Abstract
Residential radon has been found to be associated with lung cancer in epidemiological/ecological studies and the researchers have inappropriately concluded that residential radon causes lung cancer. Their conclusion relates to the linear-no-threshold (LNT) hypothesis-based, risk-assessment paradigm; however, the LNT hypothesis has been invalidated in numerous studies. It is shown in this paper that our hormetic relative risk (HRR) model is consistent with lung cancer data where detailed measurements of radon in each home were carried out. Based on the HRR model, low-level radon radioactive progeny is credited for activated natural protection (ANP) against lung cancer including smoking-related lung cancer. The proportion B(x) (benefit function) of ANP beneficiaries increases as the average radon level x increases to near the Environmental Protection Agency’s action level of 4 picocuries/L (approximately 150 Bq m−3). As the average level of radon increases to somewhat above the action level, ANP beneficiaries progressively decrease to zero (B(x) decreases to 0), facilitating the occurrence of smoking-related lung cancers as well as those related to other less important risk factors. Thus, residential radon does not appear to cause lung cancer but rather to protect, in an exposure-level-dependent manner, from its induction by other agents (e.g., cigarette-smoke-related carcinogens).
Keywords: residential radon, lung cancer, risk, hormesis
INTRODUCTION
Exposure over years to radon gas (Rn-222 and its radioactive progeny) has been found to be associated with lung cancer. Radon progeny emit both highly ionizing alpha particles and sparsely ionizing gamma rays. Rn-222 (physical half-life 3.8 d) arises as a decay product of radium-226 (Ra-226), which is widely dispersed in rocks and soil. Rn-222 migrates from rocks and soil into homes where it accumulates in confined spaces (e.g., basements). Whether radon is considered to pose an indoor health risk depends on the model used to assess health impacts and also on how data are manipulated (i.e., adjusted). Throughout this paper references to radon exposure should be interpreted to include radioactive progeny.
The linear-no-threshold (LNT) model is widely used for assessing the risk of cancer (including lung cancer) from low-level exposure to ionizing radiation and was initially adopted on the basis of high-dose data for mutation induction being misrepresented as an LNT function of radiation dose (Calabrese 2011). New low-dose data invalidate the LNT model for mutation induction (Ogura et al. 2009; Cuttler 2010). A hormetic relationship was obtained.
The BEIR VI report (USNRC 1999) analyzed pooled data from 11 cohort studies of lung-cancer from radon exposure of underground miners using an LNT model of the excess relative risk (ERR). With this approach, only positive values for ERR are allowed. Because miner exposure levels are typically much larger than for residential exposures of people, the extrapolation of risk to those lower residential exposures involves considerable uncertainty. This includes statistical uncertainty and more importantly model uncertainty. Here model uncertainty means uncertainty about what is the appropriate exposure-response model to use for low-level radon exposure.
Studies of an association of lung cancer with residential radon exposure avoid high-to-low level exposure extrapolation; however, model-uncertainty-associated issues emerge that relate to application of methods designed based on the current risk-assessment paradigm for high-level exposure where ERR > 0. For example, it is now known that exposure to low doses of gamma rays alone or in combination with low-dose alpha radiation (as is the case for radon progeny) stimulates radiation adaptive responses that suppress lung cancer induction by other risk factors (e.g., cigarette smoke) (Scott et al. 2009). Thus, the standard epidemiological study methods based on the current risk-assessment paradigm could introduce significant systematic error in the results obtained because it is then possible for ERR < 0 (i.e., negative ERR). The negative value for ERR arises because of low-dose, sparsely-ionizing-radiation activated natural protection (ANP) against cancer induction by other risk factors (e.g., smoking) can lead to RR < 1, a hormetic response (Scott et al. 2008).
Low dose radiation ANP is widely known to occur and includes DNA (deoxyribonucleic acid) double-strand break repair, p53-related apoptosis of damaged cells, epigenetically-regulated apoptosis (epiapoptosis) of precancer cells, and presumably epigenetically regulated anticancer immunity (Scott et al. 2009). In the case of smoking-related lung cancer, radiation ANP also includes suppression of cigarette-smoke-related cytokines and chemokines that promote lung cancer occurrence (Chen et al. 2011; Gonzales et al. 2011; Gott et al. 2011).
CURRENT CANCER RISK ASSESSMENT PARADIGM
Linear-No-Threshold Model
With the current radiation risk assessment paradigm, harm from radiation exposure is the main outcome. The terms “risk,” “risk function,” “hazard,” “hazard function,” “relative risk (RR),” and “ERR” are all based on the notion that harm from radiation exposure is the main outcome. This view relates closely to the LNT hypothesis and related LNT model. According to the LNT model, any radiation dose no matter how small is presumed to cause cancer (significant harm) in at least one person in a very large population when each member of the population receives a radiation dose. The ERR is presumed to increase linearly as dose increases without a threshold.
Significant harm is therefore the focus of the risk assessment paradigm and no radiation benefit is usually considered. As an example of the use of this paradigm, the U.S. Environmental Protection Agency (EPA) based their 2003 reassessment of lung cancer risk from residential radon exposure on the BEIR VI report and the LNT model (USEPA 2003). More recently, the U.S. EPA, the General Services Administration, and the departments of Agriculture, Defense, Energy, Health and Human Services, Housing and Urban Development, Interior, and Veterans Affairs joined forces to supposedly help save lives from residential radon exposure. This is based on the belief by many that residential radon exposure is a leading cause of non-smoking lung cancer and leads to an estimated 21,000 deaths each year.
Numerous studies of LNT-based ERR for lung cancer from radon exposure (including residential) have been reported for different populations (Blot et al. 1990; Stidley and Samet 1993; Alavanja et al. 1994, 1999; Letourneau et al. 1994; Cohen 1995, 1997; Auvinen et al. 1996; Lubin and Boice 1997; USNRC 1999; Fisher et al. 1998; Field et al. 2000; UN 2000, 2006; Kreuzer et al. 2003; Lubin 2003; Pavia et al. 2003; USEPA 2003; Baysson et al. 2004; Lubin et al. 2004; Bochicchio et al. 2005; Darby et al. 2005; Krewski et al. 2005, 2006; Wichmann et al. 2005; Sandler et al. 2006; Thompson et al. 2008). While many of the studies of residential exposure to relatively low-level radon report a calculated positive ERR, the 95% confidence intervals (CIs) often included ERR < 0 (implicating a possible threshold or hormetic response) (Thompson et al. 2008). A negative ERR has also been implicated for at least one study of miners exposed to radon (data evaluated in this paper).
In conducting studies of radon-associated lung cancer, researchers generally adjust for both risk factors (those factors that are known to promote lung cancer occurrence) and what is here called benefit factors (those factors known to suppress lung cancer occurrence). However, there is growing concern about analytical methods used in epidemiological and ecological studies that can distort the true shape of the dose-response curve, especially for low-level radiation exposure (e.g., residential radon exposure) where hormetic effects can occur and lead to a negative ERR.
Procedures Used in Epidemiological and Ecological Studies that Abolish Thresholds and Hormetic Responses
Recently, several procedures used in epidemiological and ecological studies of radiation-associated cancer have been recognized to abolish thresholds and hormetic responses. The procedures include the following (Scott et al. 2008):
Including persons exposed to protective doses in the unexposed or reference group (as may be the case for all studies of residential-radon-associated lung cancer).
Radiation dose (absorbed or effective dose) lagging, making smaller doses appear more harmful than they actually are.
Averaging over wide dose or exposure level intervals (as is done in most studies of radon-associated lung cancer), possibly removing non-linearity.
Including high-dose (or exposure level) data and forcing LNT-based extrapolation to low doses or low exposure levels (as is done in some studies or radon-associated lung cancer in miners).
Inappropriately attributing adaptive protection to a healthy-worker effect (Fornalski and Dobrzynski 2010).
In addition to the above, for low-dose or low-level exposure data for which ERR < 0 (i.e., hormetic response), adjusting for confounding risk factors (e.g., smoking) would be expected to reduce the magnitude of ERR attributed to radiation (Lubin 1988). This in turn would be expected to artificially increase the calculated RR since RR = 1+ ERR and ERR is negative in the hormetic zone. For the hormetic zone the indicated adjustment is equivalent to adding back risk that was removed via radiation ANP. The adding back of risk could abolish hormetic responses and thresholds as is illustrated in this paper. The risk factor adjustment problem is expected to apply to all cancer-facilitating factors other than radiation that are adjusted for in the hormetic zone when assessing radiation effects.
For low-dose (or low-level exposure) data where adaptive protection occurs and where ERR > 0, adjusting for smoking and other risk factors could also cause lung cancer RR attributed to radon to increase (a systematic error), via reintroducing risk already eliminated via natural protection. Thus, it is important to pay attention to the consequences of data adjustments. If the adjustment increases cancer RR or odds ratio (OR) or hazard ratio in the case where it should decrease (e.g., adjusting for smoking effects when radiation harm and smoking harm are additive or multiplicative), then there may be a serious problem with the adjustment procedure.
Zero Dose Group Issue for Radon
Another important issue related to studies of residential-radon-associated lung cancer is that there is no true zero exposure group (unexposed group). Everyone is exposed to some radiation from radon progeny. Thus, what one generally does is to use the lowest exposure group as a reference group (unexposed); however, for studies of an association between residential radon exposure and lung cancer, the reference group may actually be in the hormetic zone where radon ANP suppresses cancer induction by other agents. If so, a hormetic response may not be apparent, depending on the magnitude of the hormetic effect for the reference group. If the hormetic effect for the reference group is less than for higher dose groups, then a hormetic effect may be revealed as was demonstrated by Thompson et al. (2008) and Thompson (2011); otherwise, the dose-response curve can increase monotonically and appear to be of the LNT type. Ideally, one would want to use an analytical procedure that permitted treating exposure level as a continuous variable (e.g., using individual-specific exposure levels). This has been recently done with results obtained indicating a strong hormetic dose-response relationship (Thompson et al. 2008; Thompson 2011; data discussed in the next section).
HORMETIC RESPONSE FOR RESIDENTIAL-RADON-ASSOCIATED LUNG CANCER
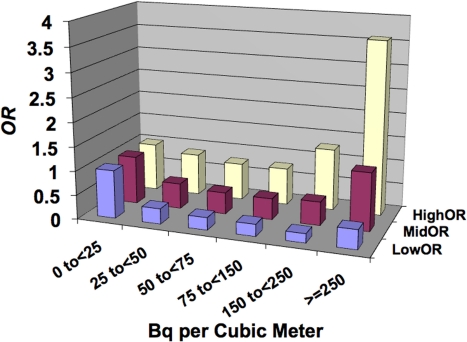
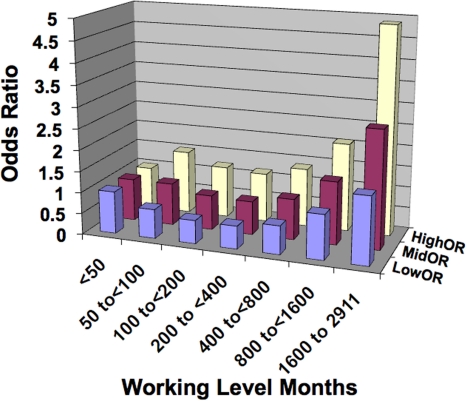
The case-control study of lung cancer risk from residential radon exposure (and its radioactive progeny) performed by Thompson et al. (2008) involved 200 cases (58% male, 42% female) and 397 controls that were matched based on age and sex. All were from the same health maintenance organization. Emphasis was placed on accurate and extensive year-long, in-home dosimetry with etch-track detectors in conjunction with careful questioning about historic patterns of in-home mobility. Here the interest is in unadjusted OR for lung cancer as reported in a more recent paper (Thompson 2011). The data are presented in Figure 1 as a bar graph with bars for the 95% CI values also shown. Note the hormetic shape to the data.
FIGURE 1.
Unadjusted odds ratio (OR) for lung cancer for residential exposure to radon progeny based on Thompson (2011). MidOR, unadjusted OR; LowOR, lower 95% confidence interval value; HighOR, upper 95% confidence interval value. Results are based on the univariate analysis of Thompson (2011) with no adjustment for other risk factors.
Thompson et al. (2008) also used conditional logistic regression in order to control for years of residency, smoking, education, income, and years of job exposure to known or potential carcinogens. Smoking was accounted for by nine categories: never smokers, four categories of current smokers, and four categories of former smokers. Radon exposure was initially divided into six categories (model 1) with break points at 25, 50, 75, 150, and 250 Bq m−3, the lowest group being the reference. The adjusted odds ratios (AORs) were respectively 1.00, 0.53, 0.31, 0.47, 0.22, and 2.50 with the third category (0.31) significantly below 1.0 (p < 0.05), and the second (0.53), fourth (0.47), and fifth (0.22) categories approaching statistical significance (p < 0.1). Thompson et al. (2008) also applied an alternate analysis using natural cubic splines which allowed calculating AORs as a continuous function of radon exposure. That analysis produced AORs that were substantially less than 1.0 (p < 0.05) for radon levels between approximately 85 and 123 Bq m−3.
The results indicated that for radon levels up to and somewhat exceeding the Environmental Protection Agency’s action level of 4 picocuries/L of air (approximately 150 Bq m−3), lung cancer OR was < 1, implicating negative values for ERR (not permitted under the LNT model). Thus, rather than finding an LNT-type response, a hormetic response was revealed as was also reported in earlier studies by Cohen (1995, 1997).
Because adjusting for other lung cancer risk factors in the hormetic zone may distort the dose-response curve shape, analysis carried out in this paper for central risk estimates are based on the unadjusted data in Figure 1. The presence of a hormetic zone indicates that beneficial effects (reduction in lung cancer risk) are far more likely than is harm. Rather than focusing on concepts such as risk, risk function, hazard, hazard function, hazard ratio, etc., for the hormetic zone, these concepts need to be replaced by concepts that relate to benefit. This is done in the next section which relates to our hormetic relative risk (HRR) model.
HORMETIC RELATIVE RISK MODEL AND RADIATION BENEFITS
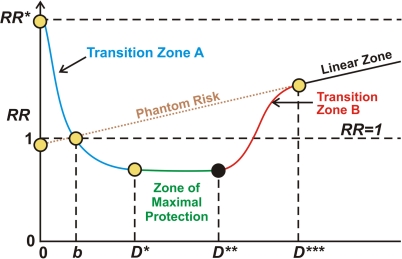
For the hormetic zone there is a radiation benefit paradigm as apposed to a radiation risk paradigm. Low doses of low-linear-energy transfer (LET) or low- plus high-LET radiation are much more likely beneficial than harmful (Scott et al. 2009). With our hormetic relative risk (HRR) model (Figure 2) for radiation related cancer, there are four different dose (or exposure level) regions of interest: Transition Zone A, Zone of Maximal Protection, Transition Zone B, and Linear Lone (Scott et al., 2008). These zones are explained in the following sections. D as used here is radiation dose or exposure level or exposure rate (e.g., for continuous lifetime exposure). The variable b is the background (residential radon excluded) radiation level at the location of interest. RR=1 at the natural background exposure level for the location of interest. RR* is the relative risk in the absence of any radiation exposure, natural or otherwise. The reference to phantom risk relates to use of the invalid LNT hypothesis to extrapolate from high to low radiation doses.
FIGURE 2.
Schematic representation of cancer relative risk (RR) under the hormetic relative risk (HRR) model. D is radiation dose or exposure level or exposure rate (for continuous lifetime exposure). The variable b is the natural background radiation level (residential radon excluded). RR = 1 at the natural background exposure level. RR* is the relative risk in the absence of any radiation exposure. The reference to phantom risk relates to use of the invalid linear-no-threshold LNT hypothesis to extrapolate from high to low radiation doses.
Transition Zone A
Transition Zone A includes the ultra low dose (or exposure level) region and allows for doses (or exposure levels) less than current natural background. Stochastic threshold doses (which vary for each person) for ANP occur in this zone and the protective processes turned on are presumed to be epigenetically activated (Scott et al. 2009). For residential radon, cancer RR (population average) can be evaluated for this zone as well as for the Zone of Maximal Protection and Transition Zone B based on the following equation that was recently introduced (Scott et al. 2011) to replace a more complicated approach initially proposed (Scott 2007):
| (1) |
B(x) is the benefit function, which represents the adaptive protection probability. The independent variable x is the dose metric (e.g., average radon concentration). The protection factor, PROFAC, is the average over the at-risk population of the individual-specific protection factor, profac, and is evaluated relative to natural background exposure. Its’ value is expected to increase when evaluated relative to the absence of any ionizing radiation (absolute zero radiation). Thus, x as used in Equation 1 represents dose (or exposure) in excess of natural background.
The individual-specific profac represents the conditional probability of cancer prevention via radiation ANP, given that ANP has been stimulated by radiation exposure. Thus, the occurrence of ANP does not guarantee cancer prevention. Because profac is assumed to depend on genetic and epigenetic characteristics of the individual, PROFAC is predicted to differ for different heterogeneous populations as has been demonstrated for radiation ANP against lung cancer (Sanders and Scott 2008). A value of PROFAC = 0.6 for example means that on average 60% of smoking-related lung cancers that would normally occur in the future would be expected to be prevented via radiation ANP when protection occurs with probability B(x). The 60% value would also apply to lung cancers caused by other risk factors. Once radon ANP occurs, the biological processes that protect against smoking-related lung cancer would be expected to also protect against other cancer types caused by smoking (e.g., oral cancer); however, PROFAC differs for each type of cancer.
The PROFAC is considered to apply only to low-LET radiation such as X-rays, gamma rays, and beta radiation. However, for combined exposure to alpha, beta, and gamma radiations (as occurs for natural radiation exposure in the home), PROFAC relates to the beta and gamma radiations involved. For residential radon exposure, it is assumed that the low-LET radiation dose is proportional to the radon level x in the home. A similar assumption also applies to the alpha radiation dose.
The benefit function B(x) increases as x increases over the Transition Zone A, causing RR(x) to decrease.
Zone of Maximal Protection
For the zone of maximal protection, stochastic thresholds for all adaptive responses are exceeded for everyone and thus full radiation benefit is expected, i.e., B(x) = 1. The cancer RR is independent of x for this zone and takes on the value 1 – PROFAC. The width of this zone is considered to be dose-rate (low-LET) dependent (Tanooka 2011). For residential radon exposure and lung cancer, the zone of maximal protection may be rather wide (Thompson 2011).
Transition Zone B
For transition zone B, stochastic thresholds for epigenetic silencing (episilencing) of adaptive-response genes occur and when exceeded leads to loss of anticancer protection (Scott et al. 2008). Suppression of anticancer immunity is expected in some but not necessarily all. In addition, induction of significant numbers of cancer-facilitating mutations is also expected for this dose zone. B(x) decreases from 1 to 0 as x increases over the dose zone. The rise in RR(x) relates at least in part to a loss of ANP against cancer induction by other agents such as cigarette smoke. The loss of ANP is manifested in a decrease in ANP beneficiaries.
High Dose Linear Zone
The high-dose linear zone is the dose regions where most previous epidemiological studies or radiation associated cancer were conducted. Stochastic thresholds for episilencing of adaptive response genes are assumed to be exceeded for everyone. Anticancer immunity is also assumed to be suppressed in everyone (and B(x) = 0), facilitating cancer occurrence (e.g., smoking related lung cancer). In addition, induction of cancer facilitating mutations increases as x increases for this zone. The ERR for induced cancer is assumed to be given by the LNT equivalent relationship:
| (2) |
The RR is therefore given by the following equation:
| (3) |
The function ERR(x) represents the ERR. The parameter K is the slope parameter and R0 is the baseline cancer frequency. Equations 2 and 3 apply to survivors of acute effects
RESULTS OF APPLYING THE HRR MODEL TO DATA
This section focuses on evaluating radon benefits so far as preventing lung cancer from cigarette smoke carcinogens and other carcinogens. The results should be viewed from the perspective that for low-level radon exposure, the gamma-ray component to the radiation dose leads to ANP over a prolonged period. This is thought to be achieved via repeatedly stimulating the transient protective processes that suppress cancer occurrence.
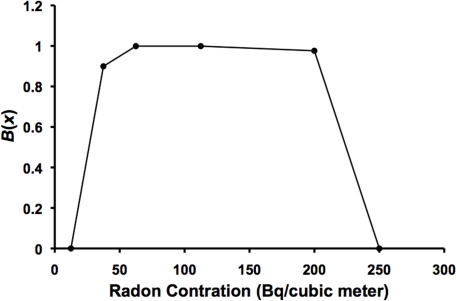
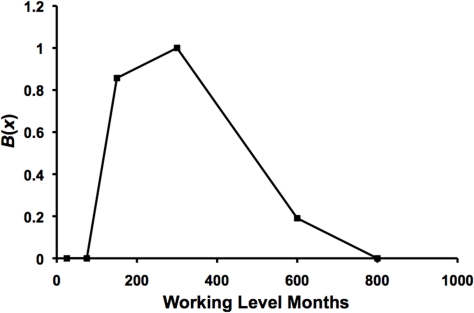
The residential radon benefit B(x) for lung cancer suppression has been estimated (point estimates) based on the univariate analysis (no adjustments for competing risk factors) results of Thompson (2011) in Figure 1. The results obtained are presented in Figure 3. B(x) was calculated as (1 – OR)/PROFAC and plotted vs. the midrange radon level in Bq m−3 except for the highest exposure level where the lowest value for x (250 Bq m−3) for the group was used since B(x) = 0. PROFAC was estimated to be 0.52 ± 0.04, based on the data in Figure 1 for OR = 0.53, 0.45, 0.44, and 0.49 for the four levels just above the reference level. These levels were assumed to be in the Zone of Maximal Protection where B(x) = 1. PROFAC was calculated as the average of 1 – OR, since OR estimates RR. B(x) increases from zero to 1 (Transition Zone A) as x increases and reaches a plateau (zone of maximal protection) and then decreases to zero (Transition Zone B) as x increases further. Interestingly, for the highest exposure group in Figure 1, OR increased by a factor of 2 when Thompson (2011) adjusted for other risk factors (including smoking), suggesting that B(x) is greater than zero even for the highest exposure group in Figure 3 (B(x) is assigned the value of zero in the figure for this group). The indicated adjustment effect is in the wrong direction (should have led to a reduced OR attributed to radon) suggesting that the adjustment reintroduced cancer risk that was eliminated via radon ANP.
FIGURE 3.
Benefit function B(x) (central estimate) for radon progeny activated natural protection (ANP) against lung cancer. Data points are based on central estimates of lung cancer odds ratio (OR) in Figure 1. Data points were connected by straight lines.
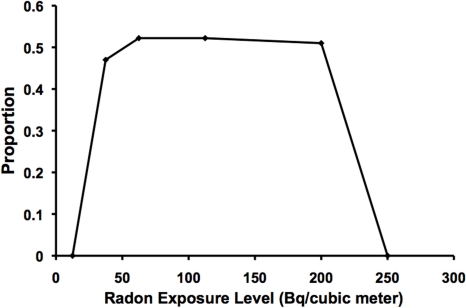
To obtain an estimate of the probability of lung cancer prevention via radon ANP, B(x) in Figure 3 should be multiplied by PROFAC (central estimate of 0.52). The results obtained are presented in Figure 4. Optimum protection from smoking (and other carcinogen) related lung cancer is inferred to occur for average residential radon levels of 50 to 200 Bq m−3. With optimum protection, > 50% of sporadic lung cancers (e.g., smoking related) that would occur in the absence of radon ANP would be expected to be prevented as a result of residential radon exposure. This finding is consistent with those reported by others (Cohen 1995, 1997; 2008; Thompson et al. 2008; Thompson 2011)
FIGURE 4.
Estimates of the proportion of sporadic lung cancer cases prevented by prolonged residential exposure to radon, based on data from Thompson (2011).
For persons residing in homes with radon levels near the United States Environmental Protection Agency’s action level of 4 picocuries/L (approximately 150 Bq m−3), eliminating radon from the home or moving into another home with a much lower radon level could substantially increase the risk of lung cancer because of the loss of radon ANP. However, for persons residing in homes with radon levels much higher than the action level (e.g., > 250 Bq m−3), eliminating radon such that the level was reduced to near the action level would be expected to substantially reduce the risk of lung cancer. The reduction would be due to added radon ANP against cancer caused by smoking and other carcinogens. However, reducing the radon level even further could then lead to a substantial increase in lung cancer risk due to a loss in radon ANP against cancer caused by other agents.
DISCUSSION
The results discussed in the above sections are based on the somewhat crude analyses used. A more formal analysis is required to obtain improved estimates of B(x) and PROFAC.
As previously indicated, the current LNT-hypothesis-based risk assessment paradigm is used for evaluating likely impacts of residential radon exposure of humans. With this paradigm, any radiation exposure would be calculated to cause harm (lung cancer) to at least one person among any large population under study. Thus, the terminology used under this paradigm includes risk, risk function, relative risk, hazard, hazard function, hazard ratio and other terminology. Further, the adjustment methodologies used in epidemiological studies focus on competing risk factors. For cases where both smoking and radon exposure are involved, one can supposedly adjust to eliminate the smoking influence. In doing so, the unadjusted ERR is expected to be positive and also to be larger before than after adjusting for smoking. Thus, after adjusting for smoking, the ERR attributed to radiation exposure should be less. This works so long as ERR is positive and ANP does not occur. However, for low-level exposure to radon, the data are supportive of a hormetic response rather than an LNT response (Thompson et al. 2008; Thompson 2011), in which case ERR can be negative because of a significant loss in the risk of sporadic cancers due to radiation ANP. In this case and for the hormetic zone, RR(x) is given by the following:
| (4) |
Adjusting for competing risk factors (e.g., smoking) would be expected to reduce the magnitude of ERR(x) and thereby increase RR(x) since ERR(x) equals – PROFAC•B(x) and is therefore negative. This is equivalent to adding back risk (e.g., from smoking related cancer) that has been eliminated via radiation ANP.
Data of Turner et al. (2011) clearly illustrate the phenomenon of adding back risk via risk-factor adjustment. They conducted a cohort study of the association between residential radon exposure and lung cancer mortality. The study involved nearly 1.2 million Cancer Prevention Study-22 participants that were recruited in 1982. Mean county-level residential radon concentrations were linked to study participants according to Zip code information ant enrollment. Multivariate Cox proportional hazard regression models were used to obtain adjusted hazard ratios and 95% CI intervals. The three categories of adjustments that were employed are as follows:
Minimally-adjusted: age, race, and gender stratified.
Fully-adjusted-1: age, race, gender stratified and adjusted for education, martial status, body mass index, body mass index squared, cigarette smoking status, cigarettes per day, cigarettes per day square, duration of smoking, duration of smoking squared, age started smoking, passive smoking, vegetable/fruit/fiber consumption, fat consumption, industrial exposures, occupation dirtiness index.
Fully-adjusted-2: same as for Fully-adjusted-1 but also state stratified.
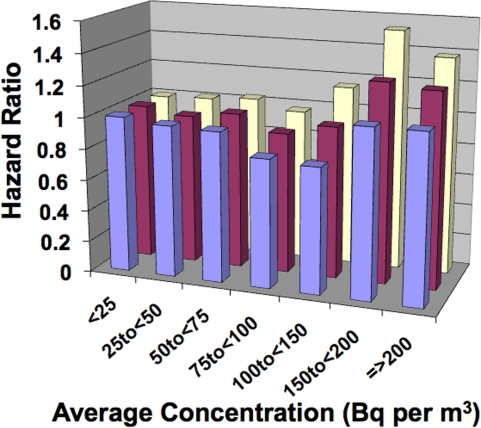
Interesting, for the minimally-adjusted group, a negative ERR was implicated (i.e., hormetic response) and the dose-response pattern (Figure 5) is similar to that presented in Figure 1. Each of the two other levels of adjustment appeared to add back lung cancer mortality risk that was removed by radon ANP. The authors attributed the increase in the hazard ratio with increasing radon level to radon-induced cancer, while based on the analysis presented in this paper, the increase may in fact relate mainly to the loss of radon progeny ANP against smoking-related lung cancer with the level of protection decreasing as the exposure level increased. This is especially true for radon exposure levels somewhat above 150 Bq m−3 (Transition Zone B in the HRR model [see Figures 3 and 4]). Thus, the increase in lung cancer incidence or mortality reported by others for residential radon exposure may not be caused by radon radioactive progeny induced damage but rather by the loss of radon ANP against lung cancer induction by smoking and/or other risk factors. The misinterpretation of the data by others appears to be linked to the LNT hypothesis whereby any amount of radiation is considered harmful, even small amounts from residential radon progeny.
FIGURE 5.
Hazard ratio for lung cancer mortality from residential radon exposure based on data from Turner et al. (2011). Front row bars (minimally-adjusted), middle bars (fully-adjusted-1), rear bars (fully-adjusted-2).
Now there is abundant evidence against the validity of the LNT hypothesis and therefore against the LNT model, especially for exposure to low doses and dose rates of low-LET radiation or low- plus high-LET radiation (as is the case for residential exposure to radon progeny). The abundant evidence against the LNT hypothesis is discussed in a number of recent papers (Mitchel et al. 2003; Sakai et al. 2003; Nowosielska et al. 2006; Liu 2007; Chen et al. 2007; Feinendegen et al. 2007; Tubiana 2008; Cohen 2008; Jaworowski 2008; Scott 2008; Scott et al. 2008; Thompson et al. 2008; Averbeck 2009; Ogura et al. 2009; Tubiana et al. 2009; Tanooka 2001, 2011; Feinendegen 2010; Sanders 2010).
It is clear from numerous studies of miners exposed to radon that chronic exposure to high levels of radon is associated with lung cancer (UN 2006). The positive associations have been interpreted to be evidence for lung cancer being caused by radon and radon-decay products; however, such associations are not proof of causality.
Several previous publications (Lubin and Boice 1997; USNRC 1999; United Nations 2000; NCRP 2004; UN 2006) describe numerous case-control studies related to an association between residential radon level and lung cancer occurrence. Where positive associations were obtained, the researchers also interpreted the results as evidence for lung cancer being caused by chronic exposure to residential radon. Again, such associations are not proof of causality, even though they are consistent with the LNT risk model.
Stidley and Samet (1993) reviewed 15 ecological studies from a number of countries of residential radon exposure. A positive association between radon concentration and lung cancer was found for 7 studies, no association was found for 6 studies, and a statistically-significant hormet-ic response was found for 2 studies. The indicated results should be sufficient for questioning both the assumption that residential radon causes lung cancer and the validity of LNT risk model. This is particularly important because with the HRR risk model, one can get a positive association, no association, or a negative association between residential radon and lung cancer occurrence, depending on how the reference exposure group (assigned unexposed group) is selected relative to the zone or maximal protection. If the radon level for the reference group is significantly below the zone of maximal protection (x < D*; Figure 2), then a negative association (hormetic effect) would be expected for a range of radon exposure levels for the test groups. If the reference group is within the zone of maximal protection (D* < x < D**), then no association may be observed for a range of exposure levels for the test groups if they also fall within the indicated zone. If the reference group is near the upper end of the zone of maximal protection or in transition zone B, then a positive association between radon level and lung cancer occurrence would be expected irrespective of the choice of test dose groups. Thus, the hormetic model may explain the different observations of Stidley and Samet (1993).
UNSCEAR (UN 2006) discusses how the hormetic response for lung cancer for residential radon exposure demonstrated by Cohen (1995) was discounted by Puskin (2003) on the basis of other smoking-related cancers also being negatively correlated with residential radon exposure (i.e., hormetic responses). Such an observation is consistent with the HRR model in that once the body’s natural defenses are activated (i.e., radon ANP), the defenses are free to protect against all forms of cancer. Further, where hormetic responses have been generated without adjusting for smoking, some have assumed the response to be an artifact of not adjusting for smoking. Interesting, smoking prevalence is generally significantly higher for lung cancer cases than for controls, so that properly adjusting for smoking would not be expected to lead to an increase in the lung cancer risk attributed to radon exposure but rather a decrease. Instances where such increases have been observed appear to relate to adding back lung cancer risk that has been eliminated via radiation ANP. Thus, smoking adjustment procedures used in epidemiological studies may introduce significant systematic error when the dose-response curve is actually hormetic. New research on developing appropriate risk factor adjustment procedures in circumstances where the dose-response curve is hormetic is therefore needed. Currently-used adjustment procedures may be appropriate for LNT-type responses but not for hormetic responses.
It is clear that radon levels significantly greater than the U.S. Environmental Protection Agency (EPA)’s action level (approximately 150 Bq m−3) are associated with an increase risk of lung cancer (although this does not prove causality). Thus, reducing residential levels in such circumstances makes sense with regards to protecting the health of the general public. Based on results of this study, for homes with radon levels > 250 Bq m−3, reducing the radon levels to near the EPA’s action level would be expected to reduce lung cancer risk mainly for those cancers caused by cigarette smoke. The reduction in cancer risk relates to and increase in radon ANP against sporadic lung cancer since B(x) would be expected to increase as the level of radon decreased (a Transition-Zone-B phenomenon). However, a further decrease in the level of radon into Transition Zone A would be expected to increase the risk of lung cancer because B(x) decreases as the level of radon decreases for this zone. In this case, home remediation to reduce radon would be expected to pose a significant threat to the home occupants. Thus, if too much radon is eliminated from the home as a result of home remediation, an increase in lung cancer risk could occur. Interestingly, for homes with levels of radon that are in Transition Zone A (i.e., levels less that about 50 Bq m−3), a remediation strategy to increase the radon level could lead to a significant reduction in the risk of lung cancer provided the increase was not too high. Ideally one would aim for a radon level that is in the Zone of Maximal Protection. Again, the protection relates to preventing smoking and other carcinogen induced lung cancer.
There is also evidence of lung cancer suppression benefit from radon ANP for German uranium miners previously employed by the Wismut Company. A case-control study was conducted by Brüske-Hohlfeld et al. (2006) to investigate the lung cancer risk in relation to attained age, time since exposure, exposure duration, and exposure rate. It consisted of 505 patients with lung cancer and 1,073 controls matched to cases according to the year of birth. The cumulative exposure to radon and radon decay products was calculated as the sum of yearly exposures and expressed in Working Level Months (WLM). The cases had a mean cumulative exposure of 552 WLM compared to 420 WLM in controls.
Figure 6 shows lung cancer OR (crude) for the former miners, adjusted only for the year of birth. Bar graphs are used for central estimates and also for the corresponding 95% CI values. Note the hormetic shape to the data. Based on the central estimates (MidOR) of the lung cancer OR in Figure 6, the PROFAC was estimated to be 0.2 ± 0.02, using the two lowest values of OR. Interestingly, when the data were adjusted for smoking and exposure to asbestos (risk factors), OR values increased indicating that the data are likely in the hormetic zone where ERR is negative. Figure 7 shows the dose-response relationship developed for B(x) based on these data with data points plotted at the midrange for the exposure levels (in WLM) in Figure 6 except for the highest exposure level where the lowest WLM value (800) for the group is used since B(x) = 0. Note that the results suggest a significant benefit of uranium mine air radon in preventing lung cancer (e.g., smoking related lung cancer). Thus, not only does residential radon exposure appear to protect from lung cancer in an exposure-level-dependent manner, but radon in mines also! In addition, these results point out the possibility that the increase in lung cancer risk seen in studies of radon exposed miners may not be solely a result of alpha radiation damage to the lung from radon progeny but may also relate to a loss of radon ANP against smoking-related cancer with increasing radon levels.
FIGURE 6.
Lung cancer odds ratio for WISMUT uranium miners based on data from Brüske-Hohlfeld et al. (2006) based on data adjusted only for year of birth. MidOR, central estimate of OR; LowOR, lower 95% confidence interval value; HighOR, upper 95% confidence interval value.
FIGURE 7.
Benefit B(x) for lung cancer suppression by radon progeny in uranium mine air based on data from Brüske-Hohlfeld et al. (2006) in Figure 6.
Results obtained in this paper are at odds with those discussed in the United Nations report on radon (UN 2006). The indicated report used methodologies that favor deriving an LNT-type relationship as has been the case in most epidemiological studies of radiation-associated cancer. As an example, Figure XV of the UN report shows summary lung cancer RR from a meta analysis of eight indoor radon studies and from a pooled analysis of underground miner studies (Lubin et al. 1997). Data were restricted to radon exposure levels < 50 WLM. For the lowest exposure group, RR =1 is plotted at zero Bq m−3 and an LNT function was forced through the data, even though the data appear to be uncorrelated with the radon exposure level, given the uncertainty in the data. In fact, the data appear to be supportive of the HRR model if it is assumed that all of the data points fall within the zone of maximal protection where the correlation coefficient is expected to be zero. A straight line with a negative slope is also included in the figure for the hormetic data of Cohen (1995). The intercept (RR = 1) at zero Bq m−3 is also indicated for Cohen’s data. Actually, nobody in the meta analysis or in Cohen’s study were in radon-free environments (i.e., zero Bq m−3). In addition, the meta analysis data were adjusted for competing risks factors and such adjustments if carried out in the hormetic zone can reintroduce risk eliminated via radon ANP as illustrated in Figure 5 of this paper. Thus, Figure XV of the UN report may involve multiple systematic errors (e.g., at least one error for each data set included in the meta analysis).
As a second example, Figure XVII of the United Nations report (UN 2006) shows summary RR from 21 studies for exposure to a radon concentration of 100 Bq m−3. The central estimates of RR from the different studies are similar and are apparently LNT-hypothesis related; however, reference group radon concentrations for each study were likely significantly different. Thus, had the 21 results been based on a common reference group the results presented may have been very different. In addition, where adjustments were made for competing risks factors, such adjustments if in the hormetic zone may have reintroduced risk eliminated via radon ANP.
CONCLUSIONS
Beware of association studies between lung cancer and radon because they do not prove causality! Finding an increase in lung cancer risk with increasing residential radon exposure for exposure levels just above the EPA’s action level (approximately 150 Bq m−3) does not prove that the increased risk was caused by damage from alpha radiation from radon progeny. The increase appears to be due to a loss of radon ANP against smoking and other causes of lung cancer. A similar loss of radon ANP may also be responsible at least in part for the increase in lung cancer risk reported for radon-exposed miners. If after adjusting for smoking and other lung cancer risk factors it is found that the lung cancer OR, RR, or hazard ratio increases, this should be a warning that something is possibly wrong with the analytical methods used in studying radon-associated cancer. Methods used for adjusting for competing risk factors were apparently designed for the risk assessment paradigm where ERR > 0. New methods appear to be needed for the low-dose radiation benefit paradigm where ERR < 0. Current methods appear to add back cancer risk that has been eliminated via radon ANP.
Acknowledgments
This research was supported by the Office of Science (BER), U.S. Department of Energy, Grant No. DE-FG02-09ER64783. I am grateful to the journal reviewers for their helpful comments provided.
REFERENCES
- Alavanja MCR, Brownson RC, Lubin JH, Berger E, Chang J, Boice JD., Jr Residential radon exposure and lung cancer among nonsmoking women. J Nat Cancer Inst. 1994;86:1829–1837. doi: 10.1093/jnci/86.24.1829. [DOI] [PubMed] [Google Scholar]
- Alavanja MCR, Lubin JH, Mahaffey JA, Brownson RC. Residential radon exposure and risk of lung cancer in Missouri. Am J Public Health. 1999;89:1042–1048. doi: 10.2105/ajph.89.7.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auvinen A, Makelainen I, Hakama M, Castren O, Pukkala E, Reisbacka H, Rytomaa T. Indoor radon exposure and risk of lung cancer: a nested case-control study in Finland. J Nat Cancer Inst. 1996;88:966–972. doi: 10.1093/jnci/88.14.966. [DOI] [PubMed] [Google Scholar]
- Averbeck D. Does scientific evidence support a change from the LNT model for low-dose radiation risk extrapolation? Health Phys. 2009;95:493–504. doi: 10.1097/HP.0b013e3181b08a20. [DOI] [PubMed] [Google Scholar]
- Baysson H, Tirmarche M, Tymen G, Gouva S, Caillaud D, Artus JC, Vergnenegre A, Ducloy F, Laurier D. Indoor radon and lung cancer in France. Epidemiol. 2004;15:709–716. doi: 10.1097/01.ede.0000142150.60556.b8. [DOI] [PubMed] [Google Scholar]
- Blot WJ, Xu ZY, Boice JD, Jr, Zhao DZ, Stone BJ, Sun J, Jing L-B, Fraumeni JF., Jr Indoor radon and lung cancer in China. J Nat Cancer Inst. 1990;82:1025–1030. doi: 10.1093/jnci/82.12.1025. [DOI] [PubMed] [Google Scholar]
- Bochicchio F, Forastiere F, Farchi S, Quarto M, Axelson O. Residential radon exposure, diet and lung cancer: a case control study in a Mediterranean region. Int J Cancer. 2005;114:983–991. doi: 10.1002/ijc.20799. [DOI] [PubMed] [Google Scholar]
- Brüske-Hohlfeld I, Rosario AS, Wölke G, Heinrich J, Kreuzer M, Krienbrock L, Wichmann H-E. Lung cancer risk among former uranium miners of the Wismut Company in Germany. Health Phys. 2006;90(3):208–216. doi: 10.1097/01.HP.0000175443.08832.84. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. Muller’s Nobel lecture on dose-response for ionizing radiation: ideology or science? Arch Toxicol. 2011 doi: 10.1007/s00204-011-0728-8. [DOI] [PubMed] [Google Scholar]
- Chen WL, Luan YC, Shieh MC, Chen ST, Kung HT, Soong KL, Yeh YC, Chou TS, Mong SH, Wu JT, Sun CP, Deng WP, Wu MF, Shen ML. Effects of cobalt-60 exposure on health of Taiwan residents suggest new approach needed in radiation protection. Dose-Response. 2007;5:63–75. doi: 10.2203/dose-response.06-105.Chen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Xu X, Bai L, Padilla MT, Tellez C, Gott KM, Leng S, Wilder JA, Belinsky SA, Scott BR, Lin Y. Biological basis for radiation adaptive responses that protect against bronchial epithelial cell transformation. Poster presentation, Low Dose Radiation Research Program Investigators’ Workshop X; May 9–11, 2011; Hyatt Regency, Bethesda, MD. 2011. [Google Scholar]
- Cohen BL. Test of the linear-no threshold theory of radiation carcinogenesis for inhaled radon decay products. Health Phys. 1995;68:157–174. doi: 10.1097/00004032-199502000-00002. [DOI] [PubMed] [Google Scholar]
- Cohen BL. Lung cancer rate vs. mean radon level in U.S. counties of various characteristics. Health Phys. 1997;72:114–119. doi: 10.1097/00004032-199701000-00016. [DOI] [PubMed] [Google Scholar]
- Cohen BL. The linear no-threshold theory of radiation carcinogenesis should be rejected. J Am Physicians Surg. 2008;13(3):70–76. [Google Scholar]
- Cuttler JM. Commentary on using LNT for radiation protection and risk assessment. Dose-Response. 2010;8(3):378–3843. doi: 10.2203/dose-response.10-003.Cuttler. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby S, Hill D, Auvinen A, Barros-Dios JM, Baysson H, Bochicchio F, Deo H, Falk R, Forastiere F, Hakama M, Heid I, Kreienbrock L, Kreuzer M, Lagarde F, Makelainen I, Muirhead C, Oberaigner W, Pershagen G, Ruano-Ravina A, Ruosteenoja E, Schaffrath Rosario A, Tirmarche M, Tomasek L, Whitley E, Wichmann H-E, Doll R. Radon in homes and risk of lung cancer: collaborative analysis of individual data from 13 European case-control studies. BMJ. 2005;330:223–228. doi: 10.1136/bmj.38308.477650.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinendegen LE, Paretzke HG, Neumann RD. Damage propagation in complex biological systems following exposure to low doses of ionizing radiation. Atoms for Peace. 2007;1:336–354. [Google Scholar]
- Feinendegen LE. Low-dose cancer risk modeling must recognize up-regulation of protection. Dose-Response. 2010;8:227–252. doi: 10.2203/dose-response.09-035.Feinendegen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field RW, Steck DJ, Smith BJ, Brus CP, Fisher EL, Neuberger JS, Platz CE, Robinson RA, Woolson RF, Lynch CF. Residential radon gas exposure and lung cancer: the Iowa radon lung cancer study. Am J Epidemiol. 2000;151:1091–1102. doi: 10.1093/oxfordjournals.aje.a010153. [DOI] [PubMed] [Google Scholar]
- Fisher EL, Field RW, Smith BJ, Lynch CF, Steck DJ, Neuberger JS. Spatial variation of residential radon concentrations: the Iowa radon lung cancer study. Health Phys. 1998;75:506–513. doi: 10.1097/00004032-199811000-00007. [DOI] [PubMed] [Google Scholar]
- Fornalski KW, Dobrzyński L. The healthy worker effect and nuclear industry workers. Dose-Response. 2010;8:125–147. doi: 10.2203/dose-response.09-019.Fornalski. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales V, Gott K, Makvandi M, Kikendall N, Monier A, Maloy E, Rietz C, Scott B, Wilder J. Low-dose, low-Let γ-radiation alters carcinogen-induced splenic cytokine production and immune cell phenotype of A/J mice. Poster presentation, Low Dose Radiation Research Program Investigators’ Workshop X; May 9–11, 2011; Hyatt Regency, Bethesda, MD. 2011. [Google Scholar]
- Gott K, Gonzales V, Makavandi M, Nikendall N, Monier A, Maloy E, Rietz C, Scott B, Wilder J. Effect of low-dose, low-LET γ-radiation and carcinogen injection on pulmonary immunity in A/J mice. Poster presentation, Low Dose Radiation Research Program Investigators’ Workshop X; May 9–11, 2011; Hyatt Regency, Bethesda, MD. 2011. [Google Scholar]
- Jaworowski A. The paradigm that failed. Int J Low Radiat. 2008;5(2):151–155. [Google Scholar]
- Kreuzer M, Heinrich J, Wolke G, Schaffrath Rosario A, Gerken M, Wellmann J, Keller G, Kreienbrock L, Wichmann HE. Residential radon and risk of lung cancer in Eastern Germany. Epidemiol. 2003;14:559–568. doi: 10.1097/01.ede.0000071410.26053.c4. [DOI] [PubMed] [Google Scholar]
- Krewski D, Lubin JH, Zielinski JM, Alavanja M, Catalan VS, Field RW, Klotz JB, Letourneau EG, Lynch CF, Lyon JI, Sandler DP, Schoenberg JB, Steck DJ, Stolwijk JA, Weinberg C, Wilcox HB. Residential radon and risk of lung cancer: a combined analysis of 7 North American case-control studies. Epidemiol. 2005;16:137–145. doi: 10.1097/01.ede.0000152522.80261.e3. [DOI] [PubMed] [Google Scholar]
- Krewski D, Lubin JH, Zielinski JM, Alavanja M, Catalan VS, Field RW, Klotz JB, Letourneau EG, Lynch CF, Lyon JI, Sandler DP, Schoenberg JB, Steck DJ, Stolwijk JA, Weinberg C, Wilcox HB. A combined analysis of North American case-control studies of residential radon and lung cancer. J Toxicol Environ Health A. 2006;69:533–597. doi: 10.1080/15287390500260945. [DOI] [PubMed] [Google Scholar]
- Letourneau EG, Krewski D, Choi NW, Goddard MJ, McGregor RG, Zielinski JM, Du J. Case-control study of residential radon and lung cancer in Winnipeg, Manitoba, Canada. Am J Epidemiol. 1994;140:310–322. doi: 10.1093/oxfordjournals.aje.a117253. [DOI] [PubMed] [Google Scholar]
- Liu S-Z. Cancer control related to stimulation of immunity by low-dose radiation. Dose-Response. 2007;5:39–47. doi: 10.2203/dose-response.06-108.Liu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin JH. Models for the analysis of radon exposed populations. The Yale Journal of Biology and Medicine. 1988;61:195–214. [PMC free article] [PubMed] [Google Scholar]
- Lubin JH, Boice JD. Lung cancer risk from residential radon: meta-analysis of eight epidemiological studies. J Natl Cancer Inst. 1997;89(2):49–57. doi: 10.1093/jnci/89.1.49. [DOI] [PubMed] [Google Scholar]
- Lubin JH, Tomásek L, Edling Hornung RW, Howe G, Kunz E, Kusiak RA, Morrison HI, Radford EP, Samet JM, Tirmarche M, Woodward A, Yao SX. Estimating lung cancer mortality from residential radon using data for low exposure of miners. Radiat Res. 1997;147(2):126–134. [PubMed] [Google Scholar]
- Lubin JH. Studies of radon and lung cancer in North America and China. Radiat Prot Dosim. 2003;104:315–319. doi: 10.1093/oxfordjournals.rpd.a006194. [DOI] [PubMed] [Google Scholar]
- Lubin JH, Wang ZY, Boice JD, Jr, Xu ZY, Blot WJ, De Wang L, Kleinerman RA. Risk of lung cancer and residential radon in China: pooled results of two studies. Int J Cancer. 2004;109:132–137. doi: 10.1002/ijc.11683. [DOI] [PubMed] [Google Scholar]
- Mitchel REJ, Jackson JS, Morrison DP, Carlisle SM. Low doses of radiation increase the latency of spontaneous lymphomas and spinal osteosarcomas in cancer prone, radiation sensitive Trp53 heterozygous mice. Radiat Res. 2003;159:320–327. doi: 10.1667/0033-7587(2003)159[0320:ldorit]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Nowosielska EM, Wrembel-Wargocka J, Cheda A, Lisiak E, Janiak MK. Enhanced cytotoxic activity of macrophages and suppressed tumor metastases in mice irradiated with low dose x-rays. J Radiat Res. 2006;47:229–236. doi: 10.1269/jrr.0572. [DOI] [PubMed] [Google Scholar]
- Ogura K, Magae J, Kawakami Y, Koana T. Reduction in mutation frequency by very low-dose gamma irradiation of Drosophila melanogaster germ cells. Radiat Res. 2009;171:1–8. doi: 10.1667/RR1288.1. [DOI] [PubMed] [Google Scholar]
- Pavia M, Bianco A, Pileggi C, Angelillo IF. Meta-analysis of residential exposure to radon gas and lung cancer. Bull World Health Org. 2003;81:732–738. [PMC free article] [PubMed] [Google Scholar]
- Puskin JS. Smoking as a confounder in ecologic correlations of cancer mortality rates with average county radon levels. Health Phys. 2003;84(4):526–532. doi: 10.1097/00004032-200304000-00012. [DOI] [PubMed] [Google Scholar]
- Sakai K, Hoshi Y, Nomura T, Oda T, Iwasaki T, Fujita K, Yamada T, Tanooka H. Suppression of carcinogenic process in mice by chronic low dose rate gamma-irradiation. Int J Low Radiat. 2003;1:142–146. [Google Scholar]
- Sanders CL, Scott BR. Smoking and hormesis as confounding factors in radiation pulmonary carcinogenesis. Dose-Response. 2008;6:53–79. doi: 10.2203/dose-response.06-003.Sanders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders CL. Radiation hormesis and the linear-no-threshold assumption. 1st ed. Heidelberg, Dordrecht, London, New York: Springer; 2010. [Google Scholar]
- Sandler DP, Weinberg CR, Shore DL, Archer VE, Stone MB, Lyon JL, Rothney-Kozlak L, Shepherd M, Stolwijk JAJ. Indoor radon and lung cancer risk in Connecticut and Utah. J Toxicol Environ Health A. 2006;69:633–654. doi: 10.1080/15287390500261117. [DOI] [PubMed] [Google Scholar]
- Scott BR. Chapter 1, Natural Background Radiation-Induced Apoptosis and the Maintenance of Mammalian Life on Earth. In: Valentino RG, editor. New Cell Apoptosis Research. Nova Science Publishers, Inc.; Hauppage, NY: 2007. pp. 1–35. [Google Scholar]
- Scott BR. Low-dose risk extrapolation fallacy associated with the linear-no-threshold model. Hum Exp Toxicol. 2008;27:163–168. doi: 10.1177/0960327107083410. [DOI] [PubMed] [Google Scholar]
- Scott BR, Sanders CL, Mitchel REJ, Boreham DR. CT scans may reduce rather than increase the risk of cancer. J Am Physicians Surg. 2008;13(1):8–11. [Google Scholar]
- Scott BR, Belinsky SA, Leng S, Lin Y, Wilder JA, Damiani LA. Radiation-stimulated epigenetic reprogramming of adaptive-response genes in the lung: An evolutionary gift for mounting adaptive protection against lung cancer. Dose-Response. 2009;7(2):104–131. doi: 10.2203/dose-response.08-016.Scott. PMCID: PMC2695570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott BR, Lin Y, Wilder JA, Chen W, Gott K, Gonzales V, Leng S, Hutt J, Tellez SC, Marshall E, Belinsky SA. Differential epigenetic changes in the lung after low and high carcinogen doses and implications for designing molecular epidemiology and other studies of radiation-induced lung cancer. Presentation, Low Dose Radiation Research Program Investigators’ Workshop X; May 9–11, 2011; Hyatt Regency, Bethesda, MD. 2011. [Google Scholar]
- Stidley CA, Samet JM. A review of ecologic studies of lung cancer and indoor radon. Health Phys. 1993;65(3):234–35. doi: 10.1097/00004032-199309000-00001. [DOI] [PubMed] [Google Scholar]
- Tanooka H. Threshold dose response in radiation carcinogenesis: an approach for chronic beta-irradiation experiments and a review of non tumor doses. Int J Radiat Biol. 2001;77:541–551. doi: 10.1080/09553000110034612. [DOI] [PubMed] [Google Scholar]
- Tanooka H. Meta-analysis of non-tumor doses for radiation-induced cancer on the basis of dose-rate. Int J Radiat Biol. 2011 doi: 10.3109/09553002.2010.545862. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RE, Nelson DF, Popkin JH, Popkin Z. Case-control study of lung cancer risk from residential radon exposure in Worchester County, Massachusetts. Health Phys. 2008;94(3):228–241. doi: 10.1097/01.HP.0000288561.53790.5f. [DOI] [PubMed] [Google Scholar]
- Thompson RE. Epidemiological evidence for possible radiation hormesis from radon exposure: A case-control study conducted in Worcester, Ma. Dose-Response. 2011;9(1):59–75. doi: 10.2203/dose-response.10-026.Thompson. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubiana M. The 2007 Marie Curie prize: the linear no threshold relationship and advances in our understanding of carcinogenesis. Int J Low Radiation. 2008;5(3):173–204. [Google Scholar]
- Tubiana MF, Feinendegen LE, Yang C, Kaminski JM. The linear no-threshold relationship is inconsistent with radiation biologic and experimental data. Radiology. 2009;251:13–22. doi: 10.1148/radiol.2511080671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner MC, Krewsski D, Chen Y, Pope CA, III, Gapstur S, Thunet MJ. Radon and lung cancer in the American Cancer Society cohort. Cancer Epidemiol Biomarkers Prev, Published OnlineFirst January. 2011;6:2011. doi: 10.1158/1055-9965.EPI-10-1153. [DOI] [PubMed] [Google Scholar]
- United Nations (UN) Sources and effects of ionizing radiation. Volume I: Sources; Volume II: Effects. United Nations Scientific Committee on the Effects of Atomic Radiation, 2000 Report to the General Assembly, with scientific annexes. United Nations; New York: 2000. [Google Scholar]
- United Nations (UN) Effects of ionizing radiation. United Nations Scientific Committee on the Effects of Atomic Radiation, 2006 Report to the General Assembly, with Scientific Annexes. United Nations; New York: 2006. [Google Scholar]
- U.S. Environmental Protection Agency (USEPA) Assessment of risks from radon in homes. Washington, DC: Office of Radiation and Indoor Air, U.S. EPA; 2003. 2003. [Google Scholar]
- U.S. National Research Council (USNRC) Health effects of exposure to radon, BEIR VI. Committee on Health Risks of Exposure to Radon, Board on Radiation Effects Research, Commission on Life Sciences. Washington, DC: National Academy Press; 1999. [Google Scholar]
- Wichmann HE, Schaffrath Rosario A, Heid IM, Kreuzer M, Heinrich J, Kreienbrock L. Increased lung cancer risk due to residential radon in a pooled and extended analysis of studies in Germany. Health Phys. 2005;88:71–79. doi: 10.1097/01.hp.0000142497.31627.86. [DOI] [PubMed] [Google Scholar]