Abstract
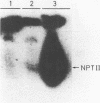
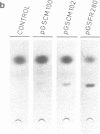
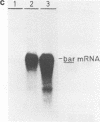
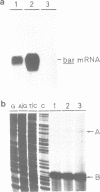
The plastid psbA promoter of tobacco was used with the aim to construct plastid specific marker genes. Upon transfer to the tobacco nuclear genome the plastid promoter fragment appeared to specify a messenger RNA. Placed 5' to the bar or nptII coding sequences the level of expression is sufficient to obtain a selectable phenotype. The transcription start site in the nucleus is site specific and is located 4 nucleotides downstream relative to the start site used in plastids. Translational fusions of the psbA coding sequence with the nptII and bar coding sequences revealed that the psbA leader sequence and the psbA translation start codon, being the second ATG codon, are recognized by the plant cytoplasmic translation apparatus. A promoter cassette utilisable in both E. coli and tobacco, was obtained by placing the CaMV 35S enhancer 5' to the psbA promoter.
Full text
PDF


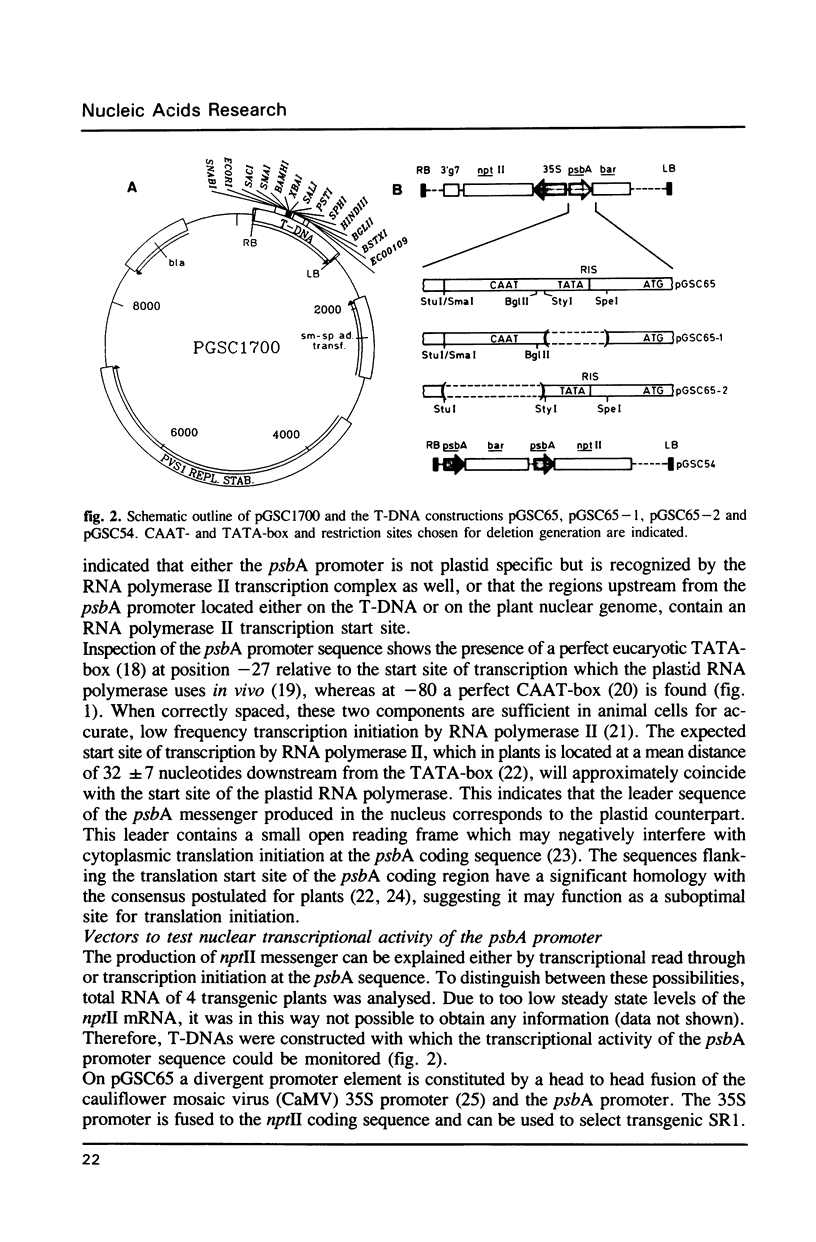
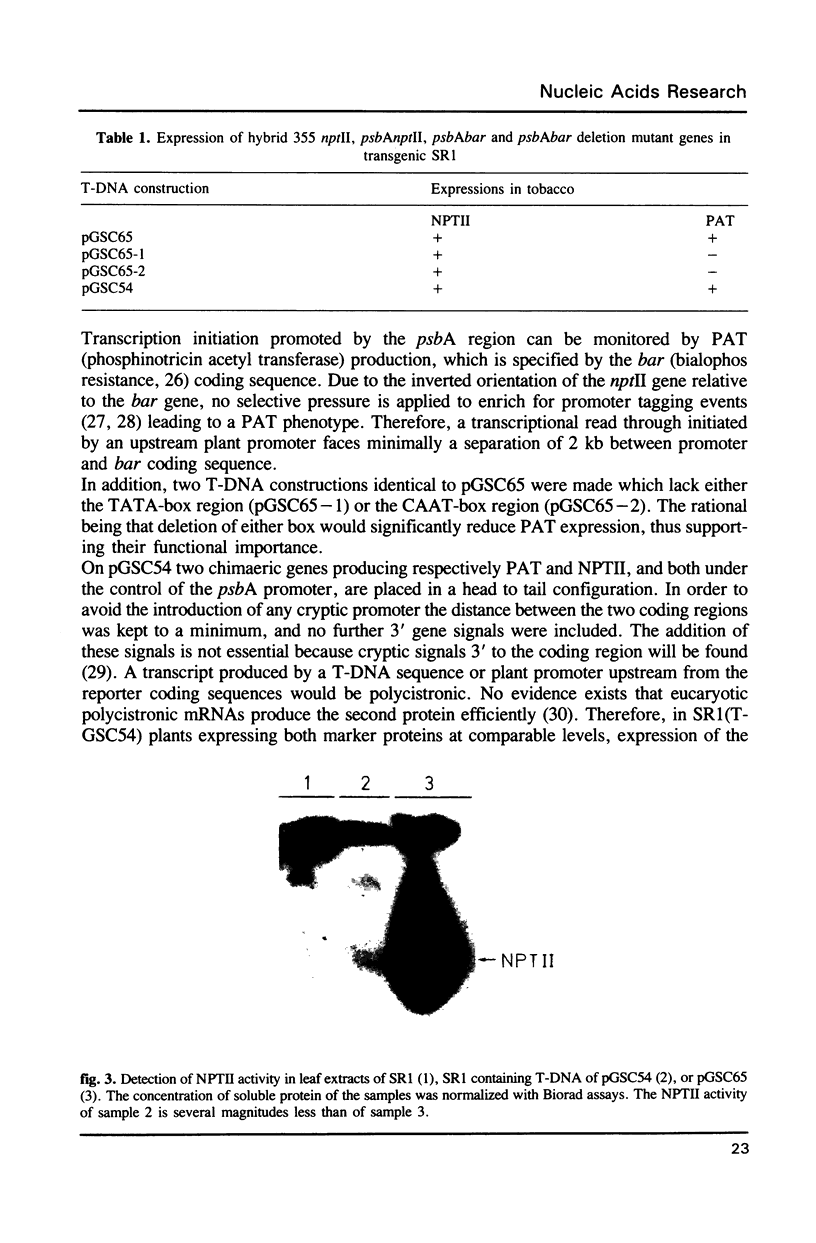
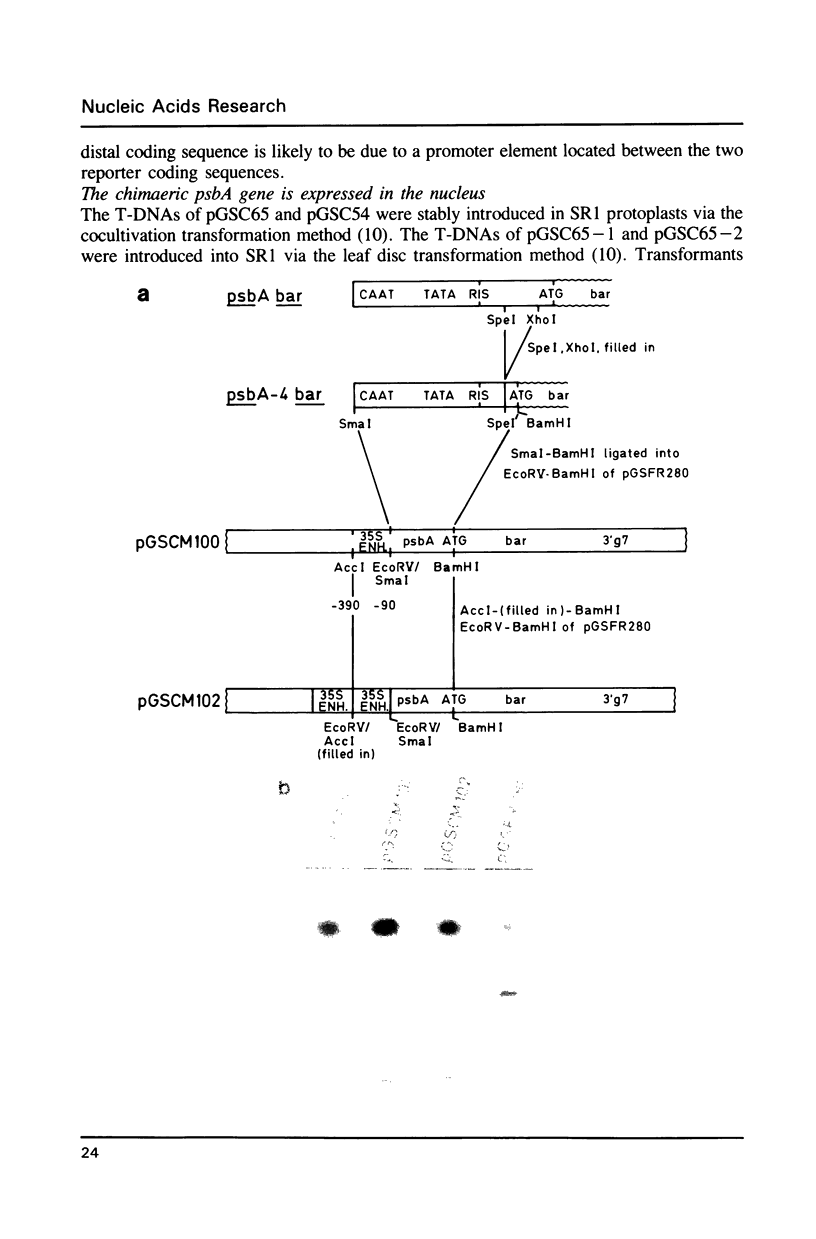





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benoist C., O'Hare K., Breathnach R., Chambon P. The ovalbumin gene-sequence of putative control regions. Nucleic Acids Res. 1980 Jan 11;8(1):127–142. doi: 10.1093/nar/8.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block M. D., Botterman J., Vandewiele M., Dockx J., Thoen C., Gosselé V., Movva N. R., Thompson C., Montagu M. V., Leemans J. Engineering herbicide resistance in plants by expression of a detoxifying enzyme. EMBO J. 1987 Sep;6(9):2513–2518. doi: 10.1002/j.1460-2075.1987.tb02537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deblaere R., Bytebier B., De Greve H., Deboeck F., Schell J., Van Montagu M., Leemans J. Efficient octopine Ti plasmid-derived vectors for Agrobacterium-mediated gene transfer to plants. Nucleic Acids Res. 1985 Jul 11;13(13):4777–4788. doi: 10.1093/nar/13.13.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrelly F., Butow R. A. Rearranged mitochondrial genes in the yeast nuclear genome. Nature. 1983 Jan 27;301(5898):296–301. doi: 10.1038/301296a0. [DOI] [PubMed] [Google Scholar]
- Jones J. D., Dunsmuir P., Bedbrook J. High level expression of introduced chimaeric genes in regenerated transformed plants. EMBO J. 1985 Oct;4(10):2411–2418. doi: 10.1002/j.1460-2075.1985.tb03949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. A., Yamamoto K. R., Tjian R. Two distinct transcription factors bind to the HSV thymidine kinase promoter in vitro. Cell. 1985 Sep;42(2):559–572. doi: 10.1016/0092-8674(85)90113-8. [DOI] [PubMed] [Google Scholar]
- Joshi C. P. An inspection of the domain between putative TATA box and translation start site in 79 plant genes. Nucleic Acids Res. 1987 Aug 25;15(16):6643–6653. doi: 10.1093/nar/15.16.6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman R. J., Murtha P., Davies M. V. Translational efficiency of polycistronic mRNAs and their utilization to express heterologous genes in mammalian cells. EMBO J. 1987 Jan;6(1):187–193. doi: 10.1002/j.1460-2075.1987.tb04737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay R., Chan A., Daly M., McPherson J. Duplication of CaMV 35S Promoter Sequences Creates a Strong Enhancer for Plant Genes. Science. 1987 Jun 5;236(4806):1299–1302. doi: 10.1126/science.236.4806.1299. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Lütcke H. A., Chow K. C., Mickel F. S., Moss K. A., Kern H. F., Scheele G. A. Selection of AUG initiation codons differs in plants and animals. EMBO J. 1987 Jan;6(1):43–48. doi: 10.1002/j.1460-2075.1987.tb04716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maliga P., Sz-Breznovits A., Márton L. Streptomycin-resistant plants from callus culture of haploid tobacco. Nat New Biol. 1973 Jul 4;244(131):29–30. doi: 10.1038/newbio244029a0. [DOI] [PubMed] [Google Scholar]
- Odell J. T., Nagy F., Chua N. H. Identification of DNA sequences required for activity of the cauliflower mosaic virus 35S promoter. 1985 Feb 28-Mar 6Nature. 313(6005):810–812. doi: 10.1038/313810a0. [DOI] [PubMed] [Google Scholar]
- Reiss B., Sprengel R., Will H., Schaller H. A new sensitive method for qualitative and quantitative assay of neomycin phosphotransferase in crude cell extracts. Gene. 1984 Oct;30(1-3):211–217. doi: 10.1016/0378-1119(84)90122-7. [DOI] [PubMed] [Google Scholar]
- Shinozaki K., Ohme M., Tanaka M., Wakasugi T., Hayashida N., Matsubayashi T., Zaita N., Chunwongse J., Obokata J., Yamaguchi-Shinozaki K. The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J. 1986 Sep;5(9):2043–2049. doi: 10.1002/j.1460-2075.1986.tb04464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D. B., Palmer J. D. Extensive and widespread homologies between mitochondrial DNA and chloroplast DNA in plants. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1946–1950. doi: 10.1073/pnas.81.7.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teeri T. H., Herrera-Estrella L., Depicker A., Van Montagu M., Palva E. T. Identification of plant promoters in situ by T-DNA-mediated transcriptional fusions to the npt-II gene. EMBO J. 1986 Aug;5(8):1755–1760. doi: 10.1002/j.1460-2075.1986.tb04423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Broeck G., Timko M. P., Kausch A. P., Cashmore A. R., Van Montagu M., Herrera-Estrella L. Targeting of a foreign protein to chloroplasts by fusion to the transit peptide from the small subunit of ribulose 1,5-bisphosphate carboxylase. 1985 Jan 31-Feb 6Nature. 313(6001):358–363. doi: 10.1038/313358a0. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]