Summary
Background and objectives
Insulin resistance is a complication of advanced CKD. Insulin resistance is less well characterized in earlier stages of CKD. The response of the pancreatic β cell, effects on glucose tolerance, and risk of diabetes are not clear.
Design, setting, participants, & measurements
The Cardiovascular Health Study included 4680 adults without baseline diabetes. The Chronic Kidney Disease Epidemiology Collaboration creatinine equation was used to obtain the estimated GFR (eGFR). Insulin resistance was evaluated as fasting insulin concentration. The insulin sensitivity index, β cell function, and glucose tolerance were assessed by oral glucose tolerance testing. Incident diabetes was defined as fasting glucose ≥126 mg/dl, nonfasting glucose ≥200 mg/dl, or use of glucose-lowering medications.
Results
Mean age was 72.5 years (range, 65–98 years). Mean eGFR was 72.2 (SD 17.1) ml/min per 1.73 m2. After adjustment, each 10 ml/min per 1.73 m2 lower eGFR was associated with a 2.2% higher fasting insulin concentration (95% confidence interval [CI], 1.4%, 2.9%; P<0.001) and a 1.1% lower insulin sensitivity index (95% CI, 0.03%, 2.2%; P=0.04). Surprisingly, eGFR was associated with an augmented β cell function index (P<0.001), lower 2-hour glucose concentration (P=0.002), and decreased risk of glucose intolerance (P=0.006). Over a median 12 years’ follow-up, 437 participants (9.3%) developed diabetes. eGFR was not associated with the risk of incident diabetes.
Conclusions
Among older adults, lower eGFR was associated with insulin resistance. However, with lower eGFR, β cell function was appropriately augmented and risks of impaired glucose tolerance and incident diabetes were not increased.
Introduction
Diabetes mellitus is associated with increased risk of myocardial infarction, stroke, and ESRD (1). Insulin resistance plays a central role in the pathogenesis of type 2 diabetes. With insulin resistance, euglycemia may initially be maintained through a compensatory increase in insulin secretion by the pancreatic β cell (2). In some individuals, however, β cell compensation fails, leading to impaired glucose tolerance and development of type 2 diabetes.
Insulin resistance is a known complication of ESRD (3). It seems that insulin resistance also occurs in early stages of CKD (4–8). Insulin resistance in CKD is due, in part, to a high prevalence of shared risk factors such as obesity, sedentary lifestyle, and diet (9). In addition, unique metabolic abnormalities contribute to insulin resistance in CKD, including uremic toxins, metabolic acidosis, and vitamin D deficiency (10–14). The site of insulin resistance in CKD is localized to skeletal muscles (3) and a postreceptor defect has been recognized as the primary abnormality in CKD (15).
Whether insulin resistance in CKD leads to β cell dysfunction and overt type 2 diabetes has not been established. We hypothesized that a lower estimated GFR (eGFR) is associated with insulin resistance as characterized by fasting insulin concentration and insulin sensitivity index. We further hypothesized that lower eGFR is associated with pancreatic β cell dysfunction, impaired glucose tolerance and increased risk of incident type 2 diabetes. We tested these hypotheses in a community-based sample of older adults, a population known to be at high risk of CKD, insulin resistance, and diabetes.
Materials and Methods
The Cardiovascular Health Study is a community-based cohort study of adults aged ≥65 years (16). In 1989–1990, 5201 men and women aged ≥65 years were recruited, and an additional 687 predominantly African-American participants were recruited in 1992–1993. Participants were recruited from Medicare eligibility lists in four US communities: Forsyth County, North Carolina (Wake Forest University School of Medicine, Winston-Salem); Sacramento County, California (University of California, Davis); Washington County, Maryland (Johns Hopkins University, Hagerstown); and Allegheny County, Pennsylvania (University of Pittsburgh, Pittsburgh). Each center’s institutional review committee approved this study and all participants provided signed informed consent.
From the original and the African-American cohorts, we excluded participants with baseline prevalent diabetes, defined by treatment with oral hypoglycemic agents or insulin or fasting blood glucose ≥126 mg/dl (Supplemental Figure 1). We also excluded participants for whom prevalent diabetes status could not be determined due to missing fasting glucose concentration, missing diabetes medication data, or missing or inadequate fasting times (<8 hours), as well as participants with missing covariate data. For cross-sectional analyses, participants without oral glucose tolerance data were excluded, leaving a final analysis sample of 4136 participants. For longitudinal analyses, participants without follow-up were excluded, leaving a final analysis sample of 4680 participants.
eGFR
Serum creatinine was measured using the Kodak Ektachem 700 Analyzer (Eastman Kodak, Rochester, NY), a colorimetric method. Mean coefficient of variation (CV) was 1.94% (range, 1.16–3.60) (17). Serum creatinine concentration was indirectly calibrated to values from the Cleveland Clinic laboratory as previously described (18). GFR was estimated using the Chronic Kidney Disease Epidemiology Collaboration equation (19). In addition, serum cystatin C concentration was measured using a BNII nephelometer (Dade Behring Inc, Deerfield, IL) that utilized a particle enhanced immunonephelometric assay (N Latex Cystatin C) for secondary analyses (20). Interassay CVs ranged from 2.3% to 3.1%. Cystatin-based GFR was estimated using the cystatin C equation (21).
Insulin Resistance, β Cell Function, and Glucose Tolerance
Serum glucose and insulin concentrations were measured before (fasting) and after administration of a 2-hour oral glucose tolerance test (OGTT), as previously described (22). The OGTT consisted of a 75-g oral bolus of glucose. Glucose was measured using the Kodak Ektachem 700 Analyzer. Plasma fasting insulin was measured using competitive RIA (Diagnostic Products Corp, Malvern, PA).
Insulin resistance was assessed by fasting insulin concentration (μU/ml) and the insulin sensitivity index (ISI). ISI was calculated as 10,000/√ (G0 × I0 × G120 × I120), where G0 is glucose concentration (mg/dl) at time 0, I0 is the insulin concentration at time 0 (µmol/ml), G120 is the glucose concentration at time 120 minutes, and I120 is the insulin concentration 120 minutes obtained from an OGTT (23). The β cell function index was calculated from OGTT data as (2503 + 6.476 × I0 − 126.5 × G120 + 0.954 × I120 − 239.3 × G0) (24). Impaired fasting glucose was defined as fasting glucose concentration between 100 and 125 mg/dl. Impaired glucose tolerance was defined as 2-hour glucose between 140 and 199 mg/dl (22).
Incident Diabetes
Glucose was measured on fasting blood samples obtained from participants during the annual clinic examinations in 1989–1990, 1992–1993, 1996–1997, 1998–1999, and 2005–2006, and on nonfasting blood samples collected in 1994–1995. Medication use was assessed at baseline and annually thereafter by medication inventory through 2007 (25). Incident diabetes was defined as fasting glucose ≥126 mg/dl, nonfasting glucose ≥200 mg/dl, or use of glucose-lowering medications.
Covariates
Age, sex, race, education, smoking status, physical activity, and alcohol consumption were assessed by self-report. Leisure-time physical activity was calculated as the weighted sum of kilocalories expended in a broad range of self-reported physical activities (26). Dietary scores were computed using quintiles of intake of dietary fiber, glycemic index, trans fat, and polysaturated fat/saturated fat ratio (27).
Prevalent CVD was defined as clinical, subclinical, or none. Clinical CVD was defined as myocardial infarction, coronary artery bypass graft or percutaneous coronary angioplasty, angina pectoris, or stroke. Subclinical cardiovascular disease was defined as presence of ankle-arm index <0.9, internal carotid wall thickness >80th percentile, carotid stenosis >25%, any major electrocardiographic abnormality, or claudication or angina reported by Rose questionnaire (28).
Statistical Analyses
Unadjusted associations of eGFR with fasting insulin concentration, ISI, β cell function, and 2-hour glucose concentration were described using spline curves. These associations were tested using linear regression. Associations of eGFR with impaired fasting glucose and impaired glucose tolerance (dichotomous outcomes) were tested using logistic regression. Nested regression models were adjusted for demographic factors including age, sex, race, site, and education. They were additionally adjusted for potential confounders of the relationships of interest, including physical activity; smoking; alcohol use; waist circumference; dietary score; use of β blockers, diuretics, steroids, or angiotensin converting enzyme inhibitors; and prevalent CVD. Distributions of fasting insulin concentration and ISI were normalized by logarithmic transformation before analysis.
Unadjusted incidence rates of diabetes were calculated by category of eGFR. Adjusted associations of eGFR with incident diabetes were tested using Cox proportional hazards models, stratified by enrollment wave (1989–1990 versus 1992–1993). Serial models were adjusted as described above and sensitivity analyses accounting for competing risk of death were performed (29).
Statistical analysis was performed using STATA 11 software. P values <0.05 were considered statistically significant.
Results
Baseline Characteristics
Mean age was 72.7 (SD 5.5) years (range, 65–98 years); 59% of participants were female and 86.4% were Caucasian. Mean eGFR was 72.2 (SD 17) ml/min per 1.73 m2. Participants who had lower eGFR were more likely to be older, had more prevalent CVD, were less physically active, and had a larger waist circumference (Table 1).
Table 1.
Baseline characteristics of 4680 participants without prevalent diabetes in the Cardiovascular Health Study
| Characteristic | Estimated GFR (ml/min per 1.73 m2) | ||||
|---|---|---|---|---|---|
| ≥90 (n=724) | 75–89 (n=1518) | 60–74 (n=1280) | 45–59 (n=874) | <45 (n=282) | |
| Age (yr) | 69 (3) | 71 (5) | 73 (5) | 74 (5) | 77 (6) |
| Female | 565 (78) | 954 (63) | 655 (51) | 455 (52) | 146 (51) |
| Caucasian | 599 (83) | 1364 (90) | 1081 (84) | 756 (86) | 242 (85) |
| Education | |||||
| < high school | 78 (10) | 183 (12) | 179 (14) | 145 (17) | 57 (20) |
| high school | 323 (45) | 638 (42) | 518 (40) | 348 (40) | 117 (41) |
| > high school | 323 (45) | 697 (46) | 583 (46) | 381 (43) | 110 (39) |
| Cardiovascular disease | |||||
| none | 298 (41) | 534 (35) | 414 (32) | 224 (26) | 40 (14) |
| subclinical | 341 (47) | 724 (48) | 624 (49) | 405 (46) | 145 (51) |
| clinical disease | 85 (12) | 260 (17) | 242 (19) | 245 (28) | 99 (35) |
| Smoking status | |||||
| current | 128 (18) | 187 (12) | 138 (11) | 77 (9) | 37 (13) |
| never and former | 596 (82) | 1331 (88) | 1142 (89) | 797 (91) | 247 (87) |
| Alcohol use | |||||
| yes | 408 (56) | 817 (54) | 696 (54) | 447 (51) | 132 (46) |
| no | 316 (44) | 701 (46) | 584 (46) | 427 (49) | 152 (54) |
| Dietary score | 3.4 (1.5) | 3.3 (1.6) | 3.3 (1.5) | 3.2 (1.5) | 3.2 (1.5) |
| Leisure time activity (kcal/wk) | 1153 (1539) | 1204 (1543) | 1301 (1673) | 1182 (1565) | 940 (1529) |
| Body mass index (kg/m2) | 26.3 (4.8) | 26.1 (4.5) | 26.5 (4.5) | 26.4 (4.5) | 26.1 (4.4) |
| Waist circumference (cm) | 91.8 (13) | 92.2 (13) | 94.2 (13) | 94.2 (13) | 94.6 (12) |
Cross-Sectional Correlations
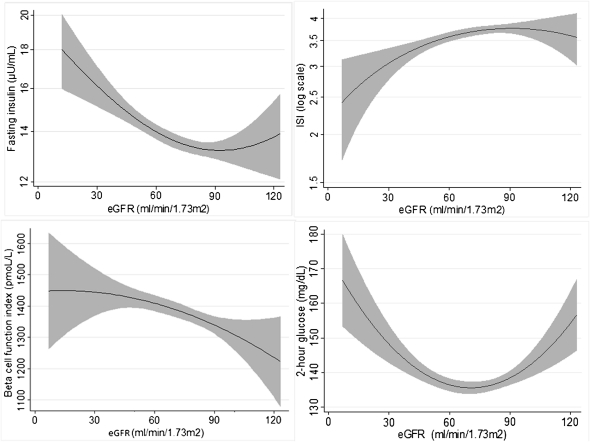
Lower eGFR was monotonically associated with insulin resistance as measured by fasting insulin concentration or ISI (Figure 1 and Table 2). In adjusted analyses, every 10 ml/min per 1.73 m2 lower eGFR was associated with a 2.2% higher fasting insulin concentration and a 1.1% lower ISI (Table 2). There was no association of lower eGFR with fasting glucose concentration (data not shown) or impaired fasting glucose after multivariate adjustment (Supplemental Table 1).
Figure 1.
Associations of creatinine-based eGFR with fasting insulin concentration, insulin sensitivity index (ISI), β cell function index, and 2-hour glucose concentration. eGFR estimated by the Chronic Kidney Disease Epidemiology Collaboration equation. eGFR, estimated GFR.
Table 2.
Associations of creatinine-based eGFR with fasting insulin, ISI, β cell function index, and 2-hour glucose concentration among 4136 participants without diabetes in the Cardiovascular Health Study
| eGFR (ml/min per 1.73 m2) | Unadjusted Mean, SD | Adjusted Difference (95% Confidence Interval)a | |
|---|---|---|---|
| Model 1 | Model 2 | ||
| Fasting insulin (μU/ml) | |||
| ≥90 | 13.3 (8.6) | 0 (reference) | 0 (reference) |
| 75–89 | 13.1 (6.5) | 3.4 (−1.0, 8.0) | 3.6 (−0.3, 7.7) |
| 60–74 | 13.8 (6.7) | 10.0 (5.1, 15.2) | 7.8 (3.5, 12.3) |
| 45–59 | 14.5 (7.6) | 15.2 (9.6, 21.0) | 10.3 (5.5, 15.3) |
| <45 | 15.1 (7.7) | 24.0 (15.8, 32.7) | 14.8 (7.9, 22.2) |
| continuousb | 3.4 (2.6, 4.2) | 2.2 (1.4, 2.9) | |
| P<0.001 | P<0.001 | ||
| ISI | |||
| ≥90 | 3.7 (2.2) | 0 (reference) | 0 (reference) |
| 75–89 | 3.7 (2.3) | −1.4 (−6.8, 4.4) | −1.6 (−0.7, 3.7) |
| 60–74 | 3.6 (2.4) | −6.8 (−0.2, −0.7) | −4.2 (−9.5, 1.4) |
| 45–59 | 3.5 (2.2) | −9.6 (−15.4, −3.4) | −3.8 (−9.5, 2.2) |
| <45 | 3.2 (1.9) | −17.3 (−24.5, −9.3) | −7.7 (−15.3, 0.6) |
| continuousb | −3.0 (−4.2, −1.8) | −1.1 (−2.2, −0.03) | |
| P<0.001 | P=0.04 | ||
| β cell function index (pmol/L) | |||
| ≥90 | 1332 (634) | 0 (reference) | 0 (reference) |
| 75–89 | 1337 (554) | 54.7 (−4.3, 113.6) | 57 (−0.1, 114.4) |
| 60–74 | 1414 (575) | 163.4 (99.8, 226.9) | 149.4 (88.4, 210.5) |
| 45–59 | 1424 (619) | 181.6 (111.9, 226.9) | 147.8 (80.3, 215.4) |
| <45 | 1439 (670) | 259.1 (157.5, 360.7) | 197.8 (99.4, 296.3) |
| continuousb | 40.0 (27.7, 52.3) | 29.9 (17.9, 41.8) | |
| P<0.001 | P<0.001 | ||
| 2-hour glucose (mg/dl) | |||
| ≥90 | 139.9 (45) | 0 (reference) | 0 (reference) |
| 75–89 | 137.4 (42) | −4.0 (−8.3, 0.2) | −4.0 (−8.2, 0.1) |
| 60–74 | 135.6 (41) | −6.5 (−11.0, −2.0) | −7.6 (−12.1, −3.2) |
| 45–59 | 137.6 (42) | −5.6 (−10.4, −0.8) | −8.3 (−13.1, −3.5) |
| <45 | 145.6 (44) | −1.4 (−8.2, 5.4) | −6.2 (−12.9, 0.6) |
| continuousb | −0.5 (−1.3, 0.3) | −1.3 (−2.1, −0.5) | |
| P=0.24 | P=0.002 | ||
Included are 607 participants with an eGFR ≥90 ml/min per 1.73 m2, 1379 participants with an eGFR of 75–89 ml/min per 1.73 m2, 1109 participants with an eGFR of 60–74 ml/min per 1.73 m2, 774 participants with an eGFR of 45–59 ml/min per 1.73 m2, and 267 participants with an eGFR <45 ml/min per 1.73 m2. Model 1 is adjusted for age, sex, race, site, and education. Model 2 is additionally adjusted for leisure time physical activity, smoking, alcohol, waist circumference, dietary score, medications, and prevalent cardiovascular disease. eGFR, estimated GFR; ISI, insulin sensitivity index.
Differences expressed in milligrams per deciliter for 2-hour glucose concentration (absolute difference), picomoles per liter for β cell function index (absolute difference), or as the percentage difference for fasting insulin concentration and ISI (relative difference).
Continuous models evaluate eGFR models per 10 ml/min per 1.73 m2 lower eGFR. P values are generated using this continuous model.
The β cell function index demonstrated an inverse hyperbolic relationship with ISI (Supplemental Figure 2). Lower eGFR was monotonically associated with a higher β cell function index (Figure 1C and Table 2). In adjusted analyses, every 10 ml/min per 1.73 m2 lower eGFR was associated with a 29.9 pmol/L higher β cell function index.
The relationship of eGFR with 2-hour glucose was U shaped in unadjusted analyses (Figure 1D). However, with multivariable adjustment, lower eGFR was associated with lower 2-hour glucose (Table 2) and a decreased risk of glucose intolerance (Supplemental Table 1).
When eGFR was estimated using cystatin C, associations with fasting insulin concentration, ISI, and β cell function were similar to those of creatinine-based eGFR but of stronger magnitude (Supplemental Table 2). In adjusted analyses, every 10 ml/min per 1.73 m2 of lower eGFR was associated with 3.5% higher fasting insulin concentration, 2.5% lower ISI, and a 38.5 pmol/L higher β cell function index. However, lower cystatin C–based eGFR was not associated with 2-hour glucose concentration or impaired glucose tolerance.
Incident Diabetes
Over 12 years’ median follow-up, 437 participants (9.3%) developed type 2 diabetes. Unadjusted incidence rates of diabetes did not differ by category of eGFR, and eGFR was not associated with risk of incident diabetes in adjusted analyses (Table 3). In addition, there was no age interaction on incident diabetes. A sensitivity analysis accounting for competing risk of death also did not show any association of low eGFR with risk of diabetes. GFR estimated from serum cystatin C was also not associated with risk of incident diabetes (Supplemental Table 3).
Table 3.
Associations of creatinine-based eGFR with incident diabetes mellitus among 4680 participants in the Cardiovascular Health Study
| eGFR (ml/min per 1.73 m2) | Incident Diabetes Mellitus, Number of Events | Incidence Rate | Adjusted Hazard Ratio (95% Confidence Interval) | |
|---|---|---|---|---|
| Model 1 | Model 2 | |||
| ≥90 | 79 | 9.0 | 1 (reference) | 1 (reference) |
| 75–89 | 151 | 8.5 | 1.03 (0.78, 1.35) | 0.98 (0.75, 1.29) |
| 60–74 | 118 | 8.5 | 1.02 (0.76, 1.36) | 0.94 (0.70, 1.26) |
| 45–59 | 74 | 8.7 | 1.08 (0.78, 1.58) | 0.92 (0.67, 1.28) |
| <45 | 15 | 7.1 | 0.97 (0.55, 1.71) | 0.75 (0.42, 1.33) |
| Continuousa | 1.00 (0.94, 1.06) | 0.97 (0.91, 1.02) | ||
| P=0.95 | P=0.28 | |||
Included are 724 participants with an eGFR ≥90 ml/min per 1.73 m2, 1518 participants with an eGFR of 75–89 ml/min per 1.73 m2, 1280 participants with an eGFR of 60–74 ml/min per 1.73 m2, 874 participants with an eGFR of 45–59 ml/min per 1.73 m2, and 284 participants with an eGFR <45 ml/min per 1.73 m2. Model 1 is adjusted for age, sex, race, site, and education. Model 2 is additionally adjusted for leisure time physical activity, smoking, alcohol, waist circumference, dietary score, medications and prevalent cardiovascular disease. eGFR, estimated GFR.
Continuous models evaluate eGFR models per 10 ml/min per 1.73 m2 lower eGFR. P values are generated using this continuous model.
Discussion
In this large cohort study of older adults, lower eGFR was associated with insulin resistance as assessed by fasting insulin concentration and ISI in cross-section. Magnitudes of association were modest but persisted with adjustment for potential confounders. β cell function increased with decreasing eGFR, suggesting intact and appropriate compensation. The net effect of insulin resistance and augmented β cell function was preserved or improved glucose tolerance with lower eGFR. Moreover, lower eGFR was not associated with increased risk of clinical diabetes during long-term follow-up.
Our findings of an association of lower eGFR with insulin resistance are consistent with previously published studies (4–7,30). Using the gold standard hyperinsulinemic euglycemic clamp, Defronzo et al. demonstrated that nondiabetic patients with ESRD are insulin resistant (31). Using fasting insulin concentrations and a homeostatic model of assessment results (HOMA) in a cross-sectional analysis of nondiabetic US adults in the National Health and Nutrition Examination Survey III, Chen et al. found that insulin resistance is independently associated with moderate CKD (30). Furthermore, among participants in the Health, Aging and Body Composition study among community-living individuals of similar age to those in our study (70–79 years), kidney function was found to be independently associated with insulin resistance assessed as upper quartile of HOMA (32).
A unique aspect of our study was the assessment of insulin resistance using both the fasting insulin concentration and the ISI calculated from OGTT data. We found that lower eGFR was associated with insulin resistance both in the fasting and dynamic states. In the fasting state, insulin sensitivity is largely determined by the ability of insulin to regulate hepatic glucose production, thereby reflecting primarily hepatic insulin resistance. Dynamic states, on the other hand, assess insulin sensitivity when the body is challenged with glucose or insulin. In this setting, glucose is primarily disposed of in skeletal muscle, and results largely reflect peripheral insulin resistance (33). Our findings of insulin resistance in dynamic states were consistent with previous published studies (8,34). Fliser et al. found that insulin resistance as measured using the frequently sampled intravenous glucose tolerance test is already present in early CKD, even when the eGFR is within the normal range (34). Nerpin et al. showed that insulin sensitivity assessed using euglycemic clamp was related to cystatin C–based GFR in a community-based cohort of elderly men (8).
Little is known about the response of the pancreatic β cell to insulin resistance in CKD. Prior studies suggest variable response in ESRD, resulting in glucose intolerance in some patients (35,36). We hypothesized that CKD-related insulin resistance would lead to glucose intolerance and increased risk of overt diabetes among older adults, who are already at increased risk of these disorders. However, to our surprise, β cell function increased appropriately in the setting of declining eGFR.
eGFR demonstrated a U-shaped relationship with 2-hour glucose concentration in unadjusted analyses. With adjustment for potential confounders, lower creatinine-based eGFR was associated with lower 2-hour glucose concentration. The difference was really between high eGFR ≥90 ml/min per 1.73 m2 versus normal eGFR ≥60 ml/min per 1.73 m2, but not with low eGFR <45 ml/min per 1.73 m2 versus normal eGFR ≥60 ml/min per 1.73 m2. It is possible that high GFR estimated from serum creatinine reflects low muscle mass rather than outstanding kidney function. Low muscle mass results in low creatinine generation, low serum creatinine concentration, and therefore high eGFR. In addition, low muscle mass may reduce an individual’s ability to dispose of a fixed-dose glucose load, because the majority of prandial glucose is disposed in muscle.
Moreover, we found no association of eGFR with risk of incident diabetes. This contrasts with results of previous studies (37,38). Lorenzo et al. found that individuals with both low or high eGFR (estimated from serum creatinine using the Modification of Diet in Renal Disease equation) were at increased risk of incident diabetes (37). Sahakyan et al. found a positive relationship of serum cystatin C concentration with the incidence of type 2 diabetes (38). One potential explanation for the different results may be differences in study populations. Our participant population was older and had lower body mass indices.
Although insulin resistance among study participants with lower eGFR did not lead to glucose intolerance or increased risk of incident diabetes, it may still contribute to adverse health outcomes. Insulin resistance is associated with endothelial dysfunction, oxidative stress, dyslipidemia, systemic inflammation, and activation of the renin-angiotensin-aldosterone system (39,40). In the general population, insulin resistance as assessed by HOMA has been associated with incident cardiovascular events (41–43). Therefore, the presence of insulin resistance among older adults with CKD could increase the risk of cardiovascular morbidity and mortality without affecting diabetes risk.
Our study has important strengths. We studied a large, community-based population at high risk of CKD, insulin resistance, and diabetes. We assessed eGFR using two complementary methods with robust results. We examined insulin resistance in static and dynamic states and tested β cell function and glucose tolerance outcomes not previously examined in this population. Incident diabetes was well ascertained over long-term follow-up. Detailed covariates were available to reduce the likelihood of confounding in all analyses. However, our study also has limitations. We do not have direct measures of GFR. The ISI and the β cell function index were calculated from the same OGTT data, potentially reducing our ability to separate these processes and preventing us from validly assessing disposition index (44). In addition, the study participants were older, with the youngest age of 65 years; therefore, the results may not be applicable to younger individuals. Moreover, the majority of the participants were Caucasian, potentially reducing the ability to generalize our findings to other racial groups.
In conclusion, among older adults, lower eGFR was associated with insulin resistance. However, with lower eGFR, β cell function was appropriately augmented and risks of impaired glucose tolerance and incident diabetes were not increased.
Disclosures
None.
Acknowledgments
This research was supported by the National Heart, Lung, and Blood Institute (Grant HL080295 and Contracts N01-HC-85239, N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, and N01-HC-45133), with additional contributions from the National Institute of Neurological Disorders and Stroke. Additional support was provided by the National Institute on Aging (Grants AG-023629, AG-15928, AG-20098, and AG-027058) and the National Institute of Diabetes and Digestive and Kidney Diseases (Grant DK-087726).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.11861111/-/DCSupplemental.
References
- 1.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS: Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 289: 76–79, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Stumvoll M, Goldstein BJ, van Haeften TW: Type 2 diabetes: Principles of pathogenesis and therapy. Lancet 365: 1333–1346, 2005 [DOI] [PubMed] [Google Scholar]
- 3.DeFronzo RA, Alvestrand A, Smith D, Hendler R, Hendler E, Wahren J: Insulin resistance in uremia. J Clin Invest 67: 563–568, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eidemak I, Feldt-Rasmussen B, Kanstrup IL, Nielsen SL, Schmitz O, Strandgaard S: Insulin resistance and hyperinsulinaemia in mild to moderate progressive chronic renal failure and its association with aerobic work capacity. Diabetologia 38: 565–572, 1995 [DOI] [PubMed] [Google Scholar]
- 5.Dengel DR, Goldberg AP, Mayuga RS, Kairis GM, Weir MR: Insulin resistance, elevated glomerular filtration fraction, and renal injury. Hypertension 28: 127–132, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Kato Y, Hayashi M, Ohno Y, Suzawa T, Sasaki T, Saruta T: Mild renal dysfunction is associated with insulin resistance in chronic glomerulonephritis. Clin Nephrol 54: 366–373, 2000 [PubMed] [Google Scholar]
- 7.Kobayashi S, Maesato K, Moriya H, Ohtake T, Ikeda T: Insulin resistance in patients with chronic kidney disease. Am J Kidney Dis 45: 275–280, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Nerpin E, Risérus U, Ingelsson E, Sundström J, Jobs M, Larsson A, Basu S, Arnlöv J: Insulin sensitivity measured with euglycemic clamp is independently associated with glomerular filtration rate in a community-based cohort. Diabetes Care 31: 1550–1555, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trirogoff ML, Shintani A, Himmelfarb J, Ikizler TA: Body mass index and fat mass are the primary correlates of insulin resistance in nondiabetic stage 3-4 chronic kidney disease patients. Am J Clin Nutr 86: 1642–1648, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Kielstein JT, Zoccali C: Asymmetric dimethylarginine: A cardiovascular risk factor and a uremic toxin coming of age? Am J Kidney Dis 46: 186–202, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Wilcken DE, Sim AS, Wang J, Wang XL: Asymmetric dimethylarginine (ADMA) in vascular, renal and hepatic disease and the regulatory role of L-arginine on its metabolism. Mol Genet Metab 91: 309–317, discussion 308, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Mak RH: Effect of metabolic acidosis on insulin action and secretion in uremia. Kidney Int 54: 603–607, 1998 [DOI] [PubMed] [Google Scholar]
- 13.de Boer IH: Vitamin D and glucose metabolism in chronic kidney disease. Curr Opin Nephrol Hypertens 17: 566–572, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cade C, Norman AW: Vitamin D3 improves impaired glucose tolerance and insulin secretion in the vitamin D-deficient rat in vivo. Endocrinology 119: 84–90, 1986 [DOI] [PubMed] [Google Scholar]
- 15.Friedman JE, Dohm GL, Elton CW, Rovira A, Chen JJ, Leggett-Frazier N, Atkinson SM, Jr, Thomas FT, Long SD, Caro JF: Muscle insulin resistance in uremic humans: Glucose transport, glucose transporters, and insulin receptors. Am J Physiol 261: E87–E94, 1991 [DOI] [PubMed] [Google Scholar]
- 16.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, O’Leary DH, Psaty B, Rautaharju P, Tracy RP, Weiler PG; Cardiovascular Health Study Research Group (CHS): The Cardiovascular Health Study: Design and rationale. Ann Epidemiol 1: 263–276, 1991 [DOI] [PubMed] [Google Scholar]
- 17.Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP: Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem 41: 264–270, 1995 [PubMed] [Google Scholar]
- 18.Shlipak MG, Fried LF, Cushman M, Manolio TA, Peterson D, Stehman-Breen C, Bleyer A, Newman A, Siscovick D, Psaty B: Cardiovascular mortality risk in chronic kidney disease: Comparison of traditional and novel risk factors. JAMA 293: 1737–1745, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erlandsen EJ, Randers E, Kristensen JH: Evaluation of the Dade Behring N Latex Cystatin C assay on the Dade Behring Nephelometer II System. Scand J Clin Lab Invest 59: 1–8, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD, 3rd, Zhang YL, Greene T, Levey AS: Estimating GFR using serum cystatin C alone and in combination with serum creatinine: A pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis 51: 395–406, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Unwin N, Shaw J, Zimmet P, Alberti KG: Impaired glucose tolerance and impaired fasting glycaemia: The current status on definition and intervention. Diabet Med 19: 708–723, 2002 [DOI] [PubMed] [Google Scholar]
- 23.DeFronzo RA, Matsuda M: Reduced time points to calculate the composite index. Diabetes Care 33: e93, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Stumvoll M, Van Haeften T, Fritsche A, Gerich J: Oral glucose tolerance test indexes for insulin sensitivity and secretion based on various availabilities of sampling times. Diabetes Care 24: 796–797, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Psaty BM, Lee M, Savage PJ, Rutan GH, German PS, Lyles M: Assessing the use of medications in the elderly: Methods and initial experience in the Cardiovascular Health Study. The Cardiovascular Health Study Collaborative Research Group. J Clin Epidemiol 45: 683–692, 1992 [DOI] [PubMed] [Google Scholar]
- 26.Taylor HL, Jacobs DR, Jr, Schucker B, Knudsen J, Leon AS, Debacker G: A questionnaire for the assessment of leisure time physical activities. J Chronic Dis 31: 741–755, 1978 [DOI] [PubMed] [Google Scholar]
- 27.Mozaffarian D, Kamineni A, Carnethon M, Djoussé L, Mukamal KJ, Siscovick D: Lifestyle risk factors and new-onset diabetes mellitus in older adults: The cardiovascular health study. Arch Intern Med 169: 798–807, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuller LH, Velentgas P, Barzilay J, Beauchamp NJ, O’Leary DH, Savage PJ: Diabetes mellitus: Subclinical cardiovascular disease and risk of incident cardiovascular disease and all-cause mortality. Arterioscler Thromb Vasc Biol 20: 823–829, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Gooley TA, Leisenring W, Crowley J, Storer BE: Estimation of failure probabilities in the presence of competing risks: New representations of old estimators. Stat Med 18: 695–706, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Chen J, Muntner P, Hamm LL, Fonseca V, Batuman V, Whelton PK, He J: Insulin resistance and risk of chronic kidney disease in nondiabetic US adults. J Am Soc Nephrol 14: 469–477, 2003 [DOI] [PubMed] [Google Scholar]
- 31.DeFronzo RA, Smith D, Alvestrand A: Insulin action in uremia. Kidney Int Suppl 16: S102–S114, 1983 [PubMed] [Google Scholar]
- 32.Landau M, Kurella-Tamura M, Shlipak MG, Kanaya A, Strotmeyer E, Koster A, Satterfield S, Simsonick EM, Goodpaster B, Newman AB, Fried LF; Health, Aging and Body Composition Study: Correlates of insulin resistance in older individuals with and without kidney disease. Nephrol Dial Transplant 26: 2814–2819, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pham H, Utzschneider KM, de Boer IH: Measurement of insulin resistance in chronic kidney disease. Curr Opin Nephrol Hypertens 20: 640–646, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fliser D, Pacini G, Engelleiter R, Kautzky-Willer A, Prager R, Franek E, Ritz E: Insulin resistance and hyperinsulinemia are already present in patients with incipient renal disease. Kidney Int 53: 1343–1347, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Allegra V, Mengozzi G, Martimbianco L, Vasile A: Glucose-induced insulin secretion in uremia: Effects of aminophylline infusion and glucose loads. Kidney Int 38: 1146–1150, 1990 [DOI] [PubMed] [Google Scholar]
- 36.DeFronzo RA, Alvestrand A: Glucose intolerance in uremia: Site and mechanism. Am J Clin Nutr 33: 1438–1445, 1980 [DOI] [PubMed] [Google Scholar]
- 37.Lorenzo C, Nath SD, Hanley AJ, Abboud HE, Gelfond JA, Haffner SM: Risk of type 2 diabetes among individuals with high and low glomerular filtration rates. Diabetologia 52: 1290–1297, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sahakyan K, Lee KE, Shankar A, Klein R: Serum cystatin C and the incidence of type 2 diabetes mellitus. Diabetologia 54: 1335–1340, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siew ED, Ikizler TA: Determinants of insulin resistance and its effects on protein metabolism in patients with advanced chronic kidney disease. Contrib Nephrol 161: 138–144, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Wheatcroft SB, Williams IL, Shah AM, Kearney MT: Pathophysiological implications of insulin resistance on vascular endothelial function. Diabet Med 20: 255–268, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Hanley AJ, Williams K, Stern MP, Haffner SM: Homeostasis model assessment of insulin resistance in relation to the incidence of cardiovascular disease: The San Antonio Heart Study. Diabetes Care 25: 1177–1184, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Jeppesen J, Hansen TW, Rasmussen S, Ibsen H, Torp-Pedersen C, Madsbad S: Insulin resistance, the metabolic syndrome, and risk of incident cardiovascular disease: A population-based study. J Am Coll Cardiol 49: 2112–2119, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Bonora E, Kiechl S, Willeit J, Oberhollenzer F, Egger G, Meigs JB, Bonadonna RC, Muggeo M: Insulin resistance as estimated by homeostasis model assessment predicts incident symptomatic cardiovascular disease in caucasian subjects from the general population: The Bruneck study. Diabetes Care 30: 318–324, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Utzschneider KM, Prigeon RL, Faulenbach MV, Tong J, Carr DB, Boyko EJ, Leonetti DL, McNeely MJ, Fujimoto WY, Kahn SE: Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels. Diabetes Care 32: 335–341, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]