Abstract
Introduction
Aldose reductase (AR) initially thought to be involved in the secondary diabetic complications because of its glucose reducing potential. However, evidence from recent studies indicates that AR is an excellent reducer of a number of lipid peroxidation-derived aldehydes as well as their glutathione conjugates, which regulate inflammatory signals initiated by oxidants such as cytokines, growth factors and bacterial endotoxins, and revealed the potential use of AR inhibition as an approach to prevent inflammatory complications.
Areas covered
An extensive Internet and Medline search was performed to retrieve information on understanding the role of AR inhibition in the pathophysiology of endotoxin-mediated inflammatory disorders. Overall, inhibition of AR appears to be a promising strategy for the treatment of endotoxemia, sepsis and other related inflammatory diseases.
Expert opinion
Current knowledge provides enough evidence to indicate that AR inhibition is a logical therapeutic strategy for the treatment of endotoxin-related inflammatory diseases. Since, AR inhibitors have already gone to Phase-iii clinical studies for diabetic complications and found to be safe for human use, their use in endotoxin–related inflammatory diseases could be expedited. However, one of the major challenges will be the discovery of AR regulated clinically-relevant biomarkers to identify susceptible individuals at risk of developing inflammatory diseases, thereby warranting future research in this area.
Keywords: Aldose Reductase, Endotoxin, Inflammation, Sepsis
1. Introduction
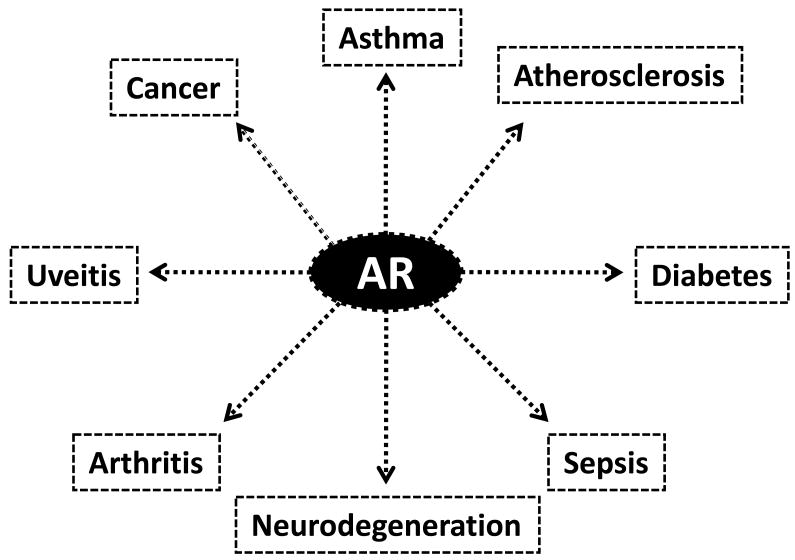
Recent years have witnessed an upsurge of interest in understanding the precise roles of aldose reductase (AR; AKR1B1) inhibition in endotoxin-related inflammatory diseases. AR, a member of the aldo-keto reductase superfamily [1], is a cytosolic protein that catalyzes the first and rate limiting step of the polyol pathway of glucose metabolism. Besides reducing glucose to sorbitol, AR also reduces a number of lipid peroxidation-derived aldehydes as well as their glutathione (GSH) conjugates [2-6]. Cellular and/or tissue inflammation is a known hallmark of most diseases ranging from asthma, cancers viz. colon, hepatic, melanoma, breast, cervical and lung cancers, atherosclerosis, sepsis, diabetes, arthritis, uveitis as well as neurodegeneration. Moreover, AR inhibition is emerging as a logical therapeutic approach in understanding the cellular and molecular mechanisms as well as the clinical sequelae associated with diseases, especially bacterial endotoxin, lipopolysaccharide (LPS)-related inflammatory disorders (Fig. 1). Inflammatory diseases are the leading causes of morbidity and mortality in populations worldwide. There is accumulating evidence regarding the role of AR inhibition in LPS-mediated inflammatory diseases such as allergic airway inflammation/asthma, sepsis and uveitis [7]; therefore, there is a growing need for re-evaluating anti-AR interventions for the treatment of endotoxin-related inflammatory disorders. AR appears to be an important metabolic route for the detoxification of lipid derived-aldehydes (LDAs) [8, 9, 10]; it is an excellent catalyst for the reduction of medium to long-chain unbranched saturated and unsaturated aldehydes, and their conjugates with glutathione (GSH) [11]. Current knowledge indicates that inhibiting AR may be efficacious in pre-clinical models; moreover, a few AR inhibitors (ARIs) have already gone through clinical trials for diabetic complications, thereby suggesting that they may be therapeutically exploited in inflammatory settings. Thus, inhibition of AR may serve as a novel therapeutic strategy for the prevention and/or treatment of inflammatory diseases, especially for patients who may be poor responders to conventional therapy. In this review, we have aimed to highlight significant advances in the role of AR inhibition in the pathophysiology of endotoxin-mediated inflammatory diseases, especially allergic airway inflammation, sepsis and uveitis.
Figure 1.
Aldose Reductase (AR) in human diseases
2. Overview of AR
Human AR is a monomeric protein (36 kDa) of 315 amino acids; AR is encoded by AKR1B1 gene which is mapped at chromosome region 7q35. AR's active site is located at the C-terminal end of the barrel which is best suited for efficient interaction with NADPH, a cofactor required for AR's reduction reactions [7]. Recent studies have demonstrated that AR is involved in oxidative stress signaling initiated by inflammatory cytokines, chemokines and growth factors [2]. Moreover, a beneficial role of AR has been suggested especially in the detoxification of toxic lipid aldehydes generated upon oxidative stress [12]. Although, in experimental animals ARIs have shown potential inhibition of secondary diabetic complications, none of the ARIs have passed the phase III clinical trial for the prevention of diabetic complications such as diabetic neuropathy, nephropathy and retinopathy [13]. However, previous studies indicate that the accelerated flux of sorbitol through the polyol pathway has been implicated in the etiopathogenesis of secondary diabetic complications, such as cataractogenesis, retinopathy, neuropathy, nephropathy as well as cardiovascular [12]. Therefore, targeted manipulation of the polyol pathway, especially the design of novel strategies to inhibit AR, has emerged as a promising avenue for the therapeutic intervention of diabetic complications.
A number of ARIs have been developed and some of them have gone through Phase-iii clinical studies to prevent diabetic complications. ARIs are categorized based on their chemical structure, either as acetic acid compounds or spirohydantoins.
Out of these two classes of ARIs tested to date in clinical trials, the carboxylic acid inhibitor such as zopolrestat has been shown to penetrate tissues poorly with lesser potency, whereas the spirohydantoin inhibitors penetrate tissues more efficiently. Many reports indicate the adverse effects of ARI such as skin reactions and liver toxicity [13, 14]. Moreover, each of the two classes of ARI has its own drawbacks in terms of selectivity, in vivo potency as well as human safety, thereby implicating that the pathogenic role of AR in diabetic complications remains controversial till date. Recently, a new structural class (pyridazinones) of ARI named ARI-809 was shown to have high selectivity for aldose versus aldehyde reductase [15].
AR inhibitor studies in the past few years have shed light on a number of crystallographic structures that help to explain the details of catalysis and potential binding sites, primarily the opening of the specificity pocket and occupation of the anionic hole in the active site cleft by the charged head of inhibitors [16, 17]. Moreover, clinical trials with ARIs such as Sorbinil, Tolrestat, Ponalrestat, Zopolrestat, Zenarestat, Epalrestat, Fidarestat and Ranirestat have yielded mixed results, showing either an apparent lack of efficacy or adverse effects. Due to lack of promising therapeutic efficacy in preventing diabetic complications during clinical studies, none of the AR inhibitors were approved by FDA in USA for the treatment of diabetic complications, specifically diabetic neuropathy. Only AR inhibitor, epalrestat, is in market in Japan to treat patients with diabetic neuropathy [18]. Recent report by Oates, 2008 has suggested that reliance on nerve sorbitol for assessment of AR inhibition likely caused underestimation of doses needed for clinical efficacy and overestimation of drug safety margins; these new insights will lead to novel ARIs that will effectively and safely slow the progression of diabetic neuropathy [19]. Further, a new paradigm defining the pathogenic role of AR, the ‘metabolic flux hypothesis’, was presented; robust inhibition of metabolic flux through the polyol pathway in peripheral nerve would likely result in substantial clinical benefit in treatment and prevention of diabetic peripheral neuropathy [20, 21].
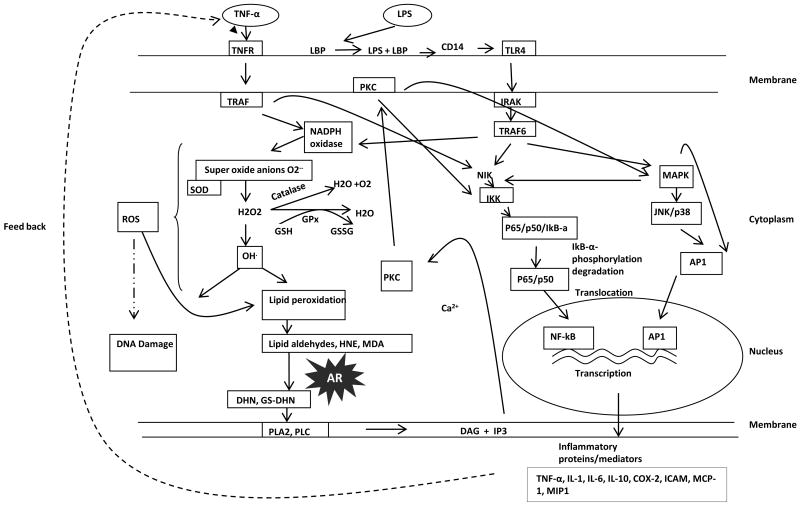
Recent studies indicate that the major physiological role of AR could be the reduction of oxidative stress generated lipid aldehydes and their GSH-conjugates [2]. This is because AR has been shown to reduce lipid aldehydes and their conjugates with GSH with a Km in μM range as compared to the reduction of glucose to sorbitol. Inagaki et al,1982 have reported that AR acts on the aldehyde form of D-glucose with a Km of 0.66 μmol/L [22]. In addition, inhibition of AR may exacerbate the toxic effects of LDAs and their associated pathological effects. Since lipid aldehydes have been shown to be involved in the mediation of cellular signaling pathways, inhibition of AR that regulates that regulates the level of these aldehydes could regulate oxidative signals. Indeed, recent studies indicate that AR inhibition prevents cytokine, growth factor and hyperglycemia–induced oxidative stress signals in cultured cells as well as animal models of inflammatory diseases. Even though the precise pathophysiological mechanism(s) underlying the susceptibility to various inflammatory diseases are indeed highly complex, the involvement of the AR-mediated signaling pathway (Fig. 2) in inflammatory diseases has provided mechanistic insights into the biochemical/physiological role of AR. Thus, AR has become an attractive pharmacologic target for inflammatory complications other than diabetes. Accordingly, results from most recent preclinical studies in experimental animals of inflammatory diseases using ARIs have received considerable attention as logical targets for inflammatory diseases, especially mediated by bacterial endotoxins.
Figure 2.
Schematic representation of Aldose Reductase (AR) in endotoxin signaling
3. Endotoxin-induced inflammation
Bacterial lipopolysaccharide (LPS), a highly proinflammatory endotoxin, is a component of the outer envelope of all Gram-negative bacteria. LPS is formed by the phosphogylcolipid A and is covalently linked to hydrophilic hetero-polysaccharide and is responsible for its toxicity [23]. It is released from the surface of replicating Gram-negative bacteria into the circulation, where it is recognized by a variety of circulating cell types, triggering gene induction of pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), and biosynthesis of prostaglandins (PGEs) [24-27]. These and other cytokines act in an autocrine or paracrine manner to induce and amplify the host cell response and defense systems that help to eliminate the bacterial infection. However, uncontrolled and excessive cytokine expression can induce acute or chronic inflammatory processes. Thus, the cytotoxic inflammatory complications due to bacterial infections are not only due to LPS, but also caused by the autocrine effects of various cytokines released by host cells. Toll-like receptor (TLR) 4 is a major receptor that is activated after LPS binds to CD14 receptor in concert with LPS-binding protein (LBP). The LPS + CD14 complex is then transferred to the membrane-bound TLR4-MD-2 (accessory protein) complex [28, 29]. Once LPS is bound to TLR4, it signals through MyD88 to activate the redox-sensitive transcription factors such as NF-kB and AP-1, via NADPH oxidase [12]. The increased transcriptional activation by NF-kB and AP1 induces the expression of several inflammatory cytokines and chemokines, which propagate the LPS-mediated cytotoxicity. Thus activation of redox-sensitive transcription factors such as NF-kB play a central and crucial role in the propagation of LPS as well as feedback signals of LPS-induced cytokines, leading to cell injury and/or dysfunction [30, 31]. LPS-induced activation of NF-kB as well as LPS-induced cytotoxicity can be prevented by antioxidants, flavinoids, over expression of superoxide dismutase (SOD) and catalase, as well as inhibition of NADPH oxidase [32-35], thereby implicating the significance of ROS in LPS-induced cytotoxic effects. However, the specific mechanisms by which ROS propagate ROS signals remain unclear. Nevertheless, there is significant evidence showing that ROS generated by LPS via NADPH oxidase can be messengers of cellular signal transduction and gene expression. Further, earlier studies have provided important links between elevated cytokines such as TNF-α and IL-1 with oxidative stress during initial inflammatory processes, and have shown to alter redox equilibrium through a thiol-dependent mechanism. Interestingly, antioxidants have been shown to down-regulate cytokine transcription and biosynthesis.
Conversely, increased oxidative stress, e.g., depletion of GSH, can augment pro-inflammatory signals by up-regulating ROS. Hence, levels of reduced GSH are a critical determinant of ROS signaling. Therefore, when oxidants overwhelm the antioxidative capacity, lipid peroxides and toxic lipid-derived aldehydes such as 4-hydroxynonenal (HNE) are formed, which may be responsible for both, ROS signaling and ROS toxicity. The lipid-derived aldehyde, HNE is highly electrophilic and exhibits numerous cellular effects [36, 37]. Recent reports support a role for this aldehyde in inflammation; indeed, HNE can be detected in inflammatory exudates, and increased HNE-proteins adducts were observed during LPS-induced apoptosis of placental cells and other inflammatory processes [38]. HNE has been shown to increase the LPS-induced production of cytokines such as IL-1, IL-10 and TNF-α in human blood mononuclear cells [39]. However, it is not clear how the lipid peroxidation-derived toxic aldehydes (such as HNE and its conjugate with GSH, GS-HNE) are involved in the inflammatory process, or what role AR (which catalyzes the detoxification of HNE) plays in the transduction of ROS signals generated by LPS and cytokines. Our studies indicate that AR inhibitors prevent HNE and GS-HNE induced inflammatory signals but not GS-DHN induced, suggesting that AR catalyzed product, GS-DHN could mediate inflammatory signals [9, 40, 41, 42 and FIG.2].
3.1. AR inhibition in endotoxin-mediated inflammatory diseases
Inflammation is a complex system of host systemic and local responses to injury and infection. Inflammation contributes to almost all disease processes, including immunological and vascular pathology, sepsis, and chemical and metabolic injury. In inflammation and the regulation of the immune response, macrophages play a central role. The prototypical inflammatory stimulus initiated by LPS has been implicated in inflammatory diseases due to bacterial infections [43]. It is well established that increased expression of cytokines elicits the cytotoxic actions of LPS signals in many inflammatory diseases. About 100,000 deaths in the U.S. each year can be endorsed to an unnecessary inflammatory response to bacterial infections. Moreover, cytokines play a critical role in several cardiovascular and neurological degenerative diseases as well as cancer. Hence elucidation of the mechanisms that mediate and regulate cytokine signals is of profound importance to understanding and managing a very large array of disease processes. Therefore, strategies focused on inhibiting endotoxin-related inflammation may hold promise for therapeutic intervention. In this respect, ARIs are emerging as pivotal players in understanding the cellular and molecular bases of several inflammatory disorders/diseases ranging from asthma, sepsis and uveitis.
3.1.1. Asthma
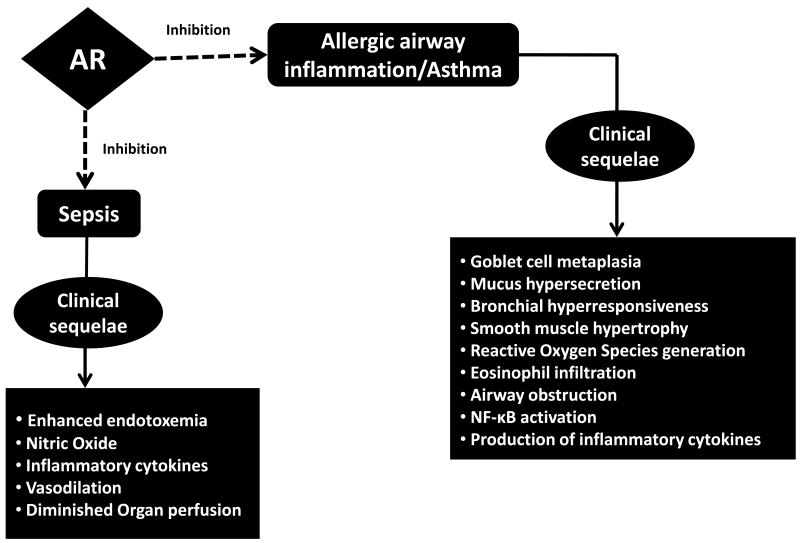
The prevalence of asthma, a complex airway inflammatory disorder, is increasing globally. Asthma is a complex disease with both a strong genetic susceptibility as well as environmental influence. Allergic asthma is one of the most common chronic conditions in Western countries [44]. Cellular and physiological responses to endotoxin have remained an area of interest in the last few decades and epidemiological studies have revealed that endotoxin has notable impacts on allergic disease [45]. Exposure to endotoxin causes chronic obstructive lung disease [46] which may lead to the development of obstructive lung disease including emphysema, chronic bronchitis and asthma [47-50]. Endotoxin has been demonstrated to cause goblet cell hypertrophy, mucus over production, thereby causing transforming growth factor (TGF)-α and TNF-α-mediated induction of epidermal growth factor receptor in epithelial cells that may lead to the proliferation of goblet cells, mucin secretion by neutrophil elastase and induction of histamine release from basophils [45]. CD14 expression is reportedly upregulated in the airways of subjects with asthma after allergen challenge; moreover eosinophilic inflammation may further aggravate the response to endotoxin [51]. Endotoxin has been implicated in increased formation of ROS and free radicals upon exposure to inhaled environmental pollutants [52, 53]. The clinical sequelae in endotoxin-related allergic airway inflammation/asthma include goblet cell metaplasia, mucus hypersecretion, bronchial hyperresponsiveness, smooth muscle hypertrophy, reactive oxygen species generation, eosinophil infiltration, airway obstruction, NF-kB activation and production of inflammatory cytokines (Fig. 3). Inhibition of AR has recently been demonstrated to prevent allergic airway inflammation and asthma [54-56].
Figure 3.
Implications of Aldose Reductase (AR) inhibition in Asthma and Sepsis
Pharmacological inhibition and/or genetic ablation of AR by signal was observed to prevent TNF-α- as well as LPS-induced apoptosis, ROS generation, synthesis of various inflammatory mediators including IL-6, IL-8, and PGE-2; moreover, AR inhibition also caused activation of transcription factors NF-kB and AP-1 in small airway epithelial cells. Murine model studies investigating the potential role of AR inhibition in asthma have revealed that AR inhibition prevents ragweed pollen extract and ovalbumin-induced allergic inflammation. The role of AR inhibition has also been demonstrated in downregulating the expression of IL-13-induced expression of mucin as well as phosphorylation of components of JAK-STAT signaling pathway, primarily JAK-1, ERK1/2 and STAT-6 [57]. These findings implicate the emerging role of AR inhibition as a promising strategy in asthma management.
Besides AR inhibition in endotoxin-mediated inflammatory diseases, there is growing evidence regarding the potential role of AR in allergen-induced airway inflammation. Murine-model studies from our laboratory have recently demonstrated that AR is a pivotal player in allergen viz. ovalbumin (OVA)-as well as ragweed pollen extract (RWE)-induced airway inflammation [12]. OVA-sensitization/challenge induced a marked perivascular and peribronchial eosinophilic infiltration into the lungs of mice; moreover, the infiltration of inflammatory cells into the airways of OVA-challenged mice was greatly reduced with AKR1B1 inhibitor [54, 57]. A significant reduction in the levels of Th2 cytokines including IL-4, 5 and 6 as well as chemokines including keratinocyte-derived chemokine (KC), granulocyte colony stimulating factor (G-CSF) and monocyte chemotactic protein (MCP)-1 in broncho-alveolar lavage (BAL) fluid was observed in our OVA-induced inflammation studies. AR inhibition studies in RWE-challenged experimental murine model system revealed robust airway inflammation that was quantified by accumulation of inflammatory cells in the BAL fluid and sub-epithelium; mice treated with AKR1B1 inhibitor showed relatively lower inflammation as determined by the eosinophil-count in the BAL fluid. In a similar manner, perivascular and peribronchial inflammation in the lavage fluid of experimental animals challenged with RWE was significantly prevented by AR inhibitor. AR inhibition was also observed to prevent RWE-induced mucin secretion and airway hyper-responsiveness in mice challenged with methacholine [55]. The above findings from our research group further strengthen the growing significance of AR inhibition in allergen-induced airway inflammation. However, these studies in murine models need to be further corroborated in human study subjects in ethnically disparate populations to fully dissect the mechanisms involved in allergen-induced airway inflammation at the cellular, molecular as well as genetic level.
3.1.2. Sepsis
Sepsis is emerging as a major cause of morbidity and mortality worldwide in recent times. Major injury due to surgical and/or trauma results in increased production of potentially profound immunological dysfunction which causes tissue injury, postoperative infection, and eventually multiple organ dysfunction syndrome (MODS) [58]. The hallmarks of sepsis include enhanced endotoxemia alongwith increased levels of nitric oxide, inflammatory cytokines, vasodilatiom and diminished organ perfusion. The initial proinflammatory immune response, or systemic inflammatory response syndrome (SIRS), is mediated primarily by the cells of the innate immune system, and therefore the use of novel strategies targeting mediators in the immune system as well as inflammatory mileu appears to be an attractive immunotherapeutic option. Furthermore, an unbalanced systemic compensatory anti-inflammatory response can result in immunosuppression, which may predispose the host to the development of opportunistic infection. In this context, targeting the inhibition of AR may be beneficial in understanding the complexities associated with sepsis (Fig. 3). Recently, AR inhibition has been implicated in LPS-endotoxemia [59, 60]. Inhibition/ablation of AR was observed to significantly attenuate LPS-induced activation of PKC, PLC, NF-kB, phosphorylation and proteolytic degradation of IkB-α in macrophages. Further, AR inhibition has been shown to prevent the macrophage death induced by LPS in cultured cells. Furthermore, in a similar study in mouse model of endotoxemia, AR inhibition has been demonstrated to alter/diminish inflammatory cytokines in response to LPS challenge in murine serum, liver, spleen and heart. AR inhibition also resulted in a blunted activation of PKC, JNK, and p38-MAPK and phosphorylation of IkB-a, IKK, and PLC [61]. Indeed, AR inhibition has been shown to prevent LPS-induced cardiomyopathy, a major of mortality in sepsis patients, in mice. Further, AR inhibition induced functional recovery in myocardial fractional shortening due to endotoxemia in vivo and preserved contractile function of isolated perfused hearts. Moreover, AR inhibitors have shown to decrease the mortality associated with LPS in mice. In addition, studies using a caecum-ligation-puncture murine model of polymicrobial sepsis demonstrated that AR inhibition prevented the levels of inflammatory cytokines and chemokines [61]. However, the efficacy of AR inhibition in preventing multi-organ toxicity, one of the major causes of septic shock and death still needs to be investigated. Overall, these studies showing that inhibition of AR prevents sepsis in clinically relevant animal models will provide tangible benefits to patients with sepsis.
3.1.3. Uveitis
Uveitis is a systemic inflammatory response syndrome, the hallmarks being excessive production of inflammatory cytokines in response to bacterial infections [62]. Moreover, the recurrent nature of uveitis may result in certain sight-threatening conditions such, as cataracts, glaucoma and even blindness [63, 64]. Endotoxin-induced uveitis is usually considered to be an inflammation of the anterior uvea, with some changes in the posterior segment (vitreous and retina) [65, 66]. The pathophysiological mechanisms underlying uveitis appear to be indeed complicated, thereby, emphasizing the need to identify potential therapeutic targets that may help in understanding the etiopathogenesis of uveitis. Recent studies suggest the growing significance of AR inhibitors as therapeutic targets in treating uveitis patients. AR has been implicated as a pivotal player in LPS-induced inflammation in human non-pigmented ciliary epithelial cells (hNPECs) as well as in lens epithelial cells. LPS-stimulation caused increased secretion of PGE (2) and NO in the culture medium as well as enhanced expression of COX-2 and iNOS proteins in cellular extracts [67]. Moreover, the findings revealed increased phosphorylation of MAPK/ERK and SAPK/JNK as well as activation of redox-sensitive transcription factors NF-kB and AP-1; however, AR inhibition by sorbinil and zopolrestat ameliorated these inflammatory alterations in hNPECs. Interestingly, LPS-induced decrease in the expression of Na/K-ATPase in hNPECs was restored by AR inhibitors. Inhibition of AR has been demonstrated to attenuate TNF-α, NO and PGE2 levels as well as downregulate the infiltration of inflammatory cells in the aqueous humor of uveitis rat eyes. Moreover, the expression of TNF-α, inducible-NOS and COX-2 proteins in the ciliary body, corneal epithelium and retinal wall was significantly prevented by AR inhibition. Additionally, naturally occurring compounds viz. benfotiamine and guggulsterone that attenuate the expression of AR as well as the activation of NF-kB have also been observed to ameliorate endotoxin-induced uveitis [68, 69]. Recent studies also indicate that AR inhibition prevents auto-immune induced uveitis in rats. Further, AR null mice were shown to be protective against endotoxin-induced uveitis, confirming the definitive role of AR in endotoxin–induced ocular inflammatory complications.
3.1.4. Vasculoproliferative disorders
Recent studies indicate that bacterial infections increase the risk of vasculoproliferative disorders. AR inhibition is emerging as an important research strategy in understanding the etiology of vasculoproliferative disorders. The cellular and molecular mechanisms underlying atherosclerosis and restenosis are not completely understood. Therefore, the identification of logical therapeutic targets in the management of vasculoproliferative disorders is currently an area of intense study worldwide. Atherosclerosis is a chronic inflammatory condition resulting from the interaction between modified lipoproteins and various cell types of the blood and the vessel wall, including monocytes and macrophages, platelets and vascular smooth muscle cells. Apart from inflammation, atherosclerotic plaque development involves the phenotypic modulation of vascular smooth muscle cells to proliferating and dedifferentiated cells. In contrast, restenosis is the process of luminal narrowing in an atherosclerotic artery after intra-arterial intervention, such as balloon angioplasty and stent placement [70]. The hallmarks of restenosis include the migration and proliferation of vascular smooth muscle cells and accumulation of extracellular matrix. AR inhibitors have been implicated in the delay and/or prevention of the onset of major cardiovascular complications such as ischemic injury, restenosis and atherosclerosis [71]. The cardioprotective effects of ARIs have been demonstrated in ischaemia-reperfusion injury in diabetic myocardium. Calderone et al, 2010 have shown that ARI, epalrestat is cardioprotective in the diabetic hearts submitted to ischaemia-reperfusion where the AR activity is high but not in the non-diabetic hearts where AR activity is low [72]. Similarly, inhibition of AR by zopolrestat was demonstrated to improve the function of sacro/endoplasmic reticulum Ca (2+)-ATPase (SERCA) and ryanodine receptor by protecting them from irreversible oxidation in the ischemia/reperfusion (I/R) of hearts [73]. Treatment of apoE-null mice with ARIs sorbinil or tolrestat increased early lesion formation but did not affect the formation of advanced lesions, thus suggesting that AR is upregulated in atherosclerotic lesions and has a protective effect against early stages of atherogenesis by removal of toxic aldehydes generated in oxidized lipids [74]. Pharmacological blockade of AR has also been implicated in significant reduction of ROS generation and mitochondrial permeability transition (MPT) pore opening in mitochondria of aldose reductase transgenic (ARTg) mice hearts exposed to I/R stress, thereby suggesting that inhibition of AR pathway protects mitochondria and therefore may be a valuable adjunct for salvaging ischemic myocardium [75]. Moreover, AR inhibition resulted in reduction of oxLDL-induced intracellular oxidative stress; oxLDL-induced upregulation of AR in human macrophages was observed to be proinflammatory in foam cells and may be a potential link between the clinical sequelae associated with hyperlipidemia, atherosclerosis, and diabetes mellitus [76]. Recently, atherosclerosis development was quantified in transgenic mice expressing human AR (hAR) double crossed with the apoE knockout; the transgenes were used to increase the normally low levels of this enzyme in wild-type mice [77]. This study demonstrated that hAR expression in apoE null mice is atherogenic and that expression specifically in endothelial cells also leads to a significant disease progression. Further, both generalized hAR overexpression as well as hAR expression via the Tie 2 promoter increased lesion size in streptozotocin diabetic mice. Much needed investigations on how AR inhibition prevents these vasculoproliferative disorders specifically initiated by bacterial infection or endotoxins are still awaited.
4. Conclusions
Endotoxin has significant impact on inflammation-based disorders such as asthma, sepsis and uveitis. There has been considerable enthusiasm in the research community for assessing the clinical relevance of AR inhibition in endotoxin-related disease models. However, the design of bioavailable and more specific inhibitors of aldose reductase remains a challenge. Development and assessment of physiological activity of novel inhibitors of aldose reductase, the key enzyme in the polyol pathway, is currently an area of extensive research globally. The efficacy of ARIs is usually assessed using electrophysiological recordings that incorporate measurements of three key nerves: median and tibial motor nerves and the median sensory nerve [78]. Although ARI-targeted therapies are currently being evaluated in phase I/II and III studies for diabetes, yet, till date, they have not achieved worldwide clinical use in disease management because of limited efficacy and/or unfavorable adverse effects [79]. However, recent findings indicate that Fidarestat appears to be promising inhibitor with high specificity and minimal irreversible side effects. Recent 52 week clinical studies with fidarestat for diabetic neuropathy indicate that it is safe to use in human, hence this drug could be developed as potential anti-inflammatory agent in preventing inflammatory diseases. However, additional studies investigating the biological roles of fidarestat in clinical trial settings is essential to fully validate the potential market availability of this drug as well as determine their overall success in disease management worldwide. Future research exploring the role of ARIs in cellular and molecular mechanisms underlying endotoxin-related inflammatory diseases may significantly contribute in identifying and/or designing novel diagnostic approaches and intervention strategies to aid in the overall management of cellular/tissue inflammation in patients worldwide. In conclusion, AR inhibition appears to be an attractive therapeutic strategy for future anti-inflammatory therapy and translational research.
5. Expert opinion
Current knowledge indicates that AR inhibition is a logical therapeutic strategy for the treatment of endotoxin-related inflammatory diseases. The role of AR in the prevention of NF-kB activation, inflammation, cellular signaling leading to apoptosis or proliferation has been now extensively confirmed by our studies as well as others (80-88). The precise role of inhibitors of AR in altering/mediating endotoxin-related inflammatory diseases and their local/systemic effect(s) in both experimental as well as clinical settings warrants further investigation. The in vitro and in vivo findings in murine model systems need to be further corroborated in preclinical settings using genetically altered animal models and clinical settings with human patients to fully understand the precise role(s) of AR inhibition in tissue damage and organ dysfunction in patients with endotoxin-related inflammatory complications. Further, one of the major challenges in the AR-field is the relative paucity of clinically-relevant biomarkers to identify and accordingly stratify susceptible individuals at risk of developing inflammatory diseases. Thus, targeting interrelated biochemical pathways, in addition to aldose reductase inhibition, may be beneficial in the elucidation of the precise cellular/molecular mechanisms underlying endotoxin-related inflammation in the near future.
Article highlights.
Aldose Reductase (AR) catalyzes the rate limiting step of the polyol pathway of glucose metabolism; besides reducing glucose to sorbitol, AR also reduces a number of lipid peroxidation-derived aldehydes as well as their glutathione (GSH) conjugates.
Endotoxin has significant impact on inflammation-based disorders such as asthma, sepsis and uveitis.
AR inhibitors are emerging as pivotal players in understanding the cellular and molecular bases of endotoxin-related inflammatory disorders/diseases.
As AR inhibitors have already gone to Phase III clinical trials for diabetic complications and found to be safe for human use, their use in endotoxin-related inflammatory diseases could be expedited.
AR inhibition appears to be an attractive therapeutic strategy for future anti-inflammatory therapy and translational research.
Acknowledgments
The studies in the Authors laboratory were supported by National Institutes of Health (NIH) grants DK36118 (SKS) and GM 71036 (KVR).
Abbreviations
- AR
Aldose reductase
- ARIs
AR inhibitors
- BAL
broncho-alveolar lavage
- GSH
glutathione
- GS-DHN
Glutathionyl-1,4-dihydroxynonene
- G-CSF
granulocyte colony stimulating factor
- hNPECs
human non-pigmented ciliary epithelial cells
- HNE
hydroxynonenal
- IL-1
interleukin-1
- I/R
ischemia/reperfusion
- KC
keratinocyte-derived chemokine
- LPS
lipopolysaccharide
- LBP
LPS-binding protein
- MPT
mitochondrial permeability transition
- MCP-1
monocyte chemotactic protein
- MODS
multiple organ dysfunction syndrome
- OVA
ovalbumin
- PGE2
prostaglandins
- PPO
pyrido[1,2-a]-pyrimidin-4-one derivative
- RWE
ragweed pollen extract
- SERCA
sacro/endoplasmic reticulum Ca(2+)-ATPase
- SOD
superoxide dismutase
- SIRS
systemic inflammatory response syndrome
- TLR
toll-like receptor
- TGF
transforming growth factor
- TNF
tumor necrosis factor
Footnotes
Author contributions: SP researched data to include in the manuscript and drafted the manuscript; SKS provided critical comments and reviewed the manuscript; KVR conceived the idea, reviewed and edited the manuscript before submission.
Disclosures: The authors state no conflict of interest and have received no payment in preparation of this manuscript.
Contributor Information
Dr Saumya Pandey, Email: sapandey@utmb.edu, Uiversity of Texas Medical Branch, Biochemistry and Molecular Biology, Galveston, 77555 United States.
Dr Satish K Srivastava, Email: ssrivast@utmb.edu, Uiversity of Texas Medical Branch, Biochemistry and Molecular Biology, Galveston, 77555 United States.
Dr Kota V Ramana, Email: kvramana@utmb.edu, University of Texas Medical Branch, Biochemistry and Molecular Biology, 301 University Blvd, Galveston, 77555 United States.
References
- 1.Jez JM, Flynn TG, Penning TM. A new nomenclature for the aldo-keto reductase superfamily. Biochem Pharmacol. 1997;54:639–47. doi: 10.1016/s0006-2952(97)84253-0. [DOI] [PubMed] [Google Scholar]
- 2*.Srivastava SK, Ramana KV, Bhatnagar A. Role of aldose reductase and oxidative damage in diabetes and the consequent potential for therapeutic options. Endocr Rev. 2005;26:380–92. doi: 10.1210/er.2004-0028. This is a useful review that describes role of aldose reductase in diabetic complications. [DOI] [PubMed] [Google Scholar]
- 3.Srivastava S, Watowich SJ, Petrash JM, et al. Structural and kinetic determinants of aldehyde reduction by aldose reductase. Biochemistry. 1999;38:42–54. doi: 10.1021/bi981794l. [DOI] [PubMed] [Google Scholar]
- 4.Srivastava S, Spite M, Trent JO, et al. Aldose reductase-catalyzed reduction of aldehyde phospholipids. J Biol Chem. 2004;279:53395–406. doi: 10.1074/jbc.M403416200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dixit BL, Balendiran GK, Watowich SJ, et al. Kinetic and structural characterization of the glutathione-binding site of aldose reductase. J Biol Chem. 2000;275:21587–95. doi: 10.1074/jbc.M909235199. [DOI] [PubMed] [Google Scholar]
- 6.Ramana KV, Dixit BL, Srivastava S, et al. Selective recognition of glutathiolated aldehydes by aldose reductase. Biochemistry. 2000;39:12172–80. doi: 10.1021/bi000796e. [DOI] [PubMed] [Google Scholar]
- 7.Ramana KV. Aldose reductase: new insights for an old enzyme. BioMol Concepts. 2011;2:103–114. doi: 10.1515/BMC.2011.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srivastava S, Chandra A, Bhatnagar A, Srivastava SK, Ansari NH. Lipid peroxidation product, 4-hydroxynonenal and its conjugate with GSH are excellent substrates of bovine lens aldose reductase. Biochem Biophys Res Commun. 1995;217:741–746. doi: 10.1006/bbrc.1995.2835. [DOI] [PubMed] [Google Scholar]
- 9.Srivastava S, Dixit BL, Cai J, et al. Metabolism of lipid peroxidation product, 4-hydroxynonenal (HNE) in rat erythrocytes: role of aldose reductase. Free Radic Biol Med. 2000;29:642–651. doi: 10.1016/s0891-5849(00)00351-8. [DOI] [PubMed] [Google Scholar]
- 10.Vander Jagt DL, Kolb NS, Vander Jagt TJ, et al. Substrate specificity of human aldose reductase: identification of 4-hydroxynonenal as an endogenous substrate. Biochim Biophys Acta. 1995;1249:117–126. doi: 10.1016/0167-4838(95)00021-l. [DOI] [PubMed] [Google Scholar]
- 11.Bhatnagar A, Liu SQ, Ueno N, Chakrabarti B, Srivastava SK. Human placental aldose reductase: role of Cys-298 in substrate and inhibitor binding. Biochim Biophys Acta. 1994;1205:207–214. doi: 10.1016/0167-4838(94)90235-6. [DOI] [PubMed] [Google Scholar]
- 12.Srivastava SK, Yadav UC, Reddy AB, et al. Aldose reductase inhibition suppresses oxidative stress-induced inflammatory disorders. Chem Biol Interac. 2011;191:330–8. doi: 10.1016/j.cbi.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzen S, Buyukbingol E. Recent studies of aldose reductase enzyme inhibition for diabetic complications. Curr Med Chem. 2003;10:1329–1352. doi: 10.2174/0929867033457377. [DOI] [PubMed] [Google Scholar]
- 14.Sorbinil Retinopathy Trial Research Group. A randomized trial of sorbinil, an aldose reductase inhibitor, in diabetic retinopathy. Arch Ophthalmol. 1990;108:1234–1244. doi: 10.1001/archopht.1990.01070110050024. [DOI] [PubMed] [Google Scholar]
- 15.Sun W, Oates PJ, Coutcher JB, Gerhardinger C, Lorenzi M. A Selective Aldose Reductase Inhibitor of a New Structural Class Prevents or Reverses Early Retinal Abnormalities in Experimental Diabetic Retinopathy. Diabetes. 2006;55:2757–2762. doi: 10.2337/db06-0138. [DOI] [PubMed] [Google Scholar]
- 16.Podjarny A, Cachau RE, Schneider T, et al. Subatomic and atomic crystallographic studies of aldose reductase: implications for inhibitor binding. Cell Mol Life Sci. 2004;61:763–73. doi: 10.1007/s00018-003-3404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Kabbani O, Podjarny A. Selectivity determinants of the aldose and aldehyde reductase inhibitor-binding sites. Cell Mol Life Sci. 2007;64:1970–8. doi: 10.1007/s00018-007-6514-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cumbie BC, Hermayer KL. Current concepts in targeted therapies for the pathophysiology of diabetic microvascular complications. Vasc Health Risk Manag. 2007;3:823–832. [PMC free article] [PubMed] [Google Scholar]
- 19.Oates PJ. Aldose reductase, still a compelling target for diabetic neuropathy. Curr Drug Targets. 2008;9:14–36. doi: 10.2174/138945008783431781. [DOI] [PubMed] [Google Scholar]
- 20.Oates PJ. Aldose reductase inhibitors and diabetic kidney disease. Curr Opin Investig Drugs. 2010;11:402–17. [PubMed] [Google Scholar]
- 21.Oates PJ. Polyol pathway and diabetic peripheral neuropathy. Int Rev Neurobiol. 2002;50:325–92. doi: 10.1016/s0074-7742(02)50082-9. [DOI] [PubMed] [Google Scholar]
- 22.Inagaki K, Miwa I, Okuda J. Affinity purification and glucose specificity of aldose reductase from bovine lens. Arch Biochem Biophys. 1982;216:337–344. doi: 10.1016/0003-9861(82)90219-3. [DOI] [PubMed] [Google Scholar]
- 23*.Rietschel ET, Kirikae T, Schade FU, et al. Bacterial endotoxin: molecular relationships of structure to activity and function. FASEB J. 1994;8:217–25. doi: 10.1096/fasebj.8.2.8119492. This manuscript describes the molecular mechanism by which LPS interacts with the host cells. [DOI] [PubMed] [Google Scholar]
- 24.Saraf A, Larsson L, Burge H, Milton D. Quantification of ergosterol and 3-hydroxy fatty acids in settled house dust by gas chromatography-mass spectrometry: comparison with fungal culture and determination of endotoxin by a Limulus amebocyte lysate assay. Appl Environ Microbiol. 1997;63:2554–9. doi: 10.1128/aem.63.7.2554-2559.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhiping W, Malmberg P, Larsson BM, et al. Exposure to bacteria in swine-house dust and acute inflammatory reactions in humans. Am J Respir Crit Care Med. 1996;154:1261–6. doi: 10.1164/ajrccm.154.5.8912733. [DOI] [PubMed] [Google Scholar]
- 26**.Miller SI, Ernst RK, Bader MW. LPS, TLR4 and infectious disease diversity. Nat Rev Microbiol. 2005;3:36–46. doi: 10.1038/nrmicro1068. This review describes the interactions between innate immune receptor pathways and their bacterial ligands which are the important factors in the diverse manifestations of infectious diseases. [DOI] [PubMed] [Google Scholar]
- 27.Yang RB, Mark MR, Gray A, et al. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nature. 1998;395:284–88. doi: 10.1038/26239. [DOI] [PubMed] [Google Scholar]
- 28*.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–91. doi: 10.1038/nature01326. This review describes pathological mechanisms associated with sepsis complications. [DOI] [PubMed] [Google Scholar]
- 29.Landmann R, Zimmerli W, Sansano S, et al. Increased circulating soluble CD14 is associated with high mortality in gram-negative septic shock. J Infect Dis. 1995;171:639–44. doi: 10.1093/infdis/171.3.639. [DOI] [PubMed] [Google Scholar]
- 30.Karin M, Lin A. NF-kB at the crossroads of life and death. Nat Immunol. 2002;3:221–27. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- 31.Nathan C. Points of control in inflammation. Nature. 2002;420:846–52. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 32.Victor VM, Rocha M, Esplugues JV, De la Fuente M. Role of free radicals in sepsis: antioxidant therapy. Curr Pharm Res. 2005;11:3141–58. doi: 10.2174/1381612054864894. [DOI] [PubMed] [Google Scholar]
- 33.Park HS, Jung HY, Park EY, et al. Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J Immunol. 2004;173:3589–93. doi: 10.4049/jimmunol.173.6.3589. [DOI] [PubMed] [Google Scholar]
- 34.Mirochnitchenko O, Inouye M. Effect of overexpression of human Cu, Zn superoxide dismutase in transgenic mice on macrophage functions. J Immunol. 1996;156:1578–86. [PubMed] [Google Scholar]
- 35.Kim BH, Cho SM, Reddy AM, et al. Down-regulatory effect of quercitrin gallate on nuclear factor-kappa B-dependent inducible nitric oxide synthase expression in lipopolysaccharide-stimulated macrophages RAW264.7. Biochem Pharmacol. 2005;69:1577–83. doi: 10.1016/j.bcp.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 36.Awasthi YC, Sharma R, Cheng JZ, et al. Role of 4-hydroxynonenal in stress-mediated apoptosis signaling. Mol Aspects Med. 2003;24:219–30. doi: 10.1016/s0098-2997(03)00017-7. [DOI] [PubMed] [Google Scholar]
- 37.Eckl PM. Genotoxicity of HNE. Mol Aspects Med. 2003;24:161–65. doi: 10.1016/s0098-2997(03)00010-4. [DOI] [PubMed] [Google Scholar]
- 38.Hnat MD, Meadows JW, Brockman DE, et al. Heat shock protein-70 and 4-hydroxy-2-nonenal adducts in human placental villous tissue of normotensive, preeclamptic and intrauterine growth restricted pregnancies. Am J Obstet Gynecol. 2005;193:836–40. doi: 10.1016/j.ajog.2005.01.059. [DOI] [PubMed] [Google Scholar]
- 39.Kimura H, Liu S, Yamada S, Uchida K, Matsumoto K, Mukaida M, Yoshida K. Rapid increase in serum lipid peroxide 4-hydroxynonenal (HNE) through monocyte NADPH oxidase in early endo-toxemia. Free Radic Res. 2005 Aug;39(8):845–51. doi: 10.1080/10715760500161546. [DOI] [PubMed] [Google Scholar]
- 40.Ramana KV, Reddy AB, Tammali R, Srivastava SK. Aldose reductase mediates endotoxin-induced production of nitric oxide and cytotoxicity in murine macrophages. Free Radic Biol Med. 2007;42:1290–302. doi: 10.1016/j.freeradbiomed.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Srivastava S, Chandra A, Wang LF, et al. Metabolism of the lipid peroxidation product, 4-hydroxy-trans-2-nonenal, in isolated perfused rat heart. J Biol Chem. 1998;273:10893–10900. doi: 10.1074/jbc.273.18.10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raetz CRH, Reynolds CM, Trent MS, Bishop RE. Lipid A modification systems in gram-negative bacteria. Annu Rev Biochem. 2007;76:295–329. doi: 10.1146/annurev.biochem.76.010307.145803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beasley R The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. Lancet. 1998;351:1225–32. [PubMed] [Google Scholar]
- 44.Beasley R The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. Lancet. 1998;351:1225–32. [PubMed] [Google Scholar]
- 45**.Doreswamy V, Peden DB. Modulation of asthma by endotoxin. Clin Exp Allergy. 2011;41:9–19. doi: 10.1111/j.1365-2222.2010.03628.x. This paper described how endotoxin is involved asthma pathogenesis. [DOI] [PubMed] [Google Scholar]
- 46.Christiani DC, Wegman DH, Eisen EA, et al. Cotton dust and gram-negative bacterial endotoxin correlations in two cotton textile mills. Am J Ind Med. 1993;23:333–42. doi: 10.1002/ajim.4700230210. [DOI] [PubMed] [Google Scholar]
- 47.Simpson JC, Niven RM, Pickering CA, et al. Prevalence and predictors of work related respiratory symptoms in workers exposed to organic dusts. Occup Environ Med. 1998;55:668–72. doi: 10.1136/oem.55.10.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Niven RM, Fletcher AM, Pickering CA, et al. Chronic bronchitis in textile workers. Thorax. 1997;52:22–27. doi: 10.1136/thx.52.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sigsgaard T, Pedersen OF, Juul S, Gravesen S. Respiratory disorders and atopy in cotton, wool, and other textile mill workers in Denmark. Am J Ind Med. 1992;22:163–84. doi: 10.1002/ajim.4700220204. [DOI] [PubMed] [Google Scholar]
- 50.Pal TM, de Monchy JG, Groothoff JW, Post D. The clinical spectrum of humidifier disease in synthetic fiber plants. Am J Ind Med. 1997;31:682–92. doi: 10.1002/(sici)1097-0274(199706)31:6<682::aid-ajim3>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 51.Alexis N, Eldridge M, Reed W, Bromberg P, Peden DB. CD14-dependent airway neutrophil response to inhaled LPS: role of atopy. J Allergy Clin Immunol. 2001;107:31–35. doi: 10.1067/mai.2001.111594. [DOI] [PubMed] [Google Scholar]
- 52.Arimoto T, Kadiiska MB, Sato K, et al. Synergistic production of lung free radicals by diesel exhaust particles and endotoxin. Am J Respir Crit Care Med. 2005;171:379–87. doi: 10.1164/rccm.200402-248OC. [DOI] [PubMed] [Google Scholar]
- 53.Elder AC, Gelein R, Finkelstein JN, et al. Pulmonary inflammatory response to inhaled ultrafine particles is modified by age, ozone exposure, and bacterial toxin. Inhal Toxicol. 2000;12:227–46. doi: 10.1080/089583700750019585. [DOI] [PubMed] [Google Scholar]
- 54*.Yadav UC, Naura AS, Aguilera-Aguirre L, et al. Aldose Reductase inhibition suppresses the expression of Th2 cytokines and airway inflammation in ovalbumin-induced asthma in mice. J Immunol. 2009;183:4723–32. doi: 10.4049/jimmunol.0901177. This paper for the first time reports the role of aldose reductase in asthma pathogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yadav UC, Ramana KV, Aguilera-Aguirre L, et al. Inhibition of aldose reductase prevents experimental allergic airway inflammation in mice. PLoS One. 2009;4:e6535. doi: 10.1371/journal.pone.0006535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Srivastava SK, Ramana KV. Aldose reductase inhibition for the treatment of asthma. Expert Rev Clin Immunol. 2010;6:1–4. doi: 10.1586/eci.09.79. [DOI] [PubMed] [Google Scholar]
- 57.Yadav UC, Aguilera-Aguirre L, Ramana KV, et al. Aldose reductase inhibition prevents metaplasia of airway epithelial cells. PLoS One. 2010;5:e14440. doi: 10.1371/journal.pone.0014440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kimura F, Shimizu H, Yoshidome H, et al. Immunosuppression following surgical and traumatic injury. Surg Today. 2010;40:793–808. doi: 10.1007/s00595-010-4323-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59*.Ramana KV, Fadl AA, Tammali R, et al. Aldose reductase mediates the lipopolysaccharide-induced release of inflammatory mediators in RAW264.7 murine macrophages. J Biol Chem. 2006;281:33019–29. doi: 10.1074/jbc.M603819200. This paper describes novel role of aldose reductase in mediation of endotoxin –induced inflammatory signals. [DOI] [PubMed] [Google Scholar]
- 60.Ramana KV, Srivastava SK. Mediation of aldose reductase in lipopolysaccharide-induced inflammatory signals in mouse peritoneal macrophages. Cytokine. 2006;36:115–22. doi: 10.1016/j.cyto.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reddy AB, Srivastava SK, Ramana KV. Anti-inflammatory effect of aldose reductase inhibition in murine polymicrobial sepsis. Cytokine. 2009;48:170–6. doi: 10.1016/j.cyto.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Srivastava A, Rajappa M, Kaur J. Uveitis: mechanisms and recent advances in therapy. Clin Chim Acta. 2010;411:1165–71. doi: 10.1016/j.cca.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 63.Poulaki V, Iliaki E, Mitsiades N, et al. Inhibition of Hsp90 attenuates inflammation in endotoxin-induced uveitis. FASEB J. 2007;21:2113–23. doi: 10.1096/fj.06-7637com. [DOI] [PubMed] [Google Scholar]
- 64*.Yadav UC, Subramanyam S, Ramana KV. Prevention of endotoxin-induced uveitis in rats by benfotiamine, a lipophilic analogue of vitamin B1. Invest Ophthalmol Vis Sci. 2009;50:2276–82. doi: 10.1167/iovs.08-2816. This paper describes novel role of aldose reductase in mediation of ocular inflammatory complications. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ruiz-Moreno JM, Thillaye B, de Kozak Y. Retino-choroidal changes in endotoxin-induced uveitis in the rat. Ophthalmic Res. 1992;24:162–8. doi: 10.1159/000267163. [DOI] [PubMed] [Google Scholar]
- 66.Altan-Yaycioglu R, Akova Y, Akca S, Yilmaz G. Inflammation of the posterior uvea: findings on fundus fluorescein and indocyanine green angiography. Ocul Immunol Inflamm. 2006;14:171–9. doi: 10.1080/09273940600660524. [DOI] [PubMed] [Google Scholar]
- 67.Yadav UC, Srivastava SK, Ramana KV. Inhibition of aldose reductase attenuates endotoxin signals in human non-pigmented ciliary epithelial cells. Exp Eye Res. 2010;90:555–63. doi: 10.1016/j.exer.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kalariya NM, Shoeb M, Reddy AB, et al. Prevention of endotoxin-induced uveitis in rats by plant sterol guggulsterone. Invest Ophthalmol Vis Sci. 2010;51:5105–13. doi: 10.1167/iovs.09-4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yadav UC, Srivastava SK, Ramana KV. Understanding the role of aldose reductase in ocular inflammation. Curr Mol Med. 2010;10:540–49. doi: 10.2174/1566524011009060540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Feil S, Hofmann F. A heretical view on the role of NO and cGMP in vascular proliferative diseases. Trends Mol Med. 2005;11:71–75. doi: 10.1016/j.molmed.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 71.Reddy AB, Ramana KV. Aldose reductase inhibition: emerging drug target for the treatment of cardiovascular complications. Recent Pat Cardiovasc Drug Discov. 2010;5:25–32. doi: 10.2174/157489010790192683. [DOI] [PubMed] [Google Scholar]
- 72.Calderone V, Testai L, Martelli A, et al. Anti-ischaemic activity of an antioxidant aldose reductase inhibitor on diabetic and non-diabetic rat hearts. J Pharm Pharmacol. 2010;62:107–13. doi: 10.1211/jpp.62.01.0012. [DOI] [PubMed] [Google Scholar]
- 73.Tang WH, Kravtsov GM, Sauert M, et al. Polyol pathway impairs the function of SERCA and RyR in ischemic-reperfused rat hearts by increasing oxidative modifications of these proteins. J Mol Cell Cardiol. 2010;49:58–69. doi: 10.1016/j.yjmcc.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Srivastava S, Vladykovskaya E, Barski OA, et al. Aldose reductase protects against early atherosclerotic lesion formation in apolipoprotein E-null mice. Circ Res. 2009;105:793–802. doi: 10.1161/CIRCRESAHA.109.200568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ananthakrishnan R, Kaneko M, Hwang YC, et al. Aldose reductase mediates myocardial ischemia-reperfusion injury in part by opening mitochondrial permeability transition pore. Am J Physiol Heart Circ Physiol. 2009;296:H333–41. doi: 10.1152/ajpheart.01012.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gleissner CA, Sanders JM, Nadler J, Ley K. Upregulation of aldose reductase during foam cell formation as possible link among diabetes, hyperlipidemia, and atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:1137–43. doi: 10.1161/ATVBAHA.107.158295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vedantham S, Noh H, Ananthakrishnan R, Son N, Hallam K, Hu Y, Yu S, Shen X, Rosario R, Lu Y, Ravindranath T, Drosatos K, Huggins LA, Schmidt AM, Goldberg IJ, Ramasamy R. Human aldose reductase expression accelerates atherosclerosis in diabetic apolipoprotein E-/- mice. Arterioscler Thromb Vasc Biol. 2011 Aug;31(8):1805–13. doi: 10.1161/ATVBAHA.111.226902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schemmel KE, Padiyara RS, D'Souza JJ. Aldose reductase inhibitors in the treatment of diabetic peripheral neuropathy: a review. J Diabetes Complications. 2010;24:354–60. doi: 10.1016/j.jdiacomp.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 79.Hotta N, Sakamoto N, Shigeta Y, et al. Clinical investigation of epalrestat, an aldose reductase inhibitor, on diabetic neuropathy in Japan: multicenter study. J Diabetes Complications. 1996;10:168–72. doi: 10.1016/1056-8727(96)00113-4. [DOI] [PubMed] [Google Scholar]
- 80.Nambu H, Kubo E, Takamura Y, Tsuzuki S, Tamura M, Akagi Y. Attenuation of aldose reductase gene suppresses high-glucose-induced apoptosis and oxidative stress in rat lens epithelial cells. Diabetes Res Clin Pract. 2008;82:18–24. doi: 10.1016/j.diabres.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 81.Yang B, Hodgkinson A, Oates PJ, Millward BA, Demaine AG. High glucose induction of DNA-binding activity of the transcription factor NFkappaB in patients with diabetic nephropathy. Biochim Biophys Acta. 2008;1782:295–302. doi: 10.1016/j.bbadis.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 82.Kang ES, Kim HJ, Paek KS, Jang HS, Chang KC, Lee JH, Nishinaka T, Yabe-Nishimura C, Seo HG. Phorbol ester up-regulates aldose reductase expression in A549 cells: a potential role for aldose reductase in cell cycle modulation. Cell Mol Life Sci. 2005;62:1146–55. doi: 10.1007/s00018-005-5024-4. [DOI] [PubMed] [Google Scholar]
- 83.Suzuki T, Sekido H, Kato N, Nakayama Y, Yabe-Nishimura C. Neurotrophin-3-induced production of nerve growth factor is suppressed in Schwann cells exposed to high glucose: involvement of the polyol pathway. J Neurochem. 2004;91:1430–8. doi: 10.1111/j.1471-4159.2004.02824.x. [DOI] [PubMed] [Google Scholar]
- 84.Kang ES, Iwata K, Ikami K, Ham SA, Kim HJ, Chang KC, Lee JH, Kim JH, Park SB, Kim JH, Yabe-Nishimura C, Seo HG. Aldose reductase in keratinocytes attenuates cellular apoptosis and senescence induced by UV radiation. Free Radic Biol Med. 2011;50:680–8. doi: 10.1016/j.freeradbiomed.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 85.Ravindranath TM, Mong PY, Ananthakrishnan R, Li Q, Quadri N, Schmidt AM, Ramasamy R, Wang Q. Novel role for aldose reductase in mediating acute inflammatory responses in the lung. J Immunol. 2009;183:8128–37. doi: 10.4049/jimmunol.0900720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huang P, Zhang Y, Jiang T, Zeng W, Zhang N. Aldose reductase is a potent regulator of TGF-β1 induced expression of fibronectin in human mesangial cells. Mol Biol Rep. 2010;37:3097–103. doi: 10.1007/s11033-009-9887-6. [DOI] [PubMed] [Google Scholar]
- 87.Qiu L, Wu X, Chau JF, Szeto IY, Tam WY, Guo Z, Chung SK, Oates PJ, Chung SS, Yang JY. Aldose reductase regulates hepatic peroxisome proliferator-activated receptor alpha phosphorylation and activity to impact lipid homeostasis. J Biol Chem. 2008;283:17175–83. doi: 10.1074/jbc.M801791200. [DOI] [PubMed] [Google Scholar]
- 88.Qiu L, Lin J, Xu F, Gao Y, Zhang C, Liu Y, Luo Y, Yang JY. Inhibition of Aldose Reductase Activates Hepatic Peroxisome Proliferator-Activated Receptor-α and Ameliorates Hepatosteatosis in Diabetic db/db Mice. Exp Diabetes Res. 2012;2012:789730. doi: 10.1155/2012/789730. [DOI] [PMC free article] [PubMed] [Google Scholar]