Abstract
The tyrosine kinase c-Src and transcription factor NF-κB are considered crucial components required for normal osteoclastogenesis. Genetic ablation of either pathway leads to detrimental osteopetrotic phenotypes in mice. Similarly, obstruction of either pathway halts osteoclastogenesis and lessens various forms of bone loss. It has been shown previously that mice expressing a kinase domain-truncated c-Src, termed Src251, develop severe osteopetrosis owing to increased osteoclast apoptosis. It was further suggested that this phenomenon is associated with reduced Akt kinase activity. However, the precise mechanism underlying the osteoclast inhibitory effect of Src251 remains obscure. C-Src associates with TRAF6-p62 interacting with receptor activator of NF-κB (RANK) distal region and the complex facilitate activation of RANK down stream signal transduction cascades including NF-κB. Given this proximity between c-Src and NF-κB signaling in osteoclasts, we surmised that inhibition of osteoclastogenesis by Src251 may be achieved through inhibition of NF-κB signaling. We have demonstrated recently that NEMO, the regulatory subunit of the IKK complex, is crucial for osteoclastogenesis and interacts with c-Src in osteoclast progenitors. Transfection studies, in which we employed various forms of c-Src and NEMO, revealed that the dominant negative form of c-Src, namely Src251, mediates degradation of NEMO thus halting NF-κB signaling. Furthermore, degradation of NEMO requires its intact zinc finger domain which is located at the ubiquitination domain. This process also requires appropriate cellular localization of Src251, since deletion of its myristoylation domain ablates its degradation capacity. Buttressing these findings, the expression of NEMO and NF-κB signaling were significantly reduced in monocytes collected from Src251 transgenic mice.
Keywords: NEMO, c-Src, Src251, DEGRADATION, NF-κB
Bone homeostasis is achieved by intricate balance between bone formation by osteoblasts and bone resorption by osteoclasts. Reduced activity of osteoblasts and/or increased activity of osteoclasts lead to thinning of the bone and to increased bone fracture risk. On the other hand, decreased or absent activity of osteoclasts results with osteopetrosis featured by dense less remodeled bones and compromised bone quality [Teitelbaum and Ross, 2003; Teitelbaum, 2007].
Monocytes differentiate in response to receptor activator of NF-κB (RANK) ligand and macrophage-colony stimulating factor (M-CSF) into osteoclasts which are multi-nucleated cells that specialize in bone resorption and remodeling. Activation of RANK-mediated signaling entails mobilization and recruitment of a large number of adaptor proteins and kinases that facilitate proper activation of the osteoclast transcriptional machinery [Abu-Amer, 2005]. Specifically, upon RANK stimulation, TNF receptor associated factors primarily TNF receptor associated factor-6 (TRAF6), the tyrosine kinase c-Src, p62 (SQSTM1), TGF-activated kinase-1 (TAK1), and TAB proteins are recruited to the distal region of RANK and establish a signaling cluster. As a result, several distinct signaling cascades ensue including NF-κB pathways (classical and alternative), MAP kinase pathways (JNK, Erk, and p38), and Src/PI3K pathway [Abu-Amer, 2005, 2009]. The contribution of these pathways to osteoclast differentiation and activity has been well established.
A large number of genes have been implicated as crucial for osteoclast differentiation and function. At the precursor and pre-osteoclast stages the transcription factors PU.1, c-fms, and c-fos are absolutely required for myeloid lineage determination and commitment to the pre-osteoclast stage [Teitelbaum and Ross, 2003; Teitelbaum, 2007]. Likewise, deletion of RANK/RANKL and p50/p52 NF-κB arrest osteoclast differentiation [Iotsova et al., 1997; Hsu et al., 1999; Li et al., 2000]. However, deletion of several other genes including c-Src, beta3 integrin, TRAP, cathepsin-K, Cln7 (chloride channel), and proton ATPase subunits hindered osteoclast activity [Sly et al., 1983; Soriano et al., 1991; Schwartzberg et al., 1996; Saftig et al., 1998; Gowen et al., 1999].
Following the discovery that combined deletion of p50/p52 NF-κB impedes osteoclastogenesis and leads to osteopetrosis in mice, the role of NF-κB signaling in osteoclastogenesis has been extensively studied. NF-κB family includes the upstream IκB kinase complex which includes IKKα/IKK1, IKKβ/IKK2, and IKKγ/NF-κB essential modulator (NEMO). This family also includes IκBα (inhibitory κB), IκBβ, IκBε, p50, p52, p65/RelA, Rel-B, and c-rel [Siebenlist and Franzoso, 2001; Ghosh and Karin, 2002; Ting and Endy, 2002; Karin et al., 2004; Abu-Amer and Faccio, 2006]. In general, signaling by NF-κB has been classified into two principal pathways; classical and alternative pathways. In the former, upstream signals prompt formation of the IKK complex with emphasis on NEMO/IKK2 followed by phosphorylation and degradation of IκB. Subsequently, p65 and p50 subunits translocate to the nucleus, bind to DNA, and activate transcription [Hayden and Ghosh, 2008]. On the other hand, alternative activation of NF-κB entails activation of IKK1 by NF-κB inducing kinase (NIK) which in turn leads to activation of transcription by p52/Rel-B dimers [Sun, 2011]. Despite this signaling distinction, the functional separation between these two pathways remains elusive. Notwithstanding, genetic and biochemical studies have established that classical activation of NF-κB at the level of NEMO and IKK2 is crucial for osteoclastogenesis. Specifically, genetic ablation of IKK2 in myeloid cells, as well as knockdown of NEMO or blocking interaction of NEMO with IKK2 hindered osteoclastogenesis [Dai et al., 2004; Ruocco et al., 2005; Otero et al., 2008; Darwech et al., 2009]. These findings and many others have identified various regulatory elements in the NF-κB proteins as targets for regulating NF-κB signaling as it pertains to osteoclastogenesis and osteolysis. In this regard, NEMO which is considered as a key scaffold protein, encompasses unique structural and functional domains that facilitate IKK complex assembly and signal transduction. These include an amino terminal coiled-coil domain of NEMO which is essential for binding with IKKs; coiled-coil2 and leucine zipper domain required for NEMO trimerization; a conserved sequence located between coiled-coil2 and leucine zipper called UBAN owing to its ability to bind poly-ubiquitinated chains; and carboxyl-terminal zinc finger domain with unique stimulus-specific ubiquitin-binding activity [Rushe et al., 2008; Cordier et al., 2009]. The zinc finger has been also implicated as essential for down stream NF-κB signaling. Specifically, it recognizes upstream poly-ubiquitinated signal mediators such as TRAF6, TAK1/TAB, and facilitates protein–protein interactions required for cellular signaling.
C-Src is comprised of a short amino terminal myristoylation domain that signals for association with cytoplasmic and plasma membranes, SH2 and SH3 domains that regulate structure and binding to other proteins, a carboxyl tyrosine kinase domain that is required for c-Src kinase activity, and a short regulatory domain at the carboxyl terminus [Varmus et al., 1989; Summy et al., 2003]. Regulation of osteoclastogenesis by tyrosine kinases, chiefly c-Src, has been described [Soriano et al., 1991; Boyce et al., 1992; Horne et al., 1992]. In fact, c-Src is crucial for the activity of osteoclasts since deletion of this tyrosine kinase leads to osteopetrosis owing to dysfunctional osteoclasts [Soriano et al., 1991; Boyce et al., 1992; Horne et al., 1992]. However, the role of the kinase activity of c-Src in this phenomenon remained debatable until the generation of transgenic mice expressing c-Src devoid of its kinase domain, termed Src251. Transgenic expression of Src251 exacerbated the severity of osteopetrosis observed in c-Src-null mice owing to increased apoptosis of osteoclasts [Schwartzberg et al., 1996, 1997; Xing et al., 2001]. Further studies have described Src251 as a dominant-negative protein [Luxenburg et al., 2006]. Although decreased AKT kinase activity by Src251 was described as the underlying cause of osteoclast apoptosis, the role of the transcription factor NF-κB, which is considered the principal anti-apoptotic factor and crucial for osteoclastogenesis, was not entertained. Thus, we surmised that Src251 may inhibit osteoclastogenesis by interfering with NF-κB signaling. Indeed, we find that NF-κB activation is reduced in RANKL-treated macrophages derived from Src251 transgenic mice compared to wild-type cells. More importantly, we provide conclusive evidence that Src251, but not wild-type c-Src, induces degradation of IKKγ/NEMO in a proteosome-dependent mechanism. This activity of Src251 was independent of its kinase activity and its SH2 or SH3 domains, yet dependent on its myristoylation. Lastly, our findings implicate the zinc finger of NEMO as crucial for Src251-mediated degradation of NEMO, since deletion of this domain prevented its degradation.
MATERIALS AND METHODS
REAGENTS
All cytokines were purchased from R&D Systems (Minneapolis, MN). All antibodies were purchased from Santa Cruz Biotech (Santa Cruz, CA) and Cell Signaling Technologies, Inc. (Danvers, MA). All other chemicals are from Sigma (St. Louis, MO) unless otherwise indicated.
DNA CONSTRUCTS
c-Src and NEMO deletion mutants were generated using standard molecular biology techniques. The sequences and expression of all constructs were positively tested.
CELL TRANSFECTION
HEK293 cells were plated at 2 million cells/p100 mm plate in 10 ml DMEM + 10% heat-inactivated FBS overnight. Cells were then transfected with 2 μg DNA mixed with 250 μl Opti-MEM medium and 20 μl lipofectamine per plate. After 8 h fresh medium in the absence or presence of 10 μM MG132 (EMD, Gibbstown, NJ) was added and cells were incubated for an additional 24 h at which time cells were lysed and protein expression was analyzed. Transfection efficiency using GFP constructs as a marker exceeded 95%.
CELL ISOLATION AND PURIFICATION
Osteoclast precursors (OCPs) in the form of marrow macrophages were isolated from whole bone marrow of 4- to 6-week mice and incubated in tissue culture plates, at 37°C in 5% CO2, in the presence of 10 ng/ml M-CSF. After 24 h in culture, the nonadherent cells are collected and layered on a Ficoll–Hypaque gradient. Cells at the gradient interface are collected and plated in alpha-MEM, supplemented with 10% heat-inactivated fetal bovine serum, at 37°C in 5% CO2 in the presence of 10 ng/ml M-CSF, and plated according to each experimental conditions.
KINASE ASSAYS
Extracts prepared from the various cells under use were suspended in lysis buffer containing 40 mM Tris–HCl, pH 8.0, 500 mM NaCl, 0.1% Nonidet P-40, 6 mM EDTA, 6 mM EGTA, 5 mM β-glycerophosphate, 5 mM NaF, 1 mM NaVO4, pH 10.0, and protease inhibitor (Roche Molecular Biochemicals). The cellular lysates (500 μg) were subjected to immunoprecipitation with the relevant antibody and gamma-bind sepharose (25 μl). After washing of the immunoprecipitates, kinase assays were performed at 30°C for 30 min with buffer containing 50 mM Tris–HCl, pH 8.0, 100 mM NaCl, 10 mM MgCl2, 1 mM dithiothreitol, 10 μM ATP, 5 mM β-glycerophosphate, 5 mM NaF, 1 mM Na3VO4, pH 10.0, 5 μCi of [γ-32P]ATP, 5 μg of the GST-IκBα (aa 1–54). The kinase reaction mixtures were then resolved by SDS–polyacrylamide gel electrophoresis (PAGE), and phosphorylation of IκBα was detected by autoradiography.
WESTERN BLOT ASSAY
Total cell lysates were boiled in the presence of an equal volume of 2× SDS sample buffer consisting of (0.5 M Tris–HCl, pH 6.8, 10% (w/v) SDS, 10% glycerol, 0.05% (w/v) bromophenol blue, 3% β-mercaptoethanol, and distilled water) for 5 min and subjected to electrophoresis on 8–10% SDS–PAGE. The proteins were transferred to nitrocellulose membranes using a semi-dry blotter (Bio-Rad, Hercules, CA) and incubated in blocking solution (10% skim milk prepared in phosphate-buffered saline containing 0.05% Tween-20) to reduce nonspecific binding. The membranes were washed with phosphate-buffered saline/Tween buffer and exposed to primary antibodies (16 h at 4°C), washed again four times, and incubated with the respective secondary horseradish peroxidase-conjugated antibodies (1 h at room temperature). The membranes were washed extensively (4 × 15 min), and an ECL detection assay was performed following the manufacturer’s directions.
RESULTS
NF-KB ACTIVATION IS DIMINISHED IN MACROPHAGES EXPRESSING SRC251-TG
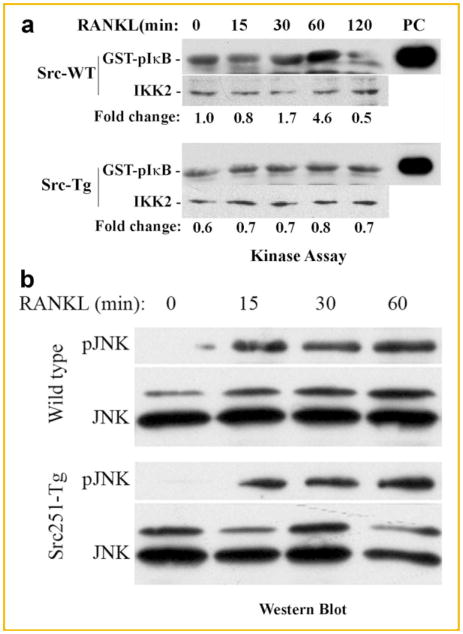
Given the fact that NF-κB activation is considered one of the crucial elements for osteoclastogenesis and cell survival, we reasoned that Src251-tg inhibition of osteoclastogenesis maybe the result of negative targeting of the NF-κB pathway by the transgene. To address this assumption, we examined expression levels and activity of IKK2 in normal and Src251-tg-bearing osteoclast progenitors. Cells were stimulated with RANKL to enable activation of NF-κB pathway. The data depicted in Figure 1a show that expression levels of IKK2 protein are similar in both cell types. In contrast, whereas IKK2 activity in wild-type cells is increased following treatment with RANKL as evident by exogenous phosphorylation of GST-IκB substrate, this activity was negligible in Src251-tg cells, suggesting that Src251-tg inhibits IKK2 activation. To address the specificity of this observation, we examined expression and activation of RANKL-inducible JNK MAP kinase which appears to be unaffected by the transgene (Fig. 1b).
Fig. 1.
IKK2 activity is reduced in Src251-expressing cells. Osteoclast progenitors from the marrow of wild-type and Src251-tg mice were allowed to adhere on tissue culture dishes then treated with RANKL as indicated. Cell lysates were immunoprecipitated with anti-IKK2 (a) or anti-JNK (b) antibodies and in vitro kinase assay was performed using GST-IκB or GST-cJun as substrates, respectively. Positive controls (PC) representing the activities of recombinant IKK2 or JNK are shown on the right. The levels of IKK2 and JNK proteins are included as input controls.
EXPRESSION LEVELS OF NEMO ARE DIMINISHED IN SRC251-TG MACROPHAGES
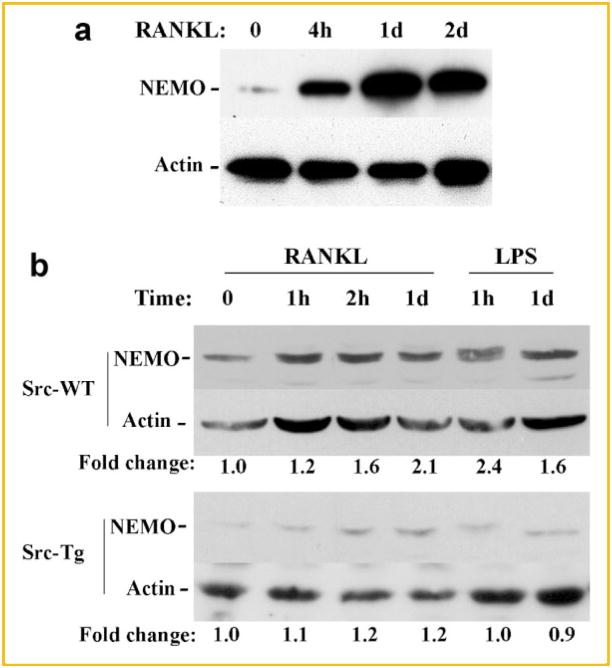
Having demonstrated defective activation of IKK2, we turned to examine proximal culprits that may contribute to this phenomenon. In this regard, it has been well documented that NEMO, which lacks catalytic activity, acts as a scaffold protein to facilitate formation of the IKK complex and distal NF-κB activation through steps of self-oligomerization and docking with IKK2 [Li et al., 2001; Ravid and Hochstrasser, 2004]. Hence, it is imperative to determine whether reduced IKK2 activity in this model is a consequence of negative regulation of NEMO by Src251-tg. First, we show that NEMO expression in RANKL-treated osteoclast progenitors is vastly increased in the pre-osteoclast phase (4 h, 1 day) and remains elevated at the early stages of osteoclast commitment (Fig. 2b). More importantly, expression levels of NEMO were significantly reduced in Src251-expressing osteoclast progenitors compared with wild-type cells (Fig. 2b). Furthermore, whereas RANKL and lipopolysaccharide (LPS) induced expression of NEMO in wild-type cells, no such induction was detected in Src251-tg cells, suggesting that Src251-tg may destabilize NEMO.
Fig. 2.
Src251 diminishes expression of NEMO. a: Wild-type osteoclast progenitors were treated with RANKL for the time points indicated and expression levels of NEMO were assessed by Western blot. Expression of osteoclast differentiation markers corresponds with this time frame as we have published previously [Otero et al., 2010]. b: Osteoclast progenitors derived from wild-type and Src251-tg mice were treated with RANKL and LPS as shown. Expression of NEMO and beta-actin proteins was measured in cell lysates using appropriate antibodies.
SRC251-TG INDUCES DEGRADATION OF NEMO
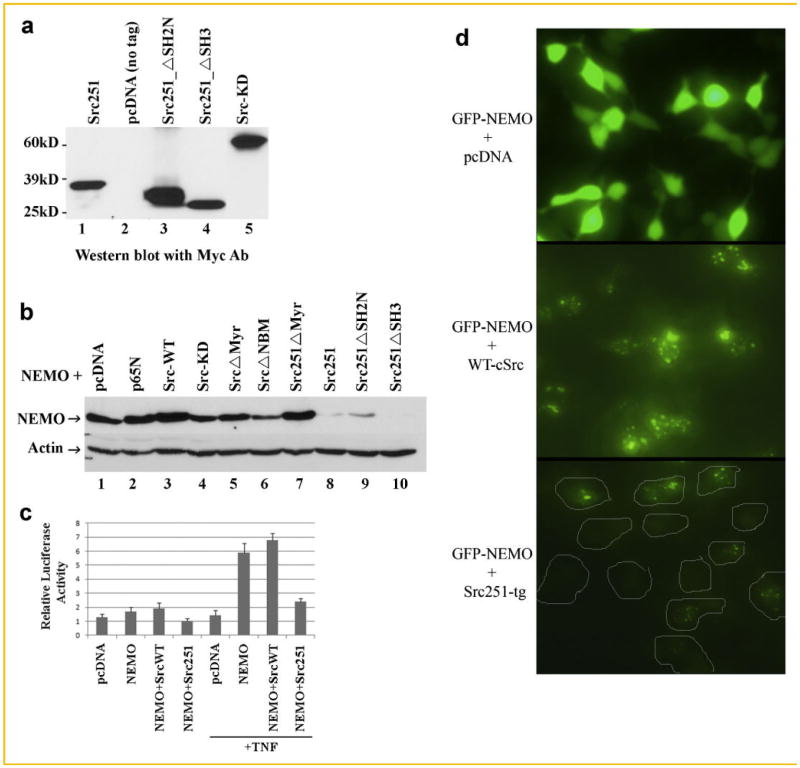
To further clarify the mechanistic details of this phenomenon, we tested the effect of various c-Src constructs on NEMO expression directly. Specifically, we generated Src251, src251SH2N (in which amino terminal SH2 domain has been deleted), Src251SH3 (lacking SH3 domain), kinase-dead c-Src (point mutation K295M) (Fig. 3a), and other forms of wild-type c-Src and Src251 in which the myristoylation sites are deleted. pcDNA and p65 constructs were used as controls. These constructs were co-transfected with NEMO in HEK293 cells and the expression of NEMO controlled by beta-actin was monitored. Consistent with the reduced NEMO expression in cells expressing endogenous Src251-tg (Fig. 2b), we demonstrate a dramatic loss of NEMO expression when co-transfected with Src251 (Fig. 3b). Deletion of the SH3 or the amino-terminal SH2 domains did not restore NEMO expression, suggesting that these domains are not required for this function of the transgene. On the other hand, similar to p65 and pcDNA, wild-type c-Src appears to not affect the expression of NEMO negatively. Surprisingly, however, deleting the myristoylation domain completely abolished the effect of Src251-tg and did not affect NEMO expression (Fig. 3b). Another interesting observation pertains to c-Src in which a putative NEMO-binding domain (NBM) has been deleted and to kinase-defective c-Src in which a point mutation has been introduced. These two constructs only partially mediated reduced NEMO expression, suggesting that c-Src kinase activity and c-Src binding to NEMO insufficient but maybe required for optimal degradation of NEMO. To provide further support for the biochemical findings, we determined NF-κB activity in HEK293 cells transfected with NEMO and wild-type c-Src or Src251 forms. Consistent with our previous observations, we demonstrate that TNF-induced activation of NF-κB reporter is hindered in the presence of Src251 (Fig. 3c). In another approach, we generated and expressed GFP-fused NEMO in HEK293 cells in the presence of pcDNA (negative control), wild-type c-Src, or Src251 constructs. The findings of this exercise confirm that whereas the control plasmid pcDNA did not affect overall cellular distribution of GFP-NEMO, wild-type c-Src induce cellular compartmentalization of NEMO in a punctate form. More importantly, expression levels of GFP-NEMO were vastly decreased in the presence of Src251, consistent with its proposed degradation (Fig. 3c).
Fig. 3.
Src251-tg induces degradation of NEMO. a: c-Src deletion mutants were generated using traditional PCR methods and cloned in pcDNA plasmid bearing amino terminal Myc tag. Constructs were expressed in HEK293 cells and detected by Western blots. b: HEK293 cells were co-transfected with NEMO and various forms of c-Src as shown. pcDNA and p65 were included as controls. Immunoblots for NEMO and beta-actin were carried out using relevant antibodies. c: HEK293 cells were transfected with pcDNA, NEMO, wild-type c-Src, Src251, or combinations as indicated. Luciferase assay to measure NF-κB activity was previously described [Otero et al., 2010]. d: HEK293 cells were transfected with GFP-fused NEMO and c-Src or Src251 as indicated. GFP-NEMO expression images were recorded using fluorescent microscope (at 10× magnification). Boundaries of cells in lower panel are marked.
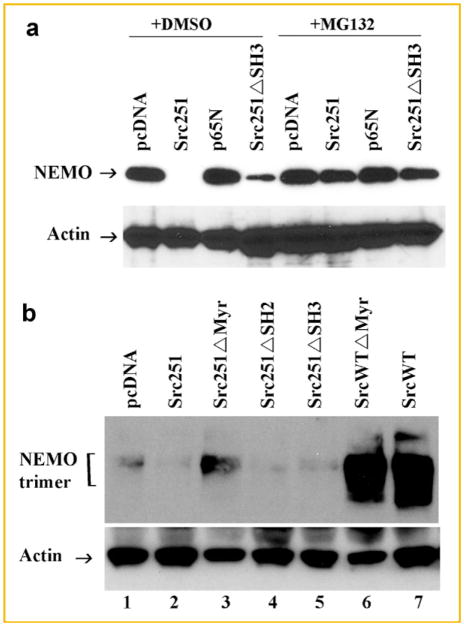
To further examine the mechanism underlying Src251-tg-mediated degradation of NEMO, we examined if this process is proteosome-dependent event. Indeed, pre-incubation of cells with the proteosome inhibitor MG132 entirely prevented degradation of NEMO by Src251-tg forms (Fig. 4a). Further insights into the mechanism underlying this event, such as lysosomal or ubiquitin-mediated degradation, are the focus of future studies.
Fig. 4.
Src251 facilitates proteosome-mediated degradation of NEMO. a: HEK293 cells were co-transfected with the constructed indicated and cells cultured in the absence or presence of 10 μM MG132 for 8 h. Expression levels of NEMO were measured by immunoblots after 24 h. b: Primary osteoclast progenitors were infected with various c-Src retroviruses as indicated. Endogenous oligomers of NEMO were detected under nonreducing conditions. Actin expression is shown as loading control.
We and others have shown recently that NEMO oligomerization is a crucial step required for stabilizing NEMO complexes and required for IKK complex assembly and ensuing signaling [Agou et al., 2004a; Fontan et al., 2007; Darwech et al., 2009]. Noticeably, obstructing NEMO oligomerization using NEMO-derived decoy peptides led to rapid degradation of NEMO, inhibition of NF-κB signaling, and blockade of osteoclastogenesis [Darwech et al., 2009]. In this study, we observed that Src251-tg, whether lacking its SH2 or its SH3 domains, impedes NEMO trimer formation leading to its instability and degradation (Fig. 4a,b). On the other hand, myristoylation-deficient Src251-tg was significantly less effective in blocking NEMO trimerization. Surprisingly, inclusion of wild-type form of c-Src greatly enhanced the formation and stability of NEMO trimers, suggesting a positive role of wild-type c-Src in stabilizing NEMO and augmenting NF-κB signal transduction.
NEMO ZINC FINGER IS REQUIRED FOR SRC251-TG-MEDIATED DEGRADATION OF NEMO
NEMO encompasses several structural motifs such as coil-coiled (CC2), leucine zipper (LZ), and zinc finger (ZF) domains which are essential for its stability and function [Huang et al., 2002; Yang et al., 2004]. To determine if Src251 targets a specific motif within NEMO, we generated deletion mutants of NEMO in which CC2, LZ, or ZF were deleted. These deletion mutants along side full length NEMO were co-transfected with Src251-tg. The results show that NEMO levels were decreased by ~82% in the presence of Src251 as expected, and levels of CC2 and LZ mutants of NEMO were entirely abolished (Fig. 5). Note that basal level expression of NEMO mutants was already reduced (in the absence of Src251) consistent with previous findings from our and other groups that these two motifs are essential for NEMO stability and trimer formation [Agou et al., 2004a,b; Fontan et al., 2007; Darwech et al., 2009]. Remarkably, deletion of the zinc finger, which is known as major target for ubiquitination events and facilitates protein–protein interactions, protects NEMO, and halts Src251-induced degradation of this protein. Moreover, it appears that basal level expression of ZF-mutated NEMO is elevated compared with wild-type NEMO, suggesting that overall degradation of NEMO is considerably halted.
Fig. 5.

The zinc finger of NEMO is required for Src251-mediated degradation of NEMO. HEK293 cells were co-transfected with various mutants and wild-type forms of NEMO in the absence or presence of Src251-tg as indicated. Cells were cultured for 24 h then lysed and expression levels of NEMO and actin were measured by immunoblots.
DISCUSSION
Osteoclast differentiation and survival are crucial for homeostasis of bone metabolism. Over the last two decades, seminal discoveries identified several molecules essential for osteoclastogenesis. The discovery that c-Src is pivotal for osteoclast activity [Soriano et al., 1991], began a new chapter focusing on the role of tyrosine kinases in osteoclastogenesis and bone metabolism. Indeed, follow-up studies have shown that inhibition of tyrosine kinase activity, especially c-Src, attenuates bone loss [Yoneda et al., 1993]. Furthermore, a flurry of studies have assigned c-Src as a component of the IKK complex and tyrosine phosphorylation of elements of the IKK complex as essential for modulating NF-κB activity [Abu-Amer et al., 2001; Huang et al., 2003; Funakoshi-Tago et al., 2005; Darwech et al., 2010]. However, the mechanisms underlying the action of c-Src were not fully elucidated and at times controversial. For example, the role of kinase activity of c-Src in osteoclastogenesis remains debatable [Schwartzberg et al., 1996, 1997; Miyazaki et al., 2004]. However, using mouse genetic models, Xing et al. [2001] provided an elegant evidence that deletion of the entire kinase domain of c-Src (referred to as Src251) rather than inactivating point mutation of c-Src (kinase-dead), inhibits osteoclast survival likely through reduction of AKT signaling. Consistently, tyrosine kinase inhibitors efficaciously inhibited osteoclastogenesis and bone resorption [Yoneda et al., 1993; Missbach et al., 2000]. Although prior evidence implicate AKT and ERK in osteoclast survival [Madge and Pober, 2000; Miyazaki et al., 2000; Lee et al., 2001], the transcription factor NF-κB is considered the principal survival, proliferation, and anti-apoptotic signal in the vast majority of cell types including osteoclasts and their myeloid progenitors [Jimi et al., 1998; Lacey et al., 2000; Papa et al., 2004; Karin, 2006]. In this regard, we and others have shown that inhibition of NF-κB signaling using dominant-negative forms of IκB halts osteoclast differentiation and survival [Abbas and Abu-Amer, 2003; Clohisy et al., 2003, 2004; Abu-Amer, 2005]. More importantly, we provided the first evidence that c-Src regulates the NF-κB pathway through tyrosine phosphorylation of IκB [Abu-Amer et al., 1998] and have recently provided novel evidence that tyrosine phosphorylation of IKK2 is a major regulator of NF-κB activation and osteoclastogenesis [Darwech et al., 2010]. Based on this information, it is conceivable to envision regulation of NF-κB by Src251. Armed with this information, we set out to examine this possibility as the mechanism underlying Src251 inhibition of osteoclastogenesis. Clearly, our observation that NF-κB activity is reduced in Src251 transgenic cells provides a strong support for this proposition.
The most intriguing observation is the novel finding that Src251 prompts degradation of NEMO. This function appears to be independent of its kinase activity as evident by stable NEMO expression in the presence of kinase-dead (K295M) c-Src. Surprisingly, deletion of the N-terminal SH2 or the SH3 domains did not alter significantly the effect of Src251 on NEMO stability suggesting that SH2 and SH3 do not play major roles in this phenomenon. We have attempted to clarify certain mechanistic aspects of Src251-induced degradation of NEMO. First, we provide clear evidence that this process is proteosome-dependent, suggesting that Src251 prompts K48-linked ubiquitination of NEMO leading to its proteosomal degradation. This proposition awaits further clarification in future studies. Second, we provide evidence that while wild-type c-Src supports NEMO trimer formation and stability, Src251 impedes formation of such trimer, a process that ultimately leads to NEMO instability and degradation. This finding is supported by our recent findings in which we show that administration of decoy small molecules that interfere with NEMO oligomerization destabilizes it [Darwech et al., 2009]. Thus, it remains possible that Src251, ***by a yet to be identified mechanism, obstructs NEMO trimer formation rendering the protein instable.
Interestingly, it appears that myristoylation of Src251 is crucial for its destructive activity toward NEMO evident by restoration of NEMO expression in the presence of Src251 lacking its myristoylation motif. N-myristoylation, a post-translational modification of proteins, is necessary for anchoring proteins to membranes. It has been shown that myristoylated proteins in cells regulate the signal transduction between membranes and cytoplasmic fractions. More importantly, a recent study have shown that N(alpha)-myristoylation of several brain proteins regulates certain protein–protein interactions that may affect signaling pathways in brain [Sharma, 2004; Hayashi and Titani, 2010]. This property is not restricted to brain proteins but has been widely described in other tissues. More importantly, myristoylated c-Src is anchored to membrane fractions and mediates signal transduction. Our finding that deletion of the N-myristoylation motif of Src251 restores NEMO stability, suggests that membrane localization of Src251 is crucial for signaling its degradative activity.
NEMO is a scaffold protein essential for formation of the IKK complex and crucial for staging NF-κB signal transduction. We have shown recently that NEMO is pivotal for RANKL and inflammatory signal induction in osteoclasts and their progenitors. For example, inhibition of NEMO binding to IKK2, interruption of NEMO oligomerization, and knockdown of NEMO in macrophages/osteoclast progenitors inhibit osteoclastogenesis in vitro and inflammatory bone loss, in vivo [Dai et al., 2004; Clohisy et al., 2006; Abu-Amer et al., 2007; Darwech et al., 2009]. Of special mention, we have documented recently that blocking oligomerization of NEMO, a step required for its assembly and stabilization, led to its rapid degradation [Darwech et al., 2009]. Our finding that deletion of the coil-coiled and leucine zipper domains did not prevent Src251-induced degradation of NEMO is consistent with this notion. Remarkably, we find that deletion of the zinc finger of NEMO blunts Src251-mediated degradation of NEMO. Given the fact that this domain is essential for NF-κB activation and is required to recognize upstream signals, our observation suggests that NEMO zinc finger domain is required for recognition of Src251-mediated destructive action. Our study does not, however, identify if poly-ubiquitination events mediate the action of Src251. Further studies are essential to clarify the precise mechanism.
Acknowledgments
Grant sponsor: National Institutes of Health; Grant numbers: AR049192, AR054326; Grant sponsor: Shriners Hospital for Children; Grant numbers: 8570, 8510.
This work is supported by National Institutes of Health Grants: AR049192, AR054326 and by grants #8570, #8510 from the Shriners Hospital for Children (to Y. A.-A).
References
- Abbas S, Abu-Amer Y. Dominant-negative IkappaB facilitates apoptosis of osteoclasts by tumor necrosis factor-alpha. J Biol Chem. 2003;278(22):20077–20082. doi: 10.1074/jbc.M208619200. [DOI] [PubMed] [Google Scholar]
- Abu-Amer Y. Advances in osteoclast differentiation and function. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5(3):347–355. doi: 10.2174/1568008054863808. [DOI] [PubMed] [Google Scholar]
- Abu-Amer Y. Inflammation, cancer, and bone loss. Curr Opin Pharmacol. 2009;9(4):427–433. doi: 10.1016/j.coph.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Amer Y, Faccio R. Therapeutic approaches in bone pathogeneses: Targeting the IKK/NF-kB axis. Future Med. 2006;1(1):133–146. [Google Scholar]
- Abu-Amer Y, Ross FP, McHugh KP, Livolsi A, Peyron JF, Teitelbaum SL. Tumor necrosis factor-alpha activation of nuclear transcription factor-kappaB in marrow macrophages is mediated by c-Src tyrosine phosphorylation of IkBa. J Biol Chem. 1998;273(45):29417–29423. doi: 10.1074/jbc.273.45.29417. [DOI] [PubMed] [Google Scholar]
- Abu-Amer Y, Dowdy SF, Ross FP, Clohisy JC, Teitelbaum SL. TAT fusion proteins containing tyrosine 42-deleted IkappaBalpha arrest osteoclastogenesis. J Biol Chem. 2001;276(32):30499–33503. doi: 10.1074/jbc.M104725200. [DOI] [PubMed] [Google Scholar]
- Abu-Amer Y, Darwech D, Otero J. Role of the NF-B axis in immune modulation of osteoclasts and bone loss. Autoimmunity. 2007;8:1–8. doi: 10.1080/08916930701694543. [DOI] [PubMed] [Google Scholar]
- Agou F, Traincard F, Vinolo E, Courtois G, Yamaoka S, Israel A, Veron M. The trimerization domain of NEMO is composed of the interacting C-terminal CC2 and LZ coiled-coil subdomains. J Biol Chem. 2004a;279(27):27861–27869. doi: 10.1074/jbc.M314278200. [DOI] [PubMed] [Google Scholar]
- Agou F, Courtois G, Chiaravalli J, Baleux F, Coic Y-M, Traincard F, Israel A, Veron M. Inhibition of NF-{kappa}B activation by peptides targeting NF-{kappa}B essential modulator (NEMO) oligomerization. J Biol Chem. 2004b;279(52):54248–54257. doi: 10.1074/jbc.M406423200. [DOI] [PubMed] [Google Scholar]
- Boyce BF, Yoneda T, Lowe C, Soriano P, Mundy GR. Requirement of pp60c-src expression for osteoclasts to form ruffled borders and resorb bone in mice. J Clin Invest. 1992;90:1622–1627. doi: 10.1172/JCI116032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clohisy J, Roy B, Biondo C, Frazier E, Willis D, Teitelbaum S, Abu-Amer Y. Direct inhibition of NF-kB blocks bone erosion associated with inflammatory arthritis. J Immunol. 2003;171(10):5547–5553. doi: 10.4049/jimmunol.171.10.5547. [DOI] [PubMed] [Google Scholar]
- Clohisy JC, Hirayama T, Frazier E, Han SK, Abu-Amer Y. NF-kB signaling blockade abolishes implant particle-induced osteoclastogenesis. J Orthop Res. 2004;22(1):13–20. doi: 10.1016/S0736-0266(03)00156-6. [DOI] [PubMed] [Google Scholar]
- Clohisy JC, Yamanaka Y, Faccio R, Abu-Amer Y. Inhibition of IKK activation, through sequestering NEMO, blocks PMMA-induced osteoclastogenesis and calvarial inflammatory osteolysis. J Orthop Res. 2006;24(7):1358–1365. doi: 10.1002/jor.20184. [DOI] [PubMed] [Google Scholar]
- Cordier F, Grubisha O, Traincard F, Veron M, Delepierre M, Agou F. The zinc finger of NEMO is a functional ubiquitin-binding domain. J Biol Chem. 2009;284(5):2902–2907. doi: 10.1074/jbc.M806655200. [DOI] [PubMed] [Google Scholar]
- Dai S, Hirayama T, Abbas S, Abu-Amer Y. The IkappaB kinase (IKK) inhibitor, NEMO-binding domain peptide, blocks osteoclastogenesis and bone erosion in inflammatory arthritis. J Biol Chem. 2004;279(36):37219–37222. doi: 10.1074/jbc.C400258200. [DOI] [PubMed] [Google Scholar]
- Darwech I, Otero J, Alhawagri M, Dai S, Abu-Amer Y. Impediment of NEMO oligomerization inhibits osteoclastogenesis and osteolysis. J Cell Biochem. 2009;108(6):1337–1345. doi: 10.1002/jcb.22364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwech I, Otero JE, Alhawagri MA, Abu-Amer Y. Tyrosine phosphorylation is required for IkappaB kinase-beta (IKKbeta) activation and function in osteoclastogenesis. J Biol Chem. 2010;285(33):25522–25530. doi: 10.1074/jbc.M110.121533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontan E, Traincard F, Levy SG, Yamaoka S, Veron M, Agou F. NEMO oligomerization in the dynamic assembly of the IkappaB kinase core complex. FEBS J. 2007;274(10):2540–2551. doi: 10.1111/j.1742-4658.2007.05788.x. [DOI] [PubMed] [Google Scholar]
- Funakoshi-Tago M, Tago K, Andoh K, Sonoda Y, Tominaga S, Kasahara T. Functional role of c-Src in IL-1-induced NF-kappa B activation: c-Src is a component of the IKK complex. J Biochem. 2005;137(2):189–197. doi: 10.1093/jb/mvi018. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Karin M. Missing pieces in the NF-kB puzzle. Cell. 2002;109(2 Supplement 1):S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- Gowen M, Lazner F, Dodds R, Kapadia R, Feild J, Tavaria M, Bertoncello I, Drake F, Zavarselk S, Tellis I, Hertzog P, Debouck C, Kola I. Cathepsin K knockout mice develop osteopetrosis due to a deficit in matrix degradation but not demineralization. J Bone Miner Res. 1999;14(10):1654–1663. doi: 10.1359/jbmr.1999.14.10.1654. [DOI] [PubMed] [Google Scholar]
- Hayashi N, Titani K. N-myristoylated proteins, key components in intracellular signal transduction systems enabling rapid and flexible cell responses. Proc Japan Acad B Phys Biol Sci. 2010;86(5):494–508. doi: 10.2183/pjab.86.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Shared principles in NF-[kappa]B signaling. Cell. 2008;132(3):344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Horne WC, Neff L, Chatterjee D, Lomri A, Levy JB, Baron R. Osteoclasts express high levels of pp60c-src in association with intracellular membranes. J Cell Biol. 1992;119:1003–1013. doi: 10.1083/jcb.119.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H, Lacey DL, Dunstan CR, Solovyev I, Colombero A, Timms E, Tan HL, Elliott G, Kelley MJ, Sarosi I, Wang L, Xia XZ, Elliott R, Chiu L, Black T, Scully S, Capparelli C, Morony S, Shimamoto G, Bass MB, Boyle WJ. Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc Natl Acad Sci USA. 1999;96(7):3540–3545. doi: 10.1073/pnas.96.7.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TT, Feinberg SL, Suryanarayanan S, Miyamoto S. The zinc finger domain of NEMO is selectively required for NF-kB activation by UV radiation and topoisomerase inhibitors. Mol Cell Biol. 2002;22(16):5813–5825. doi: 10.1128/MCB.22.16.5813-5825.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang WC, Chen JJ, Inoue H, Chen CC. Tyrosine phosphorylation of I-kB kinase-alpha/beta by protein kinase C-dependent c-Src activation is involved in TNF-alpha-induced cyclooxygenase-2 expression. J Immunol. 2003;170(9):4767–4775. doi: 10.4049/jimmunol.170.9.4767. [DOI] [PubMed] [Google Scholar]
- Iotsova V, Caamaäno J, Loy J, Yang Y, Lewin A, Bravo R. Osteopetrosis in mice lacking NF-kappaB1 and NF-kappaB2. Nat Med. 1997;3(11):1285–1289. doi: 10.1038/nm1197-1285. [DOI] [PubMed] [Google Scholar]
- Jimi E, Nakamura I, Ikebe T, Akiyama S, Takahashi N, Suda T. Activation of NF-kB is involved in the survival of osteoclasts promoted by interleukin-1. J Biol Chem. 1998;273(15):8799–8805. doi: 10.1074/jbc.273.15.8799. [DOI] [PubMed] [Google Scholar]
- Karin M. NF-kappaB and cancer: Mechanisms and targets. Mol Carcinog. 2006;45(6):355–361. doi: 10.1002/mc.20217. [DOI] [PubMed] [Google Scholar]
- Karin M, Yamamoto Y, Wang M. The IKK NF-kB system: A treasure trove for drug development. Nat Rev. 2004;3:17–26. doi: 10.1038/nrd1279. [DOI] [PubMed] [Google Scholar]
- Lacey DL, Tan HL, Lu J, Kaufman S, Van G, Qiu W, Rattan A, Scully S, Fletcher F, Juan T, Kelley M, Burgess TL, Boyle WJ, Polverino AJ. Osteoprotegerin ligand modulates murine osteoclast survival in vitro and in vivo. Am J Pathol. 2000;157(2):435–448. doi: 10.1016/S0002-9440(10)64556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SE, Chung WJ, Kwak HB, Chung CH, Kwack K, Lee ZH, Kim HH. Tumor necrosis factor-alpha supports the survival of osteoclasts through the activation of Akt and ERK. J Biol Chem. 2001;276(52):49343–49349. doi: 10.1074/jbc.M103642200. [DOI] [PubMed] [Google Scholar]
- Li J, Sarosi I, Yan XQ, Morony S, Capparelli C, Tan HL, McCabe S, Elliott R, Scully S, Van G, Kaufman S, Juan SC, Sun Y, Tarpley J, Martin L, Christensen K, McCabe J, Kostenuik P, Hsu H, Fletcher F, Dunstan CR, Lacey DL, Boyle WJ. RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastogenesis and regulation of bone mass and calcium metabolism. Proc Natl Acad Sci. 2000;97(4):1566–1571. doi: 10.1073/pnas.97.4.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XH, Fang X, Gaynor RB. Role of IKK-gamma/NEMO in assembly of the IkB kinase complex. J Biol Chem. 2001;276(6):4494–4500. doi: 10.1074/jbc.M008353200. [DOI] [PubMed] [Google Scholar]
- Luxenburg C, Parsons JT, Addadi L, Geiger B. Involvement of the Src-cortactin pathway in podosome formation and turnover during polarization of cultured osteoclasts. J Cell Sci. 2006;119(23):4878–4888. doi: 10.1242/jcs.03271. [DOI] [PubMed] [Google Scholar]
- Madge LA, Pober JS. A phosphatidylinositol 3-kinase/Akt pathway, activated by tumor necrosis factor or interleukin-1, inhibits apoptosis but does not activate NF-kB in human endothelial cells. J Biol Chem. 2000;275(20):15458–15465. doi: 10.1074/jbc.M001237200. [DOI] [PubMed] [Google Scholar]
- Missbach M, Altmann E, Susa M. Tyrosine kinase inhibition in bone metabolism. Curr Opin Drug Discov Dev. 2000;3(5):541–548. [PubMed] [Google Scholar]
- Miyazaki T, Katagiri H, Kanegae Y, Takayanagi H, Sawada Y, Yamamoto A, Pando MP, Asano T, Verma IM, Oda H, Nakamura K, Tanaka S. Reciprocal role of ERK and NF-kB pathways in survival and activation of osteoclasts. J Cell Biol. 2000;148(2):333–342. doi: 10.1083/jcb.148.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki T, Sanjay A, Neff L, Tanaka S, Horne WC, Baron R. Src kinase activity is essential for osteoclast function. J Biol Chem. 2004;279(17):17660–17666. doi: 10.1074/jbc.M311032200. [DOI] [PubMed] [Google Scholar]
- Otero JE, Dai S, Foglia D, Alhawagri M, Vacher J, Pasparakis M, Abu-Amer Y. Defective osteoclastogenesis by IKKbeta-null precursors is a result of receptor activator of NF-kappaB ligand (RANKL)-induced JNK-dependent apoptosis and impaired differentiation. J Biol Chem. 2008;283(36):24546–24553. doi: 10.1074/jbc.M800434200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero JE, Dai S, Alhawagri MA, Darwech I, Abu-Amer Y. IKKbeta activation is sufficient for RANK-independent osteoclast differentiation and osteolysis. J Bone Miner Res. 2010;25(6):1282–1294. doi: 10.1002/jbmr.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa S, Zazzeroni F, Pham CG, Bubici C, Franzoso G. Linking JNK signaling to NF-kappaB: A key to survival. J Cell Sci. 2004;117(22):5197–5208. doi: 10.1242/jcs.01483. [DOI] [PubMed] [Google Scholar]
- Ravid T, Hochstrasser M. NF-kB signaling: Flipping the switch with polyubiquitin chains. Curr Biol. 2004;14(20):R898–R900. doi: 10.1016/j.cub.2004.09.074. [DOI] [PubMed] [Google Scholar]
- Ruocco MG, Maeda S, Park JM, Lawrence T, Hsu L-C, Cao Y, Schett G, Wagner EF, Karin M. IkB kinase-beta, but not IKK-alpha, is a critical mediator of osteoclast survival and is required for inflammation-induced bone loss. J Exp Med. 2005;201(10):1677–1687. doi: 10.1084/jem.20042081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushe M, Silvian L, Bixler S, Chen LL, Cheung A, Bowes S, Cuervo H, Berkowitz S, Zheng T, Guckian K, Pellegrini M, Lugovskoy A. Structure of a NEMO/IKK-associating domain reveals architecture of the interaction site. Structure. 2008;16(5):798–808. doi: 10.1016/j.str.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Saftig P, Hunziker E, Wehmeyer O, Jones S, Boyde A, Rommerskirch W, Moritz JD, Schu P, von Figura K. Impaired osteoclastic bone resorption leads to osteopetrosis in cathepsin-K-deficient mice. Proc Natl Acad Sci USA. 1998;95(23):13453–13458. doi: 10.1073/pnas.95.23.13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzberg P, Xing L, Lowell CA, Lee E, Garrett L, Reddy S, Roodman GD, Boyce B, Varmus HE. Complementation of osteopetrosis in src−/− mice does not require src kinase activity. J Bone Miner Res. 1996;11:S135. [Google Scholar]
- Schwartzberg PL, Xing L, Hoffmann O, Lowell CA, Garrett L, Boyce BF, Varmus HE. Rescue of osteoclast function by transgenic expression of kinase-deficient Src in src−/− mutant mice. Genes Dev. 1997;11(21):2835–2844. doi: 10.1101/gad.11.21.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma RK. Potential role of N-myristoyltransferase in pathogenic conditions. Can J Physiol Pharmacol. 2004;82(10):849–859. doi: 10.1139/y04-099. [DOI] [PubMed] [Google Scholar]
- Siebenlist U, Franzoso G. Structure, regulation and function of NF-kB. Proc Natl Acad Sci USA. 2001;89(10):4333–4337. [Google Scholar]
- Sly WS, Hewett-Emmett D, Whyte MP, Yu Y-S, Tashian RE. Carbonic anhydrase II deficiency identified as the primary defect in the autosomal recessive syndrome of osteopetrosis with renal tubular acidosis and cerebral calcification. Proc Natl Acad Sci USA. 1983;80:2752–2756. doi: 10.1073/pnas.80.9.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P, Montgomery C, Geske R, Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991;64:693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- Summy JM, Qian Y, Jiang BH, Guappone-Koay A, Gatesman A, Shi X, Flynn DC. The SH4-unique-SH3-SH2 domains dictate specificity in signaling that differentiate c-Yes from c-Src. J Cell Sci. 2003;116(12):2585–2598. doi: 10.1242/jcs.00466. [DOI] [PubMed] [Google Scholar]
- Sun S-C. Non-canonical NF-[kappa]B signaling pathway. Cell Res. 2011;21(1):71–85. doi: 10.1038/cr.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum SL. Osteoclasts: What do they do and how do they do it? Am J Pathol. 2007;170(2):427–435. doi: 10.2353/ajpath.2007.060834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet. 2003;4(8):638–649. doi: 10.1038/nrg1122. [DOI] [PubMed] [Google Scholar]
- Ting AY, Endy D. Signal transduction: Decoding NF-kB signaling. Science. 2002;298(5596):1189–1190. doi: 10.1126/science.1079331. [DOI] [PubMed] [Google Scholar]
- Varmus H, Hirai H, Morgan D, Kaplan J, Bishop JM. Function, location, and regulation of the src protein-tyrosine kinase. Princess Takamatsu Symp. 1989;20:63–70. [PubMed] [Google Scholar]
- Xing L, Venegas AM, Chen A, Garrett-Beal L, Boyce BF, Varmus HE, Schwartzberg PL. Genetic evidence for a role for Src family kinases in TNF family receptor signaling and cell survival. Genes Dev. 2001;15(2):241–253. doi: 10.1101/gad.840301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Yamashita J, Tang E, Wang Hl, Guan K, Wang CY. The zinc finger mutation C417R of I-{kappa}B kinase-gamma impairs lipopolysaccharide- and TNF-mediated NF-kB activation through inhibiting phosphorylation of the IkB kinase-beta activation loop. J Immunol. 2004;172(4):2446–2452. doi: 10.4049/jimmunol.172.4.2446. [DOI] [PubMed] [Google Scholar]
- Yoneda T, Lowe C, Lee C-H, Gutierrez G, Niewolna M, Williams PJ, Izbicka E, Uehara Y, Mundy GR. Herbimycin A, a pp60c-src tyrosine kinase inhibitor, inhibits osteoclastic bone resorption in vitro and hypercalcemia in vivo. J Clin Invest. 1993;91:2791–2795. doi: 10.1172/JCI116521. [DOI] [PMC free article] [PubMed] [Google Scholar]