6.45±3.07 ng·mL−1 | 6.85±2.37 ng·mL−1 | |
| Dn | 5.62±2.01 ng·mL−1* | 12.31±3.88 ng·mL−1 | 8.49±5.91 ng·mL−1 | |
| DC | 6.98±3.25 ng·mL−1 | 9.00±4.83 ng·mL−1 | 9.15±3.45 ng·mL−1 | |
| En | 6.29±1.88 ng·mL−1* | 11.02±4.93 ng·mL−1 | 7.24±3.61 ng·h·mL−1 | |
| EC | 7.34±4.34 ng·mL−1 | 14.89±6.85 ng·mL−1 | 10.85±7.94 ng·h·mL−1 | |
| t½ | Cn | 4.36±1.32 h | 4.24±2.08 h | 4.29±1.55 h |
| Cc | 6.54±1.82 h | 6.36±2.82 h | 6.29±1.34 h | |
| Dn | 4.41±2.96 h | 4.56±2.81 h | 7.73±1.31 h | |
| DC | 7.13±1.96 h | 7.56±1.96 h | 6.73±1.31 h | |
| En | 3.75±1.35 h | 4.73±2.92 h | 7.77±1.23 h | |
| EC | 7.12±3.35 h | 8.12±3.35 h | 6.77±1.23 h | |
| AUC0-t | Cn | 27.189±11.36 ng·h·mL−1 | 32.08±13.21 ng·h·mL−1 | 32.56±13.27 ng·h·mL−1 |
| Cc | 28.654±13.36 ng·h·mL−1 | 27.97±14.32 ng·h·mL−1 | 24.00±13.04 ng·h·mL−1 | |
| Dn | 36.25±6.15 ng·h·mL−1 | 44.33±15.44 ng·h·mL−1 | 39.12±9.15 ng·h·mL−1 | |
| DC | 37.54±7.75 ng·h·mL−1 | 40.66±22.90 ng·h·mL−1 | 39.13±22.54 ng·h·mL−1 | |
| En | 38.17±7.61 ng·h·mL−1 | 72.08±117.96 ng·h·mL−1 | 25.01±12.24 ng·h·mL−1 | |
| EC | 39.27±5.61 ng·h·mL−1 | 48.09±31.22 ng·h·mL−1 | 47.23±36.01 ng·h·mL−1 | |
| AUC0-∞ | Cn | 20.95±7.19 ng·h·mL−1 | 47.92±19.11 ng·h·mL−1 | 47.98±15.00 ng·h·mL−1 |
| Cc | 25.77±18.56 ng·h·mL−1* | 34.95±7.19 ng·h·mL−1 | 50.98±15.00 ng·h·mL−1 | |
| Dn | 19.17±6.99 ng·h·mL−1* | 91.11±14.69 ng·h·mL−1 | 31.79±18.14 ng·h·mL−1 | |
| DC | 26.59±13.23 ng·h·mL−1 | 48.17±31.99 ng·h·mL−1 | 52.79±18.14 ng·h·mL−1 | |
| En | 19.50±3.65 ng·h·mL−1* | 97.40±16.39 ng·h·mL−1 | 37.35±10.61 ng·h·mL−1 | |
| EC | 30.50±18.54 ng·h·mL−1 | 70.50±42.65 ng·h·mL−1 | 67.35±10.61 ng·h·mL−1 | |
A significant difference in Cmax and AUC0-∞ was observed between normal and corneal transplant groups on say 1 (p<0.05).
n, Normal; c, corneal transplantation; Cn, Cc, high dose; Dn, Dc, medium dose; En, Ec, low dose.
FIG. 1.
Systemic FK506 concentration-time on day 1 after the first instillation. Cn, high dose in normal group; Cc, low dose in corneal transplantation group; Dn, medium dose in normal group; Dc, medium dose in corneal transplantation group; En, low dose in normal group; Ec, low dose in corneal transplantation group.
FIG. 2.
Systemic FK506 concentration-time on day 15. Cn, Cc, high dose; Dn, Dc, medium dose; En, Ec, low dose.
FIG. 3.
Systemic FK506 concentration-time on day 30 after the last instillation. Cn, high dose in normal group; Cc, low dose in corneal transplantation group; Dn, medium dose in normal group; Dc, medium dose in corneal transplantation group; En, low dose in normal group; Ec, low dose in corneal transplantation group.
Tissue distribution
After topical application of FK506 eye drops, FK506 distribution within various tissues at different time points was determined (Table 5). Cornea and conjunctiva have the highest FK506 concentration among tissues of all the subgroups.
Table 5.
FK506 Tissue Distribution after the Last Instillation
| Tissue | Subgroup | Concentration (ng/g) | Subgroup | Concentration (ng/g) |
|---|---|---|---|---|
| Cornea | Cn | 110.84±117.04 | Cc | 58.53±57.39 |
| Dn | 109.66±154.12 | DC | 62.02±63.79 | |
| En | 167.19±191.94 | EC | 65.87±99.20 | |
| Conjunctiva | Cn | 56.35±71.46 | Cc | 24.77±25.83 |
| Dn | 145.78±183.21 | DC | 34.36±35.80 | |
| En | 154.56±135.93 | EC | 141.28±183.74 | |
| Sclera | Cn | 31.11±32.14 | Cc | 28.95±44.86 |
| Dn | 55.34±68.20 | DC | 28.69±30.34 | |
| En | 56.60±73.03 | EC | 43.66±44.31 | |
| Iris | Cn | 8.71±10.75 | Cc | 5.86±6.50 |
| Dn | 20.87±24.20 | DC | 6.05±6.95 | |
| En | 21.90±28.49 | EC | 6.95±9.13 | |
| Vitreous Body | Cn | 0.88±1.44 | Cc | 0.90±1.59 |
| Dn | 1.84±2.80 | DC | 1.40±2.14 | |
| En | 2.07±3.69 | EC | 2.36±5.43 | |
| Retinal | Cn | 5.74±10.32 | Cc | 7.23±7.39 |
| Dn | 33.37±25.67 | DC | 12.11±16.25 | |
| En | 35.10±48.73 | EC | 13.76±15.95 |
n, Normal; c, corneal transplantation; Cn, Cc, high dose; Dn, Dc, medium dose; En, Ec, low dose.
Discussion
Rabbits are the most common species used for ocular toxicity testing because the rabbit eye is large enough for performing accurate ocular surgery or treatments. Our data in this study demonstrate that up to 0.1% FK506 [4 times per day (q.i.d.) topically] compared with the blank matrix did not display any ocular toxicity by pathological inspection and slit lamp biomicroscopy during 1 month of administration. To our knowledge, the safety of topically administrated FK506 has never been reported.
Previous studies have indicated that the effective serum concentration of FK506 in patients undergoing organ transplantation is 2–20 ng/mL.14 Pleyer et al. believed the same concentration of FK 506 might be considered as the minimal effective concentration for controlling immune-mediated eye diseases.15 However, the accurate concentration of FK506 required for controlling immune-mediated ocular diseases has not been established yet. Our previous studies have demonstrated that 0.05% FK506 eye drops were sufficient to suppress corneal allograft rejection in rabbits. Clinical investigation also suggested FK506 at this concentration at a q.i.d. interval showed satisfying outcome after corneal transplantation.
Although FK506 is 10–100 times more active than ciclosporin with regard to the inhibition of several immune responses, the side effect of FK506 is associated with nephrotoxic, diabetogenic, neurological, and cardiovascular effects.16 The pharmacokinetic parameters of FK506 show high inter- and intraindividual variability and, given that this drug has a narrow therapeutic index, therapeutic drug monitoring is necessary to optimize treatments of solid organ transplantation.16
Contrary to the results of Fujita et al.,17 we detected much higher concentrations of FK506 in whole-blood samples in different time points, with a minimum concentration of 4.31±0.79 ng/mL and a maximum concentration of 14.89±6.85 ng/mL in all the subgroups. Although the concentration of the FK506 eye drop is much higher in Fujita's study, Fujita et al. detected a maximum concentration of 3.7±0.5 ng/mL in the 1% FK506 ophthalmic suspension group. The discrepancy between the 2 studies may be due to different choices of detection methods in which the FK506 concentration of Fujita et al. was measured by enzyme-linked immunosorbent assay (ELISA), whereas in our study it was measured by LC-MS/MS.
On the basis of our study, Cmax, AUC0-t, and AUC0-∞increased in a dose-dependent way. However, as is shown in Table 4, there was no statistical difference between the 3 time points within each subgroup, i.e., no accumulation was detected during the study period. However, the increase from medium dose to high dose in the aforementioned parameters was not as large as that from low dose to medium dose. The reason may be that the amount of permeated FK506 is limited by the corneal barrier. This study indicates that the system absorption after topical instillation ranged from 4.31±0.79 ng/mL to 14.89±6.85 ng/mL, which have both reached the systemic minimum effective concentration in whole blood; i.e., systemic adverse effects are possible under the current administration regime, although we did not find any organ abnormity in this study.
In terms of ocular tissue distribution, concentrations of FK506 in the corneal transplant group were significantly lower compared to normal group in cornea, conjunctiva, and sclera (p<0.05). The reason might be that the corneal barrier was damaged in the corneal transplantation group; thus, the absorption would be much higher than the normal group. So in the anterior segment of the eye, concentrations of FK506 in the normal group would be much higher than in the transplantation group. Most of the FK506 molecule was detained in the conjunctiva sac; the anterior segment including the cornea, conjunctiva, and sclera served as “reservoirs” of FK506, resulting in the significantly higher concentration compared to the transplantation group. We have investigated single-dose and multiple-dose pharmacokinetics of FK506 in aqueous humor in normal rabbits previously.12 The Cmax values of aqueous humor after single- and multiple-dose administration were 31.40±9.32 ng·mL−1 and 37.73±11.25 ng·mL−1, respectively. The concentration of FK506 in the cornea at 60, 100, and 240 min after single-dose administration was 402.0 ng/g, 363.8 ng/g, and 220 ng/g, respectively. These results further support the idea that when the cornea is intact many FK506 molecules are detained in the anterior segment and the amount of systemic absorption is lowered.
This study was performed in normal rabbit eyes as well as in corneal-transplanted rabbit eyes to determine whether the absorption of FK506 is altered after corneal transplantation. On the basis of our study, the differences in Cmax and AUC0-∞ were significant on day 1 but not on days 15 and 30. The possible interpretation is that the corneal graft was almost healed with the corneal recipient bed 15 days after the corneal transplantation; the FK506 penetration would be similar to the normal group. In conclusion, our study suggests that up to 0.1% FK506 administered q.i.d. topically is safe for the rabbit eye.
Acknowledgements
This work was supported by Fundamental Research of Funds of State of Key Lab (grant number: 303060202400413) and National Natural Sciences Foundation of China (grant number: 30801263).
References
- 1.Kino T. Hatanake H. Hashimoto M., et al. FK-506, a novelimmunosuppressant isolated from a Streptomyces. I. Fermentation,isolation, and physico-chemical and biological characteristics. J. Antibiot. (Tokyo) 1987;40:1249–1255. doi: 10.7164/antibiotics.40.1249. [DOI] [PubMed] [Google Scholar]
- 2.Jensik S.C. Tacrolimus (FK 506) in kidney transplantation: Three-year survival results of the US multicenter, randomized, comparative trial. FK 506 Kidney Transplant Study Group. Transplant Proc. 1998;30:1216–1218. doi: 10.1016/s0041-1345(98)00216-4. [DOI] [PubMed] [Google Scholar]
- 3.O'Grady J.G. Burroughs A. Hardy P., et al. Tacrolimus versus microemulsified ciclosporin in liver transplantation: The TMC randomised controlled trial. Lancet. 2002;360:1119–1125. doi: 10.1016/s0140-6736(02)11196-2. [DOI] [PubMed] [Google Scholar]
- 4.Griffith B.P. Bando K. Hardesty R.L., et al. A prospective randomized trial of FK506 versus cyclosporine after human pulmonary transplantation. Transplantation. 1994;57:848–851. doi: 10.1097/00007890-199403270-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ligtenberg G. Hene R.J. Blankestijn P.J., et al. Cardiovascular risk factors in renal transplant patients: Cyclosporin A versus tacrolimus. J. Am. Soc. Nephrol. 2001;12:368–373. doi: 10.1681/ASN.V122368. [DOI] [PubMed] [Google Scholar]
- 6.Textor S.C. Weisner R. Wilson D.J., et al. Systemic and renal hemodynamic differences between FK506 and cyclosporine in liver transplant recipients. Transplantation. 1993;55:1332–1339. doi: 10.1097/00007890-199306000-00023. [DOI] [PubMed] [Google Scholar]
- 7.Friemann S. Feuring E. Padberg W., et al. Improvement of nephrotoxicity, hypertension, and lipid metabolism after conversion of kidney transplant recipients from cyclosporine to tacrolimus. Transplant Proc. 1998;30:1240–1242. doi: 10.1016/s0041-1345(98)00226-7. [DOI] [PubMed] [Google Scholar]
- 8.Shaw K.T.Y. Ho A.M. Raghavan A., et al. Immunosuppressive drugs prevent a rapid dephosphorylation of transcription factor NFAT 1 in stimulated immune cells. Proc. Natl. Acad. Sci. USA. 1995;92:11205–11209. doi: 10.1073/pnas.92.24.11205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruff V.A. Leach KL. Direct demonstration of NFATp dephosphorlation and nuclear localization in ctivated HT-2 cells using a specific NFATp polyclonal antibody. J. Biol. Chem. 1995;270:22602–22607. doi: 10.1074/jbc.270.38.22602. [DOI] [PubMed] [Google Scholar]
- 10.Tamura K. Fujimura T. Iwasaki K., et al. Interaction of tacrolimus(FK506) and its metabolites with FKBP and calcineurin. Biochem. Biophys. Res. Commun. 1994;202:437–443. doi: 10.1006/bbrc.1994.1947. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi C. Kanai A. Nakajuima A., et al. Suppression of corneal graft rejection in rabbits by a new immunosuppressive agent, FK-506. Transplant Proc. 1989;21(1 Pt 3):3156–3158. [PubMed] [Google Scholar]
- 12.Yuan J. Zhai J.J. Chen J.Q., et al. Preparation of 0.05% FK506 suspension eyedrops and its pharmacokinetics after topical ocular administration. J. Ocular Pharmacol. Ther. 2009;25:345–350. doi: 10.1089/jop.2008.0125. [DOI] [PubMed] [Google Scholar]
- 13.Yuan J. Chen J.Q. Xie Z.Y., et al. Determination of tacrolimus in rabbit aqueous humor by liquid chromatography-electrospray ionization tandem mass spectrometry. J. Chromatogr. B. 2008;868:34–41. doi: 10.1016/j.jchromb.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 14.Thomson AW. Interspecies comparison of the immunosuppressive efficacy and safety of FK506. Transplant Proc. 1990;22:100–105. [PubMed] [Google Scholar]
- 15.Pleyer U. Lutz S. Juske W.J., et al. Ocular absorption of topocally applied FK506 from liposomal and oil formulations in the rabbit eye. Invest. Ophthalmol. Vis. Sci. 1993;34:2737–2742. [PubMed] [Google Scholar]
- 16.Scott L.J. McKeage K. Keam S.J., et al. Tacrolimus: A further update of its use in the management of organ transplantation. Drugs. 2003;63:1247–1297. doi: 10.2165/00003495-200363120-00006. [DOI] [PubMed] [Google Scholar]
- 17.Fujita E. Teramura Y. Shiraga T., et al. Pharmacokinetics and tissue distribution of tacrolimus (FK506) after a single or repeated ocular instillation in rabbits. J. Ocul. Pharmacol. Ther. 2008;24:309–319. doi: 10.1089/jop.2007.0083. [DOI] [PubMed] [Google Scholar]