Summary
Background and objectives
Fibroblast growth factor 23 is a phosphate- and vitamin D–regulating hormone. The objective of this study was to determine the effect of ergocalciferol administration on fibroblast growth factor 23 levels in healthy vitamin D–deficient subjects.
Design, setting, participants, & measurements
In this 12-week trial conducted in a clinical research center, 18- to 45-year-old subjects (n=90) with 25-hydroxyvitamin D levels ≤20 ng/ml (by chemiluminescent immunoassay) were randomized to weekly ergocalciferol treatment of 50,000 international units or placebo, while consuming a self-selected diet. Changes in fibroblast growth factor 23, 25-hydroxyvitamin D (by liquid chromatography/tandem mass spectroscopy), 1,25-dihydroxyvitamin D, parathyroid hormone, and serum phosphate were measured.
Results
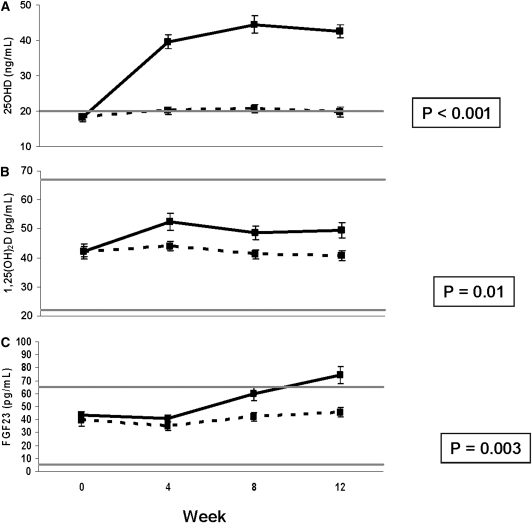
Mean 25-hydroxyvitamin D (P<0.0001), 1,25-dihydroxyvitamin D (P=0.01), and fibroblast growth factor 23 (P=0.003) increased in the treatment versus placebo group. In the treatment group, 25-hydroxyvitamin D increased from 18±7 to 40±12 ng/ml at week 4 (P<0.0001) and remained stable at 43±12 ng/ml at week 12 (P<0.0001); 1,25-dihydroxyvitamin D increased from 42±17 to 52±18 pg/ml at week 4 (P<0.001) and then remained stable, and fibroblast growth factor 23 increased from 43±17 to 60±33 pg/ml at week 8 (P=0.001) and 74±42 pg/ml at week 12 (P<0.0001). Urinary phosphate excretion increased within the treatment group, but parathyroid hormone and serum phosphate were unchanged.
Conclusions
Ergocalciferol administration increases circulating fibroblast growth factor 23. When measuring fibroblast growth factor 23, concurrent 25-hydroxyvitamin D measurements should be obtained, because vitamin D deficiency may lower circulating fibroblast growth factor 23 levels.
Introduction
Fibroblast growth factor 23 (FGF23) is a phosphate- and vitamin D–regulating hormone (1–6). FGF23 levels are elevated in patients with CKD and are independently associated with increased mortality in patients receiving hemodialysis (7,8). Additionally, elevated FGF23 levels are associated with increased cardiovascular mortality in individuals with stable coronary artery disease but normal renal function (9–11). Much is still unknown, however, about the physiologic regulation of FGF23.
1,25-Dihydroxyvitamin D [1,25(OH)2D], dietary phosphate, and circulating phosphate are key regulators of FGF23 (12–18). However, it is unknown if vitamin D (either ergocalciferol or cholecalciferol) affects FGF23 secretion. Given the increasingly common administration of vitamin D to people with normal renal function and people with CKD (19,20) and the association between FGF23 and mortality (7–9), knowledge of the effects of vitamin D on FGF23 may inform both clinical and investigative pursuits. We, thus, tested the hypothesis that weekly high-dose ergocalciferol would increase circulating FGF23 levels in subjects with normal renal function and low 25-hydroxyvitamin D (25OHD) levels.
Materials and Methods
Study Subjects
We recruited healthy, vitamin D–deficient volunteers through advertisements and mass mailings. To be eligible, subjects had to be 18–45 years old with a serum 25OHD level ≤20 ng/ml by chemiluminescent immunoassay (CLIA; CLIA was the available 25OHD assay at our institution at that time) (20). Subjects with disorders or using medications known to affect phosphate or vitamin D metabolism were excluded, as well as subjects with histories of nephrolithiasis, diabetes mellitus, malabsorption, recent ethanol abuse, or clinically significant disease. Subjects were required to have normal kidney, liver, and thyroid function; males had normal testosterone levels. Eligible females had regular menses (oral contraceptive use was allowed). Subjects self-identified as non-Hispanic or Hispanic and Asian, black/African-American, white/Caucasian, multiple races, or other. The Human Research Committee of Partners Health Care System approved the study, and subjects provided written informed consent.
Of the 651 subjects screened by telephone, 419 subjects had screening blood tests, and 136 of those tests had 25OHD levels ≤20 ng/ml; 130 subjects met the remaining inclusion criteria. Of these subjects, 92 subjects enrolled in the study, and 90 subjects completed the 12-week protocol. The season of recruitment was classified as winter (January, February, and March), spring (April, May, and June), summer (July, August, and September), or fall (October, November, and December).
Study Protocol
Subjects were randomized to receive either ergocalciferol at 50,000 international units (VIT-D) or matching placebo (PBO) weekly for 12 weeks. We used ergocalciferol, because it is available as a prescription in the United States. Randomization was stratified by sex and severity of vitamin D deficiency (25OHD ≤10 versus >10 ng/ml by CLIA). Dietary calcium and vitamin D intakes were assessed at baseline and week 12. Daily calcium intake was maintained at 1000–1500 mg in both groups through diet and/or supplements. At study completion, PBO group subjects were treated with ergocalciferol at 50,000 international units daily for 7 days.
Fasting blood and urine samples were collected between 7:00 and 9:00 AM at weeks 0, 4, 8, and 12. Fractional excretion of phosphate [FePO4; (urine PO4×serum creatinine)/(serum PO4×urine creatinine)×100] and creatinine clearance were calculated (21–23). Compliance was assessed by review of diaries and returned medication counts. Subjects were monitored for hypo- or hypercalcemia at each visit and withdrawn if necessary.
Laboratory Methods
Testing was performed on previously unthawed samples that were stored at −80°C. 25OHD was measured by CLIA (Diasorin, Stillwater, MN) with sensitivity of 2 ng/ml and intra- and interassay coefficients of variation (CVs) of 6%–8% and 12%–16%, respectively, and as prespecified in our study design by the gold-standard liquid chromatography/tandem mass spectroscopy (LC/MS/MS) with sensitivity of 6 ng/ml and interassay CV of 6%–9%. Serum 1,25(OH)2D was measured using a radioimmunoassay (Diasorin, Stillwater, MN) with intra- and interassay CVs of 7%–11% and 11%–15%, respectively. Serum FGF23 was measured using an immunometric assay (Kainos, Tokyo, Japan) with sensitivity of 3 pg/ml and intra- and interassay CVs of ≤3% and ≤4%, respectively. Serum and urine phosphate were measured using a colorimetric method (Roche Diagnostics, Indianapolis, IN) with intra- and interassay CVs of <2% and <4%, respectively. Parathyroid hormone (PTH) was measured using a two-site immunoradiometric assay (Nichols Institute Diagnostics, San Clemente, CA) with sensitivity of 1 pg/ml and intra- and interassay CVs of 2%–3% and 6%, respectively.
25OHD: CLIA Versus LC/MS/MS
At weeks 4, 8, and 12, 25OHD was measured with both methodologies. The regression line for the assays differed based on group assignment (Supplemental Figure 1). Thus, it was not possible to create a linear regression equation defining the relationship between the two assays. Supplemental Figure 2 shows the 25OHD measurements from both assays. Unless otherwise specified, the reported 25OHD levels were measured using LC/MS/MS. Supplemental Figure 3 shows on-study 25OHD2 and 25OHD3 levels.
Statistical Analyses
The primary endpoint was a comparison of the change in circulating FGF23, which is expressed as the area under the curve between the VIT-D and PBO groups. Secondary endpoints included the change in 25OHD, 1,25(OH)2D, serum phosphate, calcium, PTH, and FePO4 between the groups.
The endpoints were assessed using a mixed model ANOVA. Within-group changes in the endpoints (compared with baseline) were assessed by repeated measures ANOVA using Dunnett's test to adjust for multiple comparisons. Baseline characteristics were compared using t, Wilcoxon rank sum, chi-squared, or Fisher exact tests as appropriate. Multivariate linear regression models were used to identify which variables were independent predictors of the 12-week change in FGF23 from baseline to week 12 in the VIT-D group. The predictor variables in the models included the change from baseline to week 12 in 1,25(OH)2D, 25OHD, serum phosphate, and PTH, as well as sex and race. As a posthoc analysis, we also assessed the relationship between the 12-week absolute change in 25OHD and the 12-week absolute change in 1,25(OH)2D in the treatment group. Data are expressed as mean ± SD unless specified otherwise. Comparisons were performed by two-sided tests, and resulting P values <0.05 are considered statistically significant. SAS V9.2 was used for these analyses.
Results
Subject Characteristics
Baseline characteristics are described in Table 1. Dietary vitamin D intake was somewhat higher in the treatment than placebo group. However, the median daily intake of vitamin D was lower than the recommended daily allowance (24); less than 10% of the cohort consumed a daily multivitamin. Otherwise, there were no between-group differences. The cohort was racially diverse, and subjects were recruited equally across the four seasons.
Table 1.
Baseline characteristics
| Clinical or Biochemical Endpoints | VIT-D (n=40) | PBO (n=50) | P Value |
|---|---|---|---|
| Age (years)a | 26 (23, 32) | 27 (22, 39) | 0.82 |
| Sex (number) | 0.85 | ||
| male | 16 | 19 | |
| female | 24 | 31 | |
| Season enrolled (number) | 0.80 | ||
| fall | 15 | 14 | |
| spring | 8 | 12 | |
| summer | 10 | 15 | |
| winter | 7 | 9 | |
| Ethnicity (number) | 0.29 | ||
| non-Hispanic | 38 | 44 | |
| Hispanic | 2 | 6 | |
| Race (number) | 0.15 | ||
| white/Caucasian | 18 | 20 | |
| black/African-American | 8 | 18 | |
| Asian | 11 | 6 | |
| multiple races/other | 3 | 6 | |
| BMI (kg/m2) | 25±4 | 26±7 | 0.46 |
| MVI use | 0.46 | ||
| takes MVI | 5 | 3 | |
| does not take MVI | 35 | 47 | |
| Total VIT-D intake (international units)a | 150 (75, 231) | 65 (31, 130) | 0.01 |
| from fooda | 100 (71, 200) | 64 (31, 117) | 0.01 |
| from supplementsa | 0 (0, 43) | 0 (0, 0) | 0.06 |
| Total calcium intake (mg) | 1162±651 | 939±523 | 0.08 |
| 25OHD (ng/ml) by CLIAb | 13±3 | 15±4 | 0.54 |
| 25OHD (ng/ml) by LC/MS/MSc | 18±7 | 18±7 | 0.83 |
| 25OHD levels by LC/MS/MS | 0.17 | ||
| 25OHD ≤20 ng/mlc (n) | 25 | 35 | |
| 25OHD >20 to ≤30 ng/mlc (n) | 15 | 12 | |
| 25OHD >30 ng/mlc (n) | 0 | 3 | |
| FGF23 (pg/ml) | 43±17 | 39±29 | 0.38 |
| PTH (pg/ml) | 42±15 | 42±16 | 0.76 |
| 1,25(OH)2D (pg/ml) | 42±17 | 42±13 | 0.97 |
| Calcium (mg/dl) | 9.1±0.4 | 9.0±0.4 | 0.35 |
| Phosphate (mg/dl) | 3.3±0.5 | 3.3±0.4 | 0.88 |
| Creatinine (mg/dl) | 0.9±0.1 | 0.9±0.2 | 0.28 |
| Creatinine clearance (ml/min) | 116±29 | 112±37 | 0.53 |
| Creatinine clearance (ml/min per 1.73m2) | 97±17 | 95±17 | 0.60 |
| FePO4 (%) | 11±5 | 13±6 | 0.10 |
Values are presented as mean ± SD unless otherwise indicated. VIT-D, vitamin D; PBO, placebo; BMI, body mass index; MVI, multivitamin; 25OHD, 25-hydroxyvitamin D; CLIA, chemiluminescent immunoassay; LC/MS/MS, liquid chromatography/tandem mass spectroscopy; FGF23, fibroblast growth factor 23; PTH, parathyroid hormone; 1,25(OH)2D, 1,25-dihydroxyvitamin D; FePO4, fractional excretion of phosphate. Systeme International conversion factors: 25OHD (nM), 2.496; PTH (ng/L), 1; 1,25(OH)2D (pmol/L), 2.6; calcium (mM), 0.2495; phosphate (mM), 0.3229; and creatinine (mM), 88.4.
Age and vitamin D intake are presented as median (25th percentile, 75th percentile).
The 25OHD by CLIA was performed at screening.
The 25OHD by LC/MS/MS, similar to the other biochemical data, was performed at week 0 or baseline.
At enrollment, all subjects had 25OHD levels measured by CLIA that were ≤20 ng/ml. At visit 1 (week 0), however, 25OHD levels measured by LC/MS/MS were ≤20 ng/ml in 60 subjects, >20 to ≤30 ng/ml in 27 subjects, and >30 ng/ml in 3 subjects. At visit 1 (week 0), we observed a negative association between 25OHD and PTH levels (R=−0.28, P=0.006); 11 subjects had PTH levels >60 pg/ml.
Subject Withdrawal and Compliance
One subject was withdrawn after the baseline visit because of medication noncompliance, and one subject was withdrawn because of pregnancy (their data were not included). No subject developed hypercalcemia; one subject receiving placebo had asymptomatic hypocalcemia at week 12. Five subjects (two subjects receiving VIT-D and three subjects receiving PBO) took 85% of the study medication; the remaining subjects were 100% compliant. Dietary vitamin D did not increase during the study or differ between groups (data not shown).
FGF23, 25OHD, and 1,25(OH)2D
Figure 1 shows FGF23, 25OHD, and 1,25(OH)2D at week 0 and weeks 4, 8, and 12 after ergocalciferol administration. FGF23 (P=0.003), 25OHD (P<0.0001), and 1,25(OH)2D (P=0.01) increased more in the subjects treated with ergocalciferol than in the subjects in the PBO group. In the VIT-D group, 25OHD increased from 18±7 ng/ml at baseline to 40±12 ng/ml at week 4 (P<0.0001), 45±15 ng/ml at week 8 (P<0.0001), and 43±12 ng/ml at week 12 (P<0.0001) (Figure 1A); 1,25(OH)2D increased from 42±17 pg/ml at baseline to 52±18 pg/ml at week 4 (P<0.001), 49±15 pg/ml at week 8 (P=0.03), and 49±17 pg/ml at week 12 (P=0.01) (Figure 1B), and FGF23 increased from 43±17 pg/ml at baseline to 60±33 pg/ml at week 8 (P=0.001) and 74±42 pg/ml at week 12 (P<0.0001) (Figure 1C). In the PBO group, there was no significant change in 25OHD, 1,25(OH)2D, or FGF23.
Figure 1.
Changes in 25-hydroxyvitamin D (25OHD), 1,25-dihydroxyvitamin D [1,25(OH)2D], and fibroblast growth factor 23 (FGF23) with ergocalciferol administration. Mean (±SEM) of (A) 25OHD measured by liquid chromatography/tandem mass spectroscopy (the horizontal line represents the lower limit of normal), (B) 1,25(OH)2D (the horizontal lines represent the normal range), and (C) FGF23 (the horizontal lines represent the normal range based on our prior data) at weeks 0, 4, 8, and 12 in the vitamin D (solid line) and placebo (dashed line) groups. P value assesses change over time by repeated-measures ANOVA compared with placebo. Systeme International conversion factors are 25OHD (nM), 2.496; and 1,25(OH)2D (pmol/L), 2.6.
As a posthoc analysis, we assessed the relationship between the 12-week absolute change in 25OHD and the 12-week absolute change in 1,25(OH)2D in the treatment group. There was a trend for a positive relationship with R=0.308 (P=0.05). The equation describing this relationship was 12-week change in 1,25(OH)2D=0.4022×(12-week change in 25OHD)−2.6776.
FePO4, Serum Phosphate, PTH, and Calcium
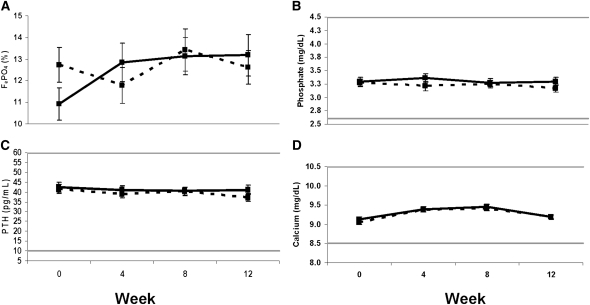
Figure 2 shows FePO4, serum phosphate, PTH, and calcium at week 0 and weeks 4, 8, and 12 after ergocalciferol administration. There were no between-group differences in these parameters; however, FePO4 increased from 11%±5% at baseline to 13%±6% at week 12 in the subjects treated with VIT-D (P=0.05).
Figure 2.
Changes in fractional excretion of phosphate (FePO4), phosphate, parathyroid hormone (PTH), and calcium with ergocalciferol administration. Mean (±SEM) of (A) FePO4, (B) serum phosphate, (C) PTH, and (D) serum calcium at weeks 0, 4, 8, and 12 in the vitamin D (solid line) and placebo (dashed line) groups. The horizontal lines represent the normal range for the variables. None of the changes over time by repeated measures ANOVA compared with placebo were statistically significant. Systeme International conversion factors are serum phosphate (mM), 0.2495; PTH (ng/L), 1; and serum calcium (mM), 0.3229.
Multivariate Analyses
To differentiate the effects of 25OHD from 1,25(OH)2D on FGF23, we created four multivariate regression models with 12-week change in FGF23 as the response variable in the VIT-D–treated group. The predictor variables were 12-week change in 1,25(OH)2D, 25OHD, serum phosphate, and PTH, as well as sex and race (in the largest model) and 12-week change in 1,25(OH)2D and 25OHD (in the most parsimonious model). The 12-week change in FGF23 was not independently predicted by any variable in any of the models.
Discussion
In this study, we have shown that administration of weekly high-dose ergocalciferol for 12 weeks increases circulating FGF23 levels in a cohort of otherwise healthy subjects with low 25OHD levels. The observed increase in FGF23 levels occurred in the context of significant increases in circulating 25OHD and 1,25(OH)2D levels. Although FePO4 increased within the VIT-D group, there were no between-group differences in FePO4, serum phosphate, PTH, or calcium levels. Prior studies have shown that 1,25(OH)2D increases FGF23 synthesis and its circulating levels (13,14,25). This study is the first to show that ergocalciferol administration is associated with increased FGF23 production; however, in this model, we were unable to determine if the increase was because of ergocalciferol, 25OHD, or 1,25(OH)2D.
We have previously shown that dietary phosphate loading stimulates FGF23 production in normal volunteers (16). Additionally, we and others have shown that dietary phosphate deprivation suppresses FGF23 production (12,16,17). In this study, within the treatment group, FGF23 levels and FePO4 increased, but serum phosphate and PTH levels were unchanged. The observed increase in FePO4 is likely a direct result of the observed increase in circulating FGF23. This increase in FePO4 is likely an adaptive response to counteract VIT-D–mediated dietary phosphate absorption and thus, maintain serum phosphate levels at baseline. Because of the duration of the study, subjects consumed an ad libitum diet, which may explain our inability to show between-group changes in urinary or serum phosphate indices; additionally, our sampling schedule may have missed early and transient increases in serum phosphate in the VIT-D group. If we had obtained daily measurements during the first 4 weeks of the study or if we had obtained postprandial measurements, we may have observed changes in serum phosphate levels in the treatment group. At baseline, we observed the anticipated negative association between 25OHD and PTH levels; however, PTH levels were stable with vitamin D administration. This finding may be because of our cohort being healthier and having milder vitamin D deficiency (only 11 subjects had PTH >60 pg/ml). Alternately, our results may be because of study duration or choice of vitamin D compound (26,27). Given that our primary endpoint was the change in FGF23 with vitamin D administration and data that PTH may stimulate FGF23 (28,29) (conversely declining PTH levels may suppress FGF23 levels), the stable PTH levels in this experimental model simplify interpretation of our data. The observed changes in FGF23 are not confounded by concurrent and sustained changes in circulating PTH levels.
FGF23 and vitamin D display classic endocrine feedback signaling, where 1,25(OH)2D stimulates FGF23 and FGF23 suppresses the production of 1,25(OH)2D (13,14,18,30–32). Consistent with that physiology, we first noted increases in circulating 25OHD and 1,25(OH)2D levels and then, increases in FGF23. In contrast to the relationship between 1,25(OH)2D and FGF23, the relationship between vitamin D and FGF23 has not been well-characterized. Our study shows that the administration of pharmacologically dosed ergocalciferol is associated with increased FGF23 production. Our findings are in contrast to a recently reported study where FGF23 decreased with vitamin D repletion (33). There are, however, differences in the two studies that make comparisons challenging. Namely, we recruited 90 healthy, community-based subjects who were randomized to a placebo-controlled study, whereas the 18 subjects in the other study (33) were hospitalized and their postvitamin D repletion FGF23 levels were compared with baseline. Additionally, the studies differed in baseline 25OHD levels (18 versus 8 ng/ml), end of study 25OHD levels (43 versus 15 ng/ml), study duration (12 versus 6 weeks), vitamin D preparation (ergocalciferol versus cholecalciferol), cumulative vitamin D dose (600,000 versus 186,960 international units), and FGF23 assay (intact versus C-terminal assay). Although the differing effects of vitamin D on FGF23 in these studies may reflect differences in circulating intact FGF23 versus C-terminal fragments, alternate explanations may be differences in assay performance (16) or differences in FGF23 when measured during an acute illness versus the healthy state (10).
In a posthoc analysis, the observed increase in 1,25(OH)2D was positively associated with the increase in 25OHD in the VIT-D group. In prior studies of vitamin D repletion, there is lack of consensus regarding the effect of vitamin D administration on circulating 1,25(OH)2D levels, with some studies showing an increase in 1,25(OH)2D with vitamin D administration (34–36) and others not showing this outcome (37–44). Comparisons between those reports and our findings are made difficult by differences in study design that include the ages of the subjects, type and dose of vitamin D administered, duration of vitamin D administration, baseline 25OHD levels, and assays used. Given prior studies (13,14,18,25,32), the observed increase in FGF23 is likely caused by the observed increase in 1,25(OH)2D, although a direct effect of ergocalciferol, 25OHD, or increased dietary phosphate absorption because of increased 1,25(OH)2D cannot be excluded. Vitamin D deficiency is widespread and typically treated with oral ergocalciferol or cholecalciferol (but not calcitriol). Our data show that routine management of vitamin D deficiency may affect FGF23 levels. This observation suggests that, when FGF23 assays are approved for clinical use (similar to PTH), the measured FGF23 level will need to be interpreted in light of that patient’s concurrent 25OHD level. Whereas vitamin D deficiency may elevate PTH (20), these data suggest that vitamin D deficiency may lower circulating FGF23.
Limitations of this study deserve mention. Some reports suggest that ergocalciferol does not replete 25OHD levels as effectively as cholecalciferol (45–47), but other reports contradict that perspective (48,49). Because of assay availability at our institution at the time of subject enrollment, subjects were enrolled based on a 25OHD level as measured by CLIA; however, as part of our prespecified study design, we assessed the change in 25OHD with ergocalciferol administration using 25OHD measured by LC/MS/MS. Notably, there were differences in the data obtained from LC/MS/MS 25OHD measurement compared with CLIA, wherein the 25OHD levels were higher with the LC/MS/MS measure. These differences are likely caused by differences in assay performance, which have been previously reported (50). Notably, the majority of subjects (60/90) still had 25OHD levels ≤20 ng/ml as measured by LC/MS/MS; 27 of 90 subjects had 25OHD levels >20 and ≤30 ng/ml by LC/MS/MS. We did not assess other important factors in vitamin D metabolism such as 25OHD-24-hydroxylase activity or vitamin D binding protein levels; however, randomization should have balanced potential differences. Subjects consumed an ad libitum diet. Although we assessed calcium and vitamin D intake over the course of the study, which were both stable, we did not assess or control for dietary phosphate intake. Finally, given our sample size (n=40 for the multivariate model) and study design, we are unable to differentiate the effects of ergocalciferol, 25OHD, or 1,25(OH)2D on the associated increase in FGF23.
In summary, we have shown that, in subjects with low vitamin D levels, treatment with weekly high-dose ergocalciferol increases circulating 25OHD and 1,25(OH)2D levels and is associated with increased FGF23 production. Increasing numbers of patients, with both normal renal function and CKD, are being screened for vitamin D deficiency and repleted with oral vitamin D. Additional studies are needed to determine the short- and long-term clinical effects of increased FGF23 stimulation in health and CKD.
Disclosures
None.
Acknowledgments
We are grateful to the staff of the Harvard Clinical and Translational Science Center for the implementation of the study protocol. We thank Henry Kronenberg for thoughtful comments on this manuscript.
We are indebted to our funding sources: National Institutes of Health Grant K23-DK-073356 (to S.M.B.), Harvard Clinical and Translational Science Center from the National Center for Research Resources Grant M01-RR-01066, Massachusetts General Hospital Physician-Scientist Development Award (to S.M.B.), Boston Area Diabetes and Endocrinology Research Center Grant (to S.M.B.), and the Harvard Medical School Office for Diversity and Community Partnership Award (to S.M.B.).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.10030911/-/DCSupplemental.
References
- 1.ADHR Consortium : Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet 26: 345–348, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Jonsson KB, Zahradnik R, Larsson T, White KE, Sugimoto T, Imanishi Y, Yamamoto T, Hampson G, Koshiyama H, Ljunggren O, Oba K, Yang IM, Miyauchi A, Econs MJ, Lavigne J, Jüppner H: Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med 348: 1656–1663, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Riminucci M, Collins MT, Fedarko NS, Cherman N, Corsi A, White KE, Waguespack S, Gupta A, Hannon T, Econs MJ, Bianco P, Gehron Robey P: FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J Clin Invest 112: 683–692, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Araya K, Fukumoto S, Backenroth R, Takeuchi Y, Nakayama K, Ito N, Yoshii N, Yamazaki Y, Yamashita T, Silver J, Igarashi T, Fujita T: A novel mutation in fibroblast growth factor 23 gene as a cause of tumoral calcinosis. J Clin Endocrinol Metab 90: 5523–5527, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, Rios H, Drezner MK, Quarles LD, Bonewald LF, White KE: Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet 38: 1310–1315, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imel EA, Hui SL, Econs MJ: FGF23 concentrations vary with disease status in autosomal dominant hypophosphatemic rickets. J Bone Miner Res 22: 520–526, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Gutiérrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Jüppner H, Wolf M: Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 359: 584–592, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jüppner H, Wolf M, Salusky IB: FGF-23: More than a regulator of renal phosphate handling? J Bone Miner Res 25: 2091–2097, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parker BD, Schurgers LJ, Brandenburg VM, Christenson RH, Vermeer C, Ketteler M, Shlipak MG, Whooley MA, Ix JH: The associations of fibroblast growth factor 23 and uncarboxylated matrix Gla protein with mortality in coronary artery disease: The Heart and Soul Study. Ann Intern Med 152: 640–648, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gutiérrez OM, Wolf M, Taylor EN: Fibroblast growth factor 23, cardiovascular disease risk factors, and phosphorus intake in the health professionals follow-up study. Clin J Am Soc Nephrol 6: 2871–2878, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desjardins L, Liabeuf S, Renard C, Lenglet A, Lemke HD, Choukroun G, Drueke T, Massy Z, on behalf of the European Uremic Toxin (EUTox) Work Group : FGF23 is independently associated with vascular calcification but not bone mineral density in patients at various CKD stages [published online ahead of print November 23, 2011]. Osteoporos Int doi:10.1007/s00198-011-1838-0 [DOI] [PubMed] [Google Scholar]
- 12.Ferrari SL, Bonjour JP, Rizzoli R: Fibroblast growth factor-23 relationship to dietary phosphate and renal phosphate handling in healthy young men. J Clin Endocrinol Metab 90: 1519–1524, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Saito H, Maeda A, Ohtomo S, Hirata M, Kusano K, Kato S, Ogata E, Segawa H, Miyamoto K, Fukushima N: Circulating FGF-23 is regulated by 1alpha,25-dihydroxyvitamin D3 and phosphorus in vivo. J Biol Chem 280: 2543–2549, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Collins MT, Lindsay JR, Jain A, Kelly MH, Cutler CM, Weinstein LS, Liu J, Fedarko NS, Winer KK: Fibroblast growth factor-23 is regulated by 1alpha,25-dihydroxyvitamin D. J Bone Miner Res 20: 1944–1950, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Perwad F, Azam N, Zhang MY, Yamashita T, Tenenhouse HS, Portale AA: Dietary and serum phosphorus regulate fibroblast growth factor 23 expression and 1,25-dihydroxyvitamin D metabolism in mice. Endocrinology 146: 5358–5364, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Burnett SM, Gunawardene SC, Bringhurst FR, Jüppner H, Lee H, Finkelstein JS: Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J Bone Miner Res 21: 1187–1196, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Antoniucci DM, Yamashita T, Portale AA: Dietary phosphorus regulates serum fibroblast growth factor-23 concentrations in healthy men. J Clin Endocrinol Metab 91: 3144–3149, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Liu S, Tang W, Zhou J, Stubbs JR, Luo Q, Pi M, Quarles LD: Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol 17: 1305–1315, 2006 [DOI] [PubMed] [Google Scholar]
- 19.National Kidney Foundation : K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42[Suppl 3]: S1–S201, 2003 [PubMed] [Google Scholar]
- 20.Holick MF: Vitamin D deficiency. N Engl J Med 357: 266–281, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Cockcroft DW, Gault MH: Prediction of creatinine clearance from serum creatinine. Nephron 16: 31–41, 1976 [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Bringhurst FR, Leder BZ: Regulation of calcium and phosphate homeostasis. In: Endocrinology, 5th Ed., edited by DeGroot LJ, Jameson JL, Philadelphia, WB Saunders, 2006, p 1475 [Google Scholar]
- 24.National Academy of Sciences : National Academy of Sciences Web-Based Press Release. Institute of Medicine of the National Academies: Dietary Reference Intakes for Calcium and Vitamin D, Washington, DC, National Academy of Sciences, 2010 [Google Scholar]
- 25.Imel EA, DiMeglio LA, Hui SL, Carpenter TO, Econs MJ: Treatment of X-linked hypophosphatemia with calcitriol and phosphate increases circulating fibroblast growth factor 23 concentrations. J Clin Endocrinol Metab 95: 1846–1850, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romagnoli E, Mascia ML, Cipriani C, Fassino V, Mazzei F, D’Erasmo E, Carnevale V, Scillitani A, Minisola S: Short and long-term variations in serum calciotropic hormones after a single very large dose of ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3) in the elderly. J Clin Endocrinol Metab 93: 3015–3020, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Viljakainen HT, Väisänen M, Kemi V, Rikkonen T, Kröger H, Laitinen EK, Rita H, Lamberg-Allardt C: Wintertime vitamin D supplementation inhibits seasonal variation of calcitropic hormones and maintains bone turnover in healthy men. J Bone Miner Res 24: 346–352, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Kawata T, Imanishi Y, Kobayashi K, Miki T, Arnold A, Inaba M, Nishizawa Y: Parathyroid hormone regulates fibroblast growth factor-23 in a mouse model of primary hyperparathyroidism. J Am Soc Nephrol 18: 2683–2688, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi K, Imanishi Y, Miyauchi A, Onoda N, Kawata T, Tahara H, Goto H, Miki T, Ishimura E, Sugimoto T, Ishikawa T, Inaba M, Nishizawa Y: Regulation of plasma fibroblast growth factor 23 by calcium in primary hyperparathyroidism. Eur J Endocrinol 154: 93–99, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Saito H, Kusano K, Kinosaki M, Ito H, Hirata M, Segawa H, Miyamoto K, Fukushima N: Human fibroblast growth factor-23 mutants suppress Na+-dependent phosphate co-transport activity and 1alpha,25-dihydroxyvitamin D3 production. J Biol Chem 278: 2206–2211, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T: FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res 19: 429–435, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Ito M, Sakai Y, Furumoto M, Segawa H, Haito S, Yamanaka S, Nakamura R, Kuwahata M, Miyamoto K: Vitamin D and phosphate regulate fibroblast growth factor-23 in K-562 cells. Am J Physiol Endocrinol Metab 288: E1101–E1109, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Uzum AK, Salman S, Telci A, Boztepe H, Tanakol R, Alagol F, Ozbey NC: Effects of vitamin D replacement therapy on serum FGF23 concentrations in vitamin D-deficient women in short term. Eur J Endocrinol 163: 825–831, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Lips P, Wiersinga A, van Ginkel FC, Jongen MJM, Netelenbos JC, Hackeng WHL, Delmas PD, van der Vijgh WJF: The effect of vitamin D supplementation on vitamin D status and parathyroid function in elderly subjects. J Clin Endocrinol Metab 67: 644–650, 1988 [DOI] [PubMed] [Google Scholar]
- 35.Pfeifer M, Begerow B, Minne HW, Nachtigall D, Hansen C: Effects of a short-term vitamin D(3) and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J Clin Endocrinol Metab 86: 1633–1637, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Bischoff HA, Stähelin HB, Dick W, Akos R, Knecht M, Salis C, Nebiker M, Theiler R, Pfeifer M, Begerow B, Lew RA, Conzelmann M: Effects of vitamin D and calcium supplementation on falls: A randomized controlled trial. J Bone Miner Res 18: 343–351, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Himmelstein S, Clemens TL, Rubin A, Lindsay R: Vitamin D supplementation in elderly nursing home residents increases 25(OH)D but not 1,25(OH)2D. Am J Clin Nutr 52: 701–706, 1990 [DOI] [PubMed] [Google Scholar]
- 38.Heaney RP, Barger-Lux MJ, Dowell MS, Chen TC, Holick MF: Calcium absorptive effects of vitamin D and its major metabolites. J Clin Endocrinol Metab 82: 4111–4116, 1997 [DOI] [PubMed] [Google Scholar]
- 39.Barger-Lux MJ, Heaney RP, Dowell S, Chen TC, Holick MF: Vitamin D and its major metabolites: Serum levels after graded oral dosing in healthy men. Osteoporos Int 8: 222–230, 1998 [DOI] [PubMed] [Google Scholar]
- 40.Heikkinen A, Parviainen MT, Tuppurainen MT, Niskanen L, Komulainen MH, Saarikoski S: Effects of postmenopausal hormone replacement therapy with and without vitamin D3 on circulating levels of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D. Calcif Tissue Int 62: 26–30, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Kyriakidou-Himonas M, Aloia JF, Yeh JK: Vitamin D supplementation in postmenopausal black women. J Clin Endocrinol Metab 84: 3988–3990, 1999 [DOI] [PubMed] [Google Scholar]
- 42.Meier C, Woitge HW, Witte K, Lemmer B, Seibel MJ: Supplementation with oral vitamin D3 and calcium during winter prevents seasonal bone loss: A randomized controlled open-label prospective trial. J Bone Miner Res 19: 1221–1230, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Talwar SA, Aloia JF, Pollack S, Yeh JK: Dose response to vitamin D supplementation among postmenopausal African American women. Am J Clin Nutr 86: 1657–1662, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park CY, Hill KM, Elble AE, Martin BR, DiMeglio LA, Peacock M, McCabe GP, Weaver CM: Daily supplementation with 25 μg cholecalciferol does not increase calcium absorption or skeletal retention in adolescent girls with low serum 25-hydroxyvitamin D. J Nutr 140: 2139–2144, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Trang HM, Cole DE, Rubin LA, Pierratos A, Siu S, Vieth R: Evidence that vitamin D3 increases serum 25-hydroxyvitamin D more efficiently than does vitamin D2. Am J Clin Nutr 68: 854–858, 1998 [DOI] [PubMed] [Google Scholar]
- 46.Armas LA, Hollis BW, Heaney RP: Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab 89: 5387–5391, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Heaney RP, Recker RR, Grote J, Horst RL, Armas LA: Vitamin D(3) is more potent than vitamin D(2) in humans. J Clin Endocrinol Metab 96: E447–E452, 2011 [DOI] [PubMed] [Google Scholar]
- 48.Holick MF, Biancuzzo RM, Chen TC, Klein EK, Young A, Bibuld D, Reitz R, Salameh W, Ameri A, Tannenbaum AD: Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J Clin Endocrinol Metab 93: 677–681, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pietras SM, Obayan BK, Cai MH, Holick MF: Vitamin D2 treatment for vitamin D deficiency and insufficiency for up to 6 years. Arch Intern Med 169: 1806–1808, 2009 [DOI] [PubMed] [Google Scholar]
- 50.Herrmann M, Harwood T, Gaston-Parry O, Kouzios D, Wong T, Lih A, Jimenez M, Janu M, Seibel MJ: A new quantitative LC tandem mass spectrometry assay for serum 25-hydroxy vitamin D. Steroids 75: 1106–1112, 2010 [DOI] [PubMed] [Google Scholar]