Summary
Background and objectives
In the albumin-to-creatinine ratio (spot-ACR), urine creatinine corrects for tonicity but also reflects muscle mass. Low muscle mass is associated with cardiovascular disease (CVD). We hypothesized that the spot-ACR would be higher in women, lower-weight persons, and older individuals, independent of timed urine albumin excretion (24hr-UAE), and accordingly, that spot-ACR would be more strongly associated with CVD events than 24hr-UAE in these subgroups.
Design, setting, participants, & methods
2627 PREVEND (Prevention of Renal and Vascular End-stage Disease) participants with 24hr-UAE <30 mg/d were followed for CVD events for 11 years. Cox regression evaluated associations of spot-ACR and 24hr-UAE with CVD events by sex, weight, and age.
Results
Female sex (26%), lower weight (2% per 5 kg), and older age (4% per 5 years) were associated with higher spot-ACR independent of 24hr-UAE (P<0.001). Spot urine albumin concentration (hazard ratio [HR], 1.26 per ln-SD higher) and 1/spot urine creatinine concentration (HR, 1.16 per ln-SD higher) were associated with CVD events. Spot-ACR was more strongly associated with CVD events than either component of the ratio (HR, 1.41 per ln-SD higher). Associations of spot-ACR ≥10 mg/g versus less (HR, 2.33) and 24hr-UAE ≥10 mg/d versus less (HR, 2.09) with CVD events were similar, and there were no significant differences across subgroups (P for interactions >0.06).
Conclusions
In community-living individuals with 24hr-UAE <30 mg/d, spot-ACR is higher in women, older persons, and lower-weight persons, independent of 24hr-UAE. Low spot urine creatinine is associated with CVD risk, but high urine albumin is a stronger determinant of the association of spot-ACR with CVD than is low urine creatinine.
Introduction
Albuminuria is an established marker of CKD progression and is associated with risk for cardiovascular disease (CVD) and death in many settings (1–4). A large meta-analysis of the relationship between the albumin-to-creatinine ratio (spot-ACR) and mortality showed that even at low spot-ACR (>5 mg/g) there is a dose-response relationship between spot-ACR and all-cause and cardiovascular mortality after adjustment for cardiovascular risk factors (2). The relationship between low-grade elevations in spot-ACR and CVD events is often attributed to subclinical renal or vascular disease (2,3,5,6). This is probably the case for patients with greater albuminuria, whereas for those with low-level albuminuria, the influence of the denominator (low urine creatinine) may more easily classify an individual into the spot-ACR > 5 mg/g group. Thus, we suspected that low urinary creatinine concentration may be another important factor linking spot-ACR with CVD risk.
The gold standard to quantify albuminuria is a timed urine collection, but this is cumbersome and frequently inaccurate because of collection errors (7). Across a population, spot-ACR correlates well with timed albuminuria (8,9). Therefore, the Kidney Disease Outcomes Quality Initiative recommends spot-ACR as the standard method for screening and monitoring albuminuria (7). Urine creatinine concentration is used in the denominator to account partly for changes in urine tonicity, which depends on fluid intake (10,11), medications, and time of day (12,13). An analysis of the RENAAL (Reduction of Endpoints in Non Insulin Dependent Diabetes Mellitus with the Angiotensin II Antagonist Losartan) study found that spot-ACR had a stronger association with loss of GFR than did 24-hour urinary albumin excretion (24hr-UAE) (14). Thus, a surrogate marker was more strongly associated with disease progression than the gold standard measure. This may have resulted from inadequate timed urine collection. However, urine creatinine might also carry prognostic information beyond urine tonicity. Creatinine is generated at a constant rate by skeletal muscle and is cleared almost exclusively in the urine (15). Thus, when serum creatinine is in steady state, the quantity of creatinine produced by muscle must equal the amount excreted. Prior studies demonstrate that timed urine creatinine excretion rate is highly correlated with muscle mass (16,17), and lower levels are associated with mortality (18,19). These studies use timed urine collections, yet similar factors should influence spot urine creatinine. Thus, beyond marking tonicity, low spot urine creatinine concentration may reflect low muscle mass, which may bias the spot-ACR to higher levels in women (20) and, probably, in older individuals or those with lower weight.
To investigate this bias, we compared the relative strengths of association of spot-ACR and 24hr-UAE with incident CVD events in the PREVEND (Prevention of Renal and Vascular End-stage Disease) study. We hypothesized that spot-ACR would be more strongly associated with CVD events than would 24hr-UAE in persons with low 24-hour creatinine excretion, in women, and in lower-weight and older subgroups. We evaluated participants with 24hr-UAE less than 30 mg/d, in whom we expected this bias to be most evident.
Materials and Methods
Participants
The PREVEND study has been described elsewhere (21,22). In brief, between 1997 and 1998, all 85,421 inhabitants of Groningen, the Netherlands, age 28–75 years were mailed a questionnaire and a vial to collect first morning-void urine. Spot urine albumin concentration (spot-UAC) was measured for the 40,856 respondents. Pregnant women and persons with type 1 diabetes were excluded. All participants with spot-UAC 10 mg/L or greater (n=7768) and a random subgroup of participants with spot-UAC less than 10 mg/L (n=3395 of 30,890) were invited to participate. In the group with higher spot-UAC, 6000 agreed to participate; in the group with lower values, 2592 agreed (total n=8592).
We created a subcohort with a distribution of spot-UAC resembling that of the 40,856 initial respondents, consistent with prior PREVEND analyses (21). We included all 2592 participants with spot-UAC less than 10 mg/L and used a computer-generated random sample of 828 individuals from the group with higher spot-UAC. Patients missing spot urine creatinine concentration were excluded (n=608). Because we hypothesized that urine creatinine concentration would have the greatest effect on CVD risk in participants with lower levels of albuminuria, those with 24hr-UAE greater than 30 mg/d (n=232) were excluded. The final sample size was 2627 participants.
All participants attended two study visits. At the first visit, a detailed health and family history survey was completed, and demographic and anthropometric data and fasting venous blood samples were collected. Participants were instructed on collecting accurate 24-hour urine samples, and written instructions were provided. Two 24-hour urine samples were collected on consecutive days less than 4 days before the second visit and stored at 4°C until processed.
Measurements
Urinary Measures.
UAC was measured in spot and timed specimens by nephelometry, with intra-assay and interassay coefficients of variation of 4.3% and 4.4%, respectively (BNII, Dade Behring Diagnostica, Marburg, Germany). Urinary creatinine concentration was measured by Kodak Ektachem dry chemistry (Eastman Kodak, Rochester, NY). Intra-assay and interassay coefficients of variation were 0.9% and 2.9%, respectively. Spot-ACR was calculated in the first morning-void sample, provided before enrollment. Twenty-four-hour albumin and creatinine excretion were calculated as the mean value from the two timed urine collections.
CVD Events.
Information on CVD events was obtained from the Dutch Central Bureau for Statistics for fatal CVD events and from Prismant, the Dutch national registry of hospital discharge diagnoses. This registry has proven validity: 84% of primary diagnoses and 87% of secondary diagnoses match those recorded in the patients’ charts (23,24). All data were coded using the International Classification of Diseases, Ninth Revision. CVD events included acute myocardial infarction, acute and subacute ischemic heart disease, coronary artery bypass grafting, percutaneous transluminal coronary angioplasty, subarachnoid hemorrhage, intracerebral or other intracranial hemorrhage, occlusion or stenosis of precerebral or cerebral arteries, or other vascular interventions (including percutaneous transluminal angioplasty or bypass grafting of the aorta and peripheral vessels). Time to event was calculated as the number of days from the urine collection to first event, last contact, or study end on January 1, 2009.
Other Measures.
Systolic BP and diastolic BP were calculated as the mean of the last 2 of 10 consecutive measurements done at each visit. Measures were taken in the supine position with an automatic Dinamap XL Model 9300 series device (Johnson & Johnson Medical Inc., Arlington, TX). Hypertension was defined as systolic BP of 140 mmHg or greater, diastolic BP of 90 mmHg or greater, or antihypertensive medication use. Diabetes was defined as a fasting glucose level of at least 126 mg/dl, self-reported diabetes, or hypoglycemic medication use. Hyperlipidemia was defined as fasting total cholesterol level of 6.2 mM or greater if there was no history of coronary artery disease or 5.0 mM or greater in those with history of coronary artery disease, self-reported diagnosis of hyperlipidemia, or use of lipid-lowering medications (25). Smoking was categorized as never, previous, or current. Serum creatinine was measured by Kodak Ektachem dry chemistry, an automatic enzymatic method, and was used to estimate GFR using the Modification of Diet in Renal Disease equation (26).
Statistical Analyses
Initial analyses demonstrated that spot urine creatinine values differed considerably by sex, so sex-specific tertiles of spot urine creatinine concentration were used to compare baseline demographic characteristics, anthropometric measurements, and CVD risk using chi-squared tests, analysis of variance, and Kruskal-Wallis tests, as appropriate.
All urine measures had skewed distribution, so Spearman rank correlations were used to determine correlations between spot and 24-hour urine measurements. We used linear regression to evaluate whether 24-hour urine creatinine excretion, weight, age, and sex were associated with natural log-transformed spot-ACR independent of 24hr-UAE. Models were adjusted for 24hr-UAE and then for age, sex, and weight individually. Results are presented as the percentage difference in spot-ACR, calculated according to the following formula: (− 1) × 100.
Cox proportional hazards models determined the association of each urinary marker with CVD events. A cut-point above or below 10 mg/g was used for spot-ACR, the level considered optimal according to recent statements from Kidney Disease Improving Global Outcomes (27). A corresponding cut-point of 10 mg/d was used for 24hr-UAE. An initial model was unadjusted; a second model was adjusted for sex, age, and weight; and a final model was additionally adjusted for estimated GFR (eGFR), hypertension, diabetes, hypercholesterolemia, prevalent coronary artery disease, and tobacco use. An additional model was adjusted only for 24-hour creatinine excretion. Subsequently, all models were repeated within several prespecified subgroups (sex and tertiles of 24-hour urine creatinine excretion, weight, and age). Because of the few CVD events in the lower two age tertiles, these tertiles were collapsed and compared with the highest tertile. Finally, we created multiplicative interaction terms to evaluate effect modification by creatinine excretion rate, weight, age, and sex on the association between spot-ACR and 24hr-UAE with CVD events. All analyses were conducted using Stata SE, version 9.0 (College Station, TX). P values less than 0.05 were considered to indicate statistically significant differences for all analyses, including interaction terms.
Results
Among the 2627 study participants, the mean age ± SD was 48±12 years, and 55% were female. Mean body weight was 71±12 kg in women and 83±11 kg in men. Mean eGFR was 80±14 ml/min per 1.73 m2. All participants had 24hr-UAE less than 30 mg/d by design. Median spot-ACR was 4.7 mg/g (interquartile range, 3.5–6.9 mg/g), and median 24hr-UAE was 6.9 mg/d (interquartile range, 5.4–9.8 mg/d). During a median follow-up of 10.7 years (interquartile range, 10.1–11.1 years), 190 CVD events occurred.
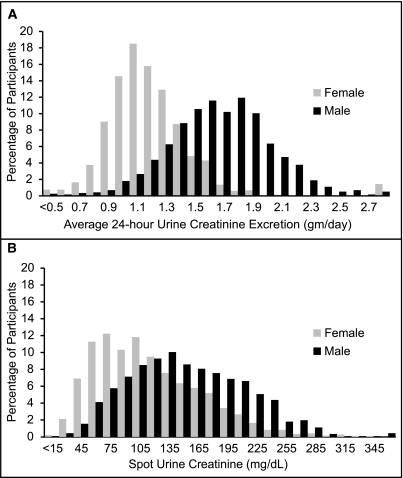
Men had higher timed and spot urine creatinine values than women (Figure 1). Compared with participants in the lowest spot urine creatinine tertile, those with higher levels were younger; had less coronary artery disease, hypertension, dyslipidemia, and smoking; and had higher eGFR (Table 1). Results were similar in men and women, although higher urine creatinine was associated with higher weight only in men. Individuals with high urine creatinine had higher spot-UAC, probably reflecting commensurate effects of urine tonicity on both measures because 24hr-UAE did not significantly differ between the urine creatinine groups. This is consistent with findings across the distribution of daily creatinine excretion; at higher levels of daily creatinine excretion, 24hr-UAE was slightly higher and spot-ACR, slightly lower.
Figure 1.
Distribution of 24-hour urine creatinine excretion and first morning spot urine creatinine concentration in women and men in PREVEND. (A) Twenty-four-hour urine creatinine excretion by sex. (B) Fiirst morning spot urine creatinine concentration by sex.
Table 1.
Baseline characteristics for all participants and by spot urinary creatinine tertile
| Variable | All Participants | Tertiles of Spot Urine Creatinine Concentration | P Value | ||
|---|---|---|---|---|---|
| I | II | III | |||
| Spot urine creatinine range (mg/dl) | |||||
| male (n=1164) | 140.3 (101.2–188.3)* | 10.2–115.4 | 116.5–170.8 | 171.9–429.9 | |
| female (n=1463) | 99.5 (65.6–141.4)* | 10.2–75.8 | 76.9–124.4 | 125.6–333.7 | |
| Mean age ± SD (yr) | |||||
| male | 49±12 | 53±12 | 50±12 | 44±10 | <0.001 |
| female | 48±12 | 54±12 | 48±12 | 44±11 | <0.001 |
| Mean weight ± SD (kg) | |||||
| male | 83±11 | 82±11 | 84±11 | 84±12 | 0.03 |
| female | 71±12 | 72±12 | 71±12 | 71±13 | 0.32 |
| Hypertension, n (%) | |||||
| male | 353 (30) | 141 (36) | 108 (28) | 104 (27) | 0.01 |
| female | 349 (24) | 147 (30) | 114 (23) | 88 (18) | <0.001 |
| Diabetes, n (%) | |||||
| male | 10 (0.9) | 6 (1.5) | 2 (0.5) | 2 (0.5) | 0.21 |
| female | 20 (1.4) | 8 (1.6) | 4 (0.8) | 8 (1.67) | 0.43 |
| Prevalent CAD, n (%) | |||||
| male | 89 (8) | 51 (13) | 21 (6) | 17 (4) | <0.001 |
| female | 53 (4) | 32 (7) | 11 (2) | 10 (2) | <0.001 |
| Dyslipidemia, n (%) | |||||
| male | 392(34) | 154 (39) | 123 (32) | 117 (31) | 0.03 |
| female | 433(30) | 192 (39) | 140 (29) | 101 (21) | <0.001 |
| Smokers, n (%) | |||||
| male | <0.001 | ||||
| current | 371 (32) | 110 (28) | 122 (32) | 139 (36) | |
| previous | 472 (41) | 190 (48) | 152 (40) | 130 (34) | |
| never | 321 (28) | 94 (24) | 109 (28) | 118 (30) | |
| female | 0.04 | ||||
| current | 412 (28) | 123 (25) | 135 (27) | 154 (32) | |
| previous | 509 (35) | 186 (38) | 180 (37) | 143 (30) | |
| never | 542 (37) | 184 (37) | 176 (36) | 182 (38) | |
| Estimated GFR ± SD (ml/min per 1.73 m2) | |||||
| male | 84±14 | 84±15 | 83±13 | 86±14 | <0.001 |
| female | 77±13 | 76±13 | 77±13 | 78±12 | 0.21 |
| 24-hr albumin excretion (mg/day)* | |||||
| male | 7.4 (5.7–10.5) | 7.2 (5.5–10.0) | 7.4 (5.7–10.7) | 7.5 (5.8–11.0) | 0.60 |
| female | 6.6 (5.2–9.2) | 6.7 (5.3–8.9) | 6.3 (5.18–9.0) | 6.8 (5.2–9.7) | 0.17 |
| Spot albumin-to-creatinine ratio (mg/g)* | |||||
| male | 4.3 (3.4–6.1) | 4.9 (3.7–8.0) | 4.1 (3.2–5.7) | 4.1 (3.2–5.5) | <0.001 |
| female | 5.1 (3.8–7.5) | 5.8 (4.4–8.8) | 4.6 (3.3–6.7) | 4.6 (3.5–6.9) | <0.001 |
| Spot urine albumin (mg/L)* | |||||
| male | 6.1 (4.0–9.4) | 4.1 (2.8–6.2) | 5.8 (4.4,8.2) | 8.9 (6.7–11.8) | <0.001 |
| female | 4.8 (2.8–8.1) | 2.8 (2.3–4.3) | 4.6 (3.3–6.6) | 7.9 (5.6–11.8) | <0.001 |
| 24-hr creatinine excretion (g)* | |||||
| male | 1.61 (1.39–1.85) | 1.52 (1.31–1.73) | 1.65 (1.41–1.88) | 1.71 (1.47–1.89) | <0.001 |
| female | 1.10 (0.97–1.27) | 1.03 (0.92–1.20) | 1.13 (0.97–1.28) | 1.16 (1.02–1.32) | <0.001 |
Median (interquartile range).
Spearman correlations between urinary measures (Supplemental Table 1) were strongest for spot-ACR and spot-UAC (r=0.59). Spot-UAC and spot urine creatinine concentration were moderately correlated (r=0.54), again probably reflecting effects of urine tonicity on both. The remaining correlations were weaker. Daily creatinine excretion and spot urine creatinine concentration were only moderately correlated (r=0.38).
We evaluated whether factors linked with lower muscle mass were associated with higher spot-ACR, independent of 24hr-UAE. We observed that persons with lower creatinine excretion rate, lower weight, older age, and female sex had significantly higher spot-ACR at any given level of 24hr-UAE (Table 2).
Table 2.
Influence of weight, sex, and age on spot urine albumin-to-creatinine ratio, independent of 24-hour urine albumin excretion
| Variable | Patients (n) | Higher Spot Urine Albumin-to-Creatinine Ratio (95% CI) (%) | P Value |
|---|---|---|---|
| Creatinine excretion | |||
| >1.49 g/d | 869 | Reference | |
| 1.13–1.49 g/d | 869 | 19 (15–23) | <0.001 |
| <1.13 g/d | 868 | 30 (26–33) | <0.001 |
| per 100 mg/d lower | 2606 | 4 (4–5) | <0.001 |
| Weight | |||
| >80.5 kg | 851 | Reference | |
| 70.5–80.5 kg | 847 | 2 (3–7) | 0.43 |
| <70.5 kg | 884 | 7 (1–12) | 0.02 |
| per 5 kg lower | 2582 | 2 (1–3) | <0.001 |
| Age | |||
| ≤53 years | 1773 | Reference | |
| >53 years | 854 | 25 (19–30) | <0.001 |
| per 5 years older | 2627 | 4 (4–5) | <0.001 |
| Sex | |||
| male | 1164 | Reference | |
| female | 1463 | 18 (14–22) | <0.001 |
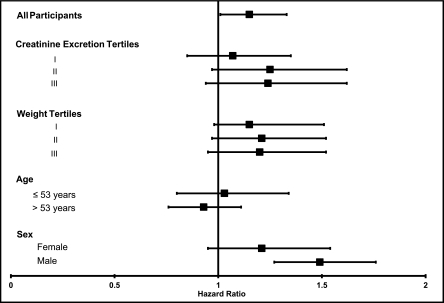
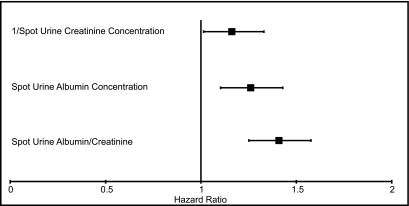
We observed an association of 1/spot urine creatinine with CVD events (hazard ratio [HR] per ln-SD higher, 1.16 [95% confidence interval (CI), 1.01–1.32]) (Figure 2). When evaluated within subgroups defined by 24-hour creatinine excretion, age, sex, and weight, 1/spot urine creatinine was similarly associated with CVD events, and 95% CIs were overlapping across subgroups. Tests for interaction were not statistically significant (P for all interactions > 0.21). Spot-UAC was associated with CVD events (HR per ln-SD higher, 1.26 [95% CI, 1.10–1.43]), and this was weaker than the association of spot-ACR with CVD events (HR per ln-SD higher, 1.41 [95% CI, 1.25–1.58]) when we compared the point estimates, although 95% CIs were overlapping (Figure 3). Results were similar across subgroups without significant interactions (P for all interactions > 0.13).
Figure 2.
Unadjusted associations per SD lower 1/spot urine creatinine (i.e., higher spot urine creatinine concentration) on the natural log scale with CVD events. Subgroups were selected a priori on the basis of hypothesized differences in muscle mass. P values for interaction were 0.47 for creatinine excretion tertiles, 0.38 for weight tertiles, 0.33 for age, and 0.21 for sex. Error bars reflect 95% confidence intervals.
Figure 3.
Unadjusted associations per SD lower spot urine creatinine and per SD higher spot urine albumin concentration and spot urine albumin-to-creatinine ratio with CVD events on the natural log scale for the entire cohort. Error bars reflect 95% confidence intervals.
Table 3 shows the unadjusted HRs for spot-ACR greater than 10 mg/g versus lower and 24hr-UAE greater than 10 mg/d versus lower in all participants and in subgroups. When all participants were evaluated together, spot-ACR greater than 10 mg/g and 24hr-UAE greater than 10 mg/d were similarly associated with CVD events. The association of high spot-ACR and 24hr-UAE with CVD events was similar across tertiles of urine creatinine excretion, age, weight, and sex, and no statistically significant interactions were detected (P for all interactions > 0.06). Results were similar when models were adjusted for age, sex, weight, kidney function, and traditional CVD risk factors (Supplemental Table 2). Finally, we evaluated whether the relative associations of spot-ACR and 24hr-UAE with CVD events were changed and rendered more similar with adjustment for 24-hour creatinine excretion (Table 4). Spot-ACR greater than 10 mg/g appeared to remain more strongly associated with CVD events in groups with low muscle mass despite this adjustment, although 95% CIs were widely overlapping.
Table 3.
Association of spot urine albumin-to-creatinine ratio >10 mg/g and 24-hour urine albumin excretion >10 mg/d with cardiovascular events*
| Variable | Events/Patients at Risk (n/n) | Spot Urine Albumin-to-Creatinine Ratio >10 mg/g versus Lower | 24-hr Urine Albumin Excretion >10 mg/d versus Lower | ||
|---|---|---|---|---|---|
| HR (95% CI) | P Value for Interaction | HR (95% CI) | P Value for Interaction | ||
| All participants | 190/2627 | 2.33 (1.67–3.24) | 2.03 (1.51–2.72) | ||
| 24-hr creatinine excretion tertiles | 0.70 | 0.45 | |||
| <1.13 g/d | 66/868 | 2.31 (1.36–3.94) | 1.83 (1.05–3.18) | ||
| 1.13–1.49 g/d | 60/869 | 2.86 (1.63–5.02) | 1.80 (1.06–3.05) | ||
| >1.49 g/d | 62/868 | 1.82 (0.90–3.68) | 2.42 (1.46–3.99) | ||
| Weight tertiles | 0.06 | 0.36 | |||
| <70.5 kg | 52/884 | 2.72 (2.10–6.59) | 1.39 (0.75–2.57) | ||
| 70.5–80.5 kg | 60/847 | 2.21 (1.22–4.02) | 2.51 (1.50–4.19) | ||
| >80.5 kg | 76/851 | 1.69 (0.94–3.03) | 2.11 (1.33–3.34) | ||
| Age | 0.28 | 0.42 | |||
| >53 yr | 128/854 | 2.01 (1.38–2.93) | 2.01 (1.41–2.86) | ||
| ≤53 yr | 62/1773 | 1.23 (0.56–2.70) | 1.53 (0.88–2.66) | ||
| Sex | 0.75 | 0.95 | |||
| female | 61/1463 | 2.91 (0.170–4.96) | 1.83 (1.06–3.14) | ||
| male | 129/1164 | 2.57 (1.67–3.96) | 1.86 (1.30–2.65) | ||
HR, hazard ratio; CI, confidence interval.
Unadjusted.
Table 4.
Association of spot urine albumin-to-creatinine ratio and 24-hour urine albumin excretion with cardiovascular events*
| Variable | Events/Patients at Risk (n/n) | HR for Spot Urine Albumin-to-Creatinine Ratio ≥10 mg/g versus <10 mg/g (95% CI) | HR for 24-hr Urine Albumin Excretion ≥10 mg/d versus <10 mg/d (95% CI) |
|---|---|---|---|
| All participants | 190/2627 | 2.35 (1.67–3.29) | 2.02 (1.50–2.73) |
| Weight tertiles | |||
| < 70.5 kg | 52/884 | 3.51 (1.96–6.30) | 1.40 (0.75–2.65) |
| 70.5–80.5 kg | 60/847 | 2.13 (1.61–3.91) | 2.61 (1.54–4.42) |
| > 80.5 kg | 76/851 | 1.71 (0.95–3.08) | 2.16 (1.35–3.46) |
| Sex | |||
| female | 61/1463 | 2.88 (1.68–4.92) | 2.04 (1.17–3.55) |
| male | 129/1164 | 2.27 (1.46–3.52) | 2.04 (1.42–2.92) |
| Age | |||
| >53 yr | 128/854 | 2.06 (1.40–3.04) | 1.93 (1.35–2.78) |
| ≤53 yr | 62/1773 | 1.31 (0.60–2.87) | 1.40 (0.80–2.46) |
Adjusted for 24-hour urine creatinine excretion.
Discussion
In a community-living population with 24hr-UAE less than 30 mg/d, we demonstrate that spot-ACR is higher in persons with lower 24-hour urine creatinine excretion (a measure of lower muscle mass), older persons, individuals with lower body weight, and women. Spot urine creatinine concentration was only moderately correlated with 24-hour urine creatinine excretion, probably reflecting the influence of urine tonicity. Despite these findings, spot-ACR and 24hr-UAE have similar associations with CVD events in all participants combined and in subgroups with different muscle mass. Both spot-UAC and 1/spot urine creatinine were associated with CVD events, and neither was as strongly associated with CVD events as was their ratio (spot-ACR).
Prior studies demonstrated that spot-ACR is more likely to misclassify patients with lower muscle mass as having microalbuminuira (28). Similarly, at the same level of albuminuria, women have higher spot-ACR than do men (20,29,30). With age, spot-ACR increases more rapidly than 24hr-UAE, whereas 24-hour creatinine excretion rate and spot urine creatinine concentration decline (21,30). These studies, in conjunction with the data presented here, are consistent with the hypothesis that muscle mass influences the relationship between spot-ACR and 24hr-UAE; spot-ACR is higher at any given level of 24hr-UAE in individuals with lower muscle mass.
Standardized measures of muscle mass (dual-energy x-ray absorptiometry, bioelectrical impedance, and mid-arm circumference measures) are highly correlated with 24-hour urinary creatinine excretion rates (17,31), and creatinine excretion rate is lower in persons who are older, in women, and in those with lower body mass index (32–34). As demonstrated here, 24-hour urine creatinine excretion rate and spot urine creatinine concentrations are moderately correlated (r=0.38), and persons with lower spot urine creatinine were older, weighed less, and were more frequently female. Thus, beyond marking dilute urine, low spot urine creatinine may also mark lower muscle mass and by extension may mark greater frailty or less physical activity, factors that themselves lead to adverse health outcomes through mechanisms independent of urine albumin excretion.
Several studies demonstrate that lower urine creatinine excretion rates on timed urine specimens are associated with greater mortality risk, CVD events, and progression of CKD, independent of albuminuria and eGFR (14,18,19). We make the novel contribution that similar associations extend to spot urine creatinine in community-living individuals. However, this association was weaker than those reported with 24-hour urine creatinine excretion (18,19). This weaker association probably reflects the joint influence of muscle mass and urine tonicity on spot urine creatinine concentration.
Despite these findings, we did not observe differences in the association of spot-ACR with CVD events across subgroups of timed urine creatinine excretion, age, sex, and weight. In this population, the association between urinary measures of albuminuria and CVD events was strongest for spot-ACR, and the relationship between 1/urine creatinine and CVD events was only modestly significant. The association of spot-UAC with CVD events was intermediate. When adjusted for daily creatinine excretion, spot-ACR was most strongly associated with events in the subgroups with low muscle mass, again suggesting that albuminuria may be a more important factor in the association of spot-ACR with events than low urinary creatinine. One explanation may be the ranges of urine albumin and creatinine concentrations observed in clinical practice. Urine albumin excretion often varies over several orders of magnitude (from less than 3 mg/d to greater than 3000 mg/d). In comparison, the range of urine creatinine values is constrained by less dramatic differences in muscle mass and urinary concentration. These physiologic constraints may result in greater effect of albuminuria on the spot-ACR and thus on its association with CVD events. To mitigate this, we excluded participants with 24hr-UAE greater than 30 mg/d; however, we still did not observe marked differences in associations of spot-ACR versus 24hr-UAE with CVD events.
Another possibility for the observed similar associations of spot-ACR and 24hr-UAE with CVD is the confounding influence of urine tonicity. In contrast to urine creatinine, in which lower levels appear to mark excess risk, higher urine tonicity measured by osmolality or specific gravity has been associated with higher rates of CVD and with increased BP (35,36). These risks may be due to the adverse effects of salt and fluid retention on BP. Thus, the association of lower spot urine creatinine concentration with CVD events may be weakened by differences in urine tonicity, which was not measured in this cohort. Finally, it is possible that our study was underpowered to detect differences in CVD events across subgroups because only 190 CVD events occurred during follow-up. Future studies with more CVD events should re-evaluate these associations across subgroups with different amounts of muscle mass.
Strengths of this study include its evaluation of community-living individuals with both first morning spot urine specimens and two timed 24-hour urine collections in series, evaluation of both men and women, and long-term follow-up.
The study also has limitations. We evaluated a European cohort, which provided little racial heterogeneity. We limited the study sample to persons with 24hr-UAE less than 30 mg/d because marked albuminuria is an established risk factor for CVD, and we hypothesized that the bias introduced by urine creatinine would be greatest in the sub-microalbuminuric range. Whether results generalize to persons with overt albuminuria or CKD are unknown. Other methods of adjusting spot-UAC for urine tonicity (i.e., urine osmolality or specific gravity) were not available in this study. We also lacked a direct measure of muscle mass (i.e., dual-energy x-ray absorptiometry or bioimpedence), which would be useful to more precisely evaluate patients with lower muscle mass. Last, because urine creatinine appears to mark muscle mass, difference in predominantly middle-aged community-living populations may reflect physiologic rather than pathologic changes in muscle. Results may differ in older cohorts or in individuals with chronic diseases.
In conclusion, among community-living individuals with 24hr-UAE less than 30 mg/d, spot-ACR is higher in women, individuals with lower body weight, and older persons, independent of 24hr-UAE. Spot urine creatinine is moderately correlated with timed urine creatinine excretion and is weakly inversely associated with CVD events. The relationship of spot-ACR with CVD events was similar to that of 24hr-UAE, even across subgroups that differ in muscle mass. We conclude that although muscle mass may influence spot urine creatinine concentrations, the major determinant of the relationship of spot-ACR with CVD events is driven by the numerator (albumin) rather than the denominator (creatinine) in community-living individuals.
Disclosures
None.
Acknowledgments
This study was supported by grants T32 HL007261 (C.E.C.) and R01HL096851 (J.H.I.) from the National Heart, Lung, and Blood Institute. The PREVEND Study was made possible by grants from the Dutch Kidney Foundation. Dade Behring (Marburg, Germany) generously supplied equipment (Behring Nephelometer II) and reagents for nephelometric measurement of urinary albumin concentration. This material is the result of work supported with resources of the Veterans Affairs San Diego Healthcare System.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.09300911/-/DCSupplemental.
Access to UpToDate on-line is available for additional clinical information at www.cjasn.org.
References
- 1.Brantsma AH, Bakker SJ, de Zeeuw D, de Jong PE, Gansevoort RT, PREVEND Study Group : Extended prognostic value of urinary albumin excretion for cardiovascular events. J Am Soc Nephrol 19: 1785–1791, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appel LJ, Wright JT, Jr, Greene T, Agodoa LY, Astor BC, Bakris GL, Cleveland WH, Charleston J, Contreras G, Faulkner ML, Gabbai FB, Gassman JJ, Hebert LA, Jamerson KA, Kopple JD, Kusek JW, Lash JP, Lea JP, Lewis JB, Lipkowitz MS, Massry SG, Miller ER, Norris K, Phillips RA, Pogue VA, Randall OS, Rostand SG, Smogorzewski MJ, Toto RD, Wang X, AASK Collaborative Research Group : Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med 363: 918–929, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, Hallé JP, Young J, Rashkow A, Joyce C, Nawaz S, Yusuf S, HOPE Study Investigators : Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 286: 421–426, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Roest M, Banga JD, Janssen WM, Grobbee DE, Sixma JJ, de Jong PE, de Zeeuw D, van Der Schouw YT: Excessive urinary albumin levels are associated with future cardiovascular mortality in postmenopausal women. Circulation 103: 3057–3061, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Arnlöv J, Evans JC, Meigs JB, Wang TJ, Fox CS, Levy D, Benjamin EJ, D’Agostino RB, Vasan RS: Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation 112: 969–975, 2005 [DOI] [PubMed] [Google Scholar]
- 6.van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey A, de Jong P, Gansevoort RT, van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey AS, de Jong PE, Gansevoort RT, Levey A, El-Nahas M, Eckardt KU, Kasiske BL, Ninomiya T, Chalmers J, Macmahon S, Tonelli M, Hemmelgarn B, Sacks F, Curhan G, Collins AJ, Li S, Chen SC, Hawaii Cohort KP, Lee BJ, Ishani A, Neaton J, Svendsen K, Mann JF, Yusuf S, Teo KK, Gao P, Nelson RG, Knowler WC, Bilo HJ, Joosten H, Kleefstra N, Groenier KH, Auguste P, Veldhuis K, Wang Y, Camarata L, Thomas B, Manley T, Chronic Kidney Disease Prognosis Consortium : Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int 79: 1341–1352, 2011 [DOI] [PubMed] [Google Scholar]
- 7.National Kidney Foundation : K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 39[Suppl 1]: S1–S266, 2002 [PubMed] [Google Scholar]
- 8.Ginsberg JM, Chang BS, Matarese RA, Garella S: Use of single voided urine samples to estimate quantitative proteinuria. N Engl J Med 309: 1543–1546, 1983 [DOI] [PubMed] [Google Scholar]
- 9.Schwab SJ, Christensen RL, Dougherty K, Klahr S: Quantitation of proteinuria by the use of protein-to-creatinine ratios in single urine samples. Arch Intern Med 147: 943–944, 1987 [PubMed] [Google Scholar]
- 10.Alstrup K, Graugaard-Jensen C, Rittig S, Jørgensen KA: Abnormal diurnal rhythm of urine output following renal transplantation: The impact of blood pressure and diuretics. Transplant Proc 42: 3529–3536, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Shirreffs SM, Maughan RJ: Urine osmolality and conductivity as indices of hydration status in athletes in the heat. Med Sci Sports Exerc 30: 1598–1602, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Armstrong LE, Pumerantz AC, Fiala KA, Roti MW, Kavouras SA, Casa DJ, Maresh CM: Human hydration indices: Acute and longitudinal reference values. Int J Sport Nutr Exerc Metab 20: 145–153, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Rittig S, Knudsen UB, Nørgaard JP, Pedersen EB, Djurhuus JC: Abnormal diurnal rhythm of plasma vasopressin and urinary output in patients with enuresis. Am J Physiol 256: F664–F671, 1989 [DOI] [PubMed] [Google Scholar]
- 14.Lambers Heerspink HJ, Gansevoort RT, Brenner BM, Cooper ME, Parving HH, Shahinfar S, de Zeeuw D: Comparison of different measures of urinary protein excretion for prediction of renal events. J Am Soc Nephrol 21: 1355–1360, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heymsfield SB, Arteaga C, McManus C, Smith J, Moffitt S: Measurement of muscle mass in humans: validity of the 24-hour urinary creatinine method. Am J Clin Nutr 37: 478–494, 1983 [DOI] [PubMed] [Google Scholar]
- 16.Welle S, Thornton C, Totterman S, Forbes G: Utility of creatinine excretion in body-composition studies of healthy men and women older than 60 y. Am J Clin Nutr 63: 151–156, 1996 [DOI] [PubMed] [Google Scholar]
- 17.Wang ZM, Gallagher D, Nelson ME, Matthews DE, Heymsfield SB: Total-body skeletal muscle mass: evaluation of 24-h urinary creatinine excretion by computerized axial tomography. Am J Clin Nutr 63: 863–869, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Ix JH, de Boer IH, Wassel CL, Criqui MH, Shlipak MG, Whooley MA: Urinary creatinine excretion rate and mortality in persons with coronary artery disease: the Heart and Soul Study. Circulation 121: 1295–1303, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oterdoom LH, Gansevoort RT, Schouten JP, de Jong PE, Gans RO, Bakker SJ: Urinary creatinine excretion, an indirect measure of muscle mass, is an independent predictor of cardiovascular disease and mortality in the general population. Atherosclerosis 207: 534–540, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Mattix HJ, Hsu CY, Shaykevich S, Curhan G: Use of the albumin/creatinine ratio to detect microalbuminuria: implications of sex and race. J Am Soc Nephrol 13: 1034–1039, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Lambers Heerspink HJ, Brantsma AH, de Zeeuw D, Bakker SJ, de Jong PE, Gansevoort RT, PREVEND Study Group : Albuminuria assessed from first-morning-void urine samples versus 24-hour urine collections as a predictor of cardiovascular morbidity and mortality. Am J Epidemiol 168: 897–905, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Mahmoodi BK, Gansevoort RT, Veeger NJ, Matthews AG, Navis G, Hillege HL, van der Meer J, Prevention of Renal and Vascular End-stage Disease (PREVEND) Study Group : Microalbuminuria and risk of venous thromboembolism. JAMA 301: 1790–1797, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Burrowes JD, Larive B, Chertow GM, Cockram DB, Dwyer JT, Greene T, Kusek JW, Leung J, Rocco MV, Hemodialysis (HEMO) Study Group : Self-reported appetite, hospitalization and death in haemodialysis patients: Findings from the Hemodialysis (HEMO) Study. Nephrol Dial Transplant 20: 2765–2774, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Stricker BH, Herings RM: [Plea for the retention of the Dutch National Medical Registration (LMR) to provide reliable information regarding public health and healthcare]. Ned Tijdschr Geneeskd 150: 1916–1917, 2006 [PubMed] [Google Scholar]
- 25.Özyilmaz A, Bakker SJ, de Zeeuw D, de Jong PE, Gansevoort RT, PREVEND Study Group : Selection on albuminuria enhances the efficacy of screening for cardiovascular risk factors. Nephrol Dial Transplant 25: 3560–3568, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU: The definition, classification, and prognosis of chronic kidney disease: A KDIGO Controversies Conference report. Kidney Int 80: 17–28, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Cirillo M, Laurenzi M, Mancini M, Zanchetti A, De Santo NG: Low muscular mass and overestimation of microalbuminuria by urinary albumin/creatinine ratio. Hypertension 47: 56–61, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Connell SJ, Hollis S, Tieszen KL, McMurray JR, Dornan TL: Gender and the clinical usefulness of the albumin: creatinine ratio. Diabet Med 11: 32–36, 1994 [DOI] [PubMed] [Google Scholar]
- 30.Houlihan CA, Tsalamandris C, Akdeniz A, Jerums G: Albumin to creatinine ratio: A screening test with limitations. Am J Kidney Dis 39: 1183–1189, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Rule AD, Bailey KR, Schwartz GL, Khosla S, Lieske JC, Melton LJ, 3rd: For estimating creatinine clearance measuring muscle mass gives better results than those based on demographics. Kidney Int 75: 1071–1078, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He Q, Heo M, Heshka S, Wang J, Pierson RN, Jr, Albu J, Wang Z, Heymsfield SB, Gallagher D: Total body potassium differs by sex and race across the adult age span. Am J Clin Nutr 78: 72–77, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Araujo AB, Chiu GR, Kupelian V, Hall SA, Williams RE, Clark RV, McKinlay JB: Lean mass, muscle strength, and physical function in a diverse population of men: A population-based cross-sectional study. BMC Public Health 10: 508, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gallagher D, Visser M, De Meersman RE, Sepúlveda D, Baumgartner RN, Pierson RN, Harris T, Heymsfield SB: Appendicular skeletal muscle mass: Effects of age, gender, and ethnicity. J Appl Physiol 83: 229–239, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Perucca J, Bouby N, Valeix P, Jungers P, Bankir L: [Difference in urine concentration according to gender and ethnicity: Possible involvement in the different susceptibility to various renal and cardiovascular diseases]. Nephrol Ther 4: 160–172, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Bankir L, Perucca J, Weinberger MH: Ethnic differences in urine concentration: possible relationship to blood pressure. Clin J Am Soc Nephrol 2: 304–312, 2007 [DOI] [PubMed] [Google Scholar]