Abstract
This study was conducted to investigate the potential effects of α-chlorohydrin (ACH) on epididymal function and antioxidant system in male rats. The test chemical was administered to male rats by gavage at doses of 0, 3, 10, and 30 mg/kg/day for 7 days. Twenty-four male rats were randomly assigned to four experimental groups, with six rats in each group. Spermatotoxicity was assessed by measurement of reproductive organ weight, testicular sperm head count, epididymal sperm motility and morphology, histopathologic examination, and oxidative damage analysis in rats. At 30 mg/kg/day, an increase in the incidence of clinical signs, epididymis weight, and gross necropsy findings of the epididymis, a decrease in the sperm motility, and an increased incidence of histopathological changes of the epididymis were observed in a dose-dependent manner. At 10 mg/kg/day, an increased incidence of clinical signs and histopathological changes and decreased sperm motility were observed. In the oxidative damage analysis, an increase in the malondialdehyde concentration and a decrease in the glutathione content and glutathione peroxidase and catalase activities in the epididymal tissue were detected at ≥3 mg/kg/day. The results show that graded doses of ACH elicit depletion of the antioxidant defense system and that the spermatotoxicity of ACH may be due to the induction of oxidative stress.
Keywords: α-Chlorohydrin, oxidative stress, reproductive dysfunction, sperm
Recently there has been an increased concern of possible effects of environmental contaminants on male reproduction system of wildlife, domestic animal, and humans [1,2]. α-Chlorohydrin (ACH), a potential toxic metabolite of epichlorohydrin (ECH), is an industrial chemical used in the manufacture of dumps, sewers, buildings and similar man-made structure [3]. Due to ECH's increased production and wide-spread use, the potential risk caused by ACH in humans has steadily increased. Exposure to ACH can occur by inhalation, ingestion, and eye or skin contact in the general population, as well as in workers with specific occupations [3].
Multi-organ toxicity such as epididymal toxicity, testicular toxicity, nephrotoxicity, neurotoxicity, and immunotoxicity is observed at high doses of ACH in various experimental animals [3]. Previous studies demonstrated that ACH is an anti-fertility agent that acts both as an epididymal toxicant and an agent capable of directly affecting sperm motility and sperm metabolism [4-6]. For this reason, ACH was previously used as a rodent chemosterilant. Although the exact mechanism of chemical-induced sperm and epididymal toxicity is still not fully understood, chemical-induced glyceraldehyde 3-phosphate dehydrogenase (GAPDH) inhibition is believed to be a major contributing factor for sperm and epididymal toxicity [5,6]. GAPDH inhibition causes depletion of sperm ATP and subsequently decreases sperm motility and fertility [5].
Kothari et al. [7] reported that reactive oxygen species (ROS) play a critical role in the pathogenesis of reproductive disorders, especially in the pathological mechanism of male infertility. GAPDH is a glycolytic enzyme that can be easily affected by oxidative damage, resulting in the oxidation of essential cysteine residues with a complete loss of dehogydrenase activity [8]. Therefore, we hypothesized that the inhibition of GAPDH activity may result from induction of oxidative stress in rat sperm and epididymides. To test this hypothesis, we examined the spermatotoxicity and epididymal oxidative damage of ACH after 7-day repeated oral administration in male rats to better understand the possible mechanism for spermatotoxicity of ACH. Although the most likely route of exposure is by inhalation, the oral route of exposure was selected for this study because there is a possibility of oral exposure.
Materials and Methods
Animal husbandry and maintenance
A total of 24 male Sprague-Dawley rats aged 10 weeks were obtained from a specific pathogen-free colony at Samtako Co. (Osan, Republic of Korea) and used after one week of quarantine and acclimation. The Institutional Animal Care and Use Committee of Chonnam National University approved the protocols for the animal study, and the animals were cared for in accordance with the Guidelines for Animal Experiments of Chonnam National University.
Test chemical and treatment
ACH was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). The test chemical was diluted to the appropriate concentration with corn oil (Sigma-Aldrich, St. Louis, MO, USA) and was administered orally to rats for 7 days. The vehicle control rats received an equivalent volume of corn oil only. Healthy males were assigned randomly into four experimental groups of 6 rats each: three treatment groups receiving 3, 10, and 30 mg/kg/day ACH and a vehicle control group. The doses selected were on the basis of toxicity studies described earlier [5,9].
Animal observation and sperm analysis
All animals were observed daily for any clinical signs of toxicity and body weights were measured on days 0, 1, 2, and 7 of the test. At the scheduled termination day (day 7 of the study), all male rats were euthanized by carbon dioxide and exsanguination from the aorta. The absolute weights of the prostates, seminal vesicles, testes and epididymides were measured and their weights relative to body weight calculated. Sperm analysis was conducted as has been previously described in other studies [10]. The left testis was homogenized and sonicated with 12 mL distilled water for sperm head count. The sperm suspension was put into a hemacytometer (Neubauer, Germany) and the number of homogenization-resistant sperm heads was counted under a light microscope (Leica, Germany). For motility measurements, the sperm was obtained from the left cauda epididymis, placed in Hanks' balanced salt solution (pH 7.2) containing 5 mg/mL bovine serum albumin (Sigma Chemical Co., St. Louis, MO, USA) and maintained at 37℃. Motility was observed using a microscope with a stage warmer. Sperm morphology was also examined using optical microscopy of the sperm smears (sperm suspension containing 1% Eosin Y) collected from the left cauda epididymis. The left caput epididymis was fixed with Bouin's fixative. The tissues were routinely processed, embedded in paraffin and sectioned at 4 µm. These sections were stained with Hematoxylin-Eosin for histopathologic examination and then examined microscopically.
Oxidative stress analysis
The weighed frozen right epididymis was homogenized in a glass-Teflon homogenizer with 50 mM phosphate buffer (pH 7.4) to obtain 1:9 (w/v) whole homogenate. The homogenates were then centrifuged at 11,000 g for 15 min at 4℃ to discard any cell debris, and the supernatant was used for the measurement of concentration of malondialdehyde (MDA), reduced glutathione (GSH) content and activities of catalase and glutathione-peroxidase (GPx). The concentration of MDA was assayed by monitoring thiobarbituric acid reactive substance formation by the method of Berton et al. [11]. GSH was measured by the method of Moron et al. [12]. The activities of antioxidant enzymes including catalase [13] and GPx [14] were also determined. Total protein concentrations were determined by the Bradford protein assay (Bio-rad), using bovine serum albumin as a standard.
Statistical analyses
Results are expressed as mean±SD, and all statistical comparisons were made by one-way ANOVA followed by Tukey-Kramer multiple comparison test.
Results
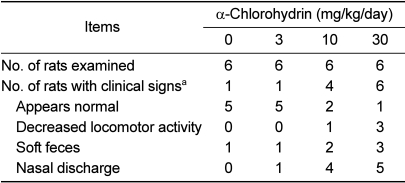
There was no treatment-related mortality and body weight change in any of the animals treated with ACH during the study period (data not shown). However, the rats treated with ACH showed treatment-related occurrence of clinical signs such as nasal discharge, soft feces, and decreased locomotor activity in a dose-dependent manner (Table 1).
Table 1.
Clinical signs of male rats treated with α-chlorohydrin for 7 days
aA single rat may be represented more than once in listing individual signs.
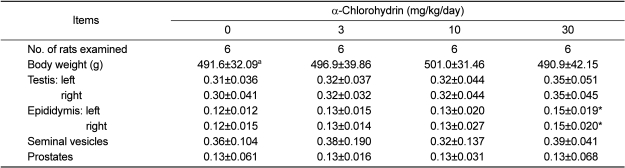
Although the difference was not statistically significant between the groups, cystic pustule of the caput epididymis was found in all animals of the high dose group (data not shown). As shown in Table 2, the rats treated with 30 mg/kg/day of ACH showed a statistically significant increase in the relative weights of both epididymides when compared to the control group.
Table 2.
Relative reproductive organ weights (organ-to-body weight ratio, %) of male rats treated with α-chlorohydrin for 7 days
aValues are expressed as mean±SD.
*Significant difference at P<0.05 level compared with the control group.
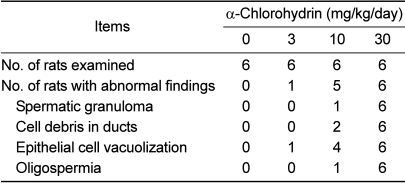
The results of the histopathological examination are summarized in Table 3. Spermatic granuloma (n=6), cell debris in the ducts (n=6), epithelial cell vacuolization (n=6), and oligospermia (n=6) were observed in caput epididymis of the 30 mg/kg group (Figure 1). Spermatic granuloma (n=1), cell debris in the ducts (n=2), epithelial cell vacuolization (n=4), and oligospermia (n=1) were also found in the 10 mg/kg group. Although the difference was not statistically significant between the groups, the incidence of histopathological lesions observed in the 10 and 30 mg/kg groups was higher than the control group.
Table 3.
Histopathological findings in epididymis of male rats treated with α-chlorohydrin for 7 days
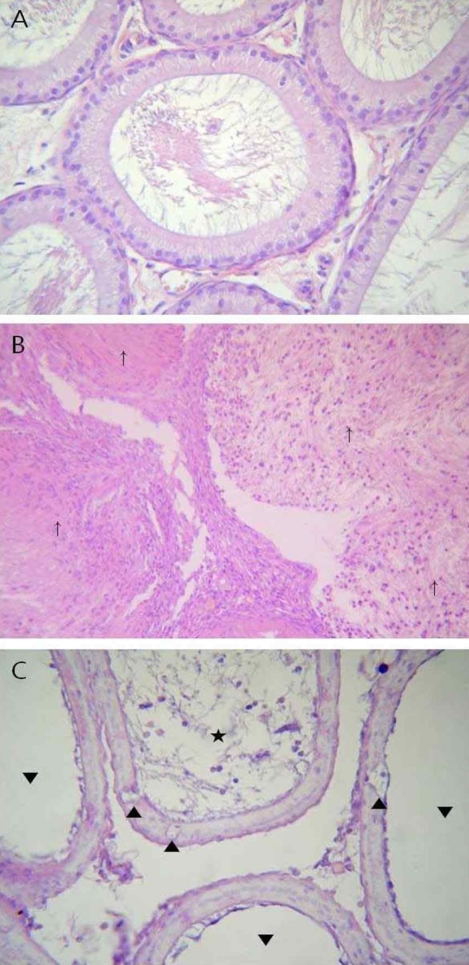
Figure 1.
Representative photographs of the epididymis sections from the control and high dose groups stained with hematoxylin and eosin. (A) Caput epididymis of control rat showing normal epithelium and luminal contents. (B, C) Caput epididymis treated with 30 mg/kg/day ACH, with cell debris in ducts (⋆), oligospermia (▾), spermatic granuloma (↑), and vacuolization of epithelial cells (▴). (A and C, ×400; B, ×100)
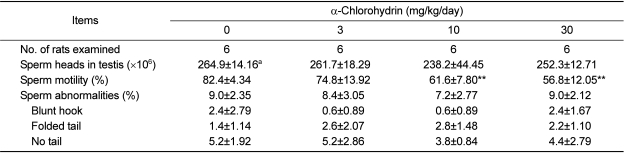
As shown in Table 4, no treatment effects were detected in terms of the number of testicular sperm heads and morphology of epididymal sperm. In contrast, the sperm motility of epididymis in the 10 and 30 mg/kg groups significantly decreased in a dose-dependent manner when compared with that of the control group.
Table 4.
Sperm analysis of male rats treated with α-chlorohydrin for 7 days
aValues are expressed as mean±SD.
**Significant difference at P<0.01 level compared with the control group.
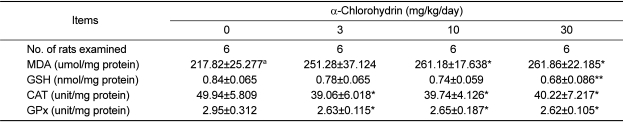
The concentration of MDA, an end product of lipid peroxidation, in the 10 and 30mg/kg groups were significantly increased in a dose-dependent manner when compared with the control (Table 5). However, the concentration of GSH were significantly decreased in the 30 mg/kg group compared to the control group. The catalase and GPx activities in all of ACH treatment groups were also significantly decreased when compared with the control group.
Table 5.
Antioxidant enzymes, reduced glutathione and lipid peroxidation of the epididymis of male rats treated with α-chlorohydrin for 7 days
Note: MDA, malondialdehyde; GSH, reduced glutathione; CAT, catalase; and GPx, glutathione peroxidase.
aValues are expressed as mean±SD.
*, **Significant difference at P<0.05 and P<0.01 levels compared with the control group, respectively.
Discussion
The toxicity of ACH has been extensively studied in short and long term animal studies over the past several decades. Previous studies demonstrated that sperm and epididymis are major targets of ACH toxicity [6,15,16]. The present study was undertaken in an attempt to evaluate the potential effects of ACH on sperm and epididymal function after 7-day repeated oral administration in rats. Treatment-related clinical signs such as nasal discharge, soft feces and decreased locomotor activity were observed in this study. These findings may be attributed to mucus and gastrointestinal irritation effects of ACH because ACH is a chemical that acts as an eye and nasal irritant [3]. On the other hand, there were no significant differences in the body weights between the groups.
A dose-dependent increase in the relative weights of epididymis may have been a consequence of the toxicity of ACH. These results are similar to the finding of Kawaguchi et al. [9], who showed that the relative epididymis weight increased when ACH was administered at 20 mg/kg/day for 19 days to rats. At the scheduled necropsy, the cystic pustule of the epididymis was observed in all cases in the high dose group. The increased incidence of the cystic pustule was considered to be a treatment-related effect, since this finding is uncommon in normal control rats [17] and was consistent with the significantly increased weight of the epididymis. Cooper et al. [4] also reported that a single dose ACH at ≥50 mg/kg/day produces large retention cysts in the ductuli efferentes and proximal caput of the epididymides in rats.
Characteristic histopathologic findings observed in the present study included spermatic granuloma, cell debris in the ducts, epithelial cell vacuolization, and oligospermia in proximal caput epididymis. Kluwe et al. [18] reported that ACH produced epididymal sperm granulomas and spermatocoeles in rats. Jelks et al. [5] and Jelks and Miller [6] also demonstrated that a single oral dose of ACH at ≥25 mg/kg/day results in a white pustule, sperm granuloma formation, sloughed epithelial cells in the lumen, and epithelial vacuolization and disruption in the rat epididymis. Recently, Kawaguchi et al. [9] reported that oral administration of ACH to rats for 18 days resulted in cell debris in the tubules of the caput epididymis and sperm granulomas in the tubules of the cauda epididymis at 20 mg/kg/day. The results of the above studies and the present study clearly show that ACH has adverse effects on epididymal histology in rats.
In the present study, although there were no obvious differences in the incidence of abnormal sperm in caudal epididymis and sperm head count in testis among the groups, epididymal sperm motility in the high dose group was significantly decreased in a dose-dependent manner. It was previously reported that evaluation of epididymal sperm motility seems to be a potential indicator of male reproductive toxicity [19]. Our results were consistent with the results of previous studies. Hoyt et al. [20] demonstrated that when rats received 5 mg/kg/day for 2 weeks follow by a 2-week withdrawal period, there was a decrease in the percentage of motile sperm. The 8-day repeated oral dose of 10 mg/kg/day to rats resulted in reductions in the mean percentage of motile sperm, curvilinear velocity, straight-line velocity, lateral head displacement, and linearity [21]. Ours and the results of previous studies strongly indicate that ACH has adverse effects on sperm function in rats.
Oxidative stress refers to a severe imbalance between ROS production and antioxidant defense systems. ROS production and oxidative stress plays a role in sperm functions during maturation and capacitation, because their plasma membranes contain high content of polyunsaturated fatty acids and their cytoplasm contains low concentrations of scavenging enzymes [22]. ROS are considered to damage spermatozoa through lipid peroxidation, resulting in altered sperm functions. In the present study, the animals treated with ACH showed decreased activities of antioxidant enzymes such as catalase and GPX, and GSH concentration in a dose-related manner, while they increased MDA concentration in the epididymis. An increase in the lipid peroxidation indicated that ACH induced oxidative stress in the epididymis by decreasing the activities of antioxidant enzymes, thereby leading to an excessive generation of ROS. Therefore, these results suggest that the administration of ACH elicits depletion of the antioxidant defense system in the epididymis, indicating that oxidative damage plays a role in sperm and epididymal toxicities.
It was reported that oxidative damage and ROS production cause sperm pathology such as ATP depletion [23]. The inhibitory effect of ACH on metabolic activity of sperm in vitro is thought to occur via ACH oxidation within the sperm to form 3-chlorolctaldehyde, which then inhibits GAPDH [24]. GAPDH, a glycolytic enzyme, is known as a major target protein in oxidative stress [25] and is an important key enzyme to generation of ATP which is necessary for sperm motility and male fertility [26]. In addition, sperm are believed to contain a more susceptible isoform of this enzyme [6]. It has been demonstrated that ACH directly affects spermatozoa by the depletion of ATP levels and the inhibition of GAPDH activity in rat sperm and epididymis [5,6]. Therefore, we can consider that oxidative damage induced by ACH administration can cause an inhibition of GAPDH activity in rat sperm and epididymis, followed by sperm ATP depletion. These results from previous studies were consistent with the decrease of sperm motility observed in our study.
Overall, it can be concluded that the 7-day repeated oral dose of ACH to rats elicits oxidative damage at ≥3 mg/kg/day and spermatotoxicity in the epididymis at ≥10 mg/kg/day, and that the adverse effects of ACH on sperm functions and epididymal histology may be at least partially due to the induction of lipid peroxidation and decrease of antioxidant activities in rats. However, further studies are needed to understand the precise mechanism of ACH toxicity.
Acknowledgments
This study was financially supported by Chonnam National University, 2010. This work was also supported by the grant from the Animal Medical Center, and Chonnam National University.
References
- 1.Harrison PT, Holmes P, Humfrey CD. Reproductive health in humans and wildlife: are adverse trends associated with environmental chemical exposure? Sci Total Environ. 1997;205(2-3):97–106. doi: 10.1016/s0048-9697(97)00212-x. [DOI] [PubMed] [Google Scholar]
- 2.Kavlock RJ, Daston GP, DeRosa C, Fenner-Crisp P, Gray LE, Kaattari S, Lucier G, Luster M, Mac MJ, Maczka C, Miller R, Moore J, Rolland R, Scott G, Sheehan DM, Sinks T, Tilson HA. Research needs for the risk assessment of health and environmental effects of endocrine disruptors: a report of the U.S. EPA-sponsored workshop. Environ Health Perspect. 1996;104:715–740. doi: 10.1289/ehp.96104s4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.U.S. Environmental Protection Agency. Alpha-chlorohydrin: Human health risk assessment for proposed use as a rodenticide. Washington, D.C.: USEPA; 2006. [Google Scholar]
- 4.Cooper ER, Jones AR, Jackson H. Effects of alpha-chlorohydrin and related compounds on the reproductive organs and fertility of the male rat. J Reprod Fertil. 1974;38(2):379–386. doi: 10.1530/jrf.0.0380379. [DOI] [PubMed] [Google Scholar]
- 5.Jelks K, Berger T, Horner C, Miller MG. α-chlorohydrin induced changes in sperm fertilizing ability in the rat: association with diminished sperm ATP levels and motility. Reprod Toxicol. 2001;15(1):11–20. doi: 10.1016/s0890-6238(00)00115-5. [DOI] [PubMed] [Google Scholar]
- 6.Jelks KB, Miller MG. α-Chlorohydrin inhibits glyceraldehyde-3-phosphate dehydrogenase in multiple organs as well as in sperm. Toxicol Sci. 2001;62(1):115–123. doi: 10.1093/toxsci/62.1.115. [DOI] [PubMed] [Google Scholar]
- 7.Kothari S, Thompson A, Agarwal A, du Plessis SS. Free radicals: their beneficial and detrimental effects on sperm function. Indian J Exp Biol. 2010;48(5):425–435. [PubMed] [Google Scholar]
- 8.Danshina PV, Schmalhausen EV, Avetisyan AV, Muronetz VI. Mildly oxidized glyceraldehyde-3-phosphate dehydrogenase as a possible regulator of glycolysis. IUBMB Life. 2001;51(5):309–314. doi: 10.1080/152165401317190824. [DOI] [PubMed] [Google Scholar]
- 9.Kawaguchi T, Kawachi M, Morikawa M, Kazuta H, Shibata K, Ishida M, Kitagawa N, Matsuo A, Kadota T. Key parameters of sperm motion in relation to male fertility in rats given alpha-chlorohydrin or nitrobenzene. J Toxicol Sci. 2004;29(3):217–231. doi: 10.2131/jts.29.217. [DOI] [PubMed] [Google Scholar]
- 10.Kim KH, Shin IS, Lim JH, Kim SH, Park NH, Moon C, Kim SH, Shin DH, Kim JC. Dose-response effects of epichlorohydrin on male reproductive function in rats. Toxicol Res. 2009;25:203–207. doi: 10.5487/TR.2009.25.4.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berton TR, Conti CJ, Mitchell DL, Aldaz CM, Lubet RA, Fischer SM. The effect of vitamin E acetate on ultraviolet-induced mouse skin carcinogenesis. Mol Carcinog. 1998;23(3):175–184. doi: 10.1002/(sici)1098-2744(199811)23:3<175::aid-mc6>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 12.Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. 1979;582(1):67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 13.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 14.Mannervik B, Alin P, Guthenberg C, Jensson H, Tahir MK, Warholm M, Jörnvall H. Identification of three classes of cytosolic glutathione transferase common to several mammalian species: correlation between structural data and enzymatic properties. Proc Natl Acad Sci USA. 1985;82(21):7202–7206. doi: 10.1073/pnas.82.21.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamada T, Inoue T, Sato A, Yamagishi K, Sato M. Effects of short-term administration of alpha-chlorohydrin on reproductive toxicity parameters in male Sprague-Dawley rats. J Toxicol Sci. 1995;20(3):195–205. doi: 10.2131/jts.20.195. [DOI] [PubMed] [Google Scholar]
- 16.Shin IS, Park NH, Lee JC, Kim KH, Moon C, Kim SH, Shin DH, Park SC, Kim HY, Kim JC. One-generation reproductive toxicity study of epichlorohydrin in Sprague-Dawley rats. Drug Chem Toxicol. 2010;33(3):291–301. doi: 10.3109/01480541003734030. [DOI] [PubMed] [Google Scholar]
- 17.Haschek WM, Rousseaux CG. Fundamentals of Toxicological Pathology. New York: Academic Press; 1998. Toxicological pathology: Morphological evaluation of toxicity; pp. 12–13. [Google Scholar]
- 18.Kluwe WM, Gupta BN, Lamb JC., 4th The comparative effects of 1,2-dibromo-3-chloropropane (DBCP) and its metabolites, 3-chloro-1,2-propaneoxide (epichlorohydrin), 3-chloro-1,2-propanediol (alphachlorohydrin), and oxalic acid, on the urogenital system of male rats. Toxicol Appl Pharmacol. 1983;70(1):67–86. doi: 10.1016/0041-008x(83)90180-1. [DOI] [PubMed] [Google Scholar]
- 19.Blazak WF, Ernst TL, Stewart BE. Potential indicators of reproductive toxicity: testicular sperm production and epididymal sperm number, transit time, and motility in Fischer 344 rats. Fundam Appl Toxicol. 1985;5:1097–1103. doi: 10.1016/0272-0590(85)90145-9. [DOI] [PubMed] [Google Scholar]
- 20.Hoyt JA, Fisher LF, Hoffman WP, Swisher DK, Seyler DE. Utilization of a short-term male reproductive toxicity study design to examine effects of alpha-chlorohydrin (3-chloro-1,2-propanediol) Reprod Toxicol. 1994;8(3):237–250. doi: 10.1016/0890-6238(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 21.Toth GP, Wang SR, McCarthy H, Tocco DR, Smith MK. Effects of three male reproductive toxicants on rat cauda epididymal sperm motion. Reprod Toxicol. 1992;6(6):507–515. doi: 10.1016/0890-6238(92)90035-r. [DOI] [PubMed] [Google Scholar]
- 22.Shiva M, Gautam AK, Verma Y, Shivgotra V, Doshi H, Kumar S. Association between sperm quality, oxidative stress, and seminal antioxidant activity. Clin Biochem. 2011;44(4):319–324. doi: 10.1016/j.clinbiochem.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Dokmeci D. Oxidative stress, male infertility and the role of carnitines. Folia Med (Plovdiv) 2005;47(1):26–30. [PubMed] [Google Scholar]
- 24.Cooney SJ, Jones AR. Inhibitory effects of (S)-3-chlorolactaldehyde on the metabolic activity of boar spermatozoa in vitro. J Reprod Fertil. 1988;82(1):309–317. doi: 10.1530/jrf.0.0820309. [DOI] [PubMed] [Google Scholar]
- 25.Nakajima H, Amano W, Fujita A, Fukuhara A, Azuma YT, Hata F, Inui T, Takeuchi T. The active site cysteine of the proapoptotic protein glyceraldehyde-3-phosphate dehydrogenase is essential in oxidative stress-induced aggregation and cell death. J Biol Chem. 2007;282(36):26562–26574. doi: 10.1074/jbc.M704199200. [DOI] [PubMed] [Google Scholar]
- 26.Miki K, Qu W, Goulding EH, Willis WD, Bunch DO, Strader LF, Perreault SD, Eddy EM, O'Brien DA. Glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme,is required for sperm motility and male fertility. Proc Natl Acad Sci USA. 2004;101(47):16501–16506. doi: 10.1073/pnas.0407708101. [DOI] [PMC free article] [PubMed] [Google Scholar]