Summary
Patient safety is the foundation of high-quality health care. More than 350,000 patients receive dialysis in the United States, and the safety of their care is ultimately the responsibility of the facility medical director. The medical director must establish a culture of safety in the dialysis unit and lead the quality assessment and performance improvement process. Several lines of investigation, including surveys of patients and dialysis professionals, have helped to identify important areas of safety risk in dialysis facilities. Among these are lapses in communication, medication errors, patient falls, errors in machine and membrane preparation, failure to follow established policies, and lapses in infection control. The quality assessment and performance improvement process should include a dedicated safety team to focus on specifically identified areas of risk and to establish outcome goals guided by best practices and agreed-upon measures of success. A safety questionnaire can be given to patients and staff and the responses evaluated to improve understanding of the prevailing attitudes and concerns about safety. By sharing these results, openly acknowledging the challenges, and using a blame-free root cause process to identify action plans, the facility can begin to establish a culture of safety.
Introduction
In July 2008, the New York State Department of Health received notification that over the preceding 6 months three hemodialysis patients in one unit experienced a hepatitis C seroconversion (1). In 2010, during a facility survey in California, “dummy drip chambers,” which increase the risk for air embolism, were used on the treatment floor to quickly prepare the machines for patient use (2). Both cases exemplified a departure from standard of care, and the medical directors and the governing bodies received citations.
These are examples of compromised safety in United States dialysis facilities. This review focuses on the application of safety principles to dialysis facilities. We review the data on high-risk safety areas within dialysis facilities and suggest strategies to improve patient safety.
Dialysis Safety Priorities
Dialysis facilities are complex organizations that involve providers from multiple disciplines and use advanced technology to care for patients with multiple serious illnesses. As organizations become more complex, the possibility for error increases (3), and potential risks must be identified and prioritized.
Some risks are readily apparent in dialysis facilities (4–6). Water quality, membrane reuse, and infection control are key areas of safety risk, and adverse events have been reported in each area (7–15). Infection control risks, such as design flaws (9), inadequate hand hygiene, and faulty machine and equipment disinfection (7,8,15,16), have been associated with outbreaks of unusual biopathogens (8,9,13), as well as transmission of vancomycin-resistant enterococcus and hepatitis B and C viruses. The regulations and safety guidelines for water quality, reuse, and infection control are established and readily available (14,17).
Other safety risks may not be as readily apparent, and data-driven efforts have helped to identify and prioritize safety efforts. Initial studies by Holley (4) on adverse events and medical errors in dialysis reported an error rate of 1 in 733 treatments and noted access events, medication errors, circuit clotting, and patient falls as among the most common events. A survey report from the National ESRD Patient Safety Initiative compiled a ranked list of safety issues (18). The top five safety issues were as follows: (1) patient falls, (2) medication errors (including deviation from dialysis prescription, allergic reactions, and medication omissions), (3) access-related events (clotting, infiltrates, poor blood flow, difficult cannulation), (4) dialyzer errors (incorrect dialyzer or dialysate and equipment-related sepsis), and (5) excess blood loss or prolonged bleeding.
In 2006, the Renal Physicians Association (RPA), in collaboration with dialysis patients, nurses, administrators, and the Forum of ESRD Networks, launched a nationwide survey of dialysis patients and providers (6,19). The survey focused on safety and assessed the attitudes of dialysis patients and professionals toward safety. The structure and results of this health and safety survey are available on the RPA website (http://www.renalmd.org/Patient-Safety).
The patient sample was randomly drawn from the Renal Management Information System maintained by Medicare and included patients who had been undergoing dialysis for at least 3 months. A total 1143 surveys (response rate, 32%) were available for analysis (19). The patient survey was completed on paper. Twenty-six percent of the patients required help completing the survey, which was largely provided by their spouse or family (81.8%). That help involved reading the questions and answers (43%), writing the results on the survey (33.6%), or answering the questions (12%). In a separate online survey of dialysis caregivers, responses were compiled from 649 dialysis professionals (Table 1) (6).
Table 1.
Renal Physicians Association Health and Safety Professional Survey respondents by role
| Role in Facility | Number | Percentage of Total |
|---|---|---|
| Assistant | ||
| administrative assistant or receptionist | 33 | 5.1 |
| social worker | 94 | 14.5 |
| dietitian | 37 | 5.7 |
| patient care technician | 21 | 3.2 |
| other technician (e.g., reuse, machine) | 19 | 2.9 |
| physician assistant | 0 | 0 |
| Nurse | ||
| nurse practitioner | 12 | 1.8 |
| nurse | 124 | 19.1 |
| charge nurse | 54 | 8.3 |
| nurse manager | 160 | 24.7 |
| Manager | ||
| facility administrator | 112 | 17.3 |
| area manager | 23 | 3.5 |
| Doctor | ||
| medical director | 26 | 4 |
| medical doctor | 26 | 4 |
| Total | 649 | 100 |
Reproduced from Renal Physicians Association Health and Safety Survey—professional respondents, with permission.
The surveys have limitations. Although all ESRD Networks were represented in both surveys, the numbers of responses from the East Coast exceeded those from other regions, and physicians had the lowest response rate. In addition, because there was no incentive to participate, the professional respondents may represent those most interested in safety. This bias might have yielded a more favorable view of dialysis safety as compared with the larger population of dialysis professionals. The RPA surveys do, however, demonstrate the perspective and concerns of patients and caregivers and, together with other data, offer insight into the safety hazards and events associated with dialysis therapy.
Safety Hazards
As shown in Table 2, many safety risk areas have been associated with dialysis. Safety hazards are latent risks that can jeopardize patient and facility safety. Several types of latent hazards exist, but these latent failures can largely be grouped into two broad categories: failures of communication and failures of policies. Policy failures include content errors and failures of implementation and compliance.
Table 2.
Areas of safety risk
| Patient safety hazards |
| communication, documentation, and/or training failures |
| failure to follow policy and procedure |
| poorly designed/implemented policies and procedures |
| lapses in infection control and surveillance (including access, catheter and hand hygiene) |
| machine design flaws |
| facility design flaws (including water purification system) |
| Patient safety events |
| General safety events |
| patient falls |
| medication errors |
| Access-related issues |
| clotting, poor blood flow |
| difficulty with cannulation |
| needle dislodgment/bleeding from needle site |
| prolonged bleeding |
| Equipment issues |
| failure of dialysis equipment |
| failure of water/reuse equipment |
Communication
Miscommunication has been documented as a key safety risk that has great potential for patient harm. Data from the Joint Commission suggest that approximately 63% of sentinel events are directly linked to communication failures (20). The RPA surveys (6,19) queried patients and professionals regarding their attitudes about communication issues. Among the professional respondents, 94% indicated it was “easy” or “very easy” to communicate with patients. Among professional respondents, 63% said that patients most frequently communicated about treatment issues, and about half of the respondents indicated that patients sometimes discussed concerns about safety (44%) and staff (48%).
However, when patients were asked how “comfortable they feel about discussing their problems,” about 20% were “uncomfortable to somewhat comfortable” discussing their care with a nurse, dietitian, or technician, and 20% said the instructions they received from those staff were unclear or only somewhat clear. These data are especially notable; several studies suggest that cognition and memory are impaired in patients with renal disease, particularly during dialysis (21–23). Other studies have demonstrated limited health care literacy, independently influenced by ethnicity and socioeconomic factors, among dialysis patients (24). Together, the data suggest that dialysis patients’ ability to assimilate data during dialysis may vary, making communication failures an inherent risk for advanced kidney disease. Other specialties have demonstrated that patient understanding of and participation in their care (which are key barriers to safe care) can be improved by using visual (charts, videos, graphics) and reading level–appropriate written educational tools (25,26). From a safety perspective, the creation of toolkits for patients with both written and graphic material about dialysis safety may help improve outcomes.
Miscommunication between dialysis staff and others can also contribute to safety failures (26). Care transitions between providers and care settings provide prime opportunities for communication errors. These transitions are common among dialysis patients as they undergo access procedures, hospitalizations, and specialist consultations. Facilities should evaluate scripted communication guides so that vital data are readily available and shared with providers. Patients should have copies of their problems lists, medications, allergies, and other vital information. These should be updated frequently, and patients should be educated to share copies with providers.
Failure to Follow Policies and Protocols
Failure to document and follow protocol are also key latent safety hazards (5,27,28). A recent ESRD Network quality audit found that about 4% of the dialysis records did not correctly document the dialyzer used (5). In Pennsylvania, failure to follow protocols represented almost 13% of the annual dialysis events reported (28). About 10% of patients responding to the RPA Health and Safety Survey indicated that during the prior 3 months, their BP and weight were not always measured before dialysis, and 13% of the professional respondents agreed that this had “sometimes” happened (6,19). Almost 60% of professionals said mistakes in the dialysis setup (dialyzer or bath) had occurred in the prior 3 months. Of note, patients noted these errors less frequently (6%). It is possible (although not definite from the survey questions) that the error was caught before initiation of therapy; however, patients also could have been unaware of the errors (6,19). In addition to clinical safety, failure to follow protocol has also contributed to technical mistakes and lapses in infection control (1,2,4–9). Among other risks, protocol departures can result in critical errors in dialysis reuse, dialysate composition, and water purification, with catastrophic effects on an entire facility (7–13).
Adherence to protocols can be improved by prominently posting key portions of critical policies and procedures and by using checklists, double sign-offs, and “red rules,” which must be followed exactly (29). Understanding patients’ attitudes toward their illness, educating them about machine preparation and treatments, and encouraging their participation in their care may also improve outcomes (25,30).
Dialysis Safety Events
Unlike safety hazards, safety events denote the actual occurrence of errors and mishaps that have compromised patient safety. Safety events vary in magnitude of risk, but the goal is to anticipate any such events and prevent them.
Patient Falls
The majority of dialysis patients are older than age 65 (31), making the risk for fall more prevalent. Earlier studies have demonstrated an increased risk for falls in dialysis patients (4,18,28,32–37). Age, diabetes, motor strength, medication use (including antidepressants), a failed walking test, previous fall episodes, and visual impairment are all risk factors for falls (33–39). Fall prevention is important because the incidence of hip fracture with fall and the 1-year mortality rate related to a hip fracture are increased in the dialysis population (36). Cook and colleagues (33) studied dialysis patients older than age 65 and demonstrated that 47% of patients fell during a 1-year period and 19% sustained injuries. In a separate prospective study, Desmet and colleagues (34) reported that during 12 months, 12 of 380 dialysis patients (mean age, 70.9 years) experienced a fall with fracture, and the overall fall rate was 1.18 falls/patient-year. This rate is many times higher than that in the nondialysis elderly population (0.32–0.7 falls/patient-year). Episodes of orthostatic hypotension (decrease in systolic BP > 20 mmHg) after dialysis were tracked, and a detailed report was obtained for every fall. Most falls (82%) occurred at home. Falls tended to be more common during the first half of the interdialytic interval; of note, however, neither postdialysis BP nor orthostatic hypotension was predictive of falling.
The RPA survey queried falls in hemodialysis units; 55 patients reported falls at dialysis during the prior 3 months (19). The most frequent explanations for falls provided by patients were dizziness or weakness, difficulty in transferring, and tripping within the unit. About 40% of the time the professional respondents stated they did not know the cause of the fall. Several strategies may help to reduce the risk for falls (34–39), including monitoring orthostatic BP, staff education, use of evidence-based tools for fall assessment (such as assessment of gait and vision), gait assistance for high-risk patients, controlling clutter, and use of in-floor patient weight scales.
Medications
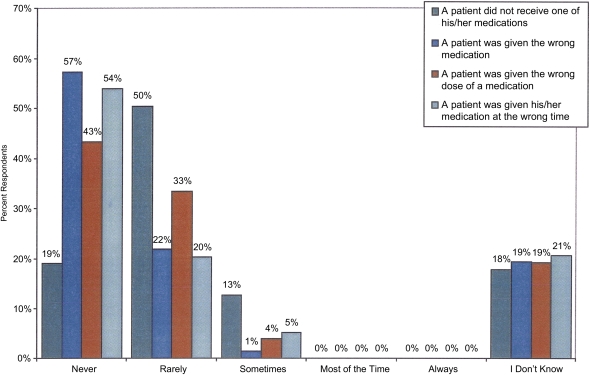
Almost half of the RPA Health Survey patients reported taking 6–10 medications daily, yet most patients reported only “sometimes” discussing all their medications with their doctor (19). In the 2008–2009 survey by the Pennsylvania Patient Safety Authority, medication errors were the most common event type (28.5%), and most of these were errors of omission (48%) (28). Other studies (4,5,19,40–42) have confirmed the risk for medication omission in dialysis. Regarding the types of errors, intravenous heparin (both omission and dosage) accounted for slightly more than 11% of the medication errors noted by the Pennsylvania Patient Safety Authority (28). Other errors involved such agents as erythropoietin, vitamin D, and antibiotics. Surprisingly, more than half of the professional respondents to the RPA safety survey believe that patients are never given the wrong medications or are given a medication at an incorrect time (Figure 1).
Figure 1.
Frequency of medication errors as reported by dialysis professionals. Used with permission from the RPA Health and Safety Survey.
In addition to medication errors at dialysis, these patients are at very high risk for medication errors when transitioning between care settings and providers (43–45). Dialysis patients require complex multidrug regimens. Non-nephrologists are often not well versed about the types of medications and the restrictions and dosing changes required by dialysis patients. For example, in a study of percutaneous cardiac interventions, despite clearly labeled warnings, 22.3% of dialysis patients received medications that were contraindicated or not recommended (enoxaparin and eptifibatide) and sustained high rates of major bleeding complications (46). Strategies to minimize medication errors include frequent review of patients’ medication lists, educating patients to share their list with each provider, medication reconciliation when care settings change, and pharmacist participation in medication review (40–45,47).
Access-Related Events
Thirty percent of patients responding to the RPA health survey indicated that staff tried more than twice to insert needles before getting assistance, and 39% reported pain at the needle site. Of note, the majority of patient care technicians and nurses indicated that they “rarely or never” had difficulties inserting needles, and two thirds indicated that after two attempts they called for assistance. Most staff said that a policy on difficult cannulation did not exist or that they were not familiar with the policy (6,19). Access infiltration (usually at the initiation of therapy) represented 6.1% of the adverse dialysis events reported to the Pennsylvania Patient Safety Authority during a 1-year period and 31 of the 88 adverse events reported over an 18-month period by Holley (4,28). In a study by Lee and colleagues, major fistula infiltrations leading to additional intervention (including catheter placement) occurred at an annualized rate of 5.2% and were more common with new fistulas (<6 months old) and in older patients (47). Strategies that include clear access policies and trained needling teams for new fistulas might reduce risk.
Dislodgement of the access needle and catheter disconnections are potentially life-threatening events (48–51). Five percent of patients in the RPA safety study reported that the needle dislodged before completion of therapy (19), and access interruptions accounted for 6% of incidents reported to the Pennsylvania Patient Safety Authority (28). Arterial and venous pressure alarms on dialysis machines are not sensitive enough to detect a partial dislodgment, and rapid blood loss can occur at the typical blood flow rate. The Veterans Affairs National Center for Patient Safety found that 40 of 47 bleeding episodes analyzed between 2002 and 2008 were related to venous needle dislodgment (51). Patient agitation and dialysis performed outside the main unit were major risk factors. Some of these resulted in patient death. As a result, all Veterans Affairs dialysis centers now use a Food and Drug Administration–approved access alarm for all patients with venous needle access undergoing hemodialysis outside the treatment unit (51). Catheter access clamps that secure the access hub to the dialysis tubing are also available (52). Other access-related safety issues include failure to cap catheter ports, failure to adequately clamp access lines, and prolonged access bleeding after dialysis (6,18,28,51). Dialysis safety teams should ensure that their policies and procedures articulate that the access must remain visible throughout the treatment. Moreover, industry should develop access needle monitors that are intrinsic to the dialysis circuit.
Hygiene Issues
Improper hand and glove hygiene were also troubling findings of the RPA safety survey. Over a 3- month period, about 10% of patients and 25% of staff reported that infection control surrounding access was not always followed. The risks engendered by this behavior are clear (14–16,53), and it is a crucial issue for the facility quality committee.
Dialysis Machine Errors and Events
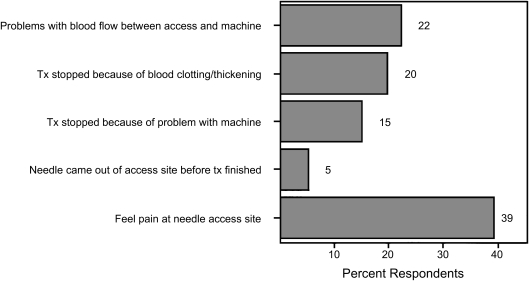
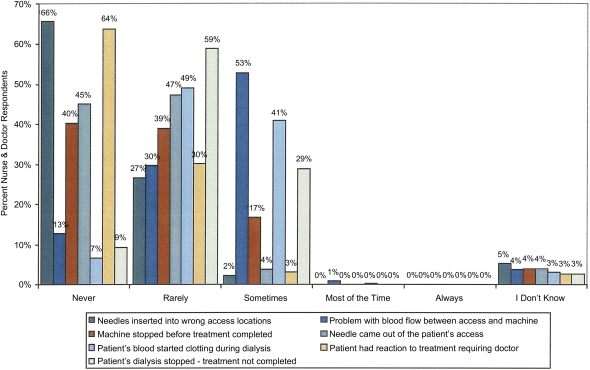
Fifteen percent of patients in the RPA safety survey reported that treatment was ended early because of problems with the dialysis equipment (Figure 2), and 20% reported that the machine clotted during treatment (19). Holley (4) reported similar data on clotting of the dialysis circuit but found that equipment failures per se were rare. Equipment problems accounted for about 4% of the adverse dialysis events reported to the Pennsylvania Department of Health (28). Surprisingly, 17% of the professionals responding indicated that in the prior 3 months a machine was stopped before the scheduled completion time (Figure 3) (6).
Figure 2.
Occurrence of each event during dialysis in past 3 months as reported by dialysis patients. Used with permission from the RPA Health and Safety Survey.
Figure 3.
Percentage of nurse and doctor respondents by frequency of events occurring during dialysis. Used with permission from the RPA Health and Safety Survey.
Attitudes toward Safety
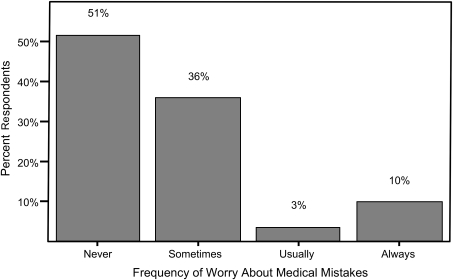
About 75% of the patients and almost 90% of professional respondents to the health and safety surveys said they would report a medical mistake, and 92% of patients indicated that it was “easy or somewhat easy” to get help whenever a problem arose. Despite these findings, almost half of the patients who responded indicated that they worry someone will make a medical mistake during their dialysis treatment (Figure 4), and 16% of respondents indicated that some things make them feel unsafe at the dialysis center. Patients were specifically asked about their level of involvement and knowledge regarding their dialysis treatments; their beliefs about the safety of their dialysis unit were not influenced by their self-reported level of involvement in their care (6,19).
Figure 4.
Percentage of patient respondents by frequency of worry about occurrence of medical mistakes. Used with permission from the RPA Health and Safety Survey.
Of professional respondents, 70% indicated that mistakes had never or rarely occurred during the past 3 months. The majority of professionals reported a “very low” likelihood that a medical mistake harmful to a patient would be made at their center (6). These findings contrast with the Pennsylvania Patient Safety Authority’s analysis of events by harm score (which assesses how often the event reached the patient and the risk for harm to the patient) (47). That analysis demonstrated that 5.5% of the events that reached their dialysis patients resulted in harm (28). As was found in other surveys (28), the majority of professionals in the RPA health and safety survey believe that medical errors were related to failure to comply with existing policies rather than to the lack of staffing, equipment, or an adequate quality program.
Developing a Culture of Safety
Elements of a Culture of Safety
The findings of the RPA health and safety surveys, summarized in Table 3, together with the other data presented, illustrate that the dialysis setting presents potential threats to patient safety. These areas offer a starting point to develop process improvements and establish a culture of safety. The key elements of a culture of safety are outlined in Table 4. Critical among these is the understanding that even a “safe” organization is not error-free. Instead, safe organizations anticipate “what-if” events and avoid blaming adverse events on an individual’s failure. Root cause analysis is used to discover the system and process issues that contribute to adverse events. The goal of a root cause analysis is to determine what happened, why it happened, and what to do to prevent it from happening again (54). This analysis examines the policies, processes, and human factors (such as staffing ratios, experience, training, distraction, and fatigue [55–59]) that can contribute to an event.
Table 3.
Summary findings of Renal Physicians Association health and safety surveys
| Safety Issues during the Prior 3 Months | Professional Staff Response (%) | Patient Response (%) |
|---|---|---|
| Patients worried or concerned about safety/staff | Patients communicated concerns sometimes or always: 63 | Sometimes or always worried: 49 |
| Ease of communication | Easy to communicate with patients: 94 | Uncomfortable/somewhat uncomfortable communicating with staff: 18 |
| BP or weight not recorded prior to dialysis | Happened sometimes: 13 | Happened sometimes: 10 |
| Mistakes in membrane or bath set up | Happened sometimes: 60 | Happened sometimes: 6 |
| Lapses in infection control (hand hygiene) | Reported event occurred: 27 | Reported event occurred: 11 |
| Medication errors | Missed or incorrect dose occurred sometimes: 23 | Always discussed all medications with staff: 23 |
| Difficulty with access needles | Rare or no difficulty inserting: 66 | Pain at access site during treatment: 39 |
| Prolonged access bleeding | Sometimes: 15 | Sometimes: 23 |
| Needle dislodgement prior to end of treatment | Sometimes occurred: 4 | Reported event occurred: 5 |
| Medical mistakes in prior 3 months | Reported no events occurred: 70 | Reported no events occurred: 73 |
Used with permission from the RPA Health and Safety Survey.
Table 4.
Elements of a culture of safety
| Acknowledge the high-risk nature of the activity |
| Establish safety as a key goal in policies and procedures |
| Evaluate errors as “system failures,” not as an individual’s failures |
| Commit needed resources, including time and technology |
| Recognize that a “safe” environment is not error free |
| Report “near misses” and events in blame- and retaliation-free environment |
| Develop processes for peer review and analysis of root cause |
A culture of safety, void of “blaming” behavior, does not obviate individual accountability for competent, appropriate care. If a dialysis caregiver neglects to follow an established process or procedure or behaves in ways that endanger patients, then peer review, remediation, and disciplinary action may be required. A robust safety system includes both individual peer review and system root cause analysis.
The Quality Improvement Process
The 2008 Centers for Medicare & Medicaid Services (CMS) Conditions for Coverage for End-Stage Renal Disease provide specific guidance concerning safety in the dialysis setting (60). The medical director is specified as the leader of the multidisciplinary quality assessment and performance improvement program (QAPI) and is charged with establishing a culture of safety and quality (CMS interpretive guideline tags V710-716) (60–62). In corporation-managed or -owned facilities, the CMS regulations hold the facility governing body responsible for allocating staff and resources for the QAPI program (CMS interpretive guideline tag V756). The medical director, who is expected to have “some authority to individualize corporate policies to address unique facility situations” (CMS interpretive guideline tag V714), is held responsible for the direct oversight and the outcomes of the quality and safety programs. A data-driven QAPI plan must review both facility- and patient-specific outcome data that focus on performance indictors that address medical errors, medical injuries, patient satisfaction, patient safety, and infection control (CMS interpretive guideline tags V626-628).
A successful QAPI plan requires that data be reliably collected and rigorously analyzed. One well accepted format for this is the Plan-Do-Check-Act cycle (63). This method begins with using available data and literature to “plan” the needed process improvements, which are then implemented (“do”). The results are evaluated to determine whether performance has improved (“check”). If outcomes improve, the results are shared and the new processes implemented throughout the organization (“act”), and outcomes are reverified to ensure that the improvements are effective and sustained. If the desired outcomes are not achieved, then alternative or additional improvements must be planned and implemented and the results reanalyzed (63).
Facility Safety Committee
CMS regulations clearly stipulate that patient and facility safety must be addressed as an integral component of the QAPI process. Facilities should create a dedicated safety team; as such teams can more reliably improve outcomes than can ad hoc efforts (64). Attitudes regarding safety can be disparate (65), and valuable information can be learned by having patients and staff complete a safety questionnaire. Such tools are available from the Agency for Healthcare Research and Quality and the Five Diamond Patient Safety Project, spearheaded by several ESRD Networks (66).
The team can evaluate safety risks and determine appropriate priorities (such as falls, medication errors, and access complications). Focused safety efforts are best accomplished when well-defined measures of success are used to monitor the effectiveness of each safety initiative. Safety teams should use specific strategies to improve care processes and reduce risk. For example, if compliance with protocols is a facility safety goal, the team might choose to implement checklists, double sign-offs, or “red rules” and then remonitor adherence. If a safety goal is not achieved, the interdisciplinary team must reevaluate the approach and seek new remedies.
Safety Resources
Excellent sites for general information focused on patient safety include the National Patient Safety Foundation (67) and the Agency for Healthcare Research and Quality (68). The Veterans Affairs National Center for Patient Safety (54) contains procedural information on root cause analysis, and the World Health Organization has online safety courses and tools for tracking patient safety risks and outcome analysis (69).
Dialysis-specific information, including education modules and a patient safety tool kit, is available at the RPA website, which also sponsors the Keeping Kidney Patients Safe website (70). The Five Diamond Patient Safety Program (66) focuses on dialysis unit safety and promotes staff and patient education aimed at creating a culture of safety. The site contains a comprehensive patient safety plan as well as questionnaires regarding attitudes toward safety. Several ESRD Network sites contain valuable examples of QAPI plans and Plan-Do-Check-Act methods (5,66,71).
Our collective appreciation and understanding of the unique safety challenges and opportunities faced by dialysis facilities would probably be enhanced by a web-based, nationwide, searchable, legally protected dialysis quality compendium. Once developed, this database could also serve as a mechanism for units to learn from each other and to share best practices.
Conclusion
The data indicate that dialysis facilities share important safety risks, and patients report more anxiety about unit safety practices than staff might predict. The data also suggest that staff believe units are safe, perhaps more safe than supported by the available data. To improve safety, the medical director and the QAPI and safety committees should prioritize goals and develop outcome-based, data-driven action plans. Staff and patients should be encouraged to express their concerns in a blame-free environment as the facility strives to create a facility culture of safety.
Disclosures
None.
Acknowledgments
The Health and Safety Survey was sponsored by the Renal Physicians Association (RPA) and the Kidney and Urology Foundation of America (KUFA), in conjunction with the Network of New England, American Association of Kidney Patients (AAKP), American Nephrology Nurses' Association (ANNA), Forum of ESRD Networks, and the National Renal Administrators Association (NRAA).
Support was provided by an educational grant from Abbott Laboratories.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Centers for Disease Control and Prevention : Hepatitis C virus transmission at an outpatient hemodialysis unit—New York, 2001-2008. MMWR Morb Mortal Wkly Rep 58: 189–194, 2009 [PubMed] [Google Scholar]
- 2.California state dialysis facility survey. Available at: http://www.qualitysafepatientcare.com/2009-dialysisfacility-surveys—calif.php Accessed April 20, 2011
- 3.Wreathall J, Nemeth C: Assessing risk: The role of probabilistic risk assessment (PRA) in patient safety improvement. Qual Saf Health Care 13: 206–212, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holley JL: A descriptive report of errors and adverse events in chronic hemodialysis units. Nephrol News Issues 29: 57–58, 60–61, 63, 2006 [PubMed] [Google Scholar]
- 5.Heartland Kidney Network. Patient safety fact sheet. Available at: http://www.network12.org/quality/patient_safety.html Accessed April 13, 2011
- 6.Report on the findings from the ESRD professional survey. Rockville, MD, Renal PhysiciansAssociation, 2007. Available at: http://www.kidneypatientsafety.org/uploadedFiles/HSSProfessionalSurveyReport_FNL_3-21-07.pdf Accessed April 1, 2011
- 7.Savey A, Simon F, Izopet J, Lepoutre A, Fabry J, Desenclos JC: A large nosocomial outbreak of hepatitis C virus infections at a hemodialysis center. Infect Control Hosp Epidemiol 26: 752–760, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Grabsch EA, Burrell LJ, Padiglione A, O’Keeffe JM, Ballard S, Grayson ML: Risk of environmental and healthcare worker contamination with vancomycin-resistant enterococci during outpatient procedures and hemodialysis. Infect Control Hosp Epidemiol 27: 287–293, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Jochimsen EM, Frenette C, Delorme M, Arduino M, Aguero S, Carson L, Ismaïl J, Lapierre S, Czyziw E, Tokars JI, Jarvis WR: A cluster of bloodstream infections and pyrogenic reactions among hemodialysis patients traced to dialysis machine waste-handling option units. Am J Nephrol 18: 485–489, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Light PD: Re-use of dialysis membranes. In: Chronic Dialysis Principles and Practice of Dialysis, edited by Henrich WL, 4th Ed., Philadelphia, Lippincott Williams & Wilkins, 2009, pp 12–25 [Google Scholar]
- 11.Ward DM: Hemodialysis water: An update on safety issues, monitoring, and adverse clinical events. ASAIO J 50: xiii–xviii, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Lacson E, Jr, Lazarus JM: Dialyzer best practice: Single use or reuse? Semin Dial 19: 120–128, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Clark T, Huhn GD, Conover C, Cali S, Arduino MJ, Hajjeh R, Brandt ME, Fridkin SK: Outbreak of bloodstream infection with the mold Phialemonium among patients receiving dialysis at a hemodialysis unit. Infect Control Hosp Epidemiol 27: 1164–1170, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention and Healthcare Infection Control Practices Advisory Committee: Guidelines for environmental infection control in healthcare facilities. Available at: http://www.cdc.gov/hicpac/pubs.html Accessed May 20, 2011
- 15.Patel PR, Thompson ND, Kallen AJ, Arduino MJ: Epidemiology, surveillance, and prevention of hepatitis C virus infections in hemodialysis patients. Am J Kidney Dis 56: 371–378, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Arenas MD, Sánchez-Payá J, Barril G, García-Valdecasas J, Gorriz JL, Soriano A, Antolin A, Lacueva J, García S, Sirvent A, Espinosa M, Angoso M: A multicentric survey of the practice of hand hygiene in haemodialysis units: Factors affecting compliance. Nephrol Dial Transplant 20: 1164–1171, 2005 [DOI] [PubMed] [Google Scholar]
- 17.American Association for the Advancement of Medical Instrumentation. Standards. Available at: http://www.AAMI.org/standards Accessed April 12, 2011
- 18.DeVivo R: National ESRD Patient Safety Initiative. Phase II Report. December 2001. A partnership between: the Renal Physicians Association, the Forum of End Stage Renal Disease Networks, and the National Patient Safety Foundation. Available at: www.renalmd.org/WorkArea/DownloadAsset.aspx?id=515 Accessed May 12, 2011
- 19.Renal Physicians Association: Health and safety survey to improve patient safety in end stage renal disease: Report of findings from the ESRD patient survey, 2007. Available at: http://www.kidneypatientsafety.org/aboutaspx. Accessed April 12, 1011
- 20.The Joint Commission. Advancing effective communication, cultural competence, and patient and family-centered care: a roadmap for hospitals. Available at: http://www.jointcommission.org/Advancing_Effective_Communication/ Accessed August 15, 2011
- 21.Kurella Tamura M, Wadley V, Yaffe K, McClure LA, Howard G, Go R, Allman RM, Warnock DG, McClellan W: Kidney function and cognitive impairment in US adults: The Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Am J Kidney Dis 52: 227–234, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurella M, Chertow GM, Fried LF, Cummings SR, Harris T, Simonsick E, Satterfield S, Ayonayon H, Yaffe K: Chronic kidney disease and cognitive impairment in the elderly: The health, aging, and body composition study. J Am Soc Nephrol 16: 2127–2133, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Elias MF, Elias PK, Seliger SL, Narsipur SS, Dore GA, Robbins MA: Chronic kidney disease, creatinine and cognitive functioning. Nephrol Dial Transplant 24: 2446–2452, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Green JA, Mor MK, Shields AM, Sevick MA, Palevsky PM, Fine MJ, Arnold RM, Weisbord SD: Prevalence and demographic and clinical associations of health literacy in patients on maintenance hemodialysis. Clin J Am Soc Nephrol 6: 1354–1360, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiss BD: Barriers to better, safer care. Health literacy and patient safety: help patients understand. Health Literacy Educational Toolkit, 2nd Ed., Chicago, American Medical Association Foundation, 2007. Available at: www.ama-assn.org/ama1/pub/upload/mm/367/healthlitclinicians.pdf Accessed August 30, 2011
- 26.Leonard M, Graham S, Bonacum D: The human factor: the critical importance of effective teamwork and communication in providing safe care. Qual Saf Health Care 13[Suppl 1]: i85–i90, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Department of Veterans Affairs: Office of the Inspector General. Healthcare inspection quality of care issues in the dialysis unit, Bay Pines VA Medical Center, Bay Pines, Florida. Available at: http://www.va.gov/oig/54/reports/VAOIG-05-02589-47.pdf Accessed September 3, 2011
- 28.Hemodialysis Administration: Strategies to ensure safe patient care. Pennsylvania Patient Safety Advisory 3:87–96. 2010. The Pennsylvania Patient Safety Authority. Available at: http://patientsafetyauthority.org/ADVISORIES/AdvisoryLibrary/2010/Sep73)/Pages/87.aspx Accessed May 3, 2011
- 29.Kliger AS: Patient safety in the dialysis facility. Blood Purif 24: 19–21, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Hagren B, Pettersen I-M, Severinsson E, Lützén K, Clyne N: Maintenance haemodialysis: Patients’ experiences of their life situation. J Clin Nurs 14: 294–300, 2005 [DOI] [PubMed] [Google Scholar]
- 31.US Renal Data System: USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2010 Available at: http://www.usrds.org/adr.htm Accessed April 21, 2011
- 32.Rubenstein LZ, Powers CM, MacLean CH: Quality indicators for the management and prevention of falls and mobility problems in vulnerable elders. Ann Intern Med 135: 686–693, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Cook WL, Tomlinson G, Donaldson M, Markowitz SN, Naglie G, Sobolev B, Jassal SV: Falls and fall-related injuries in older dialysis patients. Clin J Am Soc Nephrol 1: 1197–1204, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Desmet C, Beguin C, Swine C, Jadoul M, Université Catholique de Louvain Collaborative Group : Falls in hemodialysis patients: Prospective study of incidence, risk factors, and complications. Am J Kidney Dis 45: 148–153, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Roberts RG, Kenny RA, Brierley EJ: Are elderly haemodialysis patients at risk of falls and postural hypotension? Int Urol Nephrol 35: 415–421, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Roberts R, Jeffrey C, Carlisle G, Brierley E: Prospective investigation of the incidence of falls, dizziness and syncope in haemodialysis patients. Int Urol Nephrol 39: 275–279, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Morse J: Preventing patient falls. Thousand Oaks, CA, United States Department of Veterans Affairs, National Center for Patient Safety, 1997. Available at: http://www.patientsafety.gov/CogAids/FallPrevention/index.html Accessed April 29, 2011
- 38.Heung M, Adamowski T, Segal JH, Malani PN: A successful approach to fall prevention in an outpatient hemodialysis center. Clin J Am Soc Nephrol 5: 1775–1779, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abdel-Rahman EM, Turget F, Turkmen K, Balogun RA: Falls in elderly hemodialysis patients. Q J Med 104: 829–838, 2011 [DOI] [PubMed] [Google Scholar]
- 40.Dager WE: What are the important drug use errors in dialysis patients? Pharmacokinetic and pharmacodynamic principles. Semin Dial 23: 466–469, 2010 [DOI] [PubMed] [Google Scholar]
- 41.St Peter WL: Improving medication safety in chronic kidney disease patients on dialysis through medication reconciliation. Adv Chronic Kidney Dis 17: 413–419, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Ledger S, Choma G: Medication reconciliation in hemodialysis patients. CANNT J 18: 41–43, 2008 [PubMed] [Google Scholar]
- 43.Stephen L, Seliger SL: Min Zhan M, Van Doren Hsu VD, Lori D. Walker LD, Fink JC: Chronic kidney disease adversely influences patient safety. J Am Soc Nephrol 19: 2414–2419, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chapin E, Zhan M, Hsu VD, Seliger SL, Walker LD, Fink JC: Adverse safety events in chronic kidney disease: The frequency of “multiple hits”. Clin J Am Soc Nephrol 5: 95–101, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hug BL, Witkowski DJ, Sox CM, Keohane CA, Seger DL, Yoon C, Matheny ME, Bates DW: Occurrence of adverse, often preventable, events in community hospitals involving nephrotoxic drugs or those excreted by the kidney. Kidney Int 76: 1192–1198, 2009 [DOI] [PubMed] [Google Scholar]
- 46.Tsai TT, Maddox TM, Roe MT, Dai D, Alexander KP, Ho PM, Messenger JC, Nallamothu BK, Peterson ED, Rumsfeld JS, National Cardiovascular Data Registry : Contraindicated medication use in dialysis patients undergoing percutaneous coronary intervention. JAMA 302: 2458–2464, 2009 [DOI] [PubMed] [Google Scholar]
- 47.National Coordination Council for Medication Error Reporting and Prevention (NCC MERP): NCC MERP index for categorizing medication errors. 2001. Available at: http://www.nccmerp.org/pdf/indexColor2001-06-12.pdf Accessed May 15
- 48.Lee T, Barker J, Allon M: Needle infiltration of arteriovenous fistulae in hemodialysis: Risk factors and consequences. Am J Kidney Dis 47: 1020–1026, 2006 [DOI] [PubMed] [Google Scholar]
- 49.ECRI Institute. Hazard report: Undetected venous needle dislodgment during hemodialysis can be fatal. Health Devices 32:325–326, 2003 [PubMed]
- 50.Zeigler SA: Prevent dangerous hemodialysis catheter disconnections. Nursing 37: 70, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Veterans Health Administration Warning System Patient Safety Alert: Redsense dialysis alarm for patients undergoing needle access procedures. July 7, 2010 Available at: http://www.patientsafety.gov/alerts/AL10-13.pdf Accessed September 22, 1011.
- 52.Veterans Health Administration Warning System Patient Safety Alert: Fresenius HemaClip used during dialysis. February 3, 2010. Available at: http://www.patientsafety.gov/alerts/AL10-05WWW.pdf Accessed September 22, 2011
- 53.Association for Professionals in Infection Control and Epidemology (APIC): Guide to the elimination of infections in hemodialysis. Available at: http://www.apic.org/Content/NavigationMenu/PracticeGuidance/APICEliminationGuides/APIC_Hemodialysis_web.pdf Accessed June 2, 2011
- 54.Veterans Affairs National Center for Patient Safety: Root cause analysis training. Available at: http://www.patientsafety.gov/CogAids/RCA/index.html#page=page-1 Accessed April 12, 2011
- 55.Ulrich B: Interruptions: A danger to quality patient care. Nephrol Nurs J 37: 225–226, 2010 [PubMed] [Google Scholar]
- 56.Flynn L, Thomas-Hawkins C, Clarke SP: Organizational traits, care processes, and burnout among chronic hemodialysis nurses. West J Nurs Res 31: 569–582, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Flynn L, Thomas-Hawkins C, Clarke SP: Relationship between registered nurse staffing, processes of nursing care, and nurse-reported patient outcomes in chronic dialysis. Nephrology Nursing J 35:123–130, 145, 2008 [PMC free article] [PubMed]
- 58.Saxena AK, Panhotra BR: The impact of nurse understaffing on the transmission of hepatitis C virus in a hospital-based hemodialysis unit. Med Princ Pract 13: 129–135, 2004 [DOI] [PubMed] [Google Scholar]
- 59.Mitchell PH: Defining patient safety and quality care. In: Patient Safety and Quality an Evidence-based Handbook for Nurses, edited by Hughes RG, Rockville, MD, Agency for Healthcare Research and Quality, 2008, pp 1–5 [PubMed] [Google Scholar]
- 60.Centers for Medicare & Medicaid Services. Conditions for coverage for end-stage renal disease facilities. Code of Federal Regulations Title 42 Part 494.110. Interpretive guidelines. Available at: https://www.cms.gov/SurveyCertificationGenInfo/downloads/SCletter09-01.pdf Accessed December 13, 2011
- 61.Wish JB: What is expected of a medical director in the centers for Medicare and Medicaid Services conditions of coverage? Blood Purif 31: 61–65, 2011 [DOI] [PubMed] [Google Scholar]
- 62.DeOreo PB: The medical directorship of renal dialysis facilities under the new Medicare conditions for coverage: Challenges and opportunities. Blood Purif 27: 16–21, 2009 [DOI] [PubMed] [Google Scholar]
- 63.American Society for Quality: Project planning and implementing tools. Available at: http://asq.org/learn-about-quality/project-planning-tools/overview/pdca-cycle.html Accessed Sept.27, 2011
- 64.Clancy CM: Quality improvement: A team approach. Agency for Healthcare Research and Quality. Available at: http://www.usmedicine.com/outlook/quality-improvement-a-team-approach.html Accessed May 30, 2011
- 65.Huang DT, Clermont G, Sexton JB, Karlo CA, Miller RG, Weissfeld LA, Rowan KM, Angus DC: Perceptions of safety culture vary across the intensive care units of a single institution. Crit Care Med 35: 165–176, 2007 [DOI] [PubMed] [Google Scholar]
- 66.The Five Diamond Patient Safety Program. Available at: http://www.therenalnetwork.org/5Diamond/5DiamondSTFs.php/ Accessed May 1, 2011
- 67.National Patient Safety Foundation. Mission and vision. Available at: http://www.npsf.org/about-us/mission-and-vision/ Accessed March 30, 2011
- 68.Agency for Healthcare Research and Quality: Patient safety & medical errors. Available at: http://www.kidneypatientsafety.org/toolkit.aspx Accessed May 18, 2011
- 69.World Health Organization: Patient safety. Available at: http://www.who.int/patientsafety/en/ Accessed June2, 2011
- 70.Renal Physicians Association: Patient safety toolkit. Available at: http://www.kidneypatientsafety.org/ Accessed April 1, 2011
- 71.Intermountain End-Stage Renal Disease Available at: Condition 494:110: Quality assessment and performance improvement. Available at: http://www.esrdnet15.org/QI.htm Accessed August 20, 2011