Summary
Background and objectives
Persons with kidney disease often have cardiovascular disease, but they are less likely to use recommended medications for secondary prevention. The hypothesis was that participants with reduced estimated GFR have lower use of medications recommended for secondary prevention of cardiovascular events (antiplatelet agents, angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers, β-blockers, and statins) and lower medication adherence than participants with preserved estimated GFR.
Design, setting, participants, & measurements
In this cross-sectional analysis, we analyzed data from 6913 participants in the Reasons for Geographic and Racial Differences in Stroke study with a history of cardiovascular disease. Medication use was ascertained by an in-home pill bottle review. Medication adherence was assessed using a validated four-item scale.
Results
Among participants with a history of cardiovascular disease, 59.8% used antiplatelet agents, 49.9% used angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers, 41.6% used β-blockers, and 53.0% used statins. Compared with the referent group (estimated GFR ≥60 ml/min per 1.73 m2), participants with estimated GFR <45 ml/min per 1.73 m2 were more likely to use angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers (adjusted prevalence ratio=1.14, 95% confidence interval=1.06–1.23), β-blockers (adjusted prevalence ratio=1.20, 95% confidence interval=1.09–1.32), and statins (adjusted prevalence ratio=1.10, 95% confidence interval=1.01–1.19). Antiplatelet agent use did not differ by estimated GFR category; 30% of participants reported medication nonadherence across all categories of estimated GFR.
Conclusions
Among participants with a history of cardiovascular disease, mild to moderate reductions in estimated GFR were associated with similar and even more frequent use of medications for secondary prevention compared with participants with preserved estimated GFR. Overall medication use and adherence were suboptimal.
Introduction
The prevalence of concomitant CKD and cardiovascular disease is very high; an estimated 40% of persons with CKD have cardiovascular disease (1), and 30–60% of persons with cardiovascular disease have CKD (2,3). Given the proven benefit of antiplatelet agents, angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs), β-adrenergic receptor blockers (β-blockers), and statins on cardiovascular outcomes, current guidelines recommend their use for secondary prevention in patients with cardiovascular disease (4). The risk of adverse cardiovascular outcomes increases with progressively lower levels of estimated GFR (eGFR) (5), but persons with reduced eGFR are often less likely to be treated with recommended medications after a cardiovascular event (6–8).
In addition to appropriate medication use, another important aspect of secondary prevention of cardiovascular events is medication adherence. Persons with reduced eGFR often have a higher prevalence of comorbid conditions, such as cognitive impairment, depression, or functional status limitations, that are each associated with lower medication adherence (9–11). Medication nonadherence is associated with a higher risk of adverse outcomes (12,13). Given the high risk of recurrent cardiovascular events in persons with CKD and a history of cardiovascular disease, understanding patterns of cardioprotective medication use in this population may help identify avenues to optimize their use and improve adherence.
We analyzed data from participants in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study with a history of cardiovascular disease. Our primary objective was to test the hypothesis that participants with mild to moderate reductions in eGFR have lower use of medications recommended for secondary prevention of cardiovascular events and lower medication adherence than participants with preserved eGFR. In addition, although previous studies have shown that blacks have lower use of and adherence to medications for secondary prevention compared with whites (14,15), little is known about how race modifies the association of eGFR with medication use and adherence. We leveraged the fact that the REGARDS study has a large proportion of black participants to test the hypotheses that black participants with reduced eGFR have lower use of and adherence to medications recommended for secondary prevention than white participants and that the racial gap is wider at lower eGFR levels.
Materials and Methods
Study Cohort
The REGARDS study includes a population-based sample of adults 45 years or older from the 48 contiguous US states. Study recruitment and data collection have been described previously (16). Briefly, between January of 2003 and October of 2007, eligible participants were identified from a commercially available list of residents and recruited through an initial mailing followed by telephone contact; to be included in the cohort, participants had to complete a telephone survey, agree to an in-home visit, and agree to telephone follow-up. A modification of the standardized methods recommended in the work by Morton et al. (17) was used to determine participation rates in the REGARDS study. The response rate was 33%, and the cooperation rate was 49%, similar to the rates reported in other epidemiologic studies (18,19).
By design, 56% of the sample was recruited from eight southern US states, commonly referred to as the stroke buckle (coastal North Carolina, South Carolina, and Georgia) and stroke belt (Alabama, Mississippi, Tennessee, Arkansas, Louisiana, and the remainder of North Carolina, South Carolina, and Georgia), with the remaining cohort recruited from the other 40 contiguous US states. Additionally, black participants were purposely oversampled and comprise 42% of the final REGARDS study cohort, which included a total of 30,239 participants.
Data Collection
Trained interviewers conducted computer-assisted telephone interviews to obtain information about participants’ demographics, smoking status, marital status, annual household income, education, and self-reported prior diagnosis of major comorbid conditions. Trained health professionals conducted subsequent in-home study visits. During the in-home visit, anthropometric measurements and an electrocardiogram were obtained. Electrocardiogram reading was done centrally at Wake Forest University. Blood pressure was measured two times in a seated position, and the average of measurements used. Participants were asked to fast overnight before their in-home visit; blood was drawn, and the samples shipped to a central laboratory for determination of serum creatinine. Random urine samples were also obtained for measurement of creatinine and albumin.
Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or current pharmacological treatment for hypertension. Diabetes mellitus was defined as fasting glucose level ≥126 mg/dl, nonfasting glucose level ≥200 mg/dl, or current pharmacological treatment for diabetes mellitus. Symptoms of depression were defined as present for participants with scores greater than or equal to four on the four-item Centers for Epidemiologic Studies of Depression scale (20). Cognitive impairment was defined as scores less than five using a six-item test of global cognitive function that includes recall and temporal orientation items (21).
Participants were asked to provide bottles for all medications (including nonprescription pills such as aspirin and dietary supplements) used in the past 2 weeks, and medication names were recorded and subsequently coded into drug classes. We were specifically interested in the following drug classes, which are recommended for secondary prevention of cardiovascular disease: aspirin and/or clopidogrel (combined into a single category of antiplatelet agents), ACEI or ARBs (combined into a single category of ACEI/ARBs), β-blockers, and statins.
Medication adherence was assessed using the four-item Morisky Medication Adherence Scale, a validated questionnaire with acceptable internal consistency (Cronbach’s α=0.61) (22). The four items have response options of no or yes to these questions. (1) Do you forget to take medications? (2) Are you ever careless in taking your medications? (3) Do you ever miss taking your medications when you are feeling better? (4) Do you ever miss taking any of your medications because you are feeling sick? One point was assigned to each yes response, the points were summed, and medication adherence was grouped into three levels: zero, one, and more than or equal to two, with higher scores indicating worse adherence (23).
History of Cardiovascular Disease
Because we were interested in secondary prevention, the present analysis was limited to participants with a history of cardiovascular disease, defined as evidence of prior myocardial infarction (MI) on electrocardiogram or self-reported physician diagnosis of MI, coronary artery revascularization (percutaneous coronary intervention or coronary artery bypass surgery), stroke, aortic aneurysm repair, lower extremity bypass surgery, and/or carotid endarterectomy or carotid angioplasty. Participants who reported a history of MI or stroke were asked to estimate the number of years since their most recent event, which we categorized into the following three groups: <1, 1–5, or >5 years. We chose these time categories to have an approximately even distribution of participants within each eGFR category.
Assessment of Renal Function
Using isotope dilution mass spectrometry-traceable serum creatinine, we calculated the eGFR using the CKD Epidemiology Collaboration equation (24). We categorized participants into three eGFR groups of ≥60, 45–59 (CKD stage 3a), and <45 (CKD stage 3b and higher; in ml/min per 1.73 m2) as has been done previously (5,25). Albuminuria was assessed using a spot urine albumin to creatinine ratio (mg/g).
Statistical Analyses
Participant characteristics were described by eGFR category as mean (SD) for continuous variables and proportions for categorical variables. We calculated the distribution of antiplatelet, ACEI/ARB, β-blocker, and statin use separately and collectively by eGFR category. Similarly, we calculated the distribution of responses to each of the four questions on the adherence scale and aggregate scale scores by eGFR category.
We used log-binomial regression to calculate prevalence ratios for use of each medication class separately and collectively, comparing participants with eGFR 45–59 and <45 ml/min per 1.73 m2 with participants with eGFR ≥60 ml/min per 1.73 m2. For common outcomes, prevalence ratios are a more consistent approximation of relative risks than odds ratios derived from logistic regression (26). We also calculated prevalence ratios for medication adherence scale scores of one and more than or equal to two versus zero by eGFR category. In multivariable-adjusted models, we included the following covariates that were selected a priori: age, sex, race, region of residence (stroke buckle, stroke belt, or nonbelt), marital status (married or unmarried), education level (less than high school or high school graduate), annual income (<$20,000 or ≥$20,000), current smoking status, depression, cognitive impairment, diabetes mellitus, albuminuria (log-transformed), and hypertension. Missing data were imputed using chained equations with five datasets (27).
We conducted several companion analyses. Given the particular indication for ACEI/ARB use in participants with diabetes mellitus, we analyzed medication use for participants with and without diabetes mellitus separately. We also analyzed medication use and adherence by eGFR categories for whites and blacks separately and formally tested for interaction using a 1000-iteration bootstrap procedure. Finally, we hypothesized that medication use may decrease as the time since the last occurrence of an MI or stroke increases. To test this hypothesis, we assessed the distribution of medication use by eGFR category and time (<1, 1–5, or >5 years) since last MI or stroke, the only cardiovascular events for which we had information on time since the last event. We calculated multivariable-adjusted prevalence ratios for each medication class and eGFR category separately, with time ≤1 year as the referent category. We repeated this analysis after limiting our cohort to participants who reported a history of MI, because several previous studies of secondary medication use were conducted in this population (7,28–30).
All analyses were conducted using SAS 9.2 (SAS Institute, Cary, NC) and Stata 11.2 (StataCorp LP, College Station, TX).
Results
A total of 7726 REGARDS study participants had a history of cardiovascular disease. We excluded participants missing serum creatinine values (n=400), pill bottle review data (n=16), or adherence scale question responses (n=347). We also excluded participants with ESRD on dialysis (n=50), leaving a total of 6913 participants for the present analysis. Excluded participants were older, more often women, and more likely to be African-American. Overall, 19.8% (n=1368) of our cohort had reduced eGFR: 12.1% (n=837) had eGFR 45–59 ml/min per 1.73 m2 and 7.7% (n=531) had eGFR <45 ml/min per 1.73 m2.
Medication Use
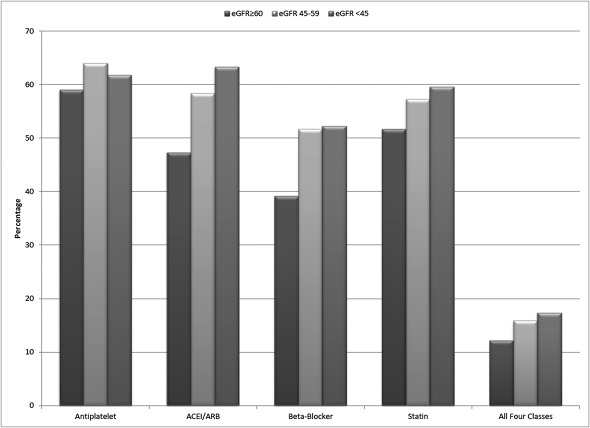
Participants with eGFR <45 ml/min per 1.73 m2 were older, more likely to be black, and more likely to have cognitive impairment, diabetes mellitus, hypertension, and albuminuria (Table 1). In the overall cohort, 59.8% of participants used antiplatelet agents, 49.9% used ACEI/ARBs, 41.6% used β-blockers, and 53.0% used statins; 13% of participants used all four medication classes (Figure 1). In unadjusted analyses, participants with eGFR levels of 45–59 and <45 ml/min per 1.73 m2 were more likely to use ACEI/ARBs, β-blockers, statins, and all four medication classes together compared with participants with eGFR ≥60 ml/min per 1.73 m2 (Table 2). After multivariable adjustment, the associations were attenuated but remained statistically significant. Antiplatelet agent use did not differ by eGFR category. For each class of medication, prevalence ratios were similar for the 45–59 and <45 ml/min per 1.73 m2 categories.
Table 1.
Characteristics of the Reasons for Geographic and Racial Differences in Stroke study participants with a history of cardiovascular disease by level of estimated GFR
| Estimated GFR (ml/min per 1.73 m2) | |||
|---|---|---|---|
| ≥60 (n=5545) | 45–59 (n=837) | <45 (n=531) | |
| Age (years) | 66.9 (8.9) | 73.0 (8.3) | 72.9 (8.5) |
| Female sex | 43.7 | 46.2 | 46.7 |
| Black race | 38.6 | 36.1 | 43.5 |
| Married | 60.9 | 53.5 | 49.5 |
| Less than high school education | 16.6 | 20.9 | 23.8 |
| Annual income ≥$20,000 | 74.4 | 72.1 | 62.6 |
| Region of residence | |||
| nonstroke belt | 43.1 | 44.0 | 49.2 |
| stroke belt | 36.5 | 33.1 | 30.1 |
| stroke buckle | 20.4 | 22.9 | 20.7 |
| Current smoker | 17.3 | 11.7 | 12.8 |
| Symptoms of depression | 14.8 | 14.3 | 12.6 |
| Cognitive impairment | 10.2 | 15.0 | 16.6 |
| Diabetes mellitus | 29.5 | 33.9 | 50.8 |
| Hypertension | 70.6 | 81.8 | 85.8 |
| Body mass index (kg/m2) | 29.4 (6.1) | 29.3 (6.4) | 29.3 (6.1) |
| Albuminuria ≥30 mg/g | 18.5 | 28.9 | 57.0 |
| Prior history of cardiovascular disease | |||
| prior myocardial infarction | 49.2 | 48.5 | 49.0 |
| coronary artery revascularization | 42.8 | 49.9 | 47.1 |
| stroke | 23.6 | 28.3 | 30.7 |
| aortic aneurysm repair | 3.4 | 5.1 | 5.5 |
| lower extremity bypass surgery | 7.4 | 9.8 | 9.4 |
| carotid endarterectomy or angioplasty | 7.3 | 11.7 | 12.1 |
All values are percentages or mean (SD).
Figure 1.
Distribution of medication use among the Reasons for Geographic and Racial Differences in Stroke study participants with a history of cardiovascular disease. Cardiovascular disease is defined as prior myocardial infarction (by electrocardiogram or self-report), coronary revascularization (percutaneous coronary intervention or coronary artery bypass surgery), stroke, aortic aneurysm repair, lower extremity bypass surgery, or carotid endarterectomy or angioplasty. ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; eGFR, estimated GFR (ml/min per 1.73 m2).
Table 2.
Prevalence ratios (95% confidence interval) for medication use associated with level of estimated GFR in the Reasons for Geographic and Racial Differences in Stroke study participants with a history of cardiovascular disease
| Estimated GFR (ml/min per 1.73 m2) | |||
|---|---|---|---|
| ≥60 (n=5545) | 45–59 (n=837) | <45 (n=531) | |
| Antiplatelet agent | |||
| unadjusted | 1.00 (ref) | 1.08 (1.00–1.15) | 1.05 (0.98–1.12) |
| multivariable-adjusteda | 1.00 (ref) | 1.04 (0.98–1.10) | 1.02 (0.95–1.10) |
| ACEI/ARB | |||
| unadjusted | 1.00 (ref) | 1.23 (1.16–1.31) | 1.34 (1.25–1.44) |
| multivariable-adjusteda | 1.00 (ref) | 1.12 (1.05–1.20) | 1.14 (1.06–1.23) |
| β-Blocker | |||
| unadjusted | 1.00 (ref) | 1.32 (1.23–1.42) | 1.33 (1.22–1.46) |
| multivariable-adjusteda | 1.00 (ref) | 1.23 (1.14–1.33) | 1.20 (1.09–1.32) |
| Statin | |||
| unadjusted | 1.00 (ref) | 1.11 (1.04–1.18) | 1.15 (1.07–1.24) |
| multivariable-adjusteda | 1.00 (ref) | 1.07 (1.00–1.14) | 1.10 (1.01–1.19) |
| All four medication classes | |||
| unadjusted | 1.00 (ref) | 1.32 (1.11–1.56) | 1.43 (1.18–1.75) |
| multivariable-adjusteda | 1.00 (ref) | 1.22 (1.02–1.46) | 1.26 (1.02–1.56) |
Cardiovascular disease is defined as prior myocardial infarction (by electrocardiogram or self-report), coronary revascularization (percutaneous coronary intervention or coronary artery bypass surgery), stroke, aortic aneurysm repair, lower extremity bypass surgery, or carotid endarterectomy or angioplasty. ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker.
Multivariable models include adjustment for age, sex, race, region of residence, marital status, education, income, current smoking, depression, cognitive impairment, diabetes mellitus, hypertension, and albuminuria.
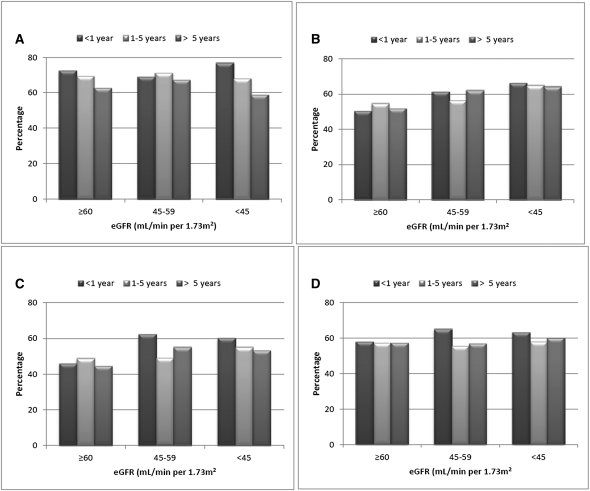
Participants with diabetes mellitus were more likely to use each class of medication than participants without diabetes mellitus, and analyses examining medication use by eGFR category were similar in participants with and without diabetes mellitus (Supplemental Table 1). We had data for 3577 participants on time since last MI or stroke. For eGFR ≥60 and <45 ml/min per 1.73 m2, we saw a trend to lower use of antiplatelet agents as time since last MI or stroke increased but no consistent trends in ACEI/ARB, β-blocker, or statin use (Figure 2). The results did not materially change after multivariable adjustment or restricting the analyses only to participants reporting a history of MI (data not shown).
Figure 2.
Distribution of medication use by time since last myocardial infarction or stroke associated with level of estimated GFR among REGARDS study participants. (A) Antiplatelet agents. (B) ACEI/ARBs. (C) β-Blockers. (D) Statins. ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; eGFR, estimated GFR (ml/min per 1.73 m2).
Medication Adherence
Responses to each adherence scale item did not differ by eGFR category (Table 3). Nearly one-third of participants had an aggregate adherence score of one or more than or equal to two, indicating some degree of medication nonadherence, but these scores did not differ by eGFR category.
Table 3.
Distribution of responses to the four-item Morisky Medication Adherence Scale and aggregate adherence scores, as well as multivariable-adjusted prevalence ratios for medication adherence scores associated with the level of estimated GFR in the Reasons for Geographic and Racial Differences in Stroke study participants with a history of cardiovascular disease
| Estimated GFR (ml/min per 1.73 m2) | |||
|---|---|---|---|
| ≥60 (n=5545) | 45–59 (n=837) | <45 (n=531) | |
| Medication adherence scale items (percent answering yes) | |||
| item 1: do you ever forget to take your medications? | 28.7 | 26.4 | 26.0 |
| item 2: are you ever careless in taking your medications? | 4.4 | 3.2 | 5.8 |
| item 3: do you ever miss taking your medications when you are feeling better? | 5.8 | 4.3 | 4.3 |
| item 4: do you ever miss taking any of your medications because you are feeling sick? | 4.4 | 3.1 | 5.3 |
| Aggregate adherence scores (%) | |||
| 0 (best adherence) | 68.5 | 72.0 | 69.9 |
| 1 | 23.4 | 21.5 | 22.8 |
| ≥2 (worst adherence) | 8.1 | 6.5 | 7.3 |
| Prevalence ratios (95% confidence interval) | |||
| 1 versus 0 | 1.00 (ref) | 0.92 (0.80–1.06) | 0.99 (0.83–1.18) |
| ≥2 versus 0 | 1.00 (ref) | 0.79 (0.60–1.03) | 0.81 (0.58–1.14) |
Multivariable models include adjustment for age, sex, race, region of residence, marital status, education, income, current smoking, depression, cognitive impairment, diabetes mellitus, hypertension, and albuminuria. Cardiovascular disease is defined as prior myocardial infarction (by electrocardiogram or self-report), coronary revascularization (percutaneous coronary intervention or coronary artery bypass surgery), stroke, aortic aneurysm repair, lower extremity bypass surgery, or carotid endarterectomy or angioplasty.
Race-Stratified Results
In unadjusted analyses, the overall use of antiplatelet agents, β-blockers, and statins was higher for white participants compared with black participants (P<0.001), but black participants had more frequent use of ACEI/ARBs (P<0.001) (Supplemental Table 2). Similar to results in the overall cohort, we observed that, in white and black participants analyzed separately, participants with eGFR levels of 45–59 and <45 ml/min per 1.73 m2 were generally more likely to use all medication classes except antiplatelet agents, although the results were not always statistically significant (Supplemental Table 2) (P interaction>0.05 for all medication classes).
For medication adherence, 69% of both white and black participants had perfect adherence scale scores of zero, but black participants more often had adherence scale scores more than or equal to two (10.2% versus 6.3%, P<0.001). No differences in medication adherence by eGFR category were observed for white participants (Supplemental Table 3). Black participants in the lowest eGFR category had better aggregate adherence scores than black participants with preserved eGFR (Supplemental Table 3).
Discussion
Our analysis shows that the use of medications recommended for secondary prevention among participants in the REGARDS study with a history of cardiovascular disease is suboptimal, regardless of eGFR category. Overall, only 40–60% of participants used antiplatelet agents, ACEI/ARBs, β-blockers, or statins, and only 13% used all four medication classes, consistent with previously reported low use of medications for treatment of chronic conditions (31). In contrast to our hypothesis, participants with lower eGFR had similar and in some cases, more frequent use of medications recommended for secondary prevention than participants with preserved eGFR. Specifically, participants with eGFR <60 ml/min per 1.73 m2 were 10–20% more likely to use ACEI/ARBs, β-blockers, and statins compared with participants with eGFR ≥60 ml/min per 1.73 m2, and they did not differ significantly in their use of antiplatelet agents. Participants in the lowest eGFR category (<45 ml/min per 1.73 m2) had medication use similar to participants with milder reductions in eGFR (45–59 ml/min per 1.73 m2).
To date, few studies have examined the association between kidney function and medication use for secondary prevention in the outpatient setting, because most previous studies were conducted at the time of hospitalization for an acute coronary event. For example, a recent study of nearly 50,000 patients hospitalized for acute MI showed that lower admission eGFR was associated with lower use of aspirin, clopidogrel, β-blockers, and statins both in hospital and at hospital discharge (28). Similarly, in an analyses of elderly patients hospitalized for acute MI, lower admission eGFR was associated with less frequent use of aspirin and β-blockers (7). However, the serum creatinine used to calculate eGFR in these studies was drawn at the time of hospital admission for the acute MI and therefore, may not represent true baseline kidney function. Rather, lower admission eGFR may reflect greater hemodynamic instability or other markers of more severe illness, which could explain a greater reluctance by providers to treat sicker patients with these medication classes. In contrast, participants in our analysis had their serum creatinine measured in a stable, outpatient setting, and many had their most recent cardiovascular event over 1 year before their study visit. We speculate that physicians may defer initiation of certain medications in persons with reduced eGFR during or soon after an acute event because of fear of exacerbating acute kidney injury or other adverse side effects. However, after a period of stability in the outpatient setting, physicians may be more likely to initiate medications such as ACEI/ARBs and β-blockers in persons with reduced eGFR for their cardioprotective, antihypertensive, or other effects. Another reason for differences between our findings and previous studies may stem from our inclusion of participants with not only a history of MI but also other types of cardiovascular diseases, such as peripheral vascular disease and carotid artery disease. We chose to include several types of cardiovascular diseases in our analysis, because current guidelines for secondary prevention are meant to be applied equally to this broader population (4). However, sensitivity analyses restricted only to participants with a reported history of MI yielded results similar to our primary analyses.
Although it is somewhat reassuring that participants with reduced eGFR had similar medication adherence scores as participants with preserved eGFR in the present analysis, it is nonetheless concerning that nearly one-third of our high-risk cohort reported forgetting to take their medications, being careless in taking their medications, or missing medications when feeling better or feeling sick. Medication nonadherence has been associated with a higher risk of death and stroke in previous studies (12,13). Given that persons with concomitant cardiovascular disease and CKD are already at very high risk for adverse cardiovascular outcomes, extra efforts aimed at improving medication adherence in this population are paramount.
One of the few previous studies to examine the association of eGFR with long-term outpatient secondary prevention medication use was unable to assess interaction by race given that 95% of their cohort was white (32). We showed that the association of eGFR category with medication use was not modified by race, because both white and black participants with reduced eGFR were more likely to use ACEI/ARBs, β-blockers, and statins compared with participants with eGFR ≥60 ml/min per 1.73 m2. White and black participants with reduced eGFR were no less likely to use antiplatelet agents than participants with preserved eGFR, although the upper bound of the confidence intervals did not exclude the possibility that there may have been small differences by eGFR category. We observed no differences in medication adherence by eGFR category for white participants, but interestingly, we noted better adherence for black participants in the lowest eGFR category. Whether our results stem from more frequent healthcare visits or other factors in black participants with reduced eGFR should be explored in future analyses.
Our analysis has several limitations. First, we did not have longitudinal information on medication use and were unable to evaluate medication persistence over time. Second, although a strength of our analysis is the availability of pill bottle review data from an in-home study visit, which allowed ascertainment of nonprescription aspirin use, we were unable to assess whether these medications were taken at the correct dose or frequency. Third, the medication adherence scale used in the REGARDS study did not explore other potential reasons for medication nonadherence, such as cost or personal beliefs, or whether participants may have had certain indications or contraindications for medication use. The scale may also be overly stringent in categorizing medication nonadherence, because it allows only for dichotomous responses. Fourth, <2% of the cohort had eGFR <30 ml/min per 1.73 m2, precluding separate analyses for this eGFR category. Finally, because the REGARDS study relied on volunteers, the cohort may have been healthier and more adherent than persons with cardiovascular disease in general.
In summary, among participants with a history of cardiovascular disease in the REGARDS study, those participants with mild to moderate reductions in eGFR had similar or even higher rates of use of medications for secondary prevention compared with participants with preserved eGFR. Our analysis also shows that, among persons with a history of cardiovascular disease, those participants with reduced eGFR have comparable levels of medication adherence as persons with preserved eGFR. However, our analysis confirmed the overall suboptimal use of medications that are of proven benefit for secondary prevention and the relatively high prevalence of medication nonadherence. Future strategies aimed at improving medication use and adherence in persons with a history of cardiovascular disease, with and without concomitant kidney disease, will be important to improve clinical outcomes in this high-risk population.
Disclosures
None.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the Reasons for Geographic and Racial Differences in Stroke study for their valuable contributions. A full list of participating Reasons for Geographic and Racial Differences in Stroke investigators and institutions can be found at http://www.regardsstudy.org.
This research project is supported by Cooperative Agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, and Department of Health and Human Service. Additional funding was provided by an investigator-initiated grant-in-aid from Amgen Corporation. T.I.C. is supported by a grant from the American Heart Association. T.M.B. is supported in part by KL2 Mentored Career Development Award 5KL2 RR025776-02 from the University of Alabama at Birmingham Center for Clinical and Translational Science with funding from the National Institutes of Health National Center for Research Resources.
Amgen did not have any role in the design and conduct of the study, the collection, management, data analysis, or interpretation of the data, or the preparation or approval of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis, or interpretation of the data. L.G. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.11441111/-/DCSupplemental.
References
- 1.National Kidney Foundation: KDOQI Clinical Practice Guidelines on Hypertension and Antihypertensive Agents in Chronic Kidney Disease, 2002. Available at http://www.kidney.org/professionals/KDOQI/guidelines_bp/index.htm Accessed May 25, 2010 [PubMed]
- 2.McClellan WM, Langston RD, Presley R: Medicare patients with cardiovascular disease have a high prevalence of chronic kidney disease and a high rate of progression to end-stage renal disease. J Am Soc Nephrol 15: 1912–1919, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Bang H, Mazumdar M, Newman G, Bomback AS, Ballantyne CM, Jaffe AS, August PA, Kshirsagar AV: Screening for kidney disease in vascular patients: SCreening for Occult REnal Disease (SCORED) experience. Nephrol Dial Transplant 24: 2452–2457, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith SC, Jr, Allen J, Blair SN, Bonow RO, Brass LM, Fonarow GC, Grundy SM, Hiratzka L, Jones D, Krumholz HM, Mosca L, Pasternak RC, Pearson T, Pfeffer MA, Taubert KA, AHA/ACC. National Heart, Lung, and Blood Institute : AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: Endorsed by the National Heart, Lung, and Blood Institute. Circulation 113: 2363–2372, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Abbott KC, Bohen EM, Yuan CM, Yeo FE, Sawyers ES, Perkins RM, Lentine KL, Oliver DK, Galey J, Sebastianelli ME, Scally JP, Taylor AJ, Boal TR: Use of beta-blockers and aspirin after myocardial infarction by patient renal function in the Department of Defense health care system. Am J Kidney Dis 47: 593–603, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Shlipak MG, Heidenreich PA, Noguchi H, Chertow GM, Browner WS, McClellan MB: Association of renal insufficiency with treatment and outcomes after myocardial infarction in elderly patients. Ann Intern Med 137: 555–562, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Chertow GM, Normand SL, McNeil BJ: “Renalism”: Inappropriately low rates of coronary angiography in elderly individuals with renal insufficiency. J Am Soc Nephrol 15: 2462–2468, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Fried LF, Lee JS, Shlipak M, Chertow GM, Green C, Ding J, Harris T, Newman AB: Chronic kidney disease and functional limitation in older people: Health, aging and body composition study. J Am Geriatr Soc 54: 750–756, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Hedayati SS, Minhajuddin AT, Toto RD, Morris DW, Rush AJ: Prevalence of major depressive episode in CKD. Am J Kidney Dis 54: 424–432, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsai CF, Wang SJ, Fuh JL: Moderate chronic kidney disease is associated with reduced cognitive performance in midlife women. Kidney Int 78: 605–610, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Gehi AK, Ali S, Na B, Whooley MA: Self-reported medication adherence and cardiovascular events in patients with stable coronary heart disease: The heart and soul study. Arch Intern Med 167: 1798–1803, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ho PM, Magid DJ, Shetterly SM, Olson KL, Maddox TM, Peterson PN, Masoudi FA, Rumsfeld JS: Medication nonadherence is associated with a broad range of adverse outcomes in patients with coronary artery disease. Am Heart J 155: 772–779, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Qato DM, Lindau ST, Conti RM, Schumm LP, Alexander GC: Racial and ethnic disparities in cardiovascular medication use among older adults in the United States. Pharmacoepidemiol Drug Saf 19: 834–842, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charles H, Good CB, Hanusa BH, Chang CC, Whittle J: Racial differences in adherence to cardiac medications. J Natl Med Assoc 95: 17–27, 2003 [PMC free article] [PubMed] [Google Scholar]
- 16.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G: The reasons for geographic and racial differences in stroke study: Objectives and design. Neuroepidemiology 25: 135–143, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Morton LM, Cahill J, Hartge P: Reporting participation in epidemiologic studies: A survey of practice. Am J Epidemiol 163: 197–203, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Jackson R, Chambless LE, Yang K, Byrne T, Watson R, Folsom A, Shahar E, Kalsbeek W, The Atherosclerosis Risk in Communities (ARIC) Study Investigators : Differences between respondents and nonrespondents in a multicenter community-based study vary by gender ethnicity. J Clin Epidemiol 49: 1441–1446, 1996 [DOI] [PubMed] [Google Scholar]
- 19.MESA: Mesa Exam 1 Participation Rate (10/13/2004), 2004. Available at http://www.mesa-nhlbi.org/participation.aspx Accessed July 5, 2011
- 20.Radloff LS: The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Meas 1: 385–401, 1977 [Google Scholar]
- 21.Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, Hendrie HC: Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care 40: 771–781, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Morisky DE, Green LW, Levine DM: Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care 24: 67–74, 1986 [DOI] [PubMed] [Google Scholar]
- 23.Shalansky SJ, Levy AR, Ignaszewski AP: Self-reported Morisky score for identifying nonadherence with cardiovascular medications. Ann Pharmacother 38: 1363–1368, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abutaleb N: Why we should sub-divide CKD stage 3 into early (3a) and late (3b) components. Nephrol Dial Transplant 22: 2728–2729, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Thompson ML, Myers JE, Kriebel D: Prevalence odds ratio or prevalence ratio in the analysis of cross sectional data: What is to be done? Occup Environ Med 55: 272–277, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.White IR, Royston P, Wood AM: Multiple imputation using chained equations: Issues and guidance for practice. Stat Med 30: 377–399, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Fox CS, Muntner P, Chen AY, Alexander KP, Roe MT, Cannon CP, Saucedo JF, Kontos MC, Wiviott SD, Acute Coronary Treatment and Intervention Outcomes Network registry : Use of evidence-based therapies in short-term outcomes of ST-segment elevation myocardial infarction and non-ST-segment elevation myocardial infarction in patients with chronic kidney disease: A report from the National Cardiovascular Data Acute Coronary Treatment and Intervention Outcomes Network registry. Circulation 121: 357–365, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winkelmayer WC, Charytan DM, Brookhart MA, Levin R, Solomon DH, Avorn J: Kidney function and use of recommended medications after myocardial infarction in elderly patients. Clin J Am Soc Nephrol 1: 796–801, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Winkelmayer WC, Levin R, Setoguchi S: Associations of kidney function with cardiovascular medication use after myocardial infarction. Clin J Am Soc Nephrol 3: 1415–1422, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Osterberg L, Blaschke T: Adherence to medication. N Engl J Med 353: 487–497, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Chang TI, Desai M, Solomon DH, Winkelmayer WC: Kidney function and long-term medication adherence after myocardial infarction in the elderly. Clin J Am Soc Nephrol 6: 864–869, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]