Abstract
Oxidized low-density lipoprotein (Ox-LDL) has been studied for over 25 years. Numerous pro- and anti-atherogenic properties have been attributed to Ox-LDL. Yet, Ox-LDL has neither been defined nor characterized, as its components and composition change depending on its source, method of preparation, storage, and use. It contains unoxidized and oxidized fatty acid derivatives both in the ester and free forms, their decomposition products, cholesterol and its oxidized products, proteins with oxidized amino acids and cross-links, and polypeptides with varying extents of covalent modification with lipid oxidation products, and many others. It seems to exist in vivo in some form not yet fully characterized. Until its pathophysiological significance, and how it is generated in vivo are determined, the nature of its true identity will be only of classical interest. In this review, its components, their biological actions and methods of preparation will be discussed.
Keywords: Atherosclerosis, oxidative stress, lipid peroxides, antioxidants, aldehydes
1. Introduction
The concept that oxidative stress and the oxidation of low-density lipoprotein (LDL) might play a role in atherosclerosis originated over 25 years ago (1–3). The concept originated from a simple observation that in vitro incubation of macrophages with oxidized LDL and not with native LDL led to cholesterol ester accumulation (2, 4). The oxidation of LDL is a complex process during which both the protein and the lipids undergo oxidative changes and form complex products. For Example, non-enzymatic oxidative changes in amino acids as well as proteolysis and cross-links of apoprotein B (apo B) occur that result in extensive alteration in the protein composition and structure (5). In addition, the peroxidized lipids decompose generating both free and core aldehydes and ketones that covalently modify ε-amino groups of lysine residues of the protein moiety (6). The latter not only generates Schiff's bases, thus modifying charges on the amino acids, but also results in both intra- and intermolecular cross-links between proteolyzed apo B. No wonder the LDL that is damaged to such an extent is avidly scavenged and degraded by macrophages. In fact, the oxidative modification is not unique to LDL as other lipoproteins such as very low-density lipoprotein (VLDL), beta very low-density lipoprotein (β-VLDL), and even high-density lipoprotein (HDL) have been suggested to undergo similar oxidative changes (7-18) with accompanying changes in their pro- or anti-atherosclerotic behavior (19-23).
What is Ox-LDL? The definition depends on the purpose of the question. The original concept defined Ox-LDL as “oxidatively modified LDL” that contained protein components that were “modified” presumably by aldehyde products creating net negative charges that were essential for its interaction and uptake by macrophages (3, 24). However, during the past 25 years myriads of protein-independent pro-atherogenic effects have been attributed to the lipid components of oxidized LDL that one has to redefine oxidized LDL. In order to define oxidized LDL, one needs to know what components are usually associated with it.
Table 24.1 is a list of products that are generated during in vitro oxidation of LDL.
Table 24.1. List of lipid/protein oxidation products generated during the oxidation of LDL.
|
The formation of all of the above products or the changes in the properties of circulating LDL are not guaranteed during the oxidation of LDL as many are secondary products of oxidation and their formation might depend on the type of oxidant, the extent of oxidation, and the presence or absence of other agents such as redox metals. Also, some products, such as malondialdehyde, readily diffuse out of Ox-LDL. More importantly, the fatty acid profile plays a major role in the formation of many of the products and of Ox-LDL (25-27). Generally, polyunsaturated fatty acids favor the oxidation of LDL while monounsaturated fatty acids are less conducive to its formation (28, 29). In addition, the protein component of the LDL (apo B) might also determine how the oxidation is propagated within the particle. Specific amino acids might generate and propagate oxidation (30-34).
In light of these, Ox-LDL can be defined as follows: A particle derived from circulating LDL that may have peroxides or their degradation products generated within the LDL molecule or elsewhere in the body associated with the particle. This would include minimally oxidized LDL that may have lipid peroxides or their degradation products (for Example, oxovaleryl PtdCho) but no apoprotein changes, MDA modified particles with MDA arising from platelets or elsewhere, and others. However, to this date, LDL particles with oxidized apo B amino acids without lipid changes have not been described. Figure 24.1 describes some of the potential Ox-LDL particles that may be of relevance.
Fig. 24.1.
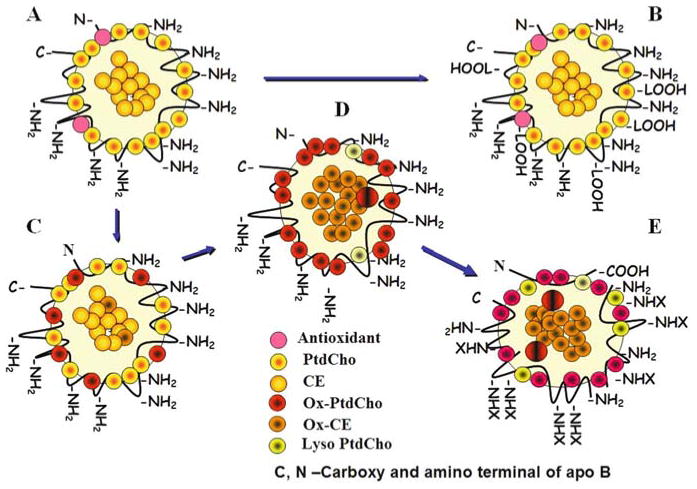
Forms of oxidized low-density lipoprotein (reproduced from Parthasarathy et al. (157)). (a)Unoxidized native LDL with amino groups of lysine residues of apo B and representative lipids. (b) Lipid peroxides generated elsewhere associated with such LDL. (c) LDL lipids might get oxidized resulting in the generation of cholesterol ester and phospholipid peroxides. (d) Such LDL might undergo extensive oxidation leading to protein changes. (e) Extensive protein changes and lipid decomposition might hallmark the end stages of oxidation.
Although not depicted in the picture, major changes in apo B might occur in addition to aldehyde modification and proteolysis, depending on the type of oxidant used. As a result, countless antigenic epitopes are possible (35, 36). As mentioned before, these changes are not unique to LDL and other lipoprotein might undergo similar changes (37, 38). However, the author has not seen any proteolysis of HDL apoproteins as a result of oxidation but has noted aggregation changes.
In summary, Ox-LDL may contain a specific oxidation product (see Table 24.2). The simplest Example may be lipoxygenase-treated LDL. The lipoprotein subjected to such treatment may contain varying amounts of phospholipid and cholesterol ester hydroperoxides. Ox-LDL may also contain limited amounts of a variety of degraded oxidized lipid products. “Minimally modified LDL” as described by Berliner, Fogelman and associates may qualify for such a criteria (39, 40). There is also Ox-LDL that is recognized by macrophages (41, 42). While it was believed for a long time that protein changes are essential for macrophage recognition, at least some studies seem to suggest that oxidized lipids might mediate the whole Ox-LDL uptake (43-45). There are, however, Ox-LDL preparations that do not share the properties of conventionally oxidized lipoproteins. For Example, LDL oxidized by photooxidation in the presence of dyes, such as Rose Bengal, showed massive increase in electrophoretic mobility as a result of oxidation of histidine residues (46). However, other than a loss of phospholipase A2 activity, it did not bear any resemblance to oxidized LDL.
Table 24.2. Suggested mechanisms for the oxidation of LDL.
| Mechanism | References |
|---|---|
| 1. Lipoxygenase reaction | (47–53) |
| 2. Copper and ceruloplasmin-mediated oxidation | (54–58) |
| 3. Iron-mediated oxidation | (54, 59, 60) |
| 4. Peroxidase-mediated oxidation including myeloperoxidase and heme | (61–63) |
| 5. Peroxynitrite-mediated oxidation | (64–66) |
| 6. Thiol-dependent oxidation | (67–70) |
| 7. Xanthine oxidase, NADPH oxidase, and other superoxide generators | (71–78) |
| 8. AAPH or other means of radical generation including cytochromes | (79–81) |
Ox-LDL, thus, might represent the elephant that is described by blind men. It is without description and yet distinct; it is a complex mixture of chemical entities and yet they are measurable, and individual investigator is responsible for setting forth the definition that suits a set of criteria. These definitions should be the basis for experimental validation and scientific reproducibility.
Not all the oxidation mechanisms are comparable or lead to similar products even under in vitro conditions. For Example, peroxidase-mediated oxidations require co-oxidants such as H2O2 or lipid peroxides. Some damage the proteins more readily than others. For Example, treatment of LDL with 2,2′-azobis(2-amidinopropane) dihydrochloride (AAPH), a radical generator or peroxynitrite, Results in more protein oxidation than lipid peroxidation (65, 80, 82, 83). Similarly, peroxidase-catalyzed oxidation generate very little aldehyde products as compared to metal-catalyzed oxidations (84). Lipoxygenase reactions which are exclusively intracellular might require additional reactions or transfers before or after lipoxygenase reactions occur (85, 86). To our knowledge, no one has characterized oxidized LDL isolated from animals or humans to the extent that mechanistic insights could be derived, although there are numerous claims of the presence of an electronegative LDL (presumably minimally oxidized LDL) in human and animal plasma (87-95).
While all these reactions are oxidative in nature, they are not uniformly amenable to inhibition by traditional antioxidants. Vitamin E or simple phenols such as tyrosine or estradiol actually enhance peroxidase-mediated oxidation of LDL (96-98). This has been taken as evidence for an intermediate prooxidant role for antioxidants. Similarly, many thiols actually enhance the oxidation of LDL depending on the peroxide content of LDL (99).
The detection of Ox-LDL, at least in vitro, has been quite easy, although earlier work, before the discovery of the involvement of oxidative process, revolved around the increase in net negative charge and increase in buoyant density. The presence and amounts of MDA has always been used as a yard stick for minimally or terminally oxidized LDL (100-103), although numerous publications concluded a short-term oxidation always resulted in a “mildly” oxidized LDL as opposed to long-term oxidations (104–107). Yet, enzymatic (lipoxygenase, peroxidase) reactions and treatment with peroxynitrite that generated lower amounts of MDA were always considered fully oxidized LDL due the ability of macrophages to engulf such LDL.
The measurement of lipid oxidation has been a great boon, not only to the understanding of the process of LDL oxidation but also in providing numerous serendipitous discoveries and methodologies. The formation of conjugated dienes during lipid peroxidation was successfully exploited to generate the “lag time” concept by Esterbauer and associates (108) that is still used as a yard stick for measuring the oxidizability of LDL. Similarly, the discovery of isoprostanes by Roberts, Morrow and others (109) also created tremendous excitement and opportunity to look for such LDL in vivo (110).
Since the discovery of oxidized LDL, over 5,000 articles have appeared on the topic with over 400 articles appearing every year during the past decade (Fig. 24.2). Combined with epidemiological evidence and success in several animal trials in a number of species using a variety of antioxidants, the hypothesis appeared to be on solid ground until recently. This euphoria of initial success led to clinical trials that were planned mostly to be the “first” to validate the hypothesis. Obviously vitamin E was the chosen antioxidant as the expectation of the general public and scientists was that a natural antioxidant (such as vitamin E) would have less undesirable effects. Accordingly a number of clinical trials were performed (111–114) using vitamin E at pharmacological doses. Some of these trials were piggyback studies on cancer and other diseases (115, 116). These trials with vitamin E have not been overwhelmingly supportive of the hypothesis. Understanding the sequence of events that led to the hypothesis, the molecular basis of antioxidant action, the potential interaction with other drugs, and the stage of the disease process to which oxidation was suggested to contribute, dosage, the transport mechanisms involved in the release and utilization of vitamin E in the body, its interaction(s) with cytochrome systems, and its function as a prooxidant in peroxidase-mediated oxidations played little role in the design of the study (117, 118). More importantly, little if any attention was paid to factors that would affect and control oxidation in vivo. There have been many reviews written dissecting out the trials that “disproved” the oxidation hypothesis (119–122).
Fig. 24.2.
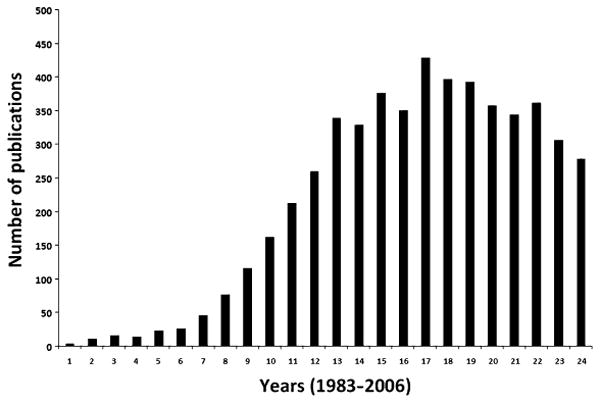
Number of publications related to Ox-LDL.
On the other hand, more and more recent publications have described the effects of oxidized lipids on atherosclerosis and associated risk factors (123–130). The prevalence of antibodies that recognize epitopes of Ox-LDL seem to correlate well with the disease process (35, 95, 131–145). These antibodies also detect such LDL in the plasma of subjects who have higher risk associated with atherosclerosis (35, 139, 145–153). Oxidative enzymes such as Myeloperoxidase seem to predict the vulnerable population and seem to correlate with the severity of the disease (154–156). Considering that vitamin E might actually enhance peroxidase-catalyzed oxidations (98), the clinical trials might have actually endorsed the hypothesis rather than shooting it down. Perhaps, when inhibitors of specific oxidases are available, additional trials might be warranted to induce the development of anti-atherosclerotic pharmacological agents.
Acknowledgments
This work was supported by funding from National Institutes of Health, HL-069038 and HL-74239 (SP) and HL74239 (NS).
References
- 1.Steinbrecher UP, Parthasarathy S, Leake DS, Witztum JL, Steinberg D. Modification of low density lipoprotein by endothelial cells involves lipid peroxidation and degradation of low density lipoprotein phospholipids. Proc Natl Acad Sci USA. 1984;81:3883–3887. doi: 10.1073/pnas.81.12.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quinn MT, Parthasarathy S, Fong LG, Steinberg D. Oxidatively modified low density lipoproteins: a potential role in recruitment and retention of monocyte/macrophages during atherogenesis. Proc Natl Acad Sci USA. 1987;84:2995–2998. doi: 10.1073/pnas.84.9.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity [see comments] N Engl J Med. 1989;320:915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 4.Parthasarathy S, Quinn MT, Steinberg D. Is oxidized low density lipoprotein involved in the recruitment and retention of monocyte/macrophages in the artery wall during the initiation of atherosclerosis? Basic Life Sci. 1988;49:375–380. doi: 10.1007/978-1-4684-5568-7_58. [DOI] [PubMed] [Google Scholar]
- 5.Fong LG, Parthasarathy S, Witztum JL, Steinberg D. Nonenzymatic oxidative cleavage of peptide bonds in apoprotein B-100. J Lipid Res. 1987;28:1466–1477. [PubMed] [Google Scholar]
- 6.Fruebis J, Parthasarathy S, Steinberg D. Evidence for a concerted reaction between lipid hydroperoxides and polypeptides. Proc Natl Acad Sci USA. 1992;89:10588–10592. doi: 10.1073/pnas.89.22.10588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parthasarathy S, Quinn MT, Schwenke DC, Carew TE, Steinberg D. Oxidative modification of beta-very low density lipoprotein. Potential role in monocyte recruitment and foam cell formation. Arteriosclerosis. 1989;9:398–404. doi: 10.1161/01.atv.9.3.398. [DOI] [PubMed] [Google Scholar]
- 8.de Rijke YB, Hessels EM, van Berkel TJ. Recognition sites on rat liver cells for oxidatively modified beta-very low density lipoproteins. Arterioscler Thromb. 1992;12:41–49. doi: 10.1161/01.atv.12.1.41. [DOI] [PubMed] [Google Scholar]
- 9.Bowry VW, Stanley KK, Stocker R. High density lipoprotein is the major carrier of lipid hydroperoxides in human blood plasma from fasting donors. Proc Natl Acad Sci USA. 1992;89:10316–10320. doi: 10.1073/pnas.89.21.10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bradamante S, Barenghi L, Giudici GA, Vergani C. Free radicals promote modifications in plasma high-density lipoprotein: nuclear magnetic resonance analysis. Free Radic Biol Med. 1992;12:193–203. doi: 10.1016/0891-5849(92)90027-e. [DOI] [PubMed] [Google Scholar]
- 11.Bonnefont-Rousselot D, Khalil A, Delattre J, Jore D, Gardes-Albert M. Oxidation of human high-density lipoproteins by OH and OH/O(-)2 free radicals. Radiat Res. 1997;147:721–728. [PubMed] [Google Scholar]
- 12.Greilberger J, Jurgens G. Oxidation of high-density lipoprotein HDL3 leads to exposure of apo-AI and apo-AII epitopes and to formation of aldehyde protein adducts, and influences binding of oxidized low-density lipoprotein to type I and type III collagen in vitro. Biochem J. 1998;331:185–191. doi: 10.1042/bj3310185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergt C, Oram JF, Heinecke JW. Oxidized HDL: the paradox-idation of lipoproteins. Arterioscler Thromb Vasc Biol. 2003;23:1488–1490. doi: 10.1161/01.ATV.0000090570.99836.9C. [DOI] [PubMed] [Google Scholar]
- 14.Asztalos BF. High-density lipoprotein metabolism and progression of atherosclerosis: new insights from the HDL Atherosclerosis Treatment Study. Curr Opin Cardiol. 2004;19:385–391. doi: 10.1097/01.hco.0000126979.41946.7e. [DOI] [PubMed] [Google Scholar]
- 15.Navab M, Ananthramaiah GM, Reddy ST, Van Lenten BJ, Ansell BJ, Fonarow GC, Vahabzadeh K, Hama S, Hough G, Kamranpour N, et al. The oxidation hypothesis of atherogenesis: the role of oxidized phospholipids and HDL. J Lipid Res. 2004;45:993–1007. doi: 10.1194/jlr.R400001-JLR200. [DOI] [PubMed] [Google Scholar]
- 16.Stojanovic N, Krilov D, Herak JN. Slow oxidation of high density lipoproteins as studied by EPR spectroscopy. Free Radic Res. 2006;40:135–140. doi: 10.1080/10715760500456789. [DOI] [PubMed] [Google Scholar]
- 17.Malle E, Marsche G, Panzenboeck U, Sattler W. Myeloperoxidase-mediated oxidation of high-density lipoproteins: fingerprints of newly recognized potential proatherogenic lipoproteins. Arch Biochem Biophys. 2006;445:245–255. doi: 10.1016/j.abb.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Ferretti G, Bacchetti T, Negre-Salvayre A, Salvayre R, Dousset N, Curatola G. Structural modifications of HDL and functional consequences. Atherosclerosis. 2006;184:1–7. doi: 10.1016/j.atherosclerosis.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Kervinen K, Horkko S, Beltz WF, Kesaniemi A. Modification of VLDL apoprotein B by acetaldehyde alters apoprotein B metabolism. Alcohol. 1995;12:189–194. doi: 10.1016/0741-8329(94)00081-n. [DOI] [PubMed] [Google Scholar]
- 20.Nagano Y, Arai H, Kita T. High density lipoprotein loses its effect to stimulate efflux of cholesterol from foam cells after oxidative modification. Proc Natl Acad Sci USA. 1991;88:6457–6461. doi: 10.1073/pnas.88.15.6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghiselli G, Giorgini L, Gelati M, Musanti R. Oxidatively modified HDLs are potent inhibitors of cholesterol biosynthesis in human skin fibroblasts. Arterioscler Thromb. 1992;12:929–935. doi: 10.1161/01.atv.12.8.929. [DOI] [PubMed] [Google Scholar]
- 22.Van Lenten BJ, Wagner AC, Nayak DP, Hama S, Navab M, Fogelman AM. High-density lipoprotein loses its anti-inflammatory properties during acute influenza a infection. Circulation. 2001;103:2283–2288. doi: 10.1161/01.cir.103.18.2283. [DOI] [PubMed] [Google Scholar]
- 23.Jaouad L, Milochevitch C, Khalil A. PON1 paraoxonase activity is reduced during HDL oxidation and is an indicator of HDL antioxidant capacity. Free Radic Res. 2003;37:77–83. doi: 10.1080/1071576021000036614. [DOI] [PubMed] [Google Scholar]
- 24.Parthasarathy S. Modified Lipoproteins in the Pathogenesis of Atherosclerosis. R.G. Landes Co.; Austin, TX: 1994. p. 152. [Google Scholar]
- 25.Lada AT, Rudel LL. Associations of low density lipoprotein particle composition with atherogenicity. Curr Opin Lipidol. 2004;15:19–24. doi: 10.1097/00041433-200402000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Moreno JJ, Mitjavila MT. The degree of unsaturation of dietary fatty acids and the development of atherosclerosis (review) J Nutr Biochem. 2003;14:182–195. doi: 10.1016/s0955-2863(02)00294-2. [DOI] [PubMed] [Google Scholar]
- 27.Kratz M, Cullen P, Kannenberg F, Kassner A, Fobker M, Abuja PM, Assmann G, Wahrburg U. Effects of dietary fatty acids on the composition and oxidizability of low-density lipoprotein. Eur J Clin Nutr. 2002;56:72–81. doi: 10.1038/sj.ejcn.1601288. [DOI] [PubMed] [Google Scholar]
- 28.Reaven P, Parthasarathy S, Grasse BJ, Miller E, Steinberg D, Witztum JL. Effects of oleate-rich and linoleaterich diets on the susceptibility of low density lipoprotein to oxidative modification in mildly hypercholesterolemic subjects. J Clin Invest. 1993;91:668–676. doi: 10.1172/JCI116247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lada AT, Rudel LL. Dietary monounsaturated versus polyunsaturated fatty acids: which is really better for protection from coronary heart disease? Curr Opin Lipidol. 2003;14:41–46. doi: 10.1097/00041433-200302000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Giessauf A, van Wickern B, Simat T, Steinhart H, Esterbauer H. Formation of N-formylkynurenine suggests the involvement of apolipoprotein B-100 centered tryptophan radicals in the initiation of LDL lipid peroxidation. FEBS Lett. 1996;389:136–140. doi: 10.1016/0014-5793(96)00546-7. [DOI] [PubMed] [Google Scholar]
- 31.Miller YI, Felikman Y, Shaklai N. Hemoglobin induced apolipoprotein B crosslinking in low-density lipoprotein peroxidation. Arch Biochem Biophys. 1996;326:252–260. doi: 10.1006/abbi.1996.0073. [DOI] [PubMed] [Google Scholar]
- 32.Alaiz M, Beppu M, Ohishi K, Kikugawa K. Modification of delipidated apoprotein B of low density lipoprotein by lipid oxidation products in relation to macrophage scavenger receptor binding. Biol Pharm Bull. 1994;17:51–57. doi: 10.1248/bpb.17.51. [DOI] [PubMed] [Google Scholar]
- 33.Picard S, Parthasarathy S, Fruebis J, Witztum JL. Aminoguanidine inhibits oxidative modification of low density lipoprotein protein and the subsequent increase in uptake by macrophage scavenger receptors. Proc Natl Acad Sci USA. 1992;89:6876–6880. doi: 10.1073/pnas.89.15.6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Antwerpen P, Legssyer I, Zouaoui Boudjeltia K, Babar S, Moreau P, Moguilevsky N, Vanhaeverbeek M, Ducobu J, Neve J. Captopril inhibits the oxidative modification of apolipoprotein B-100 caused by myeloperoxidase in a comparative in vitro assay of angiotensin converting enzyme inhibitors. Eur J Pharmacol. 2006;537:31–36. doi: 10.1016/j.ejphar.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 35.Salonen JT, Yla-Herttuala S, Yamamoto R, Butler S, Korpela H, Salonen R, Nyyssonen K, Palinski W, Witztum JL. Autoantibody against oxidised LDL and progression of carotid atherosclerosis [see comments] Lancet. 1992;339:883–887. doi: 10.1016/0140-6736(92)90926-t. [DOI] [PubMed] [Google Scholar]
- 36.Palinski W, Horkko S, Miller E, Stein-brecher UP, Powell HC, Curtiss LK, Witztum JL. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J Clin Invest. 1996;98:800–814. doi: 10.1172/JCI118853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakajima T, Sakagishi Y, Katahira T, Nagata A, Kuwae T, Nakamura H, Inoue I, Takahashi K, Katayama S, Komoda T. Characterization of a specific monoclonal antibody 9F5-3a and the development of assay system for oxidized HDL. Biochem Biophys Res Commun. 1995;217:407–411. doi: 10.1006/bbrc.1995.2791. [DOI] [PubMed] [Google Scholar]
- 38.Shao B, O'Brien KD, McDonald TO, Fu X, Oram JF, Uchida K, Heinecke JW. Acrolein modifies apolipoprotein A-I in the human artery wall. Ann NY Acad Sci. 2005;1043:396–403. doi: 10.1196/annals.1333.046. [DOI] [PubMed] [Google Scholar]
- 39.Cushing SD, Berliner JA, Valente AJ, Territo MC, Navab M, Parhami F, Gerrity R, Schwartz CJ, Fogelman AM. Minimally modified low density lipoprotein induces monocyte chemotactic protein 1 in human endothelial cells and smooth muscle cells. Proc Natl Acad Sci USA. 1990;87:5134–5138. doi: 10.1073/pnas.87.13.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liao F, Berliner JA, Mehrabian M, Navab M, Demer LL, Lusis AJ, Fogelman AM. Minimally modified low density lipoprotein is biologically active in vivo in mice [published erratum appeared in J. Clin. Invest. 88, 721, 1991] J Clin Invest. 1991;87:2253–2257. doi: 10.1172/JCI115261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parthasarathy S, Fong LG, Otero D, Steinberg D. Recognition of solubilized apoproteins from delipidated, oxidized low density lipoprotein (LDL) by the acetyl-LDL receptor. Proc Natl Acad Sci USA. 1987;84:537–540. doi: 10.1073/pnas.84.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sparrow CP, Parthasarathy S, Steinberg D. A macrophage receptor that recognizes oxidized low density lipoprotein but not acetylated low density lipoprotein. J Biol Chem. 1989;264:2599–2604. [PubMed] [Google Scholar]
- 43.Morel DW, Hessler JR, Chisolm GM. Low density lipoprotein cytotoxicity induced by free radical peroxidation of lipid. J Lipid Res. 1983;24:1070–1076. [PubMed] [Google Scholar]
- 44.Hoff HF, O'Neil J, Chisolm GMD, Cole TB, Quehenberger O, Esterbauer H, Jurgens G. Modification of low density lipoprotein with 4-hydroxynonenal induces uptake by macrophages. Arteriosclerosis. 1989;9:538–549. doi: 10.1161/01.atv.9.4.538. [DOI] [PubMed] [Google Scholar]
- 45.Tertov VV, Kaplun VV, Dvoryantsev SN, Orekhov AN. Apolipoprotein B-bound lipids as a marker for evaluation of low density lipoprotein oxidation in vivo. Biochem Biophys Res Commun. 1995;214:608–613. doi: 10.1006/bbrc.1995.2329. [DOI] [PubMed] [Google Scholar]
- 46.Parthasarathy S, Barnett J. Phospholipase A2 activity of low density lipoprotein: evidence for an intrinsic phospholipase A2 activity of apoprotein B-100. Proc Natl Acad Sci USA. 1990;87:9741–9745. doi: 10.1073/pnas.87.24.9741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sparrow CP, Parthasarathy S, Steinberg D. Enzymatic modification of low density lipoprotein by purified lipoxygenase plus phospholipase A2 mimics cell-mediated oxidative modification. J Lipid Res. 1988;29:745–753. [PubMed] [Google Scholar]
- 48.Parthasarathy S, Wieland E, Steinberg D. A role for endothelial cell lipoxygenase in the oxidative modification of low density lipoprotein. Proc Natl Acad Sci USA. 1989;86:1046–1050. doi: 10.1073/pnas.86.3.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cathcart MK, McNally AK, Chisolm GM. Lipoxygenase-mediated transformation of human low density lipoprotein to an oxidized and cytotoxic complex. J Lipid Res. 1991;32:63–70. [PubMed] [Google Scholar]
- 50.Rankin SM, Parthasarathy S, Steinberg D. Evidence for a dominant role of lipoxygenase(s) in the oxidation of LDL by mouse peritoneal macrophages. J Lipid Res. 1991;32:449–456. [PubMed] [Google Scholar]
- 51.Sigal E, Laughton CW, Mulkins MA. Oxidation, lipoxygenase, and atherogenesis. Ann NY Acad Sci. 1994;714:211–224. doi: 10.1111/j.1749-6632.1994.tb12046.x. [DOI] [PubMed] [Google Scholar]
- 52.Kuhn H, Belkner J, Suzuki H, Yamamoto S. Oxidative modification of human lipoproteins by lipoxygenases of different positional specificities. J Lipid Res. 1994;35:1749–1759. [PubMed] [Google Scholar]
- 53.Kuhn H, Chan L. The role of 15-lipoxygenase in atherogenesis: pro- and antiatherogenic actions. Curr Opin Lipidol. 1997;8:111–117. doi: 10.1097/00041433-199704000-00009. [DOI] [PubMed] [Google Scholar]
- 54.Heinecke JW, Rosen H, Chait A. Iron and copper promote modification of low density lipoprotein by human arterial smooth muscle cells in culture. J Clin Invest. 1984;74:1890–1894. doi: 10.1172/JCI111609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Quehenberger O, Jurgens G, Zadravec S, Esterbauer H. Oxidation of human low density lipoprotein initiated by copper (II) chloride. Basic Life Sci. 1988;49:387–390. doi: 10.1007/978-1-4684-5568-7_60. [DOI] [PubMed] [Google Scholar]
- 56.Parthasarathy S, Fong LG, Quinn MT, Steinberg D. Oxidative modification of LDL: comparison between cell-mediated and copper-mediated modification. Eur Heart J. 1990;11 E:83–87. doi: 10.1093/eurheartj/11.suppl_e.83. [DOI] [PubMed] [Google Scholar]
- 57.Ehrenwald E, Chisolm GM, Fox PL. Intact human ceruloplasmin oxidatively modifies low density lipoprotein. J Clin Invest. 1994;93:1493–1501. doi: 10.1172/JCI117127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lamb DJ, Leake DS. Acidic pH enables ceruloplasmin to catalyse the modification of low- density lipoprotein. FEBS Lett. 1994;338:122–126. doi: 10.1016/0014-5793(94)80348-x. [DOI] [PubMed] [Google Scholar]
- 59.Balla G, Jacob HS, Eaton JW, Belcher JD, Vercellotti GM. Hemin: a possible physiological mediator of low density lipoprotein oxidation and endothelial injury. Arterioscler Thromb Vasc Biol. 1991;11:1700–1711. doi: 10.1161/01.atv.11.6.1700. [DOI] [PubMed] [Google Scholar]
- 60.Sakurai T, Kimura S, Nakano M, Kimura H. Oxidative modification of glycated low density lipoprotein in the presence of iron. Biochem Biophys Res Commun. 1991;177:433–439. doi: 10.1016/0006-291x(91)92002-2. [DOI] [PubMed] [Google Scholar]
- 61.Wieland E, Parthasarathy S, Steinberg D. Peroxidase-dependent metal-independent oxidation of low density lipoprotein in vitro: a model for in vivo oxidation? Proc Natl Acad Sci USA. 1993;90:5929–5933. doi: 10.1073/pnas.90.13.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Savenkova ML, Mueller DM, Heinecke JW. Tyrosyl radical generated by myeloperoxidase is a physiological catalyst for the initiation of lipid peroxidation in low density lipoprotein. J Biol Chem. 1994;269:20394–20400. [PubMed] [Google Scholar]
- 63.Natella F, Nardini M, Ursini F, Scaccini C. Oxidative modification of human low-density lipoprotein by horseradish peroxidase in the absence of hydrogen peroxide. Free Radic Res. 1998;29:427–434. doi: 10.1080/10715769800300471. [DOI] [PubMed] [Google Scholar]
- 64.Darley-Usmar VM, Hogg N, O'Leary VJ, Wilson MT, Moncada S. The simultaneous generation of superoxide and nitric oxide can initiate lipid peroxidation in human low density lipoprotein. Free Radic Res Commun. 1992;17:9–20. doi: 10.3109/10715769209061085. [DOI] [PubMed] [Google Scholar]
- 65.Graham A, Hogg N, Kalyanaraman B, O'Leary V, Darley-Usmar V, Moncada S. Peroxynitrite modification of low-density lipoprotein leads to recognition by the macrophage scavenger receptor. FEBS Lett. 1993;330:181–185. doi: 10.1016/0014-5793(93)80269-z. [DOI] [PubMed] [Google Scholar]
- 66.Panasenko OM, Briviba K, Klotz LO, Sies H. Oxidative modification and nitration of human low-density lipoproteins by the reaction of hypochlorous acid with nitrite. Arch Biochem Biophys. 1997;343:254–259. doi: 10.1006/abbi.1997.0171. [DOI] [PubMed] [Google Scholar]
- 67.Parthasarathy S. Oxidation of low-density lipoprotein by thiol compounds leads to its recognition by the acetyl LDL receptor. Biochim Biophys Acta. 1987;917:337–340. doi: 10.1016/0005-2760(87)90139-1. [DOI] [PubMed] [Google Scholar]
- 68.Heinecke JW, Kawamura M, Suzuki L, Chait A. Oxidation of low density lipoprotein by thiols: superoxide-dependent and -independent mechanisms. J Lipid Res. 1993;34:2051–2061. [PubMed] [Google Scholar]
- 69.Sparrow CP, Olszewski J. Cellular oxidation of low density lipoprotein is caused by thiol production in media containing transition metal ions. J Lipid Res. 1993;34:1219–1228. [PubMed] [Google Scholar]
- 70.Wood JL, Graham A. The role of thiols in oxidation of low-density lipoprotein by macrophages. Biochem Soc Trans. 1995;23:242S. doi: 10.1042/bst023242s. [DOI] [PubMed] [Google Scholar]
- 71.Lynch SM, Frei B. Mechanisms of copper- and iron-dependent oxidative modification of human low density lipoprotein. J Lipid Res. 1993;34:1745–1753. [PubMed] [Google Scholar]
- 72.Napoli C, Ambrosio G, Palumbo G, Elia PP, Chiariello M. Human low-density lipoproteins are peroxidized by free radicals via chain reactions triggered by the superoxide radical. Cardiologia. 1991;36:527–532. [PubMed] [Google Scholar]
- 73.Jessup W, Simpson JA, Dean RT. Does superoxide radical have a role in macrophage-mediated oxidative modification of LDL? Atherosclerosis. 1993;99:107–120. doi: 10.1016/0021-9150(93)90056-z. [DOI] [PubMed] [Google Scholar]
- 74.Aviram M, Rosenblat M, Etzioni A, Levy R. Activation of NADPH oxidase required for macrophage-mediated oxidation of low-density lipoprotein. Metabolism. 1996;45:1069–1079. doi: 10.1016/s0026-0495(96)90005-0. [DOI] [PubMed] [Google Scholar]
- 75.Montgomery RR, Nathan CF, Cohn ZA. Effects of reagent and cell-generated hydrogen peroxide on the properties of low density lipoprotein. Proc Natl Acad Sci USA. 1986;83:6631–6635. doi: 10.1073/pnas.83.17.6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hiramatsu K, Rosen H, Heinecke JW, Wolfbauer G, Chait A. Super-oxide initiates oxidation of low density lipoprotein by human monocytes. Arteriosclerosis. 1987;7:55–60. doi: 10.1161/01.atv.7.1.55. [DOI] [PubMed] [Google Scholar]
- 77.Steinbrecher UP. Role of superoxide in endothelial-cell modification of low-density lipoproteins. Biochim Biophys Acta. 1988;959:20–30. doi: 10.1016/0005-2760(88)90145-2. [DOI] [PubMed] [Google Scholar]
- 78.Cathcart MK, McNally AK, Morel DW, Chisolm GMD. Superoxide anion participation in human monocyte-mediated oxidation of low-density lipoprotein and conversion of low-density lipoprotein to a cytotoxin. J Immunol. 1989;142:1963–1969. [PubMed] [Google Scholar]
- 79.Kawabe Y, Cynshi O, Takashima Y, Suzuki T, Ohba Y, Kodama T. Oxidation-induced aggregation of rabbit low-density lipoprotein by azo initiator. Arch Biochem Biophys. 1994;310:489–496. doi: 10.1006/abbi.1994.1197. [DOI] [PubMed] [Google Scholar]
- 80.Noguchi N, Gotoh N, Niki E. Effects of ebselen and probucol on oxidative modifications of lipid and protein of low density lipoprotein induced by free radicals. Biochim Biophys Acta. 1994;1213:176–182. doi: 10.1016/0005-2760(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 81.Leonhardt W, Bergmann R, Hanefeld M. Initiation of LDL oxidation by copper ions or AAPH yields different kinetic parameters which are correlated [letter] Clin Chim Acta. 1997;259:195–197. doi: 10.1016/s0009-8981(96)06476-5. [DOI] [PubMed] [Google Scholar]
- 82.Kim JG, Sabbagh F, Santanam N, Wilcox JN, Medford RM, Parthasarathy S. Generation of a polyclonal antibody against lipid peroxide-modified proteins. Free Radic Biol Med. 1997;23:251–259. doi: 10.1016/s0891-5849(96)00615-6. [DOI] [PubMed] [Google Scholar]
- 83.Dinis TC, Santosa CL, Almeida LM. The apoprotein is the preferential target for peroxynitrite-induced LDL damage protection by dietary phenolic acids. Free Radic Res. 2002;36:531–543. doi: 10.1080/10715760290025915. [DOI] [PubMed] [Google Scholar]
- 84.Heinecke JW. Pathways for oxidation of low density lipoprotein by myeloperoxidase: tyrosyl radical, reactive aldehydes, hypochlorous acid and molecular chlorine. Biofactors. 1997;6:145–155. doi: 10.1002/biof.5520060208. [DOI] [PubMed] [Google Scholar]
- 85.Cornicelli JA, Trivedi BK. 15-Lipoxygenase and its inhibition: a novel therapeutic target for vascular disease. Curr Pharm Des. 1999;5:11–20. [PubMed] [Google Scholar]
- 86.Kuhn H, Romisch I, Belkner J. The role of lipoxygenase-isoforms in atherogenesis. Mol Nutr Food Res. 2005;49:1014–1029. doi: 10.1002/mnfr.200500131. [DOI] [PubMed] [Google Scholar]
- 87.Gugliucci Creriche A, Stahl AJ. Glycation and oxidation of human low density lipoproteins reduces heparin binding and modifies charge. Scand J Clin Lab Invest. 1993;53:125–132. doi: 10.3109/00365519309088399. [DOI] [PubMed] [Google Scholar]
- 88.Sanchez-Quesada JL, Perez A, Caixas A, Ordonmez-Llanos J, Carreras G, Payes A, Gonzalez-Sastre F, de Leiva A. Electronegative low density lipoprotein subform is increased in patients with short-duration IDDM and is closely related to glycaemic control. Diabetologia. 1996;39:1469–1476. doi: 10.1007/s001250050600. [DOI] [PubMed] [Google Scholar]
- 89.Demuth K, Myara I, Chappey B, Vedie B, Pech-Amsellem MA, Haberland ME, Moatti N. A cytotoxic electronegative LDL subfraction is present in human plasma. Arterioscler Thromb Vasc Biol. 1996;16:773–783. doi: 10.1161/01.atv.16.6.773. [DOI] [PubMed] [Google Scholar]
- 90.Dai L, Zhang Z, Winyard PG, Gaffney K, Jones H, Blake DR, Morris CJ. A modified form of low-density lipoprotein with increased electronegative charge is present in rheumatoid arthritis synovial fluid. Free Radic Biol Med. 1997;22:705–710. doi: 10.1016/s0891-5849(96)00389-9. [DOI] [PubMed] [Google Scholar]
- 91.Moro E, Zambon C, Pianetti S, Cazzolato G, Pais M, Bittolo Bon G. Electronegative low density lipoprotein subform (LDL-) is increased in type 2 (non-insulin-dependent) microalbuminuric diabetic patients and is closely associated with LDL susceptibility to oxidation. Acta Diabetol. 1998;35:161–164. doi: 10.1007/s005920050123. [DOI] [PubMed] [Google Scholar]
- 92.Benitez S, Perez A, Sanchez-Quesada JL, Wagner AM, Rigla M, Arcelus R, Jorba O, Ordonez-Llanos J. Electronegative low-density lipoprotein subfraction from type 2 diabetic subjects is proatherogenic and unrelated to glycemic control. Diabetes Metab Res Rev. 2007;23:26–34. doi: 10.1002/dmrr.643. [DOI] [PubMed] [Google Scholar]
- 93.Sanchez-Quesada JL, Camacho M, Anton R, Benitez S, Vila L, Ordonez-Llanos J. Electronegative LDL of FH subjects: chemical characterization and induction of chemokine release from human endothelial cells. Atherosclerosis. 2003;166:261–270. doi: 10.1016/s0021-9150(02)00374-x. [DOI] [PubMed] [Google Scholar]
- 94.Parasassi T, Bittolo-Bon G, Brunelli R, Cazzolato G, Krasnowska EK, Mei G, Sevanian A, Ursini F. Loss of apoB-100 secondary structure and conformation in hydroperoxide rich, electronegative LDL(-) Free Radic Biol Med. 2001;31:82–89. doi: 10.1016/s0891-5849(01)00555-x. [DOI] [PubMed] [Google Scholar]
- 95.Barros MR, Bertolami MC, Abdalla DS, Ferreira WP. Identification of mildly oxidized low-density lipoprotein (electronegative LDL) and its auto-antibodies IgG in children and adolescents hypercholesterolemic offsprings. Atherosclerosis. 2006;184:103–107. doi: 10.1016/j.atherosclerosis.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 96.Yamamoto K, Niki E. Interaction of alpha-tocopherol with iron: antioxidant and prooxidant effects of alpha-tocopherol in the oxidation of lipids in aqueous dispersions in the presence of iron. Biochim Biophys Acta. 1988;958:19–23. doi: 10.1016/0005-2760(88)90241-x. [DOI] [PubMed] [Google Scholar]
- 97.Bowry VW, Ingold KU, Stocker R. Vitamin E in human low-density lipoprotein. When and how this antioxidant becomes a pro-oxidant. Biochem J. 1992;288:341–344. doi: 10.1042/bj2880341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Santanam N, Parthasarathy S. Paradoxical actions of antioxidants in the oxidation of low density lipoprotein by peroxidases. J Clin Invest. 1995;95:2594–2600. doi: 10.1172/JCI117961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Santanam N, Parthasarathy S. Cellular cysteine generation does not contribute to the initiation of LDL oxidation. J Lipid Res. 1995;36:2203–2211. [PubMed] [Google Scholar]
- 100.Palinski W, Rosenfeld ME, Yla-Herttuala S, Gurtner GC, Socher SS, Butler SW, Parthasarathy S, Carew TE, Steinberg D, Witztum JL. Low density lipoprotein undergoes oxidative modification in vivo. Proc Natl Acad Sci USA. 1989;86:1372–1376. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Aviram M. Malondialdehyde affects the physico-chemical and biological characteristics of oxidized low density lipoprotein. Atherosclerosis. 1990;84:141–143. doi: 10.1016/0021-9150(90)90084-v. [DOI] [PubMed] [Google Scholar]
- 102.Lecomte E, Artur Y, Chancerelle Y, Herbeth B, Galteau MM, Jeandel C, Siest G. Malondialdehyde adducts to, and fragmentation of, apolipoprotein B from human plasma. Clin Chim Acta. 1993;218:39–46. doi: 10.1016/0009-8981(93)90220-x. [DOI] [PubMed] [Google Scholar]
- 103.Requena JR, Fu MX, Ahmed MU, Jenkins AJ, Lyons TJ, Baynes JW, Thorpe SR. Quantification of malondialdehyde and 4-hydroxynonenal adducts to lysine residues in native and oxidized human low-density lipoprotein. Biochem J. 1997;322:317–325. doi: 10.1042/bj3220317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lyons TJ, Li W, Wells-Knecht MC, Jokl R. Toxicity of mildly modified low-density lipoproteins to cultured retinal capillary endothelial cells and pericytes. Diabetes. 1994;43:1090–1095. doi: 10.2337/diab.43.9.1090. [DOI] [PubMed] [Google Scholar]
- 105.Scaccini C, Jialal I. LDL modification by activated polymorphonuclear leukocytes: a cellular model of mild oxidative stress. Free Radic Biol Med. 1994;16:49–55. doi: 10.1016/0891-5849(94)90242-9. [DOI] [PubMed] [Google Scholar]
- 106.Sigari F, Lee C, Witztum JL, Reaven PD. Fibroblasts that over-express 15-lipoxygenase generate bioactive and minimally modified LDL. Arterioscler Thromb Vasc Biol. 1997;17:3639–3645. doi: 10.1161/01.atv.17.12.3639. [DOI] [PubMed] [Google Scholar]
- 107.Kennedy S, Fournet-Bourguignon MP, Breugnot C, Castedo-Delrieu M, Lesage L, Reure H, Briant C, Leonce S, Vilaine JP, Vanhoutte PM. Cells derived from regenerated endothelium of the porcine coronary artery contain more oxidized forms of apolipoprotein-B-100 without a modification in the uptake of oxidized LDL. J Vasc Res. 2003;40:389–398. doi: 10.1159/000072817. [DOI] [PubMed] [Google Scholar]
- 108.Esterbauer H, Striegl G, Puhl H, Rotheneder M. Continuous monitoring of in vitro oxidation of human low density lipoprotein. Free Radic Res Commun. 1989;6:67–75. doi: 10.3109/10715768909073429. [DOI] [PubMed] [Google Scholar]
- 109.Moore KP, Darley-Usmar V, Morrow J, Roberts LJ., 2nd Formation of F2-isoprostanes during oxidation of human low-density lipoprotein and plasma by peroxynitrite. Circ Res. 1995;77:335–341. doi: 10.1161/01.res.77.2.335. [DOI] [PubMed] [Google Scholar]
- 110.Moore K, Roberts LJ., 2nd Measurement of lipid peroxidation. Free Radic Res. 1998;28:659–671. doi: 10.3109/10715769809065821. [DOI] [PubMed] [Google Scholar]
- 111.Gaziano JM. Antioxidant vitamins and coronary artery disease risk. Am J Med. 1994;97:18S–21S. doi: 10.1016/0002-9343(94)90294-1. Discussion 22S–28S. [DOI] [PubMed] [Google Scholar]
- 112.Rapola JM, Virtamo J, Ripatti S, Huttunen JK, Albanes D, Taylor PR, Heinonen OP. Randomised trial of alpha-tocopherol and beta-carotene supplements on incidence of major coronary events in men with previous myocardial infraction [see comments] Lancet. 1997;349:1715–1720. doi: 10.1016/S0140-6736(97)01234-8. [DOI] [PubMed] [Google Scholar]
- 113.Porkkala-Sarataho EK, Nyyssonen MK, Kaikkonen JE, Poulsen HE, Hayn EM, Salonen RM, Salonen JT. A randomized, single-blind, placebo-controlled trial of the effects of 200 mg alpha-tocopherol on the oxidation resistance of atherogenic lipoproteins. Am J Clin Nutr. 1998;68:1034–1041. doi: 10.1093/ajcn/68.5.1034. [DOI] [PubMed] [Google Scholar]
- 114.McKechnie R, Rubenfire M, Mosca L. Antioxidant nutrient supplementation and brachial reactivity in patients with coronary artery disease. J Lab Clin Med. 2002;139:133–139. doi: 10.1067/mlc.2002.121450. [DOI] [PubMed] [Google Scholar]
- 115.Gey KF. Prospects for the prevention of free radical disease, regarding cancer and cardiovascular disease. Br Med Bull. 1993;49:679–699. doi: 10.1093/oxfordjournals.bmb.a072640. [DOI] [PubMed] [Google Scholar]
- 116.Blot WJ, Li JY, Taylor P, Guo W, Dawsey S, Wang GQ, Yang CS, Zheng SF, Gail M, Li GY, et al. Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease- specific mortality in the general population. J Natl Cancer Inst. 1993;85:1483–1492. doi: 10.1093/jnci/85.18.1483. [DOI] [PubMed] [Google Scholar]
- 117.Steinberg D. Clinical trials of antioxidants in atherosclerosis: are we doing the right thing? Lancet. 1995;346:36–38. doi: 10.1016/s0140-6736(95)92657-7. [DOI] [PubMed] [Google Scholar]
- 118.Parthasarathy S, Khan-Merchant N, Penumetcha M, Khan BV, Santanam N. Did the antioxidant trials fail to validate the oxidation hypothesis? Curr Atheroscler Rep. 2001;3:392–398. doi: 10.1007/s11883-001-0077-9. [DOI] [PubMed] [Google Scholar]
- 119.Heinecke JW. Is the emperor wearing clothes? Clinical trials of vitamin E and the LDL oxidation hypothesis. Arterioscler Thromb Vasc Biol. 2001;21:1261–1264. doi: 10.1161/hq0801.095084. [DOI] [PubMed] [Google Scholar]
- 120.Yusoff K. Vitamin E in cardiovascular disease: has the die been cast? Asia Pac J Clin Nutr. 2002;7:S443–447. doi: 10.1046/j.1440-6047.11.s.7.11.x. [DOI] [PubMed] [Google Scholar]
- 121.Heinecke JW. Clinical trials of vitamin E in coronary artery disease: is it time to reconsider the low-density lipoprotein oxidation hypothesis? Curr Atheroscler Rep. 2003;5:83–87. doi: 10.1007/s11883-003-0075-1. [DOI] [PubMed] [Google Scholar]
- 122.Ferns GA, Lamb DJ. What does the lipoprotein oxidation phenomenon mean? Biochem Soc Trans. 2004;32:160–163. doi: 10.1042/bst0320160. [DOI] [PubMed] [Google Scholar]
- 123.Mowri H, Chinen K, Ohkuma S, Takano T. Peroxidized lipids isolated by HPLC from atherosclerotic aorta. Biochem Int. 1986;12:347–352. [PubMed] [Google Scholar]
- 124.Carpenter KL, Wilkins GM, Fussell B, Ballantine JA, Taylor SE, Mitchinson MJ, Leake DS. Production of oxidized lipids during modification of low-density lipoprotein by macrophages or copper. Biochem J. 1994;304:625–633. doi: 10.1042/bj3040625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Folcik VA, Nivar-Aristy RA, Krajewski LP, Cathcart MK. Lipoxygenase contributes to the oxidation of lipids in human atherosclerotic plaques. J Clin Invest. 1995;96:504–510. doi: 10.1172/JCI118062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Staprans I, Rapp JH, Pan XM, Hard-man DA, Feingold KR. Oxidized lipids in the diet accelerate the development of fatty streaks in cholesterol-fed rabbits. Arterioscler Thromb Vasc Biol. 1996;16:533–538. doi: 10.1161/01.atv.16.4.533. [DOI] [PubMed] [Google Scholar]
- 127.Berliner J, Leitinger N, Watson A, Huber J, Fogelman A, Navab M. Oxidized lipids in atherogenesis: formation, destruction and action. Thromb Haemost. 1997;78:195–199. [PubMed] [Google Scholar]
- 128.Parthasarathy S, Santanam N, Ramachandran S, Meilhac O. Potential role of oxidized lipids and lipoproteins in antioxidant defense [In Process Citation] Free Radic Res. 2000;33:197–215. doi: 10.1080/10715760000301381. [DOI] [PubMed] [Google Scholar]
- 129.Navab M, Hama SY, Reddy ST, Ng CJ, Van Lenten BJ, Laks H, Fogelman AM. Oxidized lipids as mediators of coronary heart disease. Curr Opin Lipidol. 2002;13:363–372. doi: 10.1097/00041433-200208000-00003. [DOI] [PubMed] [Google Scholar]
- 130.Birukov KG. Oxidized lipids: the two faces of vascular inflammation. Curr Atheroscler Rep. 2006;8:223–231. doi: 10.1007/s11883-006-0077-x. [DOI] [PubMed] [Google Scholar]
- 131.Salmon S, Maziere C, Theron L, Beucler I, Ayrault-Jarrier M, Goldstein S, Polonovski J. Immunological detection of low-density lipoproteins modified by malondialdehyde in vitro or in vivo. Biochim Biophys Acta. 1987;920:215–220. doi: 10.1016/0005-2760(87)90097-x. [DOI] [PubMed] [Google Scholar]
- 132.Parums DV, Brown DL, Mitchinson MJ. Serum antibodies to oxidized low-density lipoprotein and ceroid in chronic periaortitis. Arch Pathol Lab Med. 1990;114:383–387. [PubMed] [Google Scholar]
- 133.Virella G, Virella I, Leman RB, Pryor MB, Lopes-Virella MF. Anti-oxidized low-density lipoprotein antibodies in patients with coronary heart disease and normal healthy volunteers. Int J Clin Lab Res. 1993;23:95–101. doi: 10.1007/BF02592290. [DOI] [PubMed] [Google Scholar]
- 134.Holvoet P, Perez G, Zhao Z, Brouwers E, Bernar H, Collen D. Malondialdehyde-modified low density lipoproteins in patients with atherosclerotic disease. J Clin Invest. 1995;95:2611–2619. doi: 10.1172/JCI117963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Festa A, Kopp HP, Schernthaner G, Menzel EJ. Autoantibodies to oxidised low density lipoproteins in IDDM are inversely related to metabolic control and microvascular complications. Diabetologia. 1998;41:350–356. doi: 10.1007/s001250050914. [DOI] [PubMed] [Google Scholar]
- 136.Lehtimaki T, Lehtinen S, Solakivi T, Nikkila M, Jaakkola O, Jokela H, Yla-Herttuala S, Luoma JS, Koivula T, Nikkari T. Autoantibodies against oxidized low density lipoprotein in patients with angiographically verified coronary artery disease [In Process Citation] Arterioscler Thromb Vasc Biol. 1999;19:23–27. doi: 10.1161/01.atv.19.1.23. [DOI] [PubMed] [Google Scholar]
- 137.Frostegard J, Wu R, Lemne C, Thulin T, Witztum JL, de Faire U. Circulating oxidized low-density lipoprotein is increased in hypertension. Clin Sci (London) 2003;105:615–620. doi: 10.1042/CS20030152. [DOI] [PubMed] [Google Scholar]
- 138.Herrick AL, Illingworth KJ, Hollis S, Gomez-Zumaquero JM, Tina-hones FJ. Antibodies against oxidized low-density lipoproteins in systemic sclerosis. Rheumatology (Oxford) 2001;40:401–405. doi: 10.1093/rheumatology/40.4.401. [DOI] [PubMed] [Google Scholar]
- 139.Tanaga K, Bujo H, Inoue M, Mikami K, Kotani K, Takahashi K, Kanno T, Saito Y. Increased circulating malondialdehyde-modified LDL levels in patients with coronary artery diseases and their association with peak sizes of LDL particles. Arterioscler Thromb Vasc Biol. 2002;22:662–666. doi: 10.1161/01.atv.0000012351.63938.84. [DOI] [PubMed] [Google Scholar]
- 140.Meraviglia MV, Maggi E, Bellomo G, Cursi M, Fanelli G, Minicucci F. Autoantibodies against oxidatively modified lipoproteins and progression of carotid restenosis after carotid endarterectomy. Stroke. 2002;33:1139–1141. doi: 10.1161/01.str.0000014420.15948.2e. [DOI] [PubMed] [Google Scholar]
- 141.Hsu RM, Devaraj S, Jialal I. Autoantibodies to oxidized low-density lipoprotein in patients with type 2 diabetes mellitus. Clin Chim Acta. 2002;317:145–150. doi: 10.1016/s0009-8981(01)00767-7. [DOI] [PubMed] [Google Scholar]
- 142.Tsimikas S, Bergmark C, Beyer RW, Patel R, Pattison J, Miller E, Juliano J, Witztum JL. Temporal increases in plasma markers of oxidized low-density lipoprotein strongly reflect the presence of acute coronary syndromes. J Am Coll Cardiol. 2003;41:360–370. doi: 10.1016/s0735-1097(02)02769-9. [DOI] [PubMed] [Google Scholar]
- 143.Wang J, Qiang H, Zhang C, Liu X, Chen D, Wang S. Detection of IgG-bound lipoprotein(a) immune complexes in patients with coronary heart disease. Clin Chim Acta. 2003;327:115–122. doi: 10.1016/s0009-8981(02)00342-x. [DOI] [PubMed] [Google Scholar]
- 144.Koskenmies S, Vaarala O, Widen E, Kere J, Palosuo T, Julkunen H. The association of antibodies to cardiolipin, beta 2-glycoprotein I, prothrombin, and oxidized low-density lipoprotein with thrombosis in 292 patients with familial and sporadic systemic lupus erythematosus. Scand J Rheumatol. 2004;33:246–252. doi: 10.1080/03009740410005386. [DOI] [PubMed] [Google Scholar]
- 145.Luoma JS, Kareinen A, Narvanen O, Viitanen L, Laakso M, Yla-Herttuala S. Autoantibodies against oxidized LDL are associated with severe chest pain attacks in patients with coronary heart disease. Free Radic Biol Med. 2005;39:1660–1665. doi: 10.1016/j.freeradbiomed.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 146.Maggi E, Finardi G, Poli M, Bollati P, Filipponi M, Stefano PL, Paolini G, Grossi A, Clot P, Albano E, et al. Specificity of autoantibodies against oxidized LDL as an additional marker for atherosclerotic risk. Coron Artery Dis. 1993;4:1119–1122. doi: 10.1097/00019501-199312000-00014. [DOI] [PubMed] [Google Scholar]
- 147.Bui MN, Sack MN, Moutsatsos G, Lu DY, Katz P, McCown R, Breall JA, Rackley CE. Autoantibody titers to oxidized low-density lipoprotein in patients with coronary atherosclerosis. Am Heart J. 1996;131:663–667. doi: 10.1016/s0002-8703(96)90268-9. [DOI] [PubMed] [Google Scholar]
- 148.Uusitupa MI, Niskanen L, Luoma J, Vilja P, Mercuri M, Rauramaa R, Yla-Herttuala S. Autoantibodies against oxidized LDL do not predict atherosclerotic vascular disease in non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 1996;16:1236–1242. doi: 10.1161/01.atv.16.10.1236. [DOI] [PubMed] [Google Scholar]
- 149.van de Vijver LP, Steyger R, van Poppel G, Boer JM, Kruijssen DA, Seidell JC, Princen HM. Autoantibodies against MDA-LDL in subjects with severe and minor atherosclerosis and healthy population controls. Atherosclerosis. 1996;122:245–253. doi: 10.1016/0021-9150(95)05759-5. [DOI] [PubMed] [Google Scholar]
- 150.Kim JG, Taylor WR, Parthasarathy S. Demonstration of the presence of lipid peroxide-modified proteins in human atherosclerotic lesions using a novel lipid peroxide-modified anti-peptide antibody. Atherosclerosis. 1999;143:335–340. doi: 10.1016/s0021-9150(98)00320-7. [DOI] [PubMed] [Google Scholar]
- 151.Tsai WC, Li YH, Chao TH, Chen JH. Relation between antibody against oxidized low-density lipoprotein and extent of coronary atherosclerosis. J Formos Med Assoc. 2002;101:681–684. [PubMed] [Google Scholar]
- 152.Radulescu L, Stancu C, Antohe F. Antibodies against human oxidized low-density lipoprotein (LDL) as markers for human plasma modified lipoproteins. Med Sci Monit. 2004;10:BR207–214. [PubMed] [Google Scholar]
- 153.Tsimikas S. Oxidized low-density lipoprotein biomarkers in atherosclerosis. Curr Atheroscler Rep. 2006;8:55–61. doi: 10.1007/s11883-006-0065-1. [DOI] [PubMed] [Google Scholar]
- 154.Moguilevsky N, Zouaoui Boudjeltia K, Babar S, Delree P, Legssyer I, Carpentier Y, Vanhaeverbeek M, Ducobu J. Monoclonal antibodies against LDL progressively oxidized by myeloperoxidase react with ApoB-100 protein moiety and human atherosclerotic lesions. Biochem Biophys Res Commun. 2004;323:1223–1228. doi: 10.1016/j.bbrc.2004.08.220. [DOI] [PubMed] [Google Scholar]
- 155.Nambi V. The use of myeloperoxidase as a risk marker for atherosclerosis. Curr Atheroscler Rep. 2005;7:127–131. doi: 10.1007/s11883-005-0035-z. [DOI] [PubMed] [Google Scholar]
- 156.Yamaguchi Y, Yoshikawa N, Kagota S, Nakamura K, Haginaka J, Kunitomo M. Elevated circulating levels of markers of oxidative-nitrative stress and inflammation in a genetic rat model of metabolic syndrome. Nitric Oxide. 2006;15:380–386. doi: 10.1016/j.niox.2006.04.264. [DOI] [PubMed] [Google Scholar]
- 157.Parthasarathy S, et al. J Lipid Res. 1999;40:2143–2157. [PubMed] [Google Scholar]


