Summary
Background and objectives
Hepatitis C virus (HCV) infection and kidney disease are both highly prevalent diseases. The association between HCV and GN has been supported by previous research but little is known about the relationship between HCV and kidney disease.
Design, setting, participants, & measurements
A systematic review of the published medical literature was conducted to determine if HCV is associated with increased likelihood of kidney disease in the general population. A random-effects model was used to generate a summary estimate of the relative risk for kidney disease, defined as an estimated GFR <60 ml/min per 1.73 m2 or proteinuria, with HCV across the published studies.
Results
Nine clinical studies (817,917 unique individuals) were identified. Pooling of study results demonstrated the absence of a relationship between HCV seropositive status and reduced estimated GFR (adjusted relative risk, 1.12; 95% confidence interval, 0.91, 1.38; P=0.28) according to the random-effects model. HCV seropositive serology was an independent and significant risk factor for proteinuria (defined by urine dipstick test or spot urine albumin/creatinine ratio) in the general population, with a summary estimate for adjusted relative risk of 1.47 (95% confidence interval, 1.12, 1.94; P=0.006). Significant heterogeneity was observed between studies (Ri=0.82; P value by Q test, <0.001).
Conclusions
This meta-analysis shows that HCV is independently associated with proteinuria but not with reduced GFR in the general population. Substantial heterogeneity occurred.
Introduction
Patients with long-standing hepatitis C virus (HCV) infection are at risk for progression to cirrhosis and hepatocellular carcinoma. Several extrahepatic complications, including hematologic and dermatologic, have been associated with HCV infection, as well as autoimmune disorders and renal dysfunction (1). There is increasing evidence for a relationship between HCV infection and kidney disease in both native and transplanted kidneys. Thus, novel guidelines suggest the screening for proteinuria and creatinine clearance among patients with chronic HCV (2,3). However, relatively little information is available on the prevalence of kidney disease among persons with HCV to support this recommendation (4,5). Although the prevalence of HCV among individuals with ESRD is much higher than for the general population, it is unclear whether this reflects an increased risk of viral exposure (6), a greater incidence and progression of kidney disease in individuals with HCV, or both.
Several surveys derived from large databases have recently addressed the association between HCV and kidney disease but the data are conflicting. It remains unknown whether and to what extent HCV infection affects renal function. The goal of this study was to investigate the available evidence on the relationship between HCV infection and kidney disease by performing a systematic review of the literature with a meta-analysis of observational clinical studies. This information is relevant in counseling HCV-positive patients regarding the effects of HCV on renal function.
Materials and Methods
This work was performed in accordance with published guidelines for systematic reviews, analysis, and reporting for meta-analyses of observational studies (7).
Search Strategy and Data Extraction
Two authors (F.F. and V.D.) independently reviewed English-language citations from the National Library of Medicine’s Medline database from 1989 through July 1, 2011. Data on HCV status were not available before 1989, when the first assay for anti-HCV antibody was described (2). The key words “hepatitis C,” “kidney disease,” “proteinuria,” “glomerular filtration rate,” and their synonyms were used. Four Medline database engines (Ovid, PubMed, Embase, and Grateful Med) were used. Medline searches were limited to human studies. An additional search was performed with electronic searches of the Current Contents Cochrane Library; manual searches of selected specialty journals were performed to identify all pertinent literature. Reference lists from qualitative topic reviews and published clinical studies were also searched. It was previously demonstrated that a Medline search alone might not sensitive enough (8). Because of the low prevalence of HCV infection in children, pediatric studies were not included. Unpublished studies and abstracts were not considered for inclusion in this meta-analysis. Data on study design, study period, patient characteristics, HCV prevalence, and kidney disease outcomes were abstracted. All authors of selected articles were contacted to obtain missing data and to confirm published studies. Only data from individuals with known HCV status were included in the meta-analysis. Consensus was achieved for all data. Studies were compared to eliminate duplicate reports for the same patients, which included contact with investigators when necessary. Eligibility and exclusion criteria were prespecified.
Inclusion Criteria
Studies were included if they met the following inclusion criteria: (1) they presented original data from cohort studies; (2) the outcome of interest was clearly defined as incidence or prevalence of an estimated GFR (eGFR) <60 ml/min per 1.73 m2 or frequency of proteinuria in the general population according to anti-HCV serologic status; and (3) they provided risk estimates and their confidence intervals (CIs), or provided enough data to calculate them (raw data, P value, or variance estimate). We considered both case-control studies and cohort studies as eligible for inclusion in the analysis. HCV infection was defined by testing for anti-HCV antibody in serum. Information on anti-HCV status was registered at the time of enrollment.
Ineligible Studies
Studies were excluded if they reported inadequate data on the association between kidney disease and anti-HCV seropositivity (e.g., incomplete information on HCV status or renal outcomes). Studies that were only published in abstract form or as interim reports were excluded; letters and review articles were not considered for this analysis.
End Points of Interest
Separate meta-analyses were performed according to low eGFR outcome (n=9) or proteinuria (n=4). These variables were chosen on the basis of the current National Kidney Foundation definition of CKD as kidney damage (most frequently detected as persistent proteinuria) or decreased kidney function (GFR <60 ml/min per 1.73 m2) for ≥3 months (9).
The primary end point was to provide unadjusted or adjusted estimates of the risk (and 95% CIs) of incidence (or prevalence) of reduced GFR in the general population according to anti-HCV serological status. Multivariate analysis was used to estimate the independent effect of anti-HCV–positive status on the prevalence of low eGFR after adjustment for potential confounders (e.g., age, sex, race, history of coronary artery disease or diabetes mellitus). Cox regression analysis was used to determine independent predictors of the development of reduced eGFR. An additional end point was the unadjusted or adjusted estimate of the risk (and 95% CI) of proteinuria in the healthy population according to anti-HCV serologic status. The adjusted relative risk (RR) was identified by multivariate analysis in each study.
Statistical Analyses
We calculated the unadjusted odds ratio (OR) for CKD among HCV-infected versus noninfected patients by use of a random-effects approach, as described by DerSimonian and Laird (10). Cochrane’s Q test was used for quantifying the heterogeneity (11). The I2 index, which is the percentage of total variation across studies due to heterogeneity rather than chance (12), was also calculated.
A summary estimate of the adjusted RR of CKD in the general population according to anti-HCV serological status was generated by weighting the study-specific RRs by the inverse of their variance (13). We computed fixed and random-effect estimates. The proportion of total variance due to between-study variance, Ri, was used to assess heterogeneity. Heterogeneity was also measured by a parametric version (1000 replications) of the DerSimonian and Laird Q test (10), because the number of studies to be meta-analyzed was not large. To further explore the origin of heterogeneity among the studies examining low GFR, we restricted the analysis to subgroups of studies defined by study characteristics such as country (United States or other) or criteria for definition of reduced GFR (stage ≤3 CKD/stage 5 CKD). Publication bias was assessed by examination of funnel plots. Statistical analysis was done using HEpiMA software (version 2.1.3) (14). The 5% significance level was used for α risk. Every estimate was given with its 95% CIs.
Results
Literature Review
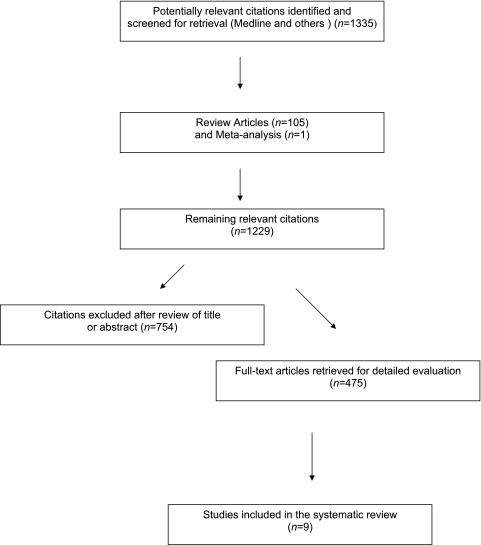
Our electronic and manual searches identified 1335 articles, of which 475 were considered potentially relevant and were selected for full text review (Figure 1). Thirty-eight observational studies were considered eligible but were excluded because outcomes from HCV-HIV co-infected participants (n=32) (15), patients with diabetes (n=2) (16,17), HCV-infected patients (n=1) (18), and chronic GN patients only (n=1) (19) were reported. Two studies did not account for important potential confounders in their analyses (20,21). Nine studies (22–30) fulfilled the inclusion criteria. The complete list of articles is available on request.
Figure 1.
Flow diagram of studies of hepatitis C virus–related kidney disease considered for inclusion.
Six cross-sectional studies (793,993 unique patients) gave information on the risk of low eGFR in HCV patients (22–27) (Table 1). Four longitudinal reports (613,368 unique patients) addressed the incidence of low eGFR in HCV-infected patients (24,25,27,28), and four cross-sectional surveys (93,919 unique patients) reported on the effect of HCV on proteinuria (22,26,29,30). The list of the 486 references is available from the authors on request. A total of 817,917 unique patients were included in our meta-analysis. There was a 100% concordance between reviewers with respect to final inclusion and exclusion of studies reviewed based on the predefined inclusion and exclusion criteria.
Table 1.
Characteristics and outcomes of studies included in the meta-analysis
| Liangpunsakul and Chalasani (29) | Tsui et al. (22) | Huang et al. (30) | Tsui et al. (24) | Dalrymple et al. (23) | Moe et al. (25) | Lee et al. (26) | Asrani et al. (27) | Butt et al. (28) | |
|---|---|---|---|---|---|---|---|---|---|
| Patients (n) | 13,990 | 15,029 | 9934 | 474,369 | 25,782 | 13,139; 7038 | 54,966 | 167,569; 88,822 | 43,139 |
| Country | US | US | Taiwan | US | US | US | Taiwan | US | US |
| Study type | CS | CS; CS | CS | CS; RC | CS | CS | CS | CS; RC | RC |
| Follow-up | NA | NA; NA | NA | 3.6 yr | NA | NA; 3.5 yr | NA; NA | NA; 25.3 mo | 3±1.3/3.15±14a yr |
| HCV positive (%) | 24 | 22 | 6.5 | 11 | 7.4 | 30; 31.8 | 9.4 | 8.7; 9.1 | 41.7 |
| Outcome | Proteinuria | Low eGFRb; proteinuria | Proteinuria | Low eGFRb; incident stage 5 CKD (HD/RT)c | Low eGFRb | Low eGFRb; incident stage 3–5 CKDc | Low eGFRb; proteinuria | Low eGFRa; incident stage 3–5 CKDc | Incident CKD stages 3–5c |
| Age (yr) | 47.6±19 | NA | 55.2±6 | 59±13/52±9a | 58±14/53±9a | 41.9±12.7/42.2±11.4a | 60.8±11.5 | 40.4±11.8/47.8±8.6a; 43.2±11.8/48.7±8.1a | 52.8±7.5/51.9±72a |
| Male, n (%) | 7192 (46.9) | 9996 (66) | 4291 (43) | 447,492 (94) | 23,462 (91) | 6858 (48); 3481 (45) | 17,168 (31) | 91,992 (54); 51,098 (57.5) | 41,974 (97) |
| Caucasian, n (%) | 10,505 (68.5) | 11,367 (76) | 0 | 318,854 (67) | 14,580 (56) | 6281 (48); 3482 (49) | 0 | NA | 24,347 (56) |
| Patients with diabetes, n (%) | 1349 (8.8) | 751 (5) | 1241 (12.5) | 220,433 (46.4) | 5533 (21.5) | 2996 (23); 1319 (18.7) | 5302 (9.6) | 11,615 (6.9); 9318 (10.4) | 10,808 (25) |
| Adjusted OR (95% CI) | 1.99 (1.38, 2.85) | 0.89 (0.49, 1.62); 1.84 (1.0, 3.37) | 1.64 (1.24, 2.17) | 0.91 (0.88, 0.95); 2.80 (2.43, 3.23) | 1.08 (0.88, 1.33) | 0.694 (0.62, 0.77); 1.024 (0.90, 1.15) | 1.3 (1.2, 1.42); 1.14 (1.0, 1.3) | 0.90 (0.36, 2.27); 0.92 (0.79, 1.08) | 1.3 (1.23, 1.37) |
| Population type | Civilians, non-institutionalized | Civilians, non-institutionalized | Metropolitan area residents | Veterans | Veterans | Urban inner city health care system users | National health insurance program users | Health insurance program Users | Veterans |
All percentages represent the proportion of the total study population. CS, cross-sectional; RC, retrospective cohort NA, not available; eGFR, estimated GFR; HD, hemodialysis; RT, renal transplant; OR, odds ratio; 95% CI, 95% confidence interval; MDRD, Modified Diet in Renal Disease.
Values expressed as HCV-negative patients/HCV-positive patients.
Low eGFR: <60 ml/min per 1.73 m2, calculated using the MDRD study equation.
Calculated using the MDRD study equation.
Patient Characteristics
Some salient demographic characteristics of participants enrolled in the included clinical trials are shown in Table 1. Seven studies were from centers in North America and two were from Asia. Baseline characteristics of the patients participating in the studies included in this meta-analysis are listed in Table 1. The mean age of patient cohorts ranged from 40.4±11.8 to 60.8±11 years. The sex distribution ranged from 31% to 97% male. In the subset of longitudinal studies, the average follow-up ranged between 25.3 months and 3.6 years.
Summary Estimates of Outcomes
eGFR Value <60 ml/min per 1.73 m2.
The unadjusted ORs for eGFR <60 ml/min per 1.73 m2 in anti-HCV-positive individuals are listed in Table 2; the pooled OR was 1.07 (95% CI, 0.92, 1.25; P=0.39).
Table 2.
Summary estimate for unadjusted ORs of reduced estimated GFR according to anti-HCV serologic status
| Study or Subcategory | Anti-HCV Positive (n/N) | Anti-HCV Negative (n/N) | OR (Random) (95% CI) | Weight (%) | OR (Random) (95% CI) |
|---|---|---|---|---|---|
| Tsui et al.(22) | 7/366 | 630/14,663 | 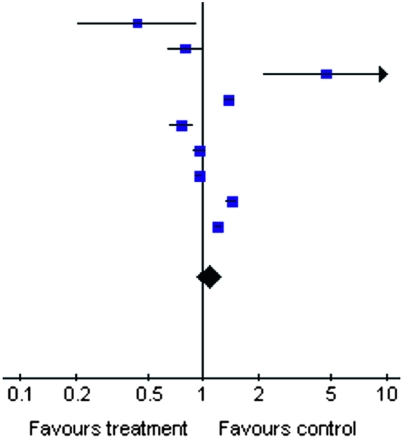 |
3.35 | 0.43 (0.20, 0.92) |
| Dalrymple et al.(23) | 93/1928 | 1423/23,854 | 11.27 | 0.80 (0.64, 0.99) | |
| Tsui et al.(24) | 10/52,874 | 17/421,495 | 3.16 | 4.69 (2.15, 10.24) | |
| Tsui et al. (retrospective) (24) | 760/52,874 | 4383/421,495 | 13.79 | 1.39 (1.28, 1.50) | |
| Moe et al.(25) | 248/3938 | 745/9201 | 12.66 | 0.76 (0.66, 0.89) | |
| Asrani et al.(27) | 682/13,384 | 8172/154,185 | 13.76 | 0.96 (0.89,1.04) | |
| Asrani et al. (retrospective) (27) | 3677/8063 | 37,957/80,759 | 14.10 | 0.95 (0.90, 0.99) | |
| Lee et al.(26) | 1066/5683 | 6872/49,283 | 13.86 | 1.42 (1.33, 1.53) | |
| Butt et al.(28) | 3140/18,002 | 3738/25,137 | 14.05 | 1.21 (1.15, 1.27) | |
| Total | 157,112 | 1,200,072 | 100.00 | 1.07 (0.92, 1.25) | |
| Total events | 9683 | 63,937 |
Test for heterogeneity: Chi square=201.96; df=8 (P<0.00001); I2=96.0%. Test for overall effect: Z=0.86 (P=0.39). OR, odds ratio; HCV, hepatitis C virus; NHANES, National Health and Nutrition Examination Survey; 95% CI, 95% confidence interval.
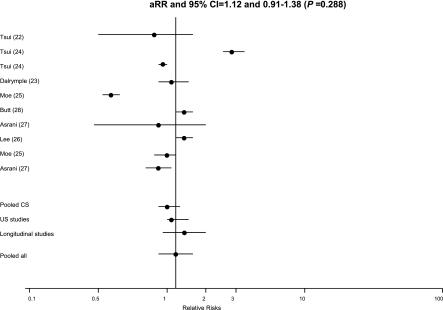
Table 1 reports the adjusted RRs and 95% CIs for kidney disease according to anti-HCV serologic status, calculated in each study. Using a random-effects model for all studies, the pooled adjusted RR of incidence (or prevalence) of low eGFR was 1.12 with a 95% CI of 0.91, 1.38 (P=0.28) (Figure 2 and Table 3). We observed significant heterogeneity between studies (Ri=0.98; P value by Q test, <0.001). We did not find asymmetry in our funnel plot (not shown); however, nine studies is not an adequate number to detect publication bias (31).
Figure 2.
Estimated relative risks and 95% confidence intervals for each study. The vertical line represents the pooled adjusted relative risk of reduced estimated GFR (random-effects model) according to positive results for anti-hepatitis C virus serology. Adjusted relative risk, 1.12; 95% confidence interval, 0.91, 1.38 (P =0.29). Heterogeneity statistics: Tau square=0.10; Ri=0.98; coefficient of variation between=4.35. Asymptotic tests: Chi square Q value=371.32; df=9; P<0.001. Bootstrap tests: Tau square boot=significant (α=0.05). Ri, proportion of total variance due to between-study variance.
Table 3.
Summary estimates for adjusted RR of CKD according to anti-HCV serologic status in various subgroups of interest
| n | Random-Effects Model Adjusted RR Estimate (95% CI) | Q Value (by Chi-Squared Test) | Ri | |
|---|---|---|---|---|
| Adjusted RR of reduced eGFRa | ||||
| all studies | 10 | 1.12 (0.91, 1.38) | 371.32 (<0.001) | 0.98 |
| US studies | 9 | 1.10 (0.87, 1.39) | 351.52 (<0.001) | 0.98 |
| studies with reduced eGFRb | 9 | 1.0 (0.85, 1.19) | 192.0 (<0.001) | 0.97 |
| cross-sectional studies | 6 | 0.96 (0.77, 1.20) | 88.48 (<0.001) | 0.97 |
| longitudinal studies | 4 | 1.36 (0.93, 1.98) | 144.61 (<0.001) | 0.99 |
| longitudinal studiesb | 3 | 1.08 (0.87, 1.34) | 26.28 (<0.001) | 0.95 |
| Adjusted RR of proteinuria | ||||
| all studies | 4 | 1.47 (1.12, 1.94) | 12.06 (<0.007) | 0.82 |
RR, relative risk; HCV, hepatitis C virus; 95% CI, 95% confidence interval; Ri, proportion of total variance due to between-study variance; eGFR, estimated GFR.
Reduced eGFR was mostly defined as a minimum eGFR value <60 ml/min per 1.73 m2 using the Modified Diet in Renal Disease study equation.
The study addressing stage 5 CKD (hemodialysis/renal transplant) was excluded. Tsui et al. (22): RR adjusted for age, sex, race/ethnicity, educational or smoking status, diabetes, and hypertension. Tsui et al. (24): RR adjusted for age, sex, race/ethnicity, diabetes, HIV status, hypertension, coronary artery disease, congestive heart failure, peripheral vascular disease, chronic obstructive pulmonary disease, cerebrovascular disease, and substance abuse. Dalrymple et al. (23): RR adjusted for age, sex, race, diabetes, and hypertension. Moe et al. (25): RR adjusted for age, sex, race/ethnicity, diabetes, hypertension, HIV, cryoglobulin, AST, and rheumatoid factor. Lee et al. (26): RR adjusted for age, sex, educational status, body mass index, albumin, hemoglobin, cholesterol, uric acid, hypertension, diabetes mellitus, and hepatitis B. Asrani et al. (27): RR adjusted for initial GFR, sex, age, drug use, HIV, diabetes, hypertension, coronary artery disease, peripheral vascular disease, cerebrovascular disease, heart failure, chronic obstructive pulmonary disease, alcohol, depression, and selected medications (diuretics or renin-angiotensin inhibitors). Butt et al. (28): RR adjusted for baseline eGFR, age, race, hypertension, smoking, chronic obstructive pulmonary disease, diabetes, dyslipidemia, anemia, alcohol, drug use, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and decompensated liver disease. Liangpunsakul and Chalasani (29): RR adjusted for age, sex, arterial hypertension, body mass index, and diabetes mellitus. Huang et al. (30): RR adjusted for age, sex, body mass index, alanine aminotransferase level, total cholesterol level, triglyceride level, diabetes mellitus, arterial hypertension, and HBsAg seropositive status.
There was small variability in the definition of low eGFR among included studies. Tsui et al. (24) defined the outcome as the onset of stage 5 CKD (hemodialysis or renal transplantation). In the other studies (22,23,25–28), reduced eGFR was defined as a minimum eGFR value <60 ml/min per 1.73 m2, using the four-variable Modified Diet in Renal Disease equation, according to the clinical practice guidelines of the National Kidney Foundation (32).
Our stratified analysis, performed in various subgroups of interest (i.e., cross-sectional or longitudinal studies), showed no significant changes in pooled adjusted RR. However, considerable heterogeneity between studies was noted, as shown by Ri and P values (Q tests) (Table 3).
Proteinuria.
Four cross-sectional studies provided data on the relationship between proteinuria and anti-HCV serologic status (Table 1) (22,26,29,30). Two studies defined proteinuria by standard dipstick urinalysis (26,30). Tsui et al. (22) and Liangpunsakul and Chalasani (29) measured albuminuria by spot urine albumin/creatinine ratio.
Table 4 shows the unadjusted ORs for proteinuria according to anti-HCV serologic status; the frequency of proteinuria was significantly higher in anti-HCV-positive patients (OR, 1.29; 95% CI, 1.11, 1.51) and no significant heterogeneity was found. After adjustment for various covariates, the summary estimate for RR of proteinuria was 1.47 (95% CI, 1.12, 1.94; P=0.006) in anti-HCV-positive patients compared with anti-HCV-negative patients. Small, although significant, heterogeneity occurred (Ri=0.82; P value by Q test, <0.001) (Table 3).
Table 4.
Summary estimate for unadjusted OR of proteinuria according to anti-HCV serologic status
| Study or Subcategory | Anti-HCV Positive (n/N) | Anti-HCV Negative (n/N) | OR (Random) (95% CI) | Weight (%) | OR (Random) (95% CI) |
|---|---|---|---|---|---|
| Liangpunsakul and Chalasani (29) | 7/362 | 20/995 | 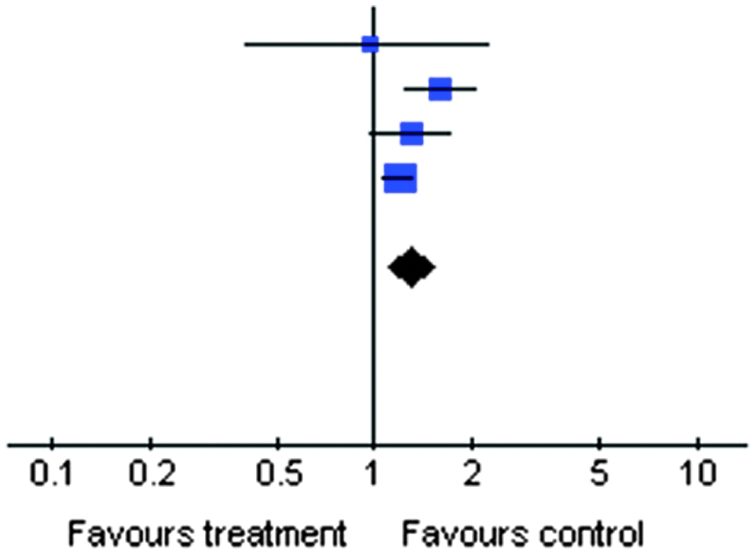 |
3.00 | 0.96 (0.40, 2.29) |
| Huang et al. (30) | 68/642 | 639/9292 | 23.40 | 1.60 (1.23, 2.09) | |
| Tsui et al. (22) | 54/366 | 1730/14,663 | 20.14 | 1.29 (0.97, 1.73) | |
| Lee et al. (26) | 360/5683 | 2634/49,283 | 53.46 | 1.20 (1.07, 1.34) | |
| Total | 7053 | 74,233 | 100.00 | 1.29 (1.11, 1.51) | |
| Total events | 489 | 5023 |
Test for heterogeneity: Chi square=4.38; df=3 (P=0.22); I2=31.5%. Test for overall effect: Z=3.29 (P=0.001). OR, odds ratio; HCV, hepatitis C virus; 95% CI, 95% confidence interval
Discussion
There is continuing controversy about the potential association of HCV infection and the development and progression of kidney disease. The results of this meta-analysis suggest that positive serologic status for anti-HCV antibody is associated with an increased risk of proteinuria but not with reduced GFR compared with anti-HCV-negative patients. The relationship between HCV and proteinuria (adjusted RR, 1.47; 95% CI, 1.12, 1.94; P=0.006) is based only on four surveys but is in keeping with results from other sources (18,33). It has been repeatedly shown that proteinuria predicts the progression of renal disease as well as cardiovascular morbidity and mortality in patients with diabetes as well as the general population (34,35).
Proteinuria clusters with the metabolic syndrome, and studies have shown a relationship between proteinuria and individual components of the metabolic syndrome (hyperglycemia, insulin resistance, dyslipidemia, abdominal obesity, and hypertension) (36). Because patients with HCV are known to have higher prevalence of components of the metabolic syndrome, it has been hypothesized that individuals with HCV may have higher prevalence of proteinuria. However, we observed the persistence of the relationship between HCV and proteinuria after correction for several confounding parameters including metabolic syndrome elements (Table 3). In addition, chronic HCV is associated with renal disease. This extrahepatic manifestation is either related to intrinsic renal disease or to cryoglobulinemia. Recent reports demonstrate that HCV increases the risk of proteinuria in native kidneys (37,38) and after liver (39) or kidney (40) transplantation.
The link between HCV and glomerular diseases, likely one part of the extrahepatic manifestations of HCV, could explain why the relationship between proteinuria and HCV occurred irrespective of diabetes mellitus and other metabolic features. Type I membranoproliferative GN associated with type II mixed cryoglobulinemia remains the most common form of kidney disease associated with HCV (37,38). Less frequently described lesions include membranoproliferative GN without cryoglobulinemia as well as membranous nephropathy (41,42). Occasional cases of FSGS, fibrillary or immunotactoid glomerulopathies (43), and thrombotic microangiopathy (44) have been reported. In addition, tubulointerstitial nephritis (45) has been associated with HCV. To date, the most conclusive survey was by El-Serag et al., who carried out a case-control study among US male veterans. They found a greater prevalence of membrano-proliferative GN among patients with HCV (0.36% versus 0.05%; P<0.001) but not membranous glomerulopathy (0.33% versus 0.19%; P=0.86) (46).
Noureddine et al. (19) evaluated a cohort (N=111) of patients with biopsy-proven chronic GN and reported an increased risk of progression of CKD in patients with positive serology for HCV compared with HCV-negative participants. These findings may explain why there is no overall association of HCV infection with lowered eGFR in our meta-analysis. Several pieces of evidence support the notion that HCV has an effect primarily on glomerular disease; thus, mixed cohorts of both nonglomerular and glomerular disease may fail to show a relationship. A recent study showed a higher proportion of HIV-HCV–infected participants showing CKD compared with those patients having HIV infection alone, suggesting that the immunologic mechanisms leading to glomerular disease may be aggravated by HCV (47). In two smaller studies (16,17), HCV positivity was found to be a significant and independent risk factor for development of ESRD in patients with diabetes, regardless of the presence of proteinuria or BP.
This meta-analysis is potentially biased by a number of issues. First, all of the clinical studies included in this meta-analysis had an observational design. Although much has been learned about the course of HCV in patients with normal kidney function or CKD, the available data are of limited nature due to the lack of controlled studies that provide baseline data, and sequential follow-up, including kidney histology, in patients with initially normal renal function and HCV. The cross-sectional design of many studies does not allow us to draw conclusions on causality. Some evidence supports an unidirectional association of HCV leading to kidney disease; however, the possibility that proteinuria predisposes to HCV infection cannot be ruled out. Second, the studies in this meta-analysis might give incomplete information on additional unmeasured confounders that could introduce bias into the analysis. A magnitude of missing data or insensitive codes for comorbidity diagnoses are inherent to clinical databases as opposed to research databases. For example, information on HCV RNA and socioeconomic status (or smoking) was incomplete. Third, individual data from each study (e.g., patient-level data) were not available; thus, it was impossible to perform our own adjustments. On the basis of the RR reported in each study, we calculated our summary estimate for RR of reduced GFR (or proteinuria) with anti-HCV across the studies. However, we used adjusted RRs obtained by the Cox model in each longitudinal study. This approach takes into account both differential follow-up time as well as differential distribution of covariates in order to isolate the effect of anti-HCV seropositive status per se. Additional limitations are that only surveys from the United States and Taiwan were enrolled and various populations (including veterans or inner city residents) have been evaluated. Finally, as with all meta-analyses, this study has the potential limitation of publication bias; negative trials are less likely to be reported. To limit the possible effect of publication bias, we used several strategies for identifying studies to include published and unpublished studies. Inclusion criteria, established a priori, were chosen to increase the likelihood that high-quality studies would be included.
In conclusion, this meta-analysis of observational studies demonstrates no relationship between HCV and incidence (or prevalence) of lowered eGFR. HCV seropositive patients have more frequently proteinuria than patients with negative serology. The higher risk of proteinuria in HCV seropositive individuals could reflect, at least partially, an increased frequency of glomerulopathies; however, a confounding effect by other factors cannot be excluded. Health care providers should be aware of this risk and future studies should better investigate the mechanisms of the observed association to allow for targeted interventions in susceptible patients.
Disclosures
None.
Acknowledgments
This work was supported in part by a Project Glomerulonephritis grant, in memory of Pippo Neglia.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Access to UpToDate on-line is available for additional clinical information at www.cjasn.org.
References
- 1.Kim WR: The burden of hepatitis C in the United States. Hepatology 36: S30–S34, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Kidney Disease: Improving Global Outcomes (KDIGO): KDIGO clinical practice guidelines for the prevention, diagnosis, evaluation, and treatment of hepatitis C in chronic kidney disease. Kidney Int 73[Suppl 109]: S1–S99, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Covic A, Abramowicz D, Bruchfeld A, Leroux-Roels G, Samuel D, Van Biesen W, Zoccali C, Zoulim F, Vanholder R, ERA-EDTA ERBP Advisory Board : Endorsement of the Kidney Disease Improving Global Outcomes (KDIGO) hepatitis C guidelines: A European Renal Best Practice (ERBP) position statement. Nephrol Dial Transplant 24: 719–727, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Daghestani L, Pomeroy C: Renal manifestations of hepatitis C infection. Am J Med 106: 347–354, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Bergman S, Accort N, Turner A, Glaze J: Hepatitis C infection is acquired pre-ESRD. Am J Kidney Dis 45: 684–689, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Fissell R, Bragg-Gresham JL, Woods J, Jadoul M, Gillespie B, Hedderwick S, Rayner H, Greenwood R, Akiba T, Young E: Patterns of hepatitis C prevalence and seroconversion in hemodialysis units from three continents: The DOPPS. Kidney Int 65: 2335–2342, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Stroup D, Berlin JA, Morton S, Olkin I, Williamson G, Rennie D, Moher D, Becker B, Sipe T, Thacker S: Meta-analysis of observational studies in epidemiology; A proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA 283: 2008–2012, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Poynard T, Conn HO: The retrieval of randomised clinical trials in liver diseases from the medical literature. A comparison of MEDLARS and manual methods. Control Clin Trials 6: 271–279, 1985 [DOI] [PubMed] [Google Scholar]
- 9.Levey A, Coresh J, Balk E, Kausz A, Levin A, Steffes M, Hogg R, Perrone R, Lau J, Eknoyan G: National Kidney Foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann Intern Med 139: 137–147, 2003 [DOI] [PubMed] [Google Scholar]
- 10.DerSimonian R, Laird N: Meta-analysis in clinical trials. Control Clin Trials 7: 177–188, 1986 [DOI] [PubMed] [Google Scholar]
- 11.Petitti DB: Approaches to heterogeneity in meta-analysis. Stat Med 20: 3625–3633, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Higgins JPT, Thompson SG, Deeks JI, Altman DG: Measuring inconsistencies in meta-analysis. BMJ 327: 557–560, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takkouche B, Cadarso-Suarez C, Spiegelman D: Evaluation of old and new tests for heterogeneity in epidemiologic meta-analysis. Am J Epidemiol 150: 206–215, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Costa-Bouzas J, Takkouche B, Cadarso-Suarez C, Spiegelman D: HepiMA: Software for the identification of heterogeneity in meta-analysis. Comput Methods Programs Biomed 64: 101–107, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Wyatt C, Malvestutto C, Coca S, Klotman P, Parikh C: The impact of hepatitis C virus co-infection on HIV-related kidney disease: A systematic review and meta-analysis. AIDS 22: 1799–1807, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soma J, Saito T, Taguma Y, Chiba S, Sato H, Sugimura K, Ogawa S, Ito S: High prevalence and adverse effects of hepatitis C virus infection in type II diabetic-related nephropathy. J Am Soc Nephrol 11: 690–699, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Crook E, Penumalee S, Gavini B, Filippova K: Hepatitis C is a predictor of poorer renal survival in diabetic patients. Diabetes Care 28: 2187–2191, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Satapathy S, Lingisetty C, Williams S: Higher prevalence of chronic kidney disease and shorter renal survival in patients with chronic hepatitis C virus infection [published online ahead of print June 23, 2011]. Hepatol Int doi:10.1007/s12072-011-9284-9 [DOI] [PubMed] [Google Scholar]
- 19.Noureddine L, Usman S, Yu Z, Moorthi R, Moe S: Hepatitis C increases the risk of progression of chronic kidney disease in patients with glomerulonephritis. Am J Nephrol 32: 311–316, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hofmann J, Torner A, Chow W, Ye W, Purdue M, Duberg A: Risk of kidney cancer and chronic kidney disease in relation to hepatitis C virus infection: A nationwide register-based cohort study in Sweden. Eur J Cancer Prev 20: 326–330, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gordon SC, Moonka D, Brown K, Rogers C, Huang M, Bhatt N, Lamerato L: Risk for renal cell carcinoma in chronic hepatitis C infection. Cancer Epidemiol Biomarkers Prev 19: 1066–1073, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Tsui J, Vittinghoff E, Shlipak M, O’Hare A: Relationship between hepatitis C and chronic kidney disease: Results from the Third National Health and Nutrition Examination Survey. J Am Soc Nephrol 17: 1168–1174, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Dalrymple L, Koepsell T, Sampson J, Louie T, Dominitz J, Young B, Kestenbaum B: Hepatitis C virus infection and the prevalence of renal insufficiency. Clin J Am Soc Nephrol 2: 715–721, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Tsui J, Vittinghoff E, Shlipak M, Berthental D, Inadomi J, Rodriguez R, O’Hare A: Association of hepatitis C seropositivity with increased risk for developing end-stage renal disease. Arch Intern Med 167: 1271–1276, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Moe S, Pampalone A, Ofner S, Rosenman M, Teal E, Hui S: Association of hepatitis C virus infection with prevalence and development of kidney disease. Am J Kidney Dis 51: 885–892, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee J, Lin M, Yang Y, Lu S, Chen H, Hwang S: Association of hepatitis C and B virus infection with chronic kidney disease in an endemic area in Taiwan: A cross-sectional study. Am J Kidney Dis 56: 23–31, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Asrani S, Buchanan P, Rey L, Schnitzler M, Kanwal F: Lack of association between hepatitis C infection and chronic kidney disease. Clin Gastroenterol Hepatol 8: 79–84, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butt A, Wang X, Fried L: HCV infection and the incidence of chronic kidney disease. Am J Kidney Dis 57: 396–402, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Liangpunsakul S, Chalasani N: Relationship between hepatitis C and microalbuminuria: Results from the NHANES III. Kidney Int 67: 285–290, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Huang J, Chuang W, Dai C, Ho C, Hwang S, Chen S, Lin Z, Wang L, Chang W, Yu M: Viral hepatitis and proteinuria in an area endemic for hepatitis B and C infections: Another chain of link? J Intern Med 260: 255–262, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Macaskill P, Walter S, Irwig L: A comparison of methods to detect publication bias in meta-analysis. Stat Med 20: 641–654, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Levey A, Bosch J, Lewis J, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Derbala M, Shebl F, Rashid A, Amer A, Bener A: Microalbuminuria in hepatitis C-genotype 4: Effect of pegylated interferon and ribavirin. World J Gastroenterol 16: 1226–1231, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mogensen CE: Microalbuminuria and hypertension with focus on type 1 and type 2 diabetes. J Intern Med 254: 45–66, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Diercks G, van Boven A, Hillege J, De Jong P, Rouleau J, Van Gilst W: The importance of microalbuminuria as a cardiovascular risk factor: A review. Can J Cardiol 18: 525–535, 2002 [PubMed] [Google Scholar]
- 36.Bakker S, Ijerman R, Teerlink T, Westerhoff H, Gans R, Heine R: Cytosolic triglycerides and oxidative stress in central obesity: The missing link between excessive atherosclerosis, endothelial dysfunction, and the beta-cell failure? Atherosclerosis 148: 17–21, 2000 [DOI] [PubMed] [Google Scholar]
- 37.D’Amico G: Renal involvement in hepatitis C infection: Cryoglobulinemic glomerulonephritis. Kidney Int 47: 650–671, 1995 [DOI] [PubMed] [Google Scholar]
- 38.Roccatello D, Fornasieri A, Giachino O, Rossi D, Beltrame A, Banfi G, Confalonieri R, Tarantino A, Pasquali S, Amoroso A, Savoldi S, Colombo V, Manno C, Ponzetto A, Moriconi A, Pani A, Rustichelli R, Di Belgiojoso G, Comotti C, Quarenghi M: Multicenter study on hepatitis C virus-related cryoglobulinemic glomerulonephritis. Am J Kidney Dis 49: 69–82, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Pascual M, Thadhani M, Chung RT, Williams W, Meehan S, Tolkoff-Rubin N, Colvin R, Cosimi A: Nephrotic syndrome after liver transplantation in a patient with hepatitis C virus associated liver transplantation. Transplantation 64: 1073–1076, 1997 [DOI] [PubMed] [Google Scholar]
- 40.Cruzado J, Carrera M, Torras J, Grinyo J: Hepatitis C virus infection and de novo glomerular lesions in renal allografts. Am J Transplant 1: 171–178, 2001 [PubMed] [Google Scholar]
- 41.Davda R, Peterson J, Weiner R, Croker B, Lau Y: Membranous glomerulonephritis in association with hepatitis C virus infection. Am J Kidney Dis 22: 452–455, 1993 [DOI] [PubMed] [Google Scholar]
- 42.Stehman-Breen C, Alpers C, Couser W, Willson R, Johnson R: Hepatitis C virus associated membranous glomerulonephritis. Clin Nephrol 44: 141–147, 1995 [PubMed] [Google Scholar]
- 43.Markowitz G, Cheng J, Colvin R, Trebbin W, D’Agati V: Hepatitis C viral infection is associated with fibrillary glomerulonephritis and immunotactoid glomerulopathy. J Am Soc Nephrol 9: 2244–2252, 1998 [DOI] [PubMed] [Google Scholar]
- 44.Baid S, Pascual M, Williams W, Tolkoff-Rubin N, Johnson S, Collins B, Chung R, Del Monico F, Cosimi A, Colvin R: Renal thrombotic microangiopathy associated with anticardiolipin antibodies in hepatitis C-positive renal allograft recipients. J Am Soc Nephrol 10: 146–153, 1999 [DOI] [PubMed] [Google Scholar]
- 45.Kasuno K, Ono T, Matsumori A, Nogaki F, Kusano H, Watanabe H, Yodoi J, Muso E: Hepatitis C virus-associated tubulo-interstitial injury. Am J Kidney Dis 41: 767–775, 2003 [DOI] [PubMed] [Google Scholar]
- 46.El-Serag H, Hampel H, Yeh C, Rabeneck L: Extrahepatic manifestations of hepatitis C among United States male veterans. Hepatology 36: 1439–1445, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Fischer M, Wyatt C, Gordon K, Giber L, Brown S, Rimland D, Rodriguez-Barradas M, Justice A, Parik C: VACS Project Team: Hepatitis C and the risk of kidney disease and mortality in veterans with HIV. J Acquir Immune Defic Syndr 53: 222–226, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]