Abstract
Alterations in arterial PaCO2 can influence local anesthetic toxicity. The objective of this study was to evaluate the effect of stress-induced changes in PaCO2 and PaO2 on the seizure threshold of lidocaine and articaine. Lidocaine (2% with 1 ∶ 100,000 epinephrine) or articaine (4% with 1 ∶ 100,000 epinephrine) was administered intravenously under rest or stress conditions to 36 rats separated into 4 groups. Propranolol and prazosin were administered preoperatively to minimize cardiovascular effects of epinephrine. Mean arterial pressure (MAP), heart rate (HR), and arterial pH, PaCO2, and PaO2 were measured. Results showed no differences in MAP, HR, or pH. Stress significantly increased the latency period for the first tonic-clonic seizure induced by a toxic dose of both lidocaine and articaine (P < .05). Seizures were brought on more rapidly by articaine. No significant difference between toxic doses of lidocaine and articaine was noted. Stress raised the seizure threshold dose for both drugs and significantly (P < .01) increased arterial PaO2 from 94.0 ± 1.90 mm Hg to 113.0 ± 2.20 mm Hg, and reduced PaCO2 from 36.0 ± 0.77 mm Hg to 27.0 ± 0.98 mm Hg. In conclusion, reduction in PaCO2 and/or increase in PaO2 raised the seizure threshold of lidocaine and articaine. This study also confirmed that lidocaine and articaine have equipotent central nervous system toxicity.
Keywords: Lidocaine, Articaine, Local anesthetic toxicity
Local anesthetics generally are safe, but systemic toxicity can occur with high plasma levels caused by overdose or accidental intravascular administration.1–7 The magnitude of side effects is dependent on inherent toxicity and the dose administered. Methods used to reduce the incidence of toxicity include aspiration, slow injection, and knowledge of the pharmacology of the drug.8,9 Toxicity manifests primarily in the central nervous system (CNS), and its signs and symptoms include light-headedness, restlessness, inarticulate speech, and diffuse muscular contractions.10–13 One classic sign of local anesthetic toxicity is tonic-clonic seizure activity, with convulsions being the first indication that a toxic reaction is occurring.10,14
Studies in cats have shown that the acid-base state is an important factor, in that acidosis increased, while alkalosis reduced, anesthetic toxicity.15,16 An increase in the levels of PaCO2 can worsen the convulsive threshold, and a decrease in PaCO2 levels tends to have the opposite effect.17,18 The duration of seizure activity is related to the local anesthetic blood level and is directly related to the arterial PaCO2 level.
Stress can lead to sympathetic hyperactivity, resulting in tachycardia and tachypnea, in turn leading to increased PaO2 and decreased PaCO2. This model can be used to study their effects on local anesthetic toxicity. Today, 2 of the most commonly used local anesthetics are lidocaine and articaine.19 Thus, by using the model of stress-induced changes in these parameters, investigators undertook this study to confirm that changes in PaCO2 and PaO2 can affect local anesthetic-induced seizure activity, and to compare the relative CNS toxicity of lidocaine and articaine.
MATERIALS AND METHODS
Studies were performed at the Laboratory of Experimental Hypertension of the Universidade Federal do Espírito Santo. Experiments were approved by the Committee on Care and Use of Experimental Animals and were conducted in accordance with the guidelines for care and use of animals of the Federation of Societies of Experimental Biology (FASEB) and of the Physiological Societies for Research Involving Animals. These experiments were conducted on 36 adult male Wistar rats weighing 250–300 g. The animals were divided into 4 groups: 2 that received lidocaine (2% with 1 ∶ 100,000 epinephrine) and 2 that received articaine (4% with 1 ∶ 100,000 epinephrine). These 2 groups were subdivided into 2 others: 1 that was submitted to a stress stimulus, and the control, which was not stressed.
Under general anesthesia induced with chloral hydrate (40 mg/100 g of body weight), the femoral vein and artery were catheterized (Polyethylene PE 50, Intramedic, Becton Dickinson, Leverton Circle, Md). These sterile catheters were filled with heparinized saline solution (100 U/mL), and the free ends were tunneled subcutaneously, exteriorized, and sutured at the dorsum of the neck. The cardiovascular parameters of heart rate (HR) and mean arterial pressure (MAP) were measured with a disposable blood pressure transducer (Cobe Laboratories, Lakewood, Colo) connected to a pressure processor amplifier and data-acquisition system (Bio Pac System, Santa Barbara, Calif). Blood samples were collected via arterial catheters for gas analysis (Radiometer ABL 555, Copenhagen, Denmark), and venous catheters were used for drug administration.
Twenty-four hours after the surgical procedures were performed, blood samples were collected (0.3 mL) to determine the pH, PaO2, and PaCO2 values. After those collections were obtained, the arterial catheters were connected to the recording setup, and a period of approximately 30 minutes was allowed for stabilization of cardiovascular parameters. After the stabilization period, MAP and HR were monitored for a period of 10 minutes, and propranolol (1 mg/kg) and prazosin (1 mg/kg) were administered to block the cardiovascular effects of the epinephrine contained in the anesthetic solution. Ten minutes after the IV infusion of beta and alpha blockers, rats in the stressed group were exposed to 5 minutes of restraint and the sound of a bell. After these 5 minutes, blood samples were drawn, and the IV infusion of anesthetic began at a rate of 0.1 mL/min delivered by infusion pump (Syringe Pump Model 341B Sage Instruments, Boston, Mass). The latency of anesthetic toxicity was measured from the beginning of the anesthetic infusion until the first generalized tonic-clonic seizure. After the seizure, the animals received a lethal dose of thiopental (80 mg/kg IV).
The primary outcome measured was the time to seizure induction. All data are expressed as mean ± SEM. Student's t test was used for comparison of independent samples. Differences were judged to be significant at a level of P < .05.
RESULTS
Before local anesthetic was administered, the sympathetic effects of epinephrine were blocked by IV injection of the alpha-1 antagonist prazosin and the nonselective beta receptor antagonist propranolol. Prazosin induced a significant reduction in MAP as compared with basal values (from 108.4 ± 1.7 to 83.3 ± 1.4 mm Hg; P < .05). Subsequent administration of propranolol returned the MAP to close to basal values (105.7 ± 2.2). HR was significantly (P < .05) increased by prazosin (from 437.16 ± 8.6 to 471.27 ± 7.2 bpm) and was returned close to basal values after propranolol. Stress and subsequent administration of anesthetic elicited no change in MAP or HR after sympathetic alpha and beta blockade.
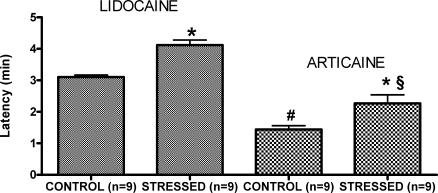
As shown in Figure 1, the time of latency, measured from the beginning of local anesthetic administration until the first generalized tonic-clonic seizure, was significantly longer with 2% lidocaine in the stressed rats (4.12 ± 0.16 min) than in controls (3.11 ± 0.06 min; P < .05). Similarly, the rats that received 4% articaine had a significant increase in latency in the stressed group (2.27 ± 0.27 min) when compared with controls (1.44 ± 0.12 min; P < .05). Independent of the group (control or stressed), seizures were induced more rapidly with articaine than with lidocaine (P < .05; see Figure 1).
Figure 1.
Time course (latency) between the beginning of the IV infusion of lidocaine 2% or articaine 4% and the first tonic-clonic contraction in control and stressed rats. Values represent the means ± SEM. *P < .05 when compared with the respective control group; #P < .05 versus lidocaine control; and §P < .05 versus lidocaine stressed groups. The sample size for each group is shown in parentheses.
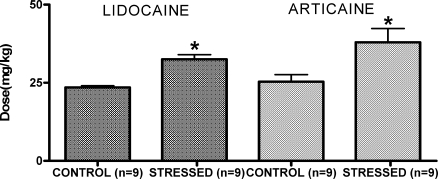
Figure 2 shows the IV doses of lidocaine and articaine required to induce the first tonic-clonic contraction in control unstressed and stressed rats. Significantly more lidocaine had to be administered to elicit the convulsion for stressed rats (32.50 ± 1.48 mg/kg) than for controls (23.54 ± 0.49 mg/kg; P < .05). Similarly, with articaine, the stressed rats needed a higher dose of anesthetic (37.94 ± 4.43 mg/kg) than was needed by controls (25.34 ± 2.28 mg/kg; P < .05). No significant difference could be seen between the doses of lidocaine and articaine needed to induce the convulsive response under unstressed or stressed conditions.
Figure 2.
Dose (IV infusion) of lidocaine or articaine to induce tonic-clonic seizure in controls and stressed rats. Values represent the means ± SEM. *P < .05 when compared with the respective control group. The sample size for each group is shown in parentheses.
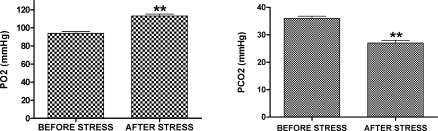
As shown in Figure 3, analysis of arterial blood demonstrated that PaO2 was higher after (113.0 ± 2.20 mm Hg) than before (94.0 ± 1.90 mm Hg) the stress period (P < .01). Consequently, the value of PaCO2 was higher before (36.0 ± 0.77 mm Hg) than after (27.0 ± 0.98 mm Hg) the stress period (P < .01). No difference was observed in blood pH (7.5 ± 0.01) measured before or after stress.
Figure 3.
Values of PaO2 and PaCO2 measured in the arterial blood of the stressed group before and after the stress period. **P < .01 when compared with the rest period. The sample size for each group is 18.
DISCUSSION
Using the model of stress-induced alterations in PaO2 and PaCO2 in conscious Wistar rats, this study investigated the role of stress in seizures induced by toxic plasma levels of lidocaine and articaine. Using the first generalized tonic-clonic seizure as the parameter for the beginning of central toxicity of local anesthetic, these results show that the time of latency was significantly shorter with 4% articaine than with 2% lidocaine. Decreased latency for the onset of seizure activity in the articaine group compared with the lidocaine group most likely can be explained by differences in concentration between the 2 local anesthetics. The rate of infusion was the same for both groups (0.1 mL/min), but because of the 4% concentration of articaine compared with 2% for lidocaine, the dose infused was doubled in the same time period for articaine. Therefore, even though the onset of seizures was more rapid with articaine than with lidocaine, the dose required to produce seizure activity was similar for both groups. Thus these results seem to demonstrate that lidocaine and articaine have equipotent CNS toxicity. These observations are in agreement with published recommendations for the same maximum doses of these 2 drugs in adult humans.13,20
Propranolol and prazosin were administered to block beta and alpha receptors and thus prevent any change in cardiovascular parameters induced by the infused epinephrine. This was confirmed by the measurement of MAP and HR. It is worth noting that with local anesthetics with epinephrine used in clinical practice in patients who are not alpha and beta blocked, cardiac output and blood flow to the brain may be increased despite a low PaCO2. Therefore the effects noted in this experimental model may not be replicated exactly in clinical practice.
In therapeutic doses, local anesthetics do not cause significant effects in the CNS, but high concentrations can induce convulsions.21–23 The initial effect on the CNS is a stimulatory phase, leading to seizure activity that is followed by an inhibitory response, which is predominant.7,12,17,21 The brief excitatory activity is the result of the removal of the selective blockade of the inhibitory neurons, leaving free the excitatory neuronal cells.24,25
Certain factors are known to affect toxicity. These include preexistent renal or hepatic failure, sex, age, blood pressure, arterial PaO2 and PaCO2, type of anesthetic and its rate of administration, and stress.26 Stress was the model used in this study. Evidence suggests that physiologic changes, secondary to stress, influence the convulsive threshold of local anesthetics.27,28 These results show that the time of latency for the beginning of the convulsion in the groups that received either lidocaine or articaine was significantly greater in stressed than in controls. This is consistent with previously reported findings by others.27 It is believed that endogenous mechanisms are involved in the inhibitory effects of stress on different seizure types.29,30 Acute stress increases the activity of the central GABAergic system31 and endogenous opioids.28 Furthermore, stress can modify the pharmacokinetic properties of the local anesthetics, resulting in alterations in free drug plasma concentration and consequently a change in its bioavailability.32
Of interest in these findings is the observation that stress increased PaO2 and decreased PaCO2, leading to a reduction of CNS toxicity for both local anesthetics. It has been shown that increased plasma PaCO2 and consequent changes in the acid-base state caused an exponential increase in the central toxicity of many local anesthetic agents.15,16 In this same way Ryan et al33 affirmed that hypercarbia decreases the convulsive threshold of local anesthetic agents. It is also important to note the cerebral vasculature response to PaCO2 as a factor affecting toxicity. Decreased PaCO2, as would be present in the stressed (hyperventilating) subject, would result in a decrease in cerebral blood flow,which, in turn, would decrease the amount of drug reaching the brain.
High values of PaCO2 reduce the convulsive threshold in the CNS, but a decrease in the levels of PaCO2 tends to stabilize the membranes of neuronal cells.17,34 Thus, acidosis increases, while alkalosis reduces, the toxicity of local anesthetics. When the cerebral pH is decreased (results from elevated PaCO2), this condition favors formation of the charged (cationic) local anesthetic species that interact with the sodium channel of inhibitory neurons in the brain, resulting in CNS toxicity.16 However, central effects observed may be dependent on changes in brain due to acidosis.10,35 Catchlove36 studied the influence of CO2 and pH on local anesthetic action applied to toad nerves, with results suggesting that CO2 augments local anesthetic action by affecting diffusion through the cellular membrane, concentrating the local anesthetic inside the axon and converting the anesthetic agent to the active cationic species.
In conclusion, these results confirm that decreases in arterial PaCO2 and increases in arterial PaO2 will increase the convulsive threshold for lidocaine and articaine. This study also demonstrated no significant difference between toxic doses of lidocaine and articaine in this experimental model. This supports the continued recommendation of equal maximum doses for these 2 commonly used local anesthetics.
REFERENCES
- 1.Daublander M, Muller R, Lipp M.D. The incidence of complications associated with local anesthesia in dentistry. Anesth Prog. 1997;44:132–141. [PMC free article] [PubMed] [Google Scholar]
- 2.De Toledo J.C. Lidocaine and seizures. Ther Drug Monit. 2000;22:320–322. doi: 10.1097/00007691-200006000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Baluga J.C, Casamayou R, Carozzi E, et al. Allergy to local anaesthetics in dentistry. Myth or reality. Allergol Immunopathol. 2002;30:14–19. doi: 10.1016/s0301-0546(02)79081-2. [DOI] [PubMed] [Google Scholar]
- 4.Finucane B.T. Allergies to local anesthetics—the real truth. Can J Anesth. 2003;50:869–874. doi: 10.1007/BF03018730. [DOI] [PubMed] [Google Scholar]
- 5.Aps C, Reynolds F. The effect of concentration on vasoactivity of bupivacaine and lignocaine. Br J Anaesth. 1976;48:1171–1174. doi: 10.1093/bja/48.12.1171. [DOI] [PubMed] [Google Scholar]
- 6.Oliveira N.E. Dissertation in Masters physiological sciences, postgraduate program in physiological sciences, Universidade Federal do Espírito Santo. Vitória: 2003. Efeitos da ropivacaína (NAROPIN®) após a anestesia pterigomandibular em humanos. 70f (Effect of ropivacaine (NAROPIN®) on pterygomandibular anesthesia in humans. 70f) [Google Scholar]
- 7.McClure H.A, Rubin A.P. Review of local anaesthetic agents. Minerva Anesthesiol. 2005;71:59–74. [PubMed] [Google Scholar]
- 8.Gall H, Kaufmann R, Kalveram C.M. Adverse reactions to local anesthetics: analysis of 197 cases. J Allergy Clin Immunol. 1996;97:933–937. doi: 10.1016/s0091-6749(96)80067-4. [DOI] [PubMed] [Google Scholar]
- 9.Brosh-Nissimov T, Ingbir M, Weintal I, Fried M, Porat R. Central nervous system toxicity following topical skin application of lidocaine. Eur J Clin Pharmacol. 2004;60:683–684. doi: 10.1007/s00228-004-0814-4. [DOI] [PubMed] [Google Scholar]
- 10.Scott D.B. Toxic effects of local anaesthetic agents on the central nervous system. Br J Anaesth. 1986;58:732–735. doi: 10.1093/bja/58.7.732. [DOI] [PubMed] [Google Scholar]
- 11.Feldman H.S, Arthur G.R, Covino B.G. Comparative systemic toxicity of convulsant and supraconvulsant doses of intravenous ropivacaine, bupivacaine and lidocaine in the conscious dog. Anesth Analg. 1989;69:794–801. [PubMed] [Google Scholar]
- 12.Malamed S.F. Handbook of Local Anesthesia. 5th ed. St Louis: Mosby; 2004. [Google Scholar]
- 13.Yagiela J.A. Pharmacology and Therapeutics for Dentistry. 5th ed. St Louis: Mosby; 2004. Local anesthetics (Chapter 16) pp. 251–270. [Google Scholar]
- 14.Endo K, Morita K, Uchiyama Y, Takada K, Tsujimoto A, Dohi T. Involvement of brain serotonergic function in lidocaine-induced convulsions in mice. Japan J Pharmacol. 1993;62:325–328. doi: 10.1254/jjp.62.325. [DOI] [PubMed] [Google Scholar]
- 15.Englesson S. The influence of acid-base changes on central nervous system toxicity of local anaesthetic agents I. Acta Anaesth Scand. 1974;18:79–87. doi: 10.1111/j.1399-6576.1974.tb00846.x. [DOI] [PubMed] [Google Scholar]
- 16.Englesson S, Grevstem S. The influence of acid-base changes on central nervous system toxicity of local anaesthetic agents II. Acta Anaesth Scand. 1974;18:88–103. doi: 10.1111/j.1399-6576.1974.tb00847.x. [DOI] [PubMed] [Google Scholar]
- 17.Englesson S. Intravenous toxicity—subjective symptoms and acid-base influences on the toxicity of local anaesthetic agents. Acta Anaesth Scand Suppl. 1966;25:28–33. [PubMed] [Google Scholar]
- 18.Bonev S, Ilouchev D, Kirin I. A study of acid-base, blood-gaseous and cerebrospinal fluid profile in cats. Folia Med. (Plovdiv) 1984;26:62–66. [PubMed] [Google Scholar]
- 19.Haas D.A, Lennon D. Local anesthetic use by dentists in Ontario. J Can Dent Assoc. 1995;61:297–304. [PubMed] [Google Scholar]
- 20.Haas D.A. An update on local anesthetics in dentistry. J Can Dent Assoc. 2002;68:546–551. [PubMed] [Google Scholar]
- 21.Pfeifer H.J, Greenblatt D.J. Koch-Weser J. Clinical use and toxicity of intravenous lidocaine: a report from the Boston Collaborative Drug Surveillance Program. Am Heart J. 1976;92:168–173. doi: 10.1016/s0002-8703(76)80252-9. [DOI] [PubMed] [Google Scholar]
- 22.Satas S, Johannessen S.I, Hoem N.O, Haaland K, Sørensen D.R, Thoresen M. Lidocaine pharmacokinetics and toxicity in newborn pigs. Anesth Analg. 1997;85:306–312. doi: 10.1097/00000539-199708000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Fujita H, Maru E, Shimada M, Suzuki H, Ogiuchi H. A decrease in seizure susceptibility to lidocaine in kindled epileptic rats. Anesth Analg. 2000;90:1129–1134. doi: 10.1097/00000539-200005000-00024. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka K, Yamasaki M. Blocking of cortical inhibitory synapses by intravenous lidocaine. Nature. 1966;209:207–208. doi: 10.1038/209207a0. [DOI] [PubMed] [Google Scholar]
- 25.Moorthy S.S, Zaffer R, Rodriguez S, Ksiazek S, Yee R.D. Apnea and seizures following retrobulbar local anesthetic injection. J Clin Anesth. 2003;15:267–270. doi: 10.1016/s0952-8180(03)00025-4. [DOI] [PubMed] [Google Scholar]
- 26.De Jong R.H, Bonin J.D. Deaths from local anaesthetic induced convulsions in mice. Anesth Analg. 1980;59:401–405. [PubMed] [Google Scholar]
- 27.Homayoun H, Khavandgar S, Dehpour A.R. The involvement of endogenous opioids and nitricoxideergic pathway in the anticonvulsant effects of foot-shock stress in mice. Epilepsy Res. 2002;49:131–142. doi: 10.1016/s0920-1211(02)00018-9. [DOI] [PubMed] [Google Scholar]
- 28.Homayoun H, Dehpour A.R. Differential contribution of cholecystokinin receptors to stress-induced modulation of seizure and nociception threshold in mice. Pharmacol Biochem Behav. 2004;78:209–215. doi: 10.1016/j.pbb.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 29.Kurt M, Bilge S.S, Kukula O, Kesim Y, Celik S. The role of nitrergic system in lidocaine-induced convulsion in the mouse. Japan J Pharmacol. 2001;85:92–94. doi: 10.1254/jjp.85.92. [DOI] [PubMed] [Google Scholar]
- 30.Shavit Y, Caldecott-Hazard S, Liebeskind J.C. Activating endogenous opioids systems by electroconvulsive shock on footshock stress inhibits recurrent kindled seizures in rats. Brain Res. 1984;305:203–207. doi: 10.1016/0006-8993(84)90426-8. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz R.D, Wess M.J, Labarca R, Skolnick P, Paul S.M. Acute stress enhances the activity of the GABA receptor-gated chloride ion channel in brain. Brain Res. 1987;411:151–155. doi: 10.1016/0006-8993(87)90692-5. [DOI] [PubMed] [Google Scholar]
- 32.Saranteas T, Mourouzis C, Dannis C, Alexopoulos C, Lolis E, Tesseromatis C. Effect of various stress models on lidocaine pharmacokinetic properties in the mandible after masseter injection. J Oral Maxillofac Surg. 2004;62:858–862. doi: 10.1016/j.joms.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 33.Ryan C.A, Robertson M, Coe J.Y. Seizures due to lidocaine toxicity in a child during cardiac catheterization. Pediatr Cardiol. 1993;14:116–118. doi: 10.1007/BF00796991. [DOI] [PubMed] [Google Scholar]
- 34.Englesson S, Matousek M. Central nervous system effects of local anaesthetic agents. Br J Anaesth. 1975;47:241–246. [PubMed] [Google Scholar]
- 35.Scott D.B. Evaluation of the toxicity of local anaesthetic agents in man. J Anaesth. 1975;47:56–61. doi: 10.1093/bja/47.1.56. [DOI] [PubMed] [Google Scholar]
- 36.Catchlove R.F.H. The influence of CO2 and pH on local anesthetic action. J Pharmacol Exp Ther. 1972;181:298–309. [PubMed] [Google Scholar]