Abstract
Aquaporins (AQPs) are small, integral membrane proteins that facilitate water transport across cell membranes in response to osmotic gradients. Water transport across epithelia and endothelia in the peripheral lung and airways occurs during airway hydration, alveolar fluid transport and submucosal gland secretion. Several AQPs are expressed in the lung and airways: AQP1 in microvascular endothelia, AQP3 and AQP4 in airway epithelia, and AQP5 in type I alveolar epithelial cells, submucosal gland acini, and a subset of airway epithelial cells. Phenotype analysis of transgenic knockout mice lacking AQPs has defined their roles in the lung and airways. AQP1 and AQP5 provide the principal route for osmotically driven water transport between airspace and capillary compartments; however, alveolar fluid clearance in the neonatal and adult lung is not affected by their deletion, nor is lung fluid accumulation in experimental models of lung injury. In the airways, though AQP3 and AQP4 facilitate osmotic water transport, their deletion does not impair airway hydration, regulation of airway surface liquid, or fluid absorption. In contrast to these negative findings, AQP5 deletion in submucosal glands reduced fluid secretion by > 50%. The substantially slower fluid transport in the lung compared to renal and secretory epithelia probably accounts for the lack of functional significance of AQPs in the lung and airways. Recent data outside of the lung implicating the involvement of AQPs in cell migration and proliferation suggests possible new roles for lung AQPs to be explored.
Keywords: Water permeability, AQP, pulmonary edema, alveolus, epithelium, transgenic mouse
1. Introduction
Fluid transport across cellular barriers results from water transport driven by osmotic gradients or hydrostatic pressure differences. In the peripheral lung and airways, fluid movement between the airspace, cellular/interstitial and vascular compartments occurs in the maintenance of airspace hydration, the absorption of airspace fluid near the time of birth and in pulmonary edema, and the secretion of fluids onto the airway surface by submucosal glands. While pressure-driven bulk fluid flow produces lung edema and pleural effusions in heart failure, osmotically-driven water transport across cell membranes is the principle mechanism of fluid transport under normal physiological conditions. Osmotic gradients produced by solute transport are dissipated by water transport across cell membranes. Some membranes contain aquaporin (AQP) water channels, which facilitate the dissipation of osmotic gradients by increasing cell membrane water permeability by 5–50 fold over that in membranes where water moves primarily through the lipid bilayer. The AQPs are a family of small (~30 kDa monomer) integral membrane proteins that function as selective water transporters, and in some cases they also transport glycerol (‘aquaglyceroporins’). There are at present 12 related AQPs in mammals, at least 4 of which are expressed in the airways and lung. This review is focused on the role of these AQPs in lung and airway physiology.
2. Barriers to fluid transport in lung and airways
There are distinct barriers to water transport between airspace, interstitial, and vascular compartments. The nasopharnyx, trachea and airways are lined by an epithelial cell layer that forms the principal barrier for water movement between the interstitial and vascular compartment and the airway surface liquid (ASL) - the thin (<50 µm) layer of liquid lining the apical surface of the epithelium. Submucosal glands, which secrete fluid and glycoproteins onto the airway surface, are present in nasopharnyx and large airways. The alveolar epithelium provides the major surface area for gas exchange. The alveolar epithelium contains type I cells, which are flat cells comprising the majority of the alveolar epithelial surface, and type II cells, which transport salt actively and produce surfactant. Water movement between the airspace and capillary compartments across the alveolar epithelium must also cross the interstitium and capillary endothelium. Exceptionally high osmotic water permeability of the airspace-capillary endothelial and epithelial barriers has been found in several mammalian species (Folkesson et al., 1994; 1996; Carter et al. 1997, 1998), as well as in alveolar and airway cells derived from lung tissues (Dobbs et al., 1998; Matsui et al., 2000; Levin et al. 2006).
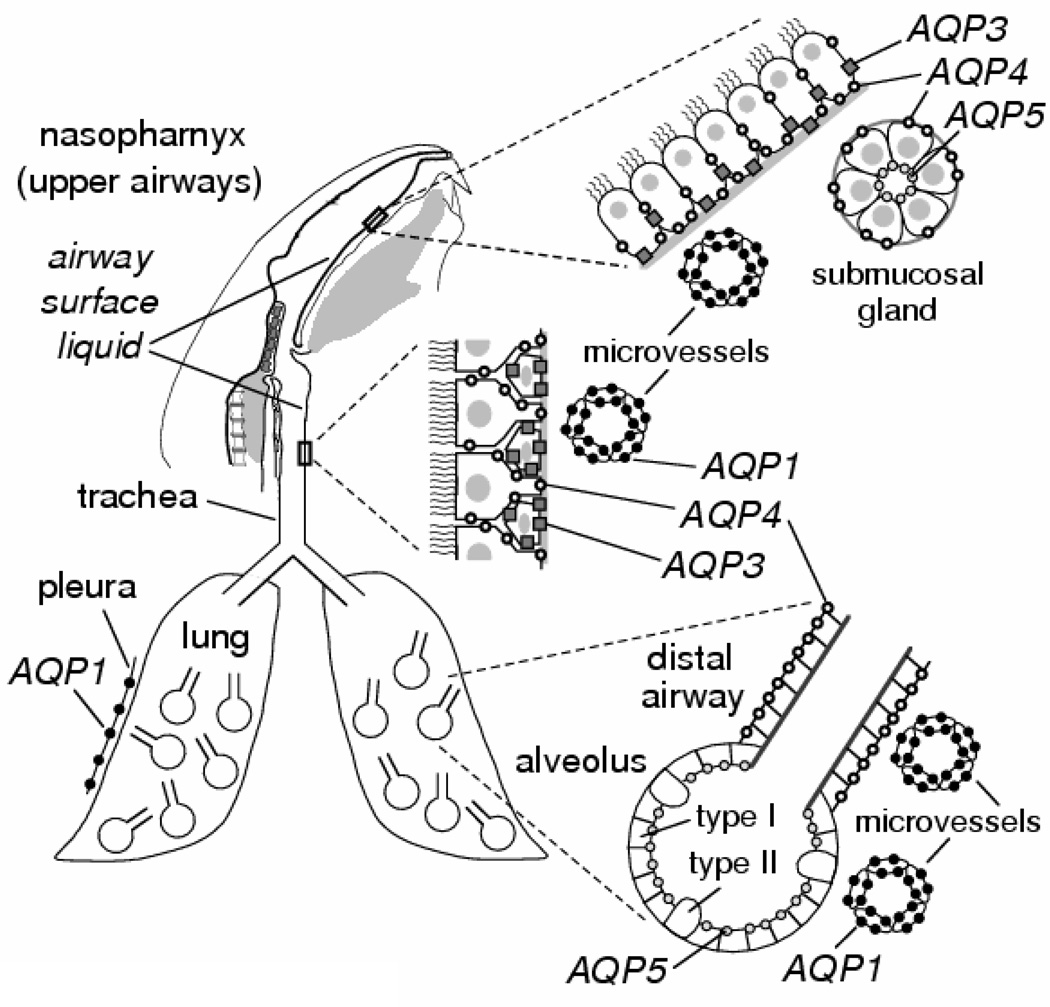
3. Expression of aquaporins in lung and airways
At least four AQPs are expressed in the respiratory tract as depicted in Fig. 1 (reviewed in Borok and Verkman (2002) with original references cited therein). AQP1 protein is expressed in microvascular endothelia associated with airways and alveoli and in microvessels and mesothelial cells of visceral and parietal pleura. AQP3 is expressed in the basolateral membranes of basal epithelial cells of large airways and nasopharynx. In human lung, one study reported AQP3 expression in small airway epithelia as well. AQP4 is expressed in the basolateral membrane of ciliated columnar cells of bronchial, tracheal and nasopharyngeal epithelium. AQP5 is expressed in the apical membrane of type I alveolar epithelial cells and at the apical membrane of acinar epithelial cells in submucosal glands, and although there are conflicting data, AQP5 may be expressed to some extent at the apical membrane of large airway epithelia. The expression pattern of AQPs provides indirect evidence for their involvement in fluid handling by the peripheral lung and airways.
Figure 1. AQP water channel expression in lung and airways.
AQPs 1, 3, 4 and 5 are expressed as indicated in epithelia and endothelia throughout the nasopharyngeal cavity, upper and lower airways, and alveoli. Information shown for mouse. See text for further explanations.
4. Indirect evidence for involvement of AQPs in lung and airway physiology
In addition to cell-specific AQP expression, several other lines of evidence suggest involvement of AQPs in fluid handling by the peripheral lung and airways. As for many proteins, the expression of lung AQPs is developmentally regulated. Rodent studies have shown developmental regulation of lung AQP expression with distinct patterns for each AQP. AQP1 is detectable just before birth in rodents, increasing several-fold perinatally and into adulthood (Umenishi et al., 1996; Ruddy et al., 1998). Functional measurements in rabbit showed significantly increased lung water permeability in the perinatal period that paralleled increasing AQP1 expression (Carter et al., 1997). AQP1 expression is also up-regulated by treatment with corticosteroids (King et al., 1996). In contrast, little AQP5 is expressed at birth and gradually increases until adulthood, whereas AQP4 expression strongly increases just after birth and is up-regulated by β-agonists and glucocorticoids. Additional indirect evidence for a physiologic role of AQPs in respiratory physiology includes regulation of AQP expression in adult lung by growth factors, inflammatory mediators and osmotic stress. AQP1 and AQP5 expression are reduced in rodent lung following adenoviral infection (Towne et al., 2002) and lipopolysaccarhide-induced lung injury (Jiao et al., 2002), and AQP5 expression is increased after bleomycin exposure (Gabazza et al., 2004). Reduced AQP5 expression was found after exposure of a mouse lung epithelial cell line to TNF-α (Towne et al., 2001), suggesting a possible mechanism for its down-regulation in viral infection in vivo. AQP5 expression is regulated in various lung cells by multiple effectors, including hypertonicity, cAMP agonists, and vallinoid receptors and others (Hoffert et al., 2000; Sidhaye et al., 2005). Although potentially interesting, the relevance of many of these observations to lung/airway physiology is unclear, since the airway/lung is probably not exposed to significant hypertonicity, and regulated AQP expression is a general phenomena not specific to the lung/airways.
5. Lessons from transgenic mouse models of AQP deletion
Analysis of the extrapulmonary phenotype of transgenic mice lacking specific AQPs has provided insight into their physiological roles (reviewed in Verkman, 2005). Mice lacking AQPs 1–4 manifest a defect in urinary concentrating ability (Verkman, 2006). Near-isosmolar fluid secretion is impaired in salivary gland in AQP5 deficiency (Ma et al., 1999). The general paradigms from these findings, and associated mechanism studies, is that high transepithelial water permeability facilitates rapid water transport in response to active transepithelial salt transport. As shown in Fig. 2A, AQP deletion impairs osmotic equilibration, resulting in secretion of a reduced volume of relatively hypertonic fluid, as found for saliva secretion in AQP5 null mice. Fig. 2B depicts a second mechanism for involvement of AQPs in mammalian physiology, in which they facilitate passive, osmotically driven water transport, as in osmotic extraction of water in kidney collecting duct. A related AQP role is in the pathophysiology of tissue fluid accumulation, as found for AQP4 in brain edema (Verkman et al., 2006) and AQP1 in corneal edema (Thiagarajah and Verkman, 2002). Here, AQPs facilitate water entry into and exit from tissues in response to clinically relevant stimuli, such as altered cellular ionic homeostasis in cytotoxic brain edema.
Figure 2. Mechanisms of AQP function outside of the lung.
A. Reduced water permeability in glandular epithelium impairs active, near-isosmolar fluid transport by slowing osmotic water transport into the acinar lumen, producing hypertonic secretion. B. Reduced transepithelial water permeability in kidney collecting duct impairs urinary concentrating ability by preventing osmotic equilibration of luminal fluid. C. AQP-facilitated water entry into protruding lamellipodia, accounting for AQP-dependent cell migration. D. Reduced steady-state glycerol content in epidermis and stratum corneum following AQP3 deletion, accounting for reduced skin hydration in AQP3 deficiency. E. Impaired AQP7-dependent glycerol escape from adipocytes resulting in intracellular glycerol accumulation and increased triglyceride content, accounting for progressive adipocyte hypertrophy in AQP7 deficiency.
Recently, we discovered a novel cellular role for AQPs in cell migration, as originally demonstrated in endothelial cells and various transfected cells (Saadoun et al., 2005), and subsequently in brain astroglial cells, kidney proximal tubule cells, and corneal epithelium cells. Fig. 2C shows our proposed mechanism for AQP involvement in cell migration in which actin cleavage and ion uptake at the tip of a lamellipodium create local osmotic gradients that drive water influx of water. Whether AQP-dependent migration of epithelial and endothelial cells is important in lung and airways in not known. Perhaps, as found for AQP1-dependent proximal tubule cell migration in recovery from acute renal tubular injury, AQP-dependent cell migration may be important in recovery from injury to the lung or airways. AQPs may also be involved in local tumor invasion and metabolic spread, as several lung and cancers have been shown to express AQPs (Hoque et al., 2006; Liu et al., 2006). We found recently that AQP-expressing tumor cells have enhanced extravasation across lung microvessels, resulting in a greater number of lung metastases, as well as greater local invasiveness (Hu and Verkman, 2006).
The aquaglyceroporins, of which airway AQP3 is an example, have unique biological roles that are related to their glycerol transporting function. AQP3-facilitated glycerol transport in skin is an important determinant of epidermal and stratum corneum hydration (Fig. 2D). Mice lacking AQP3, which is expressed in epidermal keratinocytes, have reduced stratum corneum hydration and skin elasticity, and impaired stratum corneum biosynthesis and wound healing (Hara-Chikuma and Verkman, 2006; Levin and Verkman, 2006). The mechanism for the skin phenotype involves reduced epidermal cell skin glycerol permeability in AQP3 deficiency, resulting in reduced glycerol content in the stratum corneum and epidermis. Another aquaglyceroporin, AQP7, is expressed in the plasma membrane of adipocytes. AQP7 null mice have a greater fat mass than wildtype mice as they age, with remarkable adipocyte hypertrophy, and accumulation of glycerol and triglycerides (Hara-Chikuma et al., 2005). As shown in Fig. 2E, hypertrophy of AQP7-deficient adipocytes probably results from reduced plasma membrane glycerol permeability, and consequent increased glycerol accumulation and triglyceride biosynthesis. Recently, we discovered a new role for AQP3-dependent glycerol transport in cell proliferation (Hara-Chikuma and Verkman, 2007), which is important in healing of cutaneous and corneal wounds, skin carcinogenesis, and intestinal damage in colitis. Reduced cellular glycerol content appears to alter cell metabolism, resulting in reduced ATP content and MAP kinase signaling, as well as reduced glycerol availability for biosynthesis. Whether AQP3-dependent airway cell proliferation is important following airway cell injury is unknown.
5.1. Phenotype of AQP null mice: Fluid transport in distal lung
In peripheral lung, possible AQP functions include alveolar fluid absorption at the time of birth and in the adult lung, gas (CO2) exchange, and regulation of lung water content in response to acute and subacute lung injury. The main aquaporins in peripheral lung are AQP5 in type I alveolar epithelial cells and AQP1 in endothelial cells. Initial experiments indicated that these AQPs provide the principal route for osmotically-driven water transport across the alveolar epithelial and endothelial barriers, respectively (Bai et al., 1999; Ma et al., 20002). Osmotic water permeability between the airspace-capillary barriers in isolated perfused mouse lung, as measured by a pleural surface fluorescence method (Figure 3A, Carter et al., 1997), was ~10-fold reduced by deletion of AQP5 or AQP1 separately, and >30-fold reduced by their deletion together (Fig. 3B).
Figure 3. Water permeability and fluid transport in lungs of AQP knockout mice.
A. Strategy for measurement of osmotic water permeability between the airspace/capillary barrier in isolated perfused lung as described by Carter et al. (1996). A fluorescent volume marker (F) is present in the airspace instillate, and pleural surface fluorescence is monitored in response to changes in pulmonary artery perfusate osmolality. B. Osmotically-driven water transport across the airspace/capillary barrier in lungs of wildtype mice (+/+) and knockout (−/−) mice lacking the indicated AQPs. C. Alveolar fluid clearance measured from the increased concentration of a volume marker at 15 min after instillation of isosmolar fluid at 37 °C. Where indicated, the instillate contained amiloride or mice were pre-treated with keratinocyte growth factor (KGF). Adapted from Bai et al. (1999) and Ma et al. (2000).
Unexpectedly, it was found that AQP1 deletion in mice resulted in decreased lung water accumulation in response to acute elevation in perfusate hydrostatic pressure (Bai et al., 1999). A similar observation was made in two AQP1-deficient humans compared to control subjects, where tomographic analysis of airway wall thickness after saline infusion showed a lesser increase in thickness (King et al., 2002). It is unlikely, however, that the apparent AQP1-dependent changes in fluid accumulation result from altered capillary endothelial water transport rather than to altered capillary structure and function with AQP1 deletion. Pressure-driven fluid accumulation in intact airways/lung is a very inefficient process because any solute-free water that is transported in response to a pressure difference should be absorbed rapidly by osmotic forces. Fluid accumulation occurs primarily by bulk fluid movement through transient breaches in capillary/alveolar integrity.
The various possible physiologically important functions of AQPs in peripheral lung were tested. Alveolar fluid absorption was measured from the increased concentration of an airspace volume marker (radioiodinated albumin) at 37 °C (Fig. 3C). Even with maximal stimulation of alveolar fluid absorption by beta-agonists and pre-treatment with keratinocyte growth factor (to increase the number of type II cells), there was no significant effect of AQP deletion (Bai et al., 1999; Ma et al., 2000). Further, the rapid absorption of fluid from the airspace just after birth was not impaired by AQP deletion, nor was the accumulation of lung edema in response to acid-induced epithelial cell injury, thiourea-induced endothelial cell injury, or hyperoxic subacute lung injury (Song et al., 2000). The remarkably slower rate of alveolar fluid absorption compared to proximal tubule fluid absorption in the kidney and saliva secretion probably explains the lack of effect of AQP1 and AQP5 deletion on alveolar fluid clearance.
Heterologous expression studies in Xenopus oocytes have suggested AQP1-facilitated CO2 transport (Nakhoul et al., 1998). However, abnormalities in CO2 transport were not found in AQP1 null mice in arterial blood gas measurements in anesthetized ventilated mice subjected to changes in inspired gas CO2 content (Yang et al., 2000), or in rapid changes in airspace fluid pH in isolated perfused lung subjected to sudden changes in capillary CO2 content (Fang et al., 2002). Thus, although AQP1 may be able to transport CO2 under some conditions, AQP1-facilitated CO2 transport is not of physiological importance in lung gas exchange.
5.2. Phenotype of AQP null mice: Fluid transport in the pleura
Fluid is continuously secreted into and reabsorbed from the pleural space. Little fluid is present in the pleural space (<0.2 ml/kg) despite its large surface area (4000 cm2 in man, 10 cm2 in mouse). Fluid entry into the pleural space involves filtration across microvascular endothelia near the pleural surface, and movement across a mesothelial barrier lining the pleural space, whereas fluid clearance is thought to occur primarily by lymphatic drainage. Pleural fluid can accumulate in pathological conditions such as congestive heart failure, lung infection, lung tumor, and the acute respiratory distress syndrome. AQP1 is expressed in microvascular endothelia near the visceral and parietal pleura and in mesothelial cells in visceral pleura. Osmotic water permeability across the pleural barrier, measured from the kinetics of pleural fluid osmolality after instillation of hypertonic or hypotonic fluid into the pleural space, was rapid in wildtype mice (50% osmotic equilibration in <2 min), and slowed by ~4-fold in AQP1 knockout mice (Song et al., 2001). However, the clearance of saline instilled in the pleural space was not affected by AQP1 deletion, nor was the accumulation of pleural fluid in a fluid overload model produced by intraperitoneal saline administration or in a thiourea model of acute endothelial injury. Thus, although rapid osmotic equilibration across the pleural surface is facilitated by AQP1, as in peripheral lung, AQP1 does not appear to play an important role in physiologically relevant mechanisms of pleural fluid accumulation or clearance.
5.3.1. Phenotype of AQP null mice: Fluid transport in the airways and ASL regulation
Potential functions of AQPs in the airways include humidification of inspired air, regulation of airway surface liquid (ASL) volume and composition, and absorption of fluid from the airways. Evaporative water loss in the airways is thought to drive water influx from capillaries and interstitium into the ASL through the surface epithelium by the generation of an osmotic gradient. The depth and ionic composition of the ASL should depend theoretically on the ion transporting properties of the airway epithelium and the rate of evaporative water loss, as well as the water permeability of the airway-capillary barrier. Osmotic water permeability in upper airways, measured by dilution of an airway volume marker in response to an osmotic gradient, was reduced in mice lacking AQP3 and/or AQP4 (Song et al., 2001). However, there was little effect of AQP3/AQP4 deletion on humidification of lower airways, as measured from the moisture content of expired air during mechanical ventilation with dry air through a tracheotomy, or of upper airways, as measured from the moisture content by dry air passed through the upper airways in mice breathing through a tracheotomy. Also, the depth and salt concentration of the ASL in trachea, as measured in vivo using fluorescent probes and confocal microscopy methods developed by our lab (Jayaraman et al., 2001), was not altered by AQP3/AQP4 deficiency. Finally, isosmolar fluid absorption, measured in nasopharyngeal airways (using a volume marker as done for alveolar fluid clearance) was not impaired by AQP deletion. Thus, although AQP3/AQP4 facilitate osmotic water transport in the airways, they play at most a minor role in airway humidification, ASL hydration, and isosmolar fluid absorption. One study showed increased airway reactivity in response to bronchoconstricting agents in AQP5 null mice (Krane et al., 2001). The mechanism of this phenotype was not established, but may be related to indirect effects of AQP5 deletion on agonist-induced fluid secretion from submucosal glands as described below.
5.3.2. Airway AQPs are not involved in the action of hypertonic saline in cystic fibrosis
Recent data indicate clinical benefit of nebulized hypertonic saline in cystic fibrosis lung disease, with a proposed mechanism involving sustained increase in airway surface liquid (ASL) volume. To account for the paradoxical observation in one study that amiloride suppressed the beneficial effect of hypertonic saline (Donaldson et al., 2006), it was concluded that amiloride-inhibitable AQPs in airway epithelia modulate ASL volume such that amiloride prevents water movement into the airways following hypertonic saline. To investigate this possibility, we measured water permeability and amiloride effects in well-differentiated, primary cultures of human airway epithelial cells, stably transfected Fisher Rat Thyroid epithelial cells expressing individual airway/lung AQPs, and perfused mouse lung (Levin et al., 2006). Although high, AQP-dependent water permeability was found in these systems, as expected from prior data, no inhibition by amiloride was found. Also, the airway phenotype studies described above indicating that ASL hydration does not depend on AQPs (Song et al., 2001) argues against significant involvement of AQPs in hypertonic saline therapy. A recent independent study of water permeability in airway spheroid cultures also concluded that amiloride does not inhibit water permeability of airway AQPs (Pedersen et al., 2006).
5.4. Fluid secretion by airway submucosal glands
Submucosal glands in mammalian airways secrete a mixture of water, ions and macromolecules onto the airway surface. Glandular secretions are important in establishing ASL fluid composition and volume, and in antimicrobial defense mechanisms. Abnormally viscous gland secretions in cystic fibrosis have been proposed to promote bacterial adhesion and inhibit bacterial clearance. Submucosal glands contain serous tubules, where active salt secretion into the gland lumen creates a small osmotic gradient driving water transport across a water permeable epithelium, as well as mucous cells and tubules, where viscous glycoproteins are secreted. AQP5 is expressed at the luminal membrane of the serous epithelial cells. Pilocarpine-stimulated fluid secretion was found to be reduced by >2-fold in AQP5 null mice, as determined by nasopharyngeal fluid collections and video imaging of fluid droplets (covered with mineral oil) secreted by individual submucosal glands (Song and Verkman, 2001). Analysis of secreted fluid showed a >2-fold increase of total protein concentration in AQP5 null mice as well as increased chloride concentration, suggesting intact protein and salt secretion across a relatively water impermeable epithelial barrier. There was no significant difference of submucosal gland morphology or density in wildtype vs. AQP5 knockout mice. AQP5 thus facilitates fluid secretion in submucosal glands, indicating that the luminal membrane of serous epithelial cells is the rate-limiting barrier to water movement. Modulation of AQP5 expression or function could provide a novel therapy to change the volume and viscosity of fluid secretions in cystic fibrosis and infectious or allergic rhinitis.
6. Perspective and directions
Cell-specific and regulated expression of AQPs in the lung and airways has provided indirect evidence supporting their physiological role. Water permeability measurements in mice lacking AQPs 1, 3, 4 and 5 have established that AQPs provide a major pathway for osmotically-driven water movement across epithelial and endothelial barriers in the airways, alveoli and pleura. However, except for impaired fluid secretion by airway submucosal glands in AQP5 null mice, phenotype studies in AQP-deficient mice do not support a significant role for aquaporins in the lung or airways in physiologically important functions such as alveolar fluid absorption or regulation of the airway surface liquid. The relatively slow rates of fluid transport in the lung and airways compared to organs where aquaporins are important, such as kidney, account for the conclusion that the AQPs in the lung and airways have little or no physiological significance for transepithelial water transport. However, the possibility remains that new functions of AQPs in the lung and airways will emerge, perhaps related to the new roles of AQPs in cell migration and proliferation. AQP inhibitors, when available, will be useful to study the role of AQPs in lung physiology, as ‘chemical knock-out’ by small-molecule inhibitors obviates the general concern in transgenic mouse studies about possible compensatory changes in organ function.
Figure 4. Reduced fluid secretion by airway submucosal glands in mice lacking AQP5.
A. Time course of expanding fluid droplets secreted by an airway submucosal gland after pilocarpine administration. Secretion was decreased in AQP5 null mice. B. Secretion rates from individual mice shown with mean and SE. *, P < 0.01. Adapted from Song and Verkman (2001).
ACKNOWLEDGMENTS
Supported by NIH grants HL59198, HL73856, DK35124, EB00415 and EY13574, Research and Translational Core Center grant DK72517, and Research Development Program and Drug Discovery grants from the Cystic Fibrosis Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bai C, Fukuda N, Song Y, Ma T, Matthay MA, Verkman AS. Lung fluid transport in aquaporin-1 and aquaporin-4 knockout mice. J. Clin. Invest. 1999;103:555–561. doi: 10.1172/JCI4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borok Z, Verkman AS. Lung edema clearance: 20 years of progress: Invited review: Role of aquaporin water channels in fluid transport in lung and airways. J. Appl. Physiol. 2002;93:2199–2206. doi: 10.1152/japplphysiol.01171.2001. [DOI] [PubMed] [Google Scholar]
- Carter EP, Matthay MA, Farinas J, Verkman AS. Transalveolar osmotic and diffusional water permeability in intact mouse lung measured by a novel surface fluorescence method. J. Gen. Physiol. 1996;108:133–142. doi: 10.1085/jgp.108.3.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter EP, Ölveczky BP, Matthay MA, Verkman AS. High microvascular endothelial water permeability in mouse lung measured by a pleural surface fluorescence method. Biophys. J. 1998;74:2121–2128. doi: 10.1016/S0006-3495(98)77919-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter EP, Umenishi F, Matthay MA, Verkman AS. Developmental changes in alveolar water permeability in perinatal rabbit lung. J. Clin. Invest. 1997;100:1071–1078. doi: 10.1172/JCI119617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs L, Gonzalez R, Matthay MA, Carter EP, Allen L, Verkman AS. Highly water-permeable type I alveolar epithelial cells confer high water permeability between the airspace and vasculature in rat lung. Proc. Natl. Acad. Sci. U.S.A. 1998;95:2991–2996. doi: 10.1073/pnas.95.6.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson SH, Bennett WD, Zeman KL, Knowles MR, Tarran R, Boucher RC. Mucus clearance and lung function in cystic fibrosis with hypertonic saline. N. Engl. J. Med. 2006;354:241–250. doi: 10.1056/NEJMoa043891. [DOI] [PubMed] [Google Scholar]
- Fang X, Yang B, Matthay MA, Verkman AS. Evidence against aquaporin dependent CO2 permeability in lung and kidney. J. Physiol. (London) 2002;543:63–69. doi: 10.1113/jphysiol.2001.013813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkesson H, Matthay MA, Frigeri A, Verkman AS. High transepithelial water permeability in microperfused distal airways: evidence for channel-mediated water transport. J. Clin. Invest. 1996;97:664–671. doi: 10.1172/JCI118463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkesson HG, Matthay MA, Hasegawa H, Kheradmand F, Verkman AS. Transcellular water transport in lung alveolar epithelium through mercury-sensitive water channels. Proc. Natl. Acad. Sci. U.S.A. 1994;91:4970–4974. doi: 10.1073/pnas.91.11.4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabazza EC, Kasper M, Ohta K, Keane M, D’Alessandro-Gabazza C, Fujimoto H, Nishii Y, Nakahara H, Takagi T, Menon AG, Adachi Y, Suzuki K, Taguchi O. Decreased expression of aquaporin-5 in bleomycin-induced lung fibrosis in the mouse. Pathol. Int. 2004;54:774–780. doi: 10.1111/j.1440-1827.2004.01754.x. [DOI] [PubMed] [Google Scholar]
- Hara-Chikuma M, Sohara E, Rai T, Ikawa M, Okabe S, Sasaki S, Uchida S, Verkman AS. Progressive adipocyte hypertrophy in aquaporin-7 deficient mice: adipocyte glycerol permeability as a novel regulator of fat accumulation. J. Biol. Chem. 2005;280:15493–15496. doi: 10.1074/jbc.C500028200. [DOI] [PubMed] [Google Scholar]
- Hara-Chikuma M, Verkman AS. Physiological roles of glycerol-transporting aquaporins – the aquaglyeroporins. Cell Mol. Life Sci. 2006;63:1386–1392. doi: 10.1007/s00018-006-6028-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara-Chikuma M, Verkman AS. Prevention of skin carcinogenesis by targeted aquaporin-3 gene disruption. 2007 doi: 10.1128/MCB.01482-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffert JD, Leitch V, Agre P, King LS. Hypertonic induction of aquaporin-5 expression through an ERK-dependent pathway. J. Biol. Chem. 2000;275:9070–9077. doi: 10.1074/jbc.275.12.9070. [DOI] [PubMed] [Google Scholar]
- Hoque MO, Soria JC, Woo J, Lee T, Lee J, Jang SJ, Upadhyay S, Trink B, Monittao C, Desmaze C, Mao L, Sidransky D, Moon D. Aquaporin 1 is overexpressed in lung cancer and stimulates NIH-3T3 cell proliferation and anchorage-independent growth. Am. J. Pathol. 2006;168:1345–1353. doi: 10.2353/ajpath.2006.050596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Verkman AS. Increased migration and metastatic potential of tumor cells expressing aquaporin water channels. Faseb J. 2006;20:1892–1894. doi: 10.1096/fj.06-5930fje. [DOI] [PubMed] [Google Scholar]
- Jayaraman S, Song Y, Vetrivel L, Shankar L, Verkman AS. Noninvasive in vivo fluorescence measurement of airway surface liquid depth, salt concentration and pH. J. Clin. Invest. 2001;107:317–324. doi: 10.1172/JCI11154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao G, Li E, Yu R. Decreased expression of AQP1 and AQP5 in acute injured lungs in rats. Chin. Med. J. (Engl.) 2002;115 963-867. [PubMed] [Google Scholar]
- King LS, Nielsen S, Agre P. Aquaporin-1 water channel protein in lung-ontogeny, steroid-induced expression, and distribution in rat. J. Clin. Invest. 1996;97:2183–2191. doi: 10.1172/JCI118659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King LS, Nielsen S, Agre P, Brown RH. Decreased pulmonary vascular permeability in aquaporin-1-null humans. Proc. Natl. Acad. Sci. U.S.A. 2002;99:1059–1063. doi: 10.1073/pnas.022626499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krane CM, Fortner CN, Hand AR, McGraw DW, Lorenz JN, Wert SE, Towne JE, Paul RJ, Whitsett JA, Menon AG. Aquaporin-5 deficient mouse lungs are hyperresponsive to cholinergic stimulation. Proc. Natl. Acad. Sci. U.S.A. 2001;98:14114–14119. doi: 10.1073/pnas.231273398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin MH, Sullivan S, Nielson D, Yang B, Finkbeiner WE, Verkman AS. Hypertonic saline therapy in cystic fibrosis: Evidence against the proposed mechanism involving aquaporins. J. Biol. Chem. 2006;281:25803–25812. doi: 10.1074/jbc.M604332200. [DOI] [PubMed] [Google Scholar]
- Levin MH, Verkman AS. Aquaporin-3-dependent cell migration and proliferation during corneal re-epithelialization. Invest. Opthalmol. Vis. Sci. 2006;47:4365–4372. doi: 10.1167/iovs.06-0335. [DOI] [PubMed] [Google Scholar]
- Liu YL, Matsuzaki T, Nakawara T, Murata SI, Nakamura N, Kondo T, Iwashina M, Mochizuki K, Yamane T, Takata K, Katoh R. Expression of aquaporin 3 (AQP3) in normal and neoplastic lung tissues. Hum. Pathol. 2006 doi: 10.1016/j.humpath.2006.07.015. In press. [DOI] [PubMed] [Google Scholar]
- Ma T, Fukuda N, Song Y, Matthay MA, Verkman AS. Lung fluid transport in aquaporin-5 knockout mice. J. Clin. Invest. 2000;105:93–100. doi: 10.1172/JCI8258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T, Song Y, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Defective secretion of saliva in transgenic mice lacking aquaporin-5 water channels. J. Biol. Chem. 1999;274:20071–20074. doi: 10.1074/jbc.274.29.20071. [DOI] [PubMed] [Google Scholar]
- Matsui H, Davis CW, Tarran R, Boucher RC. Osmotic water permeability of cultured, well-differentiated normal and cystic fibrosis airway epithelia. J. Clin. Invest. 2000;105:1419–1427. doi: 10.1172/JCI4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhoul NL, Davis BA, Romero MF, Boron WF. Effect of expressing the water channel aquaporin-1 on the CO2 permeability of Xenopus oocytes. Am. J. Physiol. 1998;274:C543–C548. doi: 10.1152/ajpcell.1998.274.2.C543. [DOI] [PubMed] [Google Scholar]
- Pedersen PS, Braunstein TH, Jorgensen A, Larsen PL, Holstein-Rathlou NH, Frederiksen O. Stimulation of aquaporin-5 and transepithelial water permeability in human airway epithelium by hyperosmotic stress. Pflugers Arch. 2006 doi: 10.1007/s00424-006-0157-3. In press. [DOI] [PubMed] [Google Scholar]
- Ruddy MK, Drazen JM, Pitkanen OM, Rafii B, O’Brodovich HM, Harris HW. Modulation of aquporin 4 and the amiloride-inhibitable sodium channel in perinatal rat lung epithelial cells. Am. J. Physiol. 1998;274:L1066–L1072. doi: 10.1152/ajplung.1998.274.6.L1066. [DOI] [PubMed] [Google Scholar]
- Saadoun S, Papadopoulos MC, Hara-Chikuma M, Verkman AS. Impairment of angiogenesis and cell migration by targeted of aquaporin-1 gene disruption. Nature. 2005;434:786–792. doi: 10.1038/nature03460. [DOI] [PubMed] [Google Scholar]
- Sidhaye VK, Guler AD, Schweitzer KS, D’Alessio F, Caterina MJ, King LS. Transient receptor potential vanilloid 4 regulates aquaporin-5 abundance under hypotonic conditions. Proc. Natl. Acad. Sci. U.S.A. 2006;103:4747–4752. doi: 10.1073/pnas.0511211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhaye V, Hoffert JD, King LS. cAMP has distinct acute and chronic effects on aquaporin-5 in lung epithelial cells. J. Biol. Chem. 2005;280:3590–3596. doi: 10.1074/jbc.M411038200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Fukuda N, Bai C, Ma T, Matthay MA, Verkman AS. Role of aquaporins in alveolar fluid clearance in neonatal and adult lung, and in edema formation following lung injury. J. Physiol. (Lond.) 2000;525:771–779. doi: 10.1111/j.1469-7793.2000.00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Jayaraman S, Yang B, Matthay MA, Verkman AS. Role of aquaporin water channels in airway fluid transport, humidification and surface liquid hydration. J. Gen. Physiol. 2001;117:573–582. doi: 10.1085/jgp.117.6.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Verkman AS. Aquaporin-5 dependent fluid secretion in airway submucosal glands. J. Biol. Chem. 2001;276:41288–41292. doi: 10.1074/jbc.M107257200. [DOI] [PubMed] [Google Scholar]
- Song Y, Yang B, Matthay MA, Ma T, Verkman AS. Role of aquaporin water channels in pleural fluid dynamics. Am. J. Physiol. 2001;279:C1744–C1750. doi: 10.1152/ajpcell.2000.279.6.C1744. [DOI] [PubMed] [Google Scholar]
- Thiagarajah JR, Verkman AS. Aquaporin deletion in mice reduces corneal water permeability and delays restoration of transparency after swelling. J. Biol. Chem. 2002;277:19139–19144. doi: 10.1074/jbc.M202071200. [DOI] [PubMed] [Google Scholar]
- Towne JE, Harrod KS, Crane CM, Menon AG. Decreased expression of aquaporin (AQP)1 and AQP5 in mouse lung after acute viral infection. Am. J. Respir. Cell Mol. Biol. 2000;22:34–44. doi: 10.1165/ajrcmb.22.1.3818. [DOI] [PubMed] [Google Scholar]
- Towne JE, Krane CM, Bachurski CJ, Menon AG. Tumor necrosis factor-α inhibits aquaporin 5 expression in moue lung epithelial cells. J. Biol. Chem. 2001;276:18657–18664. doi: 10.1074/jbc.M100322200. [DOI] [PubMed] [Google Scholar]
- Umenishi F, Carter EP, Yang B, Oliver B, Matthay MA, Verkman AS. Sharp increase in rat lung water channel expression in the perinatal period. Am. J. Respir. Cell Mol. Biol. 1996;15:673–769. doi: 10.1165/ajrcmb.15.5.8918374. [DOI] [PubMed] [Google Scholar]
- Verkman AS. More than just water channels: unexpected cellular roles of aquaporins. J. Cell Sci. 2005;118:3225–3232. doi: 10.1242/jcs.02519. [DOI] [PubMed] [Google Scholar]
- Verkman AS. Roles of aquaporins in kidney revealed by transgenic mice. Semin. Nephrol. 2006;26:200–208. doi: 10.1016/j.semnephrol.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Verkman AS, Binder DK, Bloch O, Auguste K, Papadopoulos MC. Three distinct roles of aquaporin-4 in brain function revealed by knockout mice. Biochem. Biophys. Acta. 2006;1758:1085–1093. doi: 10.1016/j.bbamem.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Yang B, Fukuda N, van Hoek AN, Matthay MA, Ma T, Verkman AS. Carbon dioxide permeability of aquaporin-1 measured in erythrocytes and lung of aquaporin-1 null mice and in reconstituted proteoliposomes. J. Biol. Chem. 2000;275:2686–2692. doi: 10.1074/jbc.275.4.2686. [DOI] [PubMed] [Google Scholar]
- Yang F, Kawedia JD, Menon AG. Cyclic AMP regulates aquaporin 5 expression at both transcriptional and post-transcriptional levels through a protein kinase A pathway. J. Biol. Chem. 2003;278:32173–32180. doi: 10.1074/jbc.M305149200. [DOI] [PubMed] [Google Scholar]
- Yasui M, Serlachius E, Lofgren M, Belusa R, Nielsen S, Aperia A. Perinatal changes in expression of aquaporin-4 and other water and ion transporters in rat lung. J. Physiol. (Lond.) 1997;505:3–11. doi: 10.1111/j.1469-7793.1997.003bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]