Summary
Background and objectives
This study examined differences in the concentration of markers of mineral metabolism across race in patients with advanced CKD not requiring dialysis and ESRD.
Design, setting, participants, & measurements
Concentrations of 25-hydroxyvitamin D (25(OH)D), 1,25-dihydroxyvitamin D (1,25(OH)2D), intact parathyroid hormone (iPTH), and fibroblast growth factor 23 (FGF-23) were measured in stored plasma samples of 1497 patients with advanced CKD not yet on dialysis and ESRD who participated in the Homocysteine in Kidney and End Stage Renal Disease study. Linear regression models were used to examine the relationship between race and 25(OH)D, 1,25(OH)2D, iPTH, and FGF-23 concentrations.
Results
Non-Hispanic white patients comprised 58% of the cohort, whereas non-Hispanic blacks comprised 42%. Median (interquartile range) FGF-23 concentrations were lower in blacks compared with whites with CKD (323 [181–655] versus 431 [232–1026] RU/ml; P<0.001) but not in ESRD. In adjusted linear regression models, blacks with CKD not requiring dialysis had significantly lower plasma FGF-23 concentrations (difference, −159; 95% confidence interval, −205 to −106; P<0.001) compared with whites, independent of plasma 25(OH)D, 1,25(OH)2D, and iPTH concentrations. This difference was not observed in the ESRD group. The magnitude of correlation for the relationships between 1,25(OH)2D with iPTH, FGF-23 with 1,25(OH)2D, and FGF-23 with iPTH were stronger among blacks than whites with CKD not requiring dialysis.
Conclusions
In advanced CKD not requiring dialysis, blacks have lower FGF-23 concentrations than whites. Blacks with CKD and ESRD have lower 25(OH)D and higher iPTH compared with whites, independent of FGF-23 concentrations.
Introduction
Abnormalities in 25-hydroxyvitamin D (25(OH)D), 1,25-dihydroxyvitamin D (1,25(OH)2D), intact parathyroid hormone (iPTH), and phosphate homeostasis have been linked to all-cause mortality and cardiovascular disease (CVD) including vascular calcification, left ventricular hypertrophy, and hypertension in patients with kidney disease (1–10). The recent discovery of fibroblast growth factor 23 (FGF-23) and its phosphaturic effect (11,12) has added another important variable to disordered mineral metabolism in CKD. High FGF-23 concentrations have been independently linked to death and CVD, including increased arterial medial calcification and left ventricular hypertrophy, in animal models, in the general population, and in patients with mild to moderate CKD (7,8,13).
Adding to the complexity of the abnormal mineral metabolism axis in CKD not requiring dialysis and ESRD is the observation that dissimilarities exist among different races (14,15). Black patients tend to have lower 25(OH)D levels and higher iPTH levels compared with whites (16–18). FGF-23 concentrations also seem to vary across race and kidney disease stage. A recent nested case-control analysis showed that blacks with ESRD had lower concentrations of FGF-23 compared with whites (19). These differences have not been thoroughly examined in CKD patients not requiring dialysis. Hence, understanding differences in mineral metabolism across race and kidney function strata is of pivotal importance because this may have important implications for screening and treatment.
We evaluated the distribution of plasma 25(OH)D, 1,25(OH)2D, iPTH, and FGF-23 concentrations in 1497 non-Hispanic blacks and non-Hispanic whites with either severe CKD, not yet on dialysis, or ESRD who participated in the Homocysteinemia in Kidney and End Stage Renal Disease (HOST) study (20).
Materials and Methods
Study Participants
The details of the HOST study have been described previously (20). Briefly, the study was a multicenter, prospective, randomized, double-blind, placebo controlled trial examining the effects of high doses of folic acid, pyridoxine hydrochloride (vitamin B6), and cyanocobalamin (vitamin B12) on death and cardiovascular events in patients with advanced kidney disease and elevated homocysteine concentrations. The trial enrolled 2056 participants from 36 Veterans Affairs medical centers between September 2001 and October 2003. Patients were included in the study if they were aged ≥21 years with ESRD receiving either hemodialysis or peritoneal dialysis (n=751), or with an estimated creatinine clearance (calculated by the Cockroft–Gault formula) of <30 ml/min but not yet on chronic dialysis (n=1305) and an elevated plasma homocysteine concentration of ≥15 µmol/L.
Measurements
We used stored EDTA blood samples collected from the participants 3 months after randomization for measurement of plasma 25(OH)D, 1,25(OH)2D, iPTH, and FGF-23 concentrations. We measured 25(OH)D concentrations by a commercial competitive chemiluminescent immunoassay on a Liaison analyzer and 1,25(OH)2D concentrations by a commercial competitive RIA (both from DiaSorin, Stillwater, MN). 25(OH)D measurements were not calibrated to the National Institute of Standards and Technology because reference material (SRM 972) did not become available until after the study measurements were complete.
The analytical measurement range for the 25(OH)D assay is 7–150 ng/ml. The intra-assay coefficients of variation (CVs) were 5.6% and 4.5% at 11 and 28 ng/ml, respectively. The interassay CVs were 9.1% and 5.6% at 16 and 51 ng/ml, respectively. For 1,25(OH)2D, the range of the assay was 5–200 pg/ml. The intra-assay CVs were 12.6% and 9.7% at 13 and 45 pg/ml, respectively. The interassay CVs were 21.4% and 14.7% at 25 pg/ml and 56 pg/ml, respectively. C-terminal FGF-23 concentrations were measured using a two-site second-generation ELISA kit (Immutopics, San Clemente, CA) with antibodies directed against two epitopes within the C-terminal region of the FGF-23 molecule (21). The analytical measurement range for the FGF-23 assay was 3.0–2300 RU/ml. The CVs were 2.6% and 1.4% at 32.1 and 299.2 RU/ml, respectively. The interassay CVs were 3.4% and 4.4% at 32.1 and 299.2 RU/ml, respectively. iPTH was measured using a Roche E170 electrochemiluminescent immunoassay with a reference interval of 15–65 pg/ml. The intra- and interassay CVs were both <5%.
Other Measurements
Information collected at the time of randomization included a complete history and physical examination; history of hypertension, diabetes, and CVD identified by self-report and chart review; and use of medications including angiotensin converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), β blockers, and lipid-lowering drugs. Serum creatinine was measured at local sites and was assessed by spectrophotometry analysis using the modified kinetic Jaffe reaction (22) at all sites. To note, although a creatinine clearance estimated by the Cockcroft–Gault formula was used for eligibility of the HOST study, for the purpose of this analysis, estimated GFR (eGFR) was estimated using the four-variable Modified Diet Renal Disease (MDRD) prediction equation (23).
Statistical Analyses
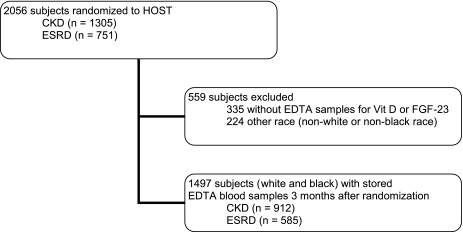
Patients were excluded if EDTA-plasma samples were not available for vitamin D and FGF-23 measurements (n=335) and/or if race was nonwhite or nonblack, categorized as other (n=224), resulting in a final sample of 1497 participants in this study (Figure 1). Two-sample t tests or Wilcoxon rank-sum tests for continuous variables and Pearson chi-squared tests for categorical variables were used to compare baseline characteristics among whites and blacks in the whole cohort. We tested for racial differences in concentrations of calcium, phosphorus, 25(OH)D, 1,25(OH)2D, iPTH, and FGF-23 on a continuous scale in groups according to CKD stage (advanced CKD or ESRD). In addition, we examined the overall and stage-specific racial differences in the prevalence of 25(OH) deficiency (<15 ng/ml), elevated iPTH (>65 pg/ml), and FGF-23 (above kidney disease stage-specific median concentration). These thresholds were chosen in keeping with the definition for 25(OH)D deficiency and elevated iPTH and FGF-23 concentrations published in previous reports (24–26). Spearman correlations and graphical methods were used to investigate the correlation of 25(OH)D, 1,25(OH)2D, iPTH, and FGF-23 concentrations with one another and with eGFR across race and kidney function strata.
Figure 1.
Cohort definition and sampling.
Multivariable linear regression models were used to examine the associations among races and plasma concentrations of 25(OH)D, 1,25(OH)2D, iPTH, and FGF-23. Given the skewed distribution of these markers of mineral metabolism, the values were transformed to the log base of 10 for all analyses. As the dependent variable (i.e., 25(OH)D, 1,25(OH)2D, iPTH, and FGF-23) of the regression was logged, model-predicted values for each dependent variable were anti-logged and the differences between blacks and whites and respective 95% confidence intervals (95% CIs) were reported for each dependent variable.
The covariates for adjustment in the final models were primarily identified if they were significantly correlated with abnormalities of mineral metabolism and were deemed to be biologically plausible. Two sequential sets of covariates were considered. In model 1, the covariates included age, sex, season, CVD, hypertension, diabetes, body mass index, serum calcium, and phosphate. Vintage and eGFR were included as potential confounders in regression models for ESRD and CKD, respectively. In model 2, the covariates included those used in model 1 plus the other three markers of mineral metabolism. All statistical analyses were performed with SAS software (version 9.13; SAS Institute, Cary, NC).
Results
Baseline Characteristics
There were a total of 1497 participants analyzed, of whom 58% (873) were white and 42% (624) were black. Baseline characteristics from the cohort are presented in Table 1. Whites were older and smoked less than blacks. Although all participants had a similar prevalence of hypertension, blacks had higher systolic and diastolic BPs. Diabetes was more prevalent among blacks, whereas a history of CVD was more prevalent among whites. To note, whites were more likely to be treated with cardioprotective medications than blacks.
Table 1.
Baseline characteristics of HOST participants by race
| Characteristic | Non-Hispanic White (n=873) | Non-Hispanic Black (n=624) | P Value |
|---|---|---|---|
| Age (yr) | 69±10.6 | 61±12.0 | <0.001 |
| Male | 856 (98) | 612 (98) | 0.80 |
| eGFR (ml/min per 1.73 m2) | |||
| >15 | 575 (66) | 280 (45) | 0.01 |
| <15 not on dialysis | 47 (5) | 10 (2) | 0.02 |
| ESRD | 251 (29) | 334 (53) | <0.001 |
| Causes of renal disease | |||
| diabetes | 326 (37) | 267 (43) | <0.001 |
| hypertension | 256 (29) | 230 (37) | |
| GN | 50 (6) | 36 (5) | |
| obstructive uropathy | 21 (3) | 7 (1) | |
| polycystic kidney disease | 38 (4) | 12(2) | |
| othera | 182 (21) | 72 (12) | |
| Current smoker | 175 (20) | 150 (24) | 0.04 |
| BMI (kg/m2) | 27.8±4.9 | 27.5±5.4 | 0.14 |
| Systolic BP (mmHg) | 141±23 | 143±24 | 0.02 |
| Diastolic BP (mmHg) | 72±13 | 78±13 | <0.001 |
| Diabetes | 437 (50) | 349 (56) | 0.02 |
| Hypertension | 838 (96) | 599 (96) | 0.96 |
| Cardiovascular disease | 533 (61) | 293 (47) | <0.001 |
| Charlson score | 4.95±2.20 | 4.92±2.21 | 0.84 |
| Medication | |||
| ACE inhibitor | 323(37) | 275(44) | 0.01 |
| ARB | 114 (13) | 69 (11) | 0.27 |
| β blocker | 559 (64) | 331 (53) | <0.001 |
| lipid-lowering agent | 498 (57) | 243 (39) | <0.001 |
All values are expressed as mean ± SD or n (%). BMI, body mass index; ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker.
Other includes tubulointerstitial disease, vascular disease, and unknown causes.
Mineral Metabolism in CKD and ESRD by Race
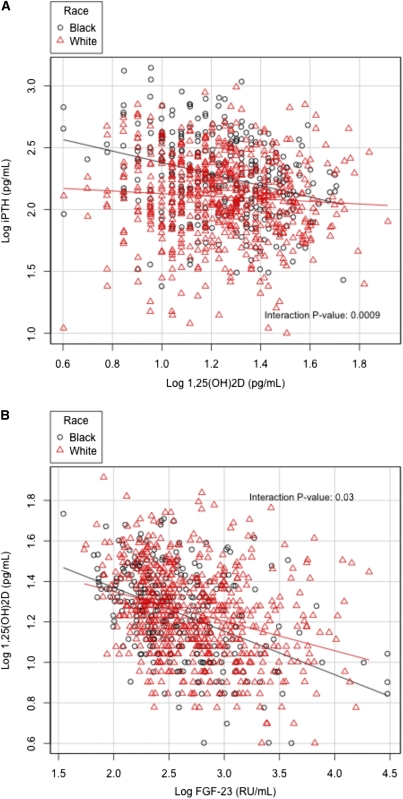
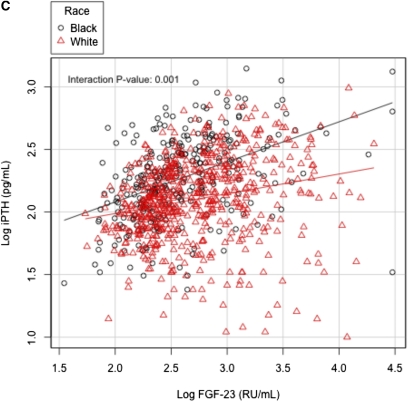
In whites with advanced CKD not requiring dialysis, 25(OH)D correlated with 1,25(OH)2D (r=0.42, P<0.001) and iPTH (r=−0.23, P<0.001). Similarly, in blacks with CKD not requiring dialysis, 25(OH)D correlated with 1,25(OH)2D (r=0.47, P<0.001) and iPTH (r=−0.23, P<0.001). Furthermore, 1,25(OH)2D correlated with iPTH in whites (r=−0.10, P=0.02) and in blacks (r=−0.31, P<0.001), as well as with FGF-23 in whites (r=−0.32, P<0.001) and in blacks (r=−0.44, P<0.001). Finally, iPTH correlated with FGF-23 in whites (r=0.30, P<0.001) and in blacks (r=0.48, P<0.001). Interestingly, there were significant interactions for the relationships of 1,25(OH)2D with iPTH, FGF-23 with 1,25(OH)2D, and FGF-23 with iPTH by race (Figure 2, A–C).
Figure 2.
Bivariate relationship of markers of mineral metabolisms in patients with CKD not requiring dialysis with interaction by race. (A) Relation between 1,25(OH)2D and iPTH, (B) FGF-23 and 1,25(OH)2D, and (C) FGF-23 and iPTH.
There were fewer correlations in ESRD. Our results showed that 25(OH)D correlated with 1,25(OH)2D in whites (r=0.40, P<0.001) and in blacks (r=0.33, P<0.001). Intact parathyroid hormone (iPTH) correlated with FGF-23 in whites (r=0.29, P<0.001) and in blacks (r=0.23, P<0.001). The stronger correlation between iPTH and FGF-23 in blacks with CKD was not observed in the ESRD group and there were no significant interactions by race for any of these bivariate relationships (not shown) (Supplemental Materials).
In the group with CKD not requiring dialysis, the relationship between each mineral metabolism variable with eGFR was also examined and was statistically significant for 1,25(OH)2D, iPTH, and FGF-23 in both whites and blacks (i.e., plasma 1,25(OH)2D decreased and iPTH and FGF-23 increased with declining eGFR similarly in whites and blacks; P<0.05 for all). For both blacks and whites, the relationship between eGFR and 25(OH)D was not significant (P>0.10 for both). We found a significant interaction between eGFR and race for iPTH (P<0.001). In further evaluation of this interaction, we noted that iPTH increased by 8.30 pg/ml and by 3.23 pg/ml for each 1 ml/min per 1.73 m2 decrease in eGFR for blacks and whites, respectively. No differences were observed between mineral metabolism variables and dialysis vintage in the ESRD group.
Table 2 shows the concentration of the mineral metabolism markers across race in HOST participants with CKD not requiring dialysis and ESRD, respectively. Among participants with CKD not on chronic dialysis (Table 2), blacks had lower concentrations of 25(OH)D than whites (14 ng/ml [9–19] versus 21 ng/ml [15–28], P<0.001) and higher concentrations of iPTH (187 pg/ml [108–328] versus 129 [77–216], P<0.001). There were no statistically significant differences in serum phosphate concentrations and kidney function across races in participants with CKD not requiring dialysis. FGF-23 concentrations were lower in blacks than nonblacks (323 RU/ml [181–655] versus 431 RU/ml [232–1026], P<0.001). In participants with ESRD (Table 2), blacks also had lower 25(OH)D concentrations (13 ng/ml [9–19] versus 17 ng/ml [12–24], P<0.001) and higher iPTH (225 pg/ml [135–430] versus 136 pg/ml [87–317], P<0.001) concentrations than non-blacks. No statistically significant differences were observed in FGF-23 concentration (3914 RU/ml [1350–13,682] versus 4036 [95% CI, 1393–12,878], P=0.73). As in CKD, there was no difference in serum phosphorus concentrations across races in patients with ESRD. To note, blacks had been receiving chronic dialysis longer (vintage) than whites. No difference in the concentrations of mineral metabolism markers were observed on the basis of randomization to either the intervention group or the placebo group in the original study across kidney function strata.
Table 2.
Distribution of markers of mineral metabolism across race in patients with CKD not requiring chronic dialysis and ESRD
| Marker | Non-Hispanic White | Non-Hispanic Black | P Value |
|---|---|---|---|
| CKD not requiring chronic dialysis | (n=622) | (n=290) | |
| serum calcium (mg/dl) | 8.9±0.7 | 9.0±0.7 | 0.02 |
| serum phosphate (mg/dl) | 4.3±1.2 | 4.4±1.7 | 0.09 |
| 25-hydroxyvitamin D (ng/ml) | 21 (15–28) | 14 (9–19) | <0.001 |
| 1,25-dihydroxyvitamin D (pg/ml) | 17 (12–26) | 18 (12–25) | 0.67 |
| intact parathyroid hormone (pg/ml) | 129 (77–216) | 187 (108–328) | <0.001 |
| fibroblast growth factor-23 (RU/ml) | 431 (232–1026) | 323 (181–655) | <0.001 |
| estimated GFR (ml/min per 1.73 m2) | 18±6 | 19±7 | 0.06 |
| ESRD | (n=251) | (n=334) | |
| serum calcium (mg/dl) | 9.3±1.0 | 9.1±0.9 | 0.001 |
| serum phosphate (mg/dl) | 5.6±1.8 | 5.5±1.8 | 0.53 |
| 25-hydroxyvitamin D (ng/ml) | 17 (12–24) | 13 (9–19) | <0.001 |
| 1,25-dihydroxyvitamin D (pg/ml) | 12 (8–16) | 12 (8–18) | 0.73 |
| intact parathyroid hormone (pg/ml) | 136 (87–317) | 225 (135–430) | <0.001 |
| fibroblast growth factor-23 (RU/ml) | 4036 (1393–12,878) | 3914 (1350–13,682) | 0.73 |
| time on dialysis (d) | 712±1018 | 950±1343 | 0.02 |
Data expressed as mean ± SD or median (interquartile range).
In general, 25(OH)D concentrations were lower in ESRD compared with CKD not requiring chronic dialysis (15 [10-–21] versus 19 [12–26] ng/ml, P<0.001). A higher percentage of blacks had a 25(OH)D concentration <15 ng/ml (59.9% of blacks with CKD versus 28.0% of whites with CKD and 63.4% of blacks with ESRD versus 41.5% of whites with ESRD; P<0.001 for both). 25(OH)D concentrations <30 ng/ml were highly prevalent among all groups. Secondary hyperparathyroidism defined as an iPTH >65 pg/ml had approximately equal prevalence in advanced CKD and ESRD and blacks had a higher prevalence in both groups than whites (91.4% of blacks with CKD versus 82.2% of whites with CKD and 89.8% of blacks with ESRD versus 83.3% of whites with ESRD; P<0.001 for both). Similar results were observed when secondary hyperparathyroidism was defined as an iPTH >110 pg/ml or an iPTH >300 pg/ml for CKD not yet on dialysis and ESRD, respectively. Among participants with CKD not requiring dialysis, FGF-23 concentrations greater than the median value (273 RU/ml) are more prevalent in whites than blacks (55.1% versus 36.6%; P<0.001). Similarly, for participants with ESRD, FGF-23 concentrations greater than the median value (563 RU/ml) were more prevalent in whites than blacks (52.7% versus 43.8%; P=0.03) but the difference between the two groups is less than in CKD.
In participants with CKD not requiring dialysis, black race was associated with significantly lower 25(OH)D concentrations than whites in both the unadjusted and fully adjusted models with a difference of −4.33 (95% CI, −5.67 to −3.48; P<0.001) in the fully adjusted model (Table 3). Black race was also associated with significantly higher iPTH concentrations than in whites in both unadjusted and adjusted models with a difference of 50.72 (95% CI, 37.00–75.18; P<0.001) in the fully adjusted model. After adjusting for eGFR, FGF-23 explained most of the variance in iPTH (7%). In the fully adjusted linear regression model, blacks had a slightly higher 1,25(OH)2D concentration than nonblacks, with a difference of 1.50 (95% CI, 0.35–2.72, P=0.01). However, this was only observed after adjustments for 25(OH)D. In CKD not requiring dialysis, FGF-23 concentration was lower in blacks than whites in both the unadjusted and adjusted models with a difference of −158.93 (95% CI, −205.26 to −106.00; P<0.001) in the fully adjusted model.
Table 3.
Relationship between race and markers of mineral metabolism in patients with CKD not requiring chronic dialysis and ESRD
| Model | 25(OH)D | 1,25(OH)2D | iPTH | FGF-23 | ||||
|---|---|---|---|---|---|---|---|---|
| Difference (95% CI)a | P Value | Difference (95% CI)b | P Value | Difference (95% CI)c | P Value | Difference (95% CI)d | P Value | |
| CKD not requiring chronic dialysis | ||||||||
| unadjusted | −5.88 (−6.82 to −4.88) | <0.001 | 0.04 (−1.17 to 1.33) | 0.95 | 58.39 (39.69–97.20) | <0.001 | −147.88 (−201.51 to −85.51) | <0.001 |
| model 1 | −5.52 (−6.49 to −4.48) | <0.001 | 0.16 (−0.99 to 1.40) | 0.79 | 61.98 (43.58–82.37) | <0.001 | −158.05 (−204.26 to −105.31) | <0.001 |
| model 2 | −4.33 (−5.67 to −3.48) | <0.001 | 1.50 (0.35–2.72) | 0.01 | 50.72 (37.00–75.18) | <0.001 | −158.93 (−205.26 to −106.00) | <0.001 |
| ESRD | ||||||||
| unadjusted | −4.09 (−5.26 to −2.81) | <0.001 | 0.46 (−0.60 to 1.61) | 0.41 | 72.49 (40.15–110.38) | <0.001 | −144.27 (−942.35 to 855.06) | 0.85 |
| model 1 | −3.93 (−5.19 to −2.54) | <0.001 | 0.80 (−0.35 to 2.06) | 0.18 | 67.74 (33.81–107.99) | <0.001 | −153.10 (−906.90 to −778.25) | 0.72 |
| model 2 | −4.11 (−2.80 to −5.29) | <0.001 | 1.90 (0.69–3.24) | 0.002 | 63.55 (29.89–103.57) | <0.001 | −432.43 (−1148.46 to 456.88) | 0.31 |
Black versus white. Model 1 adjusted for age, sex, season, cardiovascular disease, hypertension, diabetes, body mass index, estimated GFR, serum calcium, and serum phosphorus. 25(OH)D, 25-hydroxyvitamin D; 1,25(OH)2D, 1,25-dihydroxyvitamin D; iPTH, intact parathyroid hormone; FGF-23, fibroblast growth factor 23; 95% CI, 95% confidence interval.
Model 2 adjusted for model 1+1,25(OH)2D, iPTH, and FGF-23.
Model 2 adjusted for model 1+25(OH)D, iPTH, and FGF-23.
Model 2 adjusted for model 1+25(OH)D, 1,25(OH)2D, and FGF-23.
Model 2 adjusted for model 1+25(OH)D, 1,25(OH)2D, and iPTH.
Linear regression models were repeated in the ESRD group (Table 3). In patients with ESRD, black race was associated with lower 25(OH)D concentrations in unadjusted and adjusted models with a difference of −4.11 (95% CI, −2.80 to −5.29; P<0.001) in the fully adjusted model. The iPTH concentration was higher in blacks in both the unadjusted and adjusted models with a difference of 63.55 (95% CI, 29.89–103.57; P<0.001) in the fully adjusted model. Blacks with ESRD also had higher 1,25(OH)2D concentrations compared with whites only after adjustment for 25(OH)D, with a difference of 1.90 (95% CI, 0.69–3.24; P=0.002). There was no difference between races when plasma FGF-23 concentrations were modeled for the ESRD group. Finally, results remained unchanged when linear regression analyses for the CKD and ESRD group were restricted only to male patients.
Discussion
In this cross-sectional study of 1497 patients with kidney disease, we found that blacks have significantly lower 25(OH)D concentrations and higher iPTH concentrations than whites. FGF-23 was significantly lower in blacks than in whites in patients with CKD, whereas there was no association between race and FGF-23 concentrations in multivariate linear regression models in patients with ESRD. To our knowledge, this is the first study to evaluate racial differences in FGF-23 concentrations in predialysis CKD and ESRD patients, independent of 25(OH)D, 1,25(OH)2D, and iPTH concentrations.
FGF-23 has received attention recently for its potential role in the increased risk of CVD and death in patients with CKD. Racial differences in FGF-23 concentrations have been reported in the dialysis population, but not in predialysis CKD patients. Gutierrez et al. found that the median FGF-23 concentrations were significantly lower in blacks initiating dialysis than in whites and Hispanics (19). We found no significant racial differences in FGF-23 concentrations in dialysis patients; however, in contrast to the study by Gutierrez et al. our dialysis cohort was not new to dialysis and most had been receiving renal replacement therapy for ≥2 years. In patients with severe CKD not yet requiring dialysis, blacks had significantly lower FGF-23 concentrations than whites independent of other makers of mineral metabolism. These results are consistent with the findings of Gutierrez et al. and suggest that FGF-23 concentrations are different across race in severe CKD. In addition, we observed that the correlation of higher FGF-23 with lower 1,25(OH)2D and higher iPTH concentrations was stronger in blacks than in whites with CKD not yet on dialysis.
Similar to other studies (16–18), we found that blacks with CKD not yet on dialysis and ESRD have lower 25(OH)D concentrations and higher iPTH concentrations compared with whites with comparable levels of kidney function and independent of FGF-23 concentrations. We also found that secondary hyperparathyroidism, defined as iPTH >65 pg/ml, was more prevalent in blacks compared with whites with CKD not requiring dialysis and ESRD. Blacks with CKD not yet on dialysis had a more robust increase in iPTH with declining kidney function than nonblacks. In addition, blacks with CKD not requiring dialysis and ESRD were noted to have slightly higher 1,25(OH)2D concentrations, which was only evident after adjustment for 25(OH)D concentrations. These findings suggest that there may be a unique mechanism by which blacks develop secondary hyperparathyroidism, which is independent of 25(OH)D and FGF-23 (27).
Future studies in the CKD population are needed to evaluate whether racial differences exist in skeletal resistance to PTH or in the expression or activation of the calcium-sensing receptor in the parathyroid gland, both of which are possible explanations for the increased prevalence of secondary hyperparathyroidism in blacks. Furthermore, although current guidelines for the management of CKD recommend screening for and treating abnormalities in mineral metabolism (28), none take into account racial differences in mineral metabolism. According to the findings of this study and others (16), current screening recommendations may not be appropriate for all races, especially blacks.
We recognize several limitations to our study. First, we only had laboratory values obtained at one point in time and were unable to analyze the changes in mineral metabolism across races over time. Second, we did not have information on patient use of active vitamin D analogs or vitamin D supplement use. However, given the time period in which the HOST study was performed, active vitamin D analogs, vitamin D supplementation, and phosphate binders were not likely routinely given to CKD patients not yet on dialysis. Third, most of the patients were male with advanced CKD and caution should be used when extrapolating these results to female patients and patients with less advanced CKD not requiring dialysis.
In conclusion, we found that blacks with CKD not yet on dialysis have lower 25(OH)D and FGF-23 concentrations and higher 1,25(OH)2D and iPTH concentrations compared with whites when controlled for mineral metabolism markers. Blacks with ESRD have similar differences in mineral metabolism except for FGF-23. This study confirms racial differences in mineral metabolism in patients with kidney disease. It is imperative to further evaluate potential reasons for the racial differences seen in kidney disease and to evaluate whether current screening and treatment guidelines are adequate for all races/ethnicities.
Disclosures
None.
Acknowledgments
We thank William E. Owen for assistance with the plasma FGF-23 assay.
The research reported in this study was supported by the Department of Veterans Affairs Cooperative Studies Program and the HOST Executive Committee members Rex L. Jamison, Pamela Hartigan, James Kaufman, David S. Goldfarb, Stuart R. Warren, Peter D. Guarino, and J. Michael Gaziano. Additional support came from the National Institute of Diabetes and Digestive and Kidney Disease Grants K23DK087859-01A1, R01 DK081473, and R01 DK078112, as well as an Amgen fellowship grant and an investigator initiated proposal funded by Genzyme and Abbott.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.07020711/-/DCSupplemental.
References
- 1.Rostand SG, Drüeke TB: Parathyroid hormone, vitamin D, and cardiovascular disease in chronic renal failure. Kidney Int 56: 383–392, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Wolf M, Shah A, Gutiérrez O, Ankers E, Monroy M, Tamez H, Steele D, Chang Y, Camargo CA, Jr, Tonelli M, Thadhani R: Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int 72: 1004–1013, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM: Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15: 2208–2218, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Tentori F, Blayney MJ, Albert JM, Gillespie BW, Kerr PG, Bommer J, Young EW, Akizawa T, Akiba T, Pisoni RL, Robinson BM, Port FK: Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: The Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 52: 519–530, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Wald R, Sarnak MJ, Tighiouart H, Cheung AK, Levey AS, Eknoyan G, Miskulin DC: Disordered mineral metabolism in hemodialysis patients: An analysis of cumulative effects in the Hemodialysis (HEMO) Study. Am J Kidney Dis 52: 531–540, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Wang AY, Lam CW, Sanderson JE, Wang M, Chan IH, Lui SF, Sea MM, Woo J: Serum 25-hydroxyvitamin D status and cardiovascular outcomes in chronic peritoneal dialysis patients: A 3-y prospective cohort study. Am J Clin Nutr 87: 1631–1638, 2008 [DOI] [PubMed] [Google Scholar]
- 7.El-Abbadi MM, Pai AS, Leaf EM, Yang HY, Bartley BA, Quan KK, Ingalls CM, Liao HW, Giachelli CM: Phosphate feeding induces arterial medial calcification in uremic mice: Role of serum phosphorus, fibroblast growth factor-23, and osteopontin. Kidney Int 75: 1297–1307, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gutiérrez OM, Januzzi JL, Isakova T, Laliberte K, Smith K, Collerone G, Sarwar A, Hoffmann U, Coglianese E, Christenson R, Wang TJ, deFilippi C, Wolf M: Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation 119: 2545–2552, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teng M, Wolf M, Ofsthun MN, Lazarus JM, Hernán MA, Camargo CA, Jr, Thadhani R: Activated injectable vitamin D and hemodialysis survival: A historical cohort study. J Am Soc Nephrol 16: 1115–1125, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Shoben AB, Rudser KD, de Boer IH, Young B, Kestenbaum B: Association of oral calcitriol with improved survival in nondialyzed CKD. J Am Soc Nephrol 19: 1613–1619, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber TJ, Liu S, Indridason OS, Quarles LD: Serum FGF23 levels in normal and disordered phosphorus homeostasis. J Bone Miner Res 18: 1227–1234, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Gutiérrez O, Isakova T, Rhee E, Shah A, Holmes J, Collerone G, Jüppner H, Wolf M: Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol 16: 2205–2215, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Parker BD, Schurgers LJ, Brandenburg VM, Christenson RH, Vermeer C, Ketteler M, Shlipak MG, Whooley MA, Ix JH: The associations of fibroblast growth factor 23 and uncarboxylated matrix Gla protein with mortality in coronary artery disease: The Heart and Soul Study. Ann Intern Med 152: 640–648, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robinson BM, Joffe MM, Pisoni RL, Port FK, Feldman HI: Revisiting survival differences by race and ethnicity among hemodialysis patients: The Dialysis Outcomes and Practice Patterns Study. J Am Soc Nephrol 17: 2910–2918, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Mehrotra R, Kermah D, Fried L, Adler S, Norris K: Racial differences in mortality among those with CKD. J Am Soc Nephrol 19: 1403–1410, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gutiérrez OM, Isakova T, Andress DL, Levin A, Wolf M: Prevalence and severity of disordered mineral metabolism in blacks with chronic kidney disease. Kidney Int 73: 956–962, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Dawson-Hughes B: Racial/ethnic considerations in making recommendations for vitamin D for adult and elderly men and women. Am J Clin Nutr 80[Suppl]: 1763S–1766S, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Nesby-O’Dell S, Scanlon KS, Cogswell ME, Gillespie C, Hollis BW, Looker AC, Allen C, Doughertly C, Gunter EW, Bowman BA: Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: Third National Health and Nutrition Examination Survey, 1988-1994. Am J Clin Nutr 76: 187–192, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Gutiérrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Jüppner H, Wolf M: Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 359: 584–592, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jamison RL, Hartigan P, Kaufman JS, Goldfarb DS, Warren SR, Guarino PD, Gaziano JM, Veterans Affairs Site Investigators : Effect of homocysteine lowering on mortality and vascular disease in advanced chronic kidney disease and end-stage renal disease: A randomized controlled trial. JAMA 298: 1163–1170, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Jonsson KB, Zahradnik R, Larsson T, White KE, Sugimoto T, Imanishi Y, Yamamoto T, Hampson G, Koshiyama H, Ljunggren O, Oba K, Yang IM, Miyauchi A, Econs MJ, Lavigne J, Jüppner H: Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med 348: 1656–1663, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Walser M: Assessing renal function from creatinine measurements in adults with chronic renal failure. Am J Kidney Dis 32: 23–31, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Mehrotra R, Kermah DA, Salusky IB, Wolf MS, Thadhani RI, Chiu YW, Martins D, Adler SG, Norris KC: Chronic kidney disease, hypovitaminosis D, and mortality in the United States. Kidney Int 76: 977–983, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muntner P, Jones TM, Hyre AD, Melamed ML, Alper A, Raggi P, Leonard MB: Association of serum intact parathyroid hormone with lower estimated glomerular filtration rate. Clin J Am Soc Nephrol 4: 186–194, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Isakova T, Wahl P, Vargas GS, Gutiérrez OM, Scialla J, Xie H, Appleby D, Nessel L, Bellovich K, Chen J, Hamm L, Gadegbeku C, Horwitz E, Townsend RR, Anderson CA, Lash JP, Hsu CY, Leonard MB, Wolf M: Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 79: 1370–1378, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scragg R, Sowers M, Bell C, Third National Health and Nutrition Examination Survey : Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care 27: 2813–2818, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group : KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 113: S1–S130, 2009 [DOI] [PubMed] [Google Scholar]