Abstract
Estrogens may influence gastric cancer risk but published studies are inconclusive. We therefore performed a meta-analysis addressing the associations of gastric cancer in women with menstrual and reproductive factors, and with use of estrogen- and antiestrogen-related therapies. Searches of PubMed up to June, 2011 and review of citations yielded a total of 28 independent studies including at least one exposure of interest. Random effects pooled estimates of relative risk (RR) and corresponding 95% confidence intervals (CI) were calculated for eight exposures reported in at least five studies, including: age at menarche, age at menopause, years of fertility, parity, age at first birth, oral contraceptive use, hormone replacement therapy (HRT), and tamoxifen treatment. Longer years of fertility (RR= 0.74; 95% CI= 0.63 to 0.86) and HRT (RR= 0.77, 95% CI= 0.64 to 0.92) were each associated with decreased gastric cancer risk. Conversely, tamoxifen treatment was associated with increased risk (RR= 1.82, 95% CI= 1.39 to 2.38). The other five exposures were not significantly associated. Our analysis supports the hypothesis that longer exposure to estrogen effects of either ovarian or exogenous origin may decrease risk of gastric cancer. Additional studies are warranted to extend this finding and to identify the underlying mechanisms.
Keywords: Gastric Cancer, Hormone Therapy, Menstrual Factors, Reproductive Factors, Tamoxifen
BACKGROUND
Gastric cancer represents the fourth most common cancer and the second leading cause of cancer death worldwide (1). Notably, for most populations in both high and low incidence regions, the overall incidence in males is approximately double that of females (2, 3). Since these sex differences cannot be totally explained by variations in sociodemographic characteristics, environmental factors or Helicobacter pylori (H. pylori) infection (4, 5), female sex hormones have been proposed to be protective (6). This hypothesis has been previously evaluated by examining associations of gastric cancer risk in women with sex hormone -related exposures, but most individual studies have been inconclusive. To more precisely characterize the reported associations, we have performed a meta-analysis of these data.
MATERIALS AND METHODS
We searched for studies published in any language before June 30, 2011 evaluating the associations of sex hormone-related exposures with gastric cancer incidence or mortality, using PubMed® software to search Medline (U.S. National Library of Medicine, Bethesda, MD).
To identify studies of menstrual and reproductive factors, as well as exogenous estrogens, the following search strategy was used: (gastric cancer OR stomach cancer OR stomach neoplasms) AND (reproductive factors OR menstrual factors OR age at menarche OR menarche OR menstruation OR parity OR pregnancy OR breastfeeding OR miscarriage OR abortion OR fertility OR age at menopause OR estrogens OR sex hormones OR ovariectomy OR oophorectomy OR hysterectomy OR sex differences OR male predominance OR exogenous hormones OR oral contraceptives OR hormone replacement therapy OR menopausal hormone therapy OR climacteric OR reproductive history) AND (risk assessment OR risk OR risk factors OR epidemiology) AND (case-control studies OR case-control OR cohort studies OR cohort). Reference lists of the selected papers were also screened for other potential articles that may have been missed in the database search. If necessary, we attempted to contact the authors to request additional information.
For the search of tamoxifen studies the following strategy was used: tamoxifen AND (gastric cancer OR stomach cancer OR stomach neoplasms OR gastrointestinal neoplasms) AND (case-control studies OR case-control OR cohort studies OR cohort OR longitudinal studies OR longitudinal OR retrospective studies OR retrospective OR prospective studies OR prospective OR follow-up studies OR epidemiologic studies). We also searched for data on primary gastric cancer in randomized clinical trials of tamoxifen therapy for breast cancer treatment or prevention by combining the following terms: tamoxifen AND (gastric cancer OR stomach cancer OR stomach neoplasms OR gastrointestinal neoplasms OR “second cancers” OR “second malignancies” OR neoplasms, second primary[MeSH Terms]) AND (breast cancer OR breast neoplasms OR breast malignancy) AND (randomized controlled trial[Publication Type] OR (randomized[Title/Abstract] AND controlled[Title/Abstract] AND trial[Title/Abstract]) OR random*[Title/Abstract] OR random allocation[MeSH Terms]). We also reviewed the reference lists of identified articles and of two previous meta-analyses addressing the associations of tamoxifen therapy with breast cancer recurrence and with adverse effects (7, 8).
Two investigators in our team independently reviewed the articles and extracted the data; any disagreement was resolved by consulting a third reviewer. For inclusion in this re-analysis, the studies had to present adjusted estimates of relative risk (or similar measures of association including odds ratios; RR), and corresponding 95% confidence intervals (CIs). If anatomical subsite-specific RRs were reported, we extracted data on noncardia gastric cancer only. While gastric cancer risk was purportedly increased with tamoxifen exposure presumed from date of diagnosis in two cancer registry studies (9, 10), we only included data for known exposure status.
The following information was recorded for each study: first author, journal, country where conducted, year of publication, study design, studied outcome, exposure variables and categories, number of gastric cancer cases, number of controls, cohort size (if applicable), age range, menopausal status of the participants, duration of follow-up (if applicable), total person-years of observation (if applicable), treatment regimen (if applicable), adjusted-RR estimates and 95% CI for incident gastric cancer, and confounding variables controlled.
Pooled risk estimates were calculated for exposure variables that were reported in at least five studies, which included: age at menarche, age at menopause, years of fertility (defined as years between menarche and menopause in all but one study, which also omitted periods of pregnancy (11)), parity, age at first birth, oral contraceptive (OC) use, hormone replacement therapy (HRT), and tamoxifen treatment.
Other sex hormone-related variables reported in fewer than five studies included: menstrual regularity, number of pregnancies, age at first pregnancy, breastfeeding of offspring, spontaneous abortion, induced abortion, oophorectomy, hysterectomy, menopausal status, intrauterine device use, parenteral contraceptive use, tubal sterilization, duration of OC use, duration of HRT, and history of endometriosis or vaginosis.
Exposures to exogenous estrogens and tamoxifen therapy were analyzed as dichotomous variables. Since some studies reported associations for varying durations of OC use as compared to never use, we pooled those risk estimates using random effects meta-analysis to estimate the overall effect for ever versus never use. Because the categories of other exposure measures varied across studies, we performed a meta-analysis of the comparison of the highest versus the lowest category (or the inverse of the comparison of the lowest versus the highest category, as applicable) in each study. For two instances in which an adjusted RR for this comparison was unavailable, we calculated a crude RR (with Fisher exact 95% CI) from the reported data. Based on the 95% CI, we calculated the standard error (se) for the ln(RR) by the formula: se=(ln(upper limit) – ln(lower limit))/(2*Z1-α/2), where for a 95% CI, Z1-α/2 equal to 1.96 (12). Pooled RRs with corresponding 95% CI were then obtained using the random effects method of DerSimonian and Laird, with inverse variance weights (13). Between-study heterogeneity was assessed for statistical significance using the Q statistic and quantified with the I2 metric, classified as low (<25%), moderate (25–50%) and high (>50%) following Higgins et al. (14, 15). If moderate or high heterogeneity was identified for a given variable, meta-regression techniques were used to examine the extent to which one or more of the following covariates might be explanatory: study design (cohort, case-control or randomized clinical trial), continent where conducted (Asia, Europe or North America), studied outcome (incidence or mortality), menopausal status of the participants (all post-menopausal or both pre- and post-menopausal), and whether or not the study adjusted for a proxy variable related to socioeconomic status (SES) such as education, income or occupation. Galbraith plots were used to identify studies which were major contributors to heterogeneity (16). Given that SES is inversely associated with gastric cancer risk (17) and is also an important predictor of HRT use (18), we tried to minimize confounding with an alternative meta-analysis which excluded three studies that did not adjust for any SES-related variables.
Since some studies of tamoxifen reported no gastric cancers in one of the treatment groups, we could not compute individual RR estimates. We therefore summed the gastric cancers and corresponding person-time for tamoxifen treated and untreated groups, separately for randomized trials and observational studies. Summary RRs (with Fisher exact 95% CI) were derived for the two marginal analyses, and then pooled using a random effects meta-analysis.
Publication bias was investigated by visual inspection of Begg’s funnel plots and formally tested using Egger's regression asymmetry method (19, 20). The influence of individual studies on the overall meta-analysis RR was assessed by sequentially dropping each one before pooling study-specific RRs. A priori, we considered an influential study to be one for which its exclusion altered the overall pooled RR by more than 10%.
Statistical analyses were performed with Stata version 11 (StataCorp, College Station, TX) using a combination of published macros for meta-analysis, including metan, metainf, metareg, galbr and metabias (21). A p-value ≤ 0.05 was considered statistically significant for all tests except the heterogeneity and Egger regression tests, for which p<0.1 was considered significant. All statistical tests were two-sided.
RESULTS
Literature search for menstrual and reproductive factors and exogenous estrogens
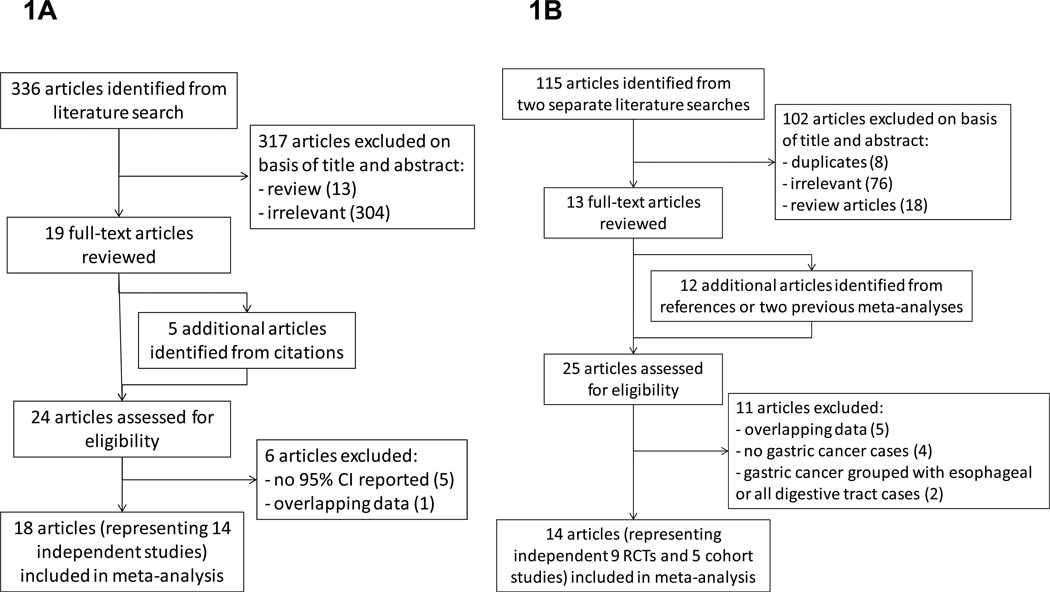
The literature search identified 336 publications, for which the titles and abstracts were scanned to determine potential eligibility for inclusion. Of the 336, 19 were retrieved for further evaluation that also led to identification of five more citations from their collective references (Figure 1A). Thus, 24 articles (23 written in English and 1 in Japanese) reported associations of at least one sex hormone-related variable with gastric cancer risk (11, 22–44). However, we excluded the articles by Miller et al. (22), Plesko et al. (23), Tsukuma et al. (24), La Vecchia et al. (25) and Kvale et al. (28) because only point estimates were reported without 95% CI. Two articles reported partially overlapping data from the Japanese Collaborative Cohort Study (31, 37); we extracted data from Sakauchi et al. (37), the more recent reference, for all sex-related variables except years of fertility, which was only available from Kaneko et al. (31). Two articles from the Shanghai Women’s Health Study reported overlapping results on OC use (36, 40), so those results were extracted from the more recent reference (40); other sex-related variables were extracted from Freedman et al. (36). Two articles reported risk estimates based on the same Italian hospital based case-control study, so data from the larger sample of Fernandez et al. (32) were used for HRT whereas other sex-related variables were only available from La Vecchia et al. (26). Two reports based on the UK General Practice Research Database overlapped (34, 44), so data from the more recent reference were used (44). Exogenous estrogen exposure in the Japan Public Health Center-based Prospective Study reported by Persson et al. (38) was excluded because OC use and HRT were not distinguished. Therefore, a total of 18 articles, representing 14 independent studies, were included in the meta-analysis (Table 1). All but two articles reported on gastric cancer incidence as the outcome; the exceptions being two articles on gastric cancer mortality by Sakauchi et al. (37) and Chang et al. (43). Six studies had been carried out in Europe, six in Asia, and two in North America. Seven studies reported case-control comparisons and seven were analyses of cohorts. Five studies were restricted to post-menopausal women (26, 27, 30, 37, 44) and the remaining nine studies included both pre- and post-menopausal women. Studies differed with respect to the risk factors controlled in the original analyses: all studies adjusted for age, seven controlled for SES-related variables (11, 26, 27, 35, 36, 39, 43), six controlled for body mass index (11, 27, 32, 36, 41, 44), six controlled for smoking (11, 30, 32, 36, 41, 44), four controlled for family history of gastric cancer (26, 27, 30, 38), six controlled for diet-related variables (11, 26, 27, 30, 35, 41), and five controlled for multiple menstrual or reproductive-related variables simultaneously (26, 29, 35, 39, 40). However, Frise et al. (35) used premenopausal as the referent category for age at menopause and Freedman et al. (41) used nulliparous as the referent category for age at first birth. Hence, these two adjusted RRs could not be pooled with others comparing the highest versus the lowest categories, so we calculated and used crude RRs instead.
Figure 1.
Flow diagram of the literature search for studies of (A) menstrual and reproductive factors and exogenous hormones and (B) tamoxifen.
Table 1.
Characteristics of observational studies addressing the association of gastric cancer with menstrual and reproductive factors, and with use of estrogen-related therapies.
| Authors, year (reference) |
Country where conducted |
Exposure(s) studied | Type of study |
Cases/controls (overall follow-up time) |
Comparison group |
Age range (menopausal status) |
Histologic type of cases | Anatomic site of cases | Confounders controlled |
|---|---|---|---|---|---|---|---|---|---|
| La Vecchia et al., 1994 (26) |
Italy | Age at menopause, age at menarche, years of fertility, parity, age at first birth, OC use, HRT use, history of spontaneous and induced abortions. |
Case- control |
229/614 | Hospital- based controls |
35–79 (all post menopausal) |
No data | No data | Age, education, area of residence, family history of gastric cancer, total calories, beta- carotene and vitamin C intake, and other hormone-related exposure variables |
| Palli et al., 1994 (27) |
Italy | Age at menopause a, age at menarche, years of fertility a, parity, age at first birth, number of spontaneous/induced abortions. |
Case- control |
339/515 | Population- based controls |
<75 (all post menopausal) |
No data | No data | Age, geographic area, place of residence, migration from the south, SES, family history of gastric cancer, BMI, total caloric intake and protein and ascorbic acid intake. |
| Heuch et al., 2000 (29) and Heuch et al., 2003 (33) |
Norway | Age at menopause, age at menarche, parity, age at first birth, number of abortions and duration of breastfeeding. |
Cohort | 572/~ 62,518 (1,442,514 p- yr) |
Participants in a screening program for breast cancer (1956– 1959) |
32–79 (pre and post- menopausal) |
No data | Cardia and fundus:58; corpus: 58; antrum and pylorus: 180; unspecified: 276 |
Age, birth cohort, area of residency and county. Parity and age at delivery were included in some models. |
| Inoue et al., 2002 (30) |
Japan | Age at menopause, age at menarche, menstrual irregularity, years of fertility, pregnancy, parity, age at first birth, number of children breastfed and duration of breastfeeding a. |
Case- control |
365/1,825 | Hospital- based controls |
39–82 (all post- menopausal) |
Differentiated: 133; undifferentiated: 232. |
Upper third: 72; middle third: 155; lower third: 127. |
Age, year and season of interview, family history of gastric cancer, smoking status and raw vegetable and cooked fish intake. |
| Kaneko et al., 2003 (31) |
Japan | Age at menopause, age at menarche, years of fertility, parity, age at first pregnancy, HRT use. |
Cohort | 156/~ 40,379 (330,786 p- yr) |
Participants in the JACC |
40–79 (all post- menopausal) |
No data | No data | Age. |
| Fernandez et al., 2003 (32) |
Italy | HRT use. | Case- control |
258/6,976 | Hospital- based controls |
45–79 | No data | No data | Age, study center, year of interview, education, smoking, drinking, type of menopause, age at menopause and BMI. |
| Frise et al., 2006 (35) |
Canada | Age at menopause, menopausal status, age at menarche a, years of fertility, parity, age at first pregnancy, OC use, use and duration of HRT, history of hysterectomy or oophorectomy. |
Case- control |
326/326 | Population- based controls |
20–74 (pre and post- menopausal) |
Intestinal: 55; diffuse: 106; mixed: 15; unknown: 150. |
Proximal: 49; distal: 176; overlapping: 13; unknown: 88. |
Age, education, meat consumption, and other hormone-related exposure variables. |
| Freedman et al., 2007 (36) |
China | Age at menopause a, menopausal status, age at menarche, years of fertility a, years since menopause, pregnancies, parity, age at first pregnancy, oral and injectable contraceptives use, IUD use a, HRT use, history of hysterectomy a or ovariectomy a, duration of breastfeeding. |
Cohort | 154/~73,288 (419,260 p- yr) |
Participants in the Shanghai Women’s Health Study |
40–70 (pre and post- menopausal) |
No data | No data | Age, BMI, education, income, cigarette smoking status, and smoking dose. |
| Sakauchi, 2007 (37) |
Japan | Age at menopause, age at menarche, type of menopause, years of fertility, pregnancies, parity, age at first delivery, use and duration of HRT. |
Cohort | 386/no data (~750,619 p- yr) |
Participants in the JACC |
all post- menopausal |
No data | No data | Age and area of study. |
| Persson et al., 2008 (38) |
Japan | Age at menopause, age at menarche, years of fertility, menstruation status menopause status, regularity of menstruation, age at first delivery, age at first pregnancy, pregnancies, number of deliveries, any hormone intake, breast-feeding, history of endometritis or vaginitis. |
Cohort | 368/~ 44,085 (541,862) |
Participants in the JPHC |
40–69 (pre and post- menopausal) |
Differentiated: 97; undifferentiated: 242, unclassified: 29. |
Proximal: 26, distal: 265, overlapping: 11, unspecified: 66. |
Age, family history of gastric cancer, and study area. |
| Bahmanyar et al., 2008 (39) |
Sweden | Age at first birth and parity. |
Nested case- control |
2,498/12,490 | Participants in a cohort of women born in 1925 or later |
≥30 (pre and post- menopausal) |
No data | Noncardia: 2498 | Year of birth, occupational class, education level, age at first birth and parity. |
| Dorjgochoo et al., 2009 (40) |
China | OC use, IDU use, tubal sterilization. |
Cohort | 168/~ 66,493 | Participants in the Shanghai Women’s Health Study |
40–70 (pre and post- menopausal) |
No data | No data | Age, education, age at menarche, number of live births, cumulative breast feeding months, BMI, exercised regularly in past 5 years, smoking, menopausal status, first- degree family history of cancer and other contraceptive methods |
| Freedman et al., 2010 (41) |
US | Age at menopause, age at menarche, parity, age at first birth, history of hysterectomy or oophorectomy, duration of OC use, use and duration of HRT. |
Cohort | 97/~ 201,409 | Participants in the NIH- AARP Diet and Health Study |
50–71 (pre and post- menopausal) |
No data | Noncardia: 97 | Age, BMI, fruit and vegetable consumption, smoking use, alcohol intake, physical activity, and total energy intake. |
| Duell et al., 2010 (11) |
European countries |
Age at menarche and menopause, duration of OC use, HRT use, parity, age at first full-term pregnancy, breastfeeding, miscarriage, induced abortion, ovariectomy, hysterectomy, and cumulative duration of menstrual cycling. |
Cohort | 181/~ 335,035 (2,927,994 p- yr) |
Participants in the EPIC |
35–70 (pre and post- menopausal) |
Intestinal: 48; diffuse: 82; mixed/unclassified/unknown: 53. |
Noncardia: 101; cardia: 31; overlapping/unknown: 49. |
Age, center, smoking status, education, BMI, and calorie- adjusted vegetable, fruit, red meat, and processed meat intakes. |
| Chung et al., 2011 (42) |
Korea | Age at menarche, age at first pregnancy, parity, history of lactation, and OC use. |
Case- control |
1,495/1,350 | Hospital- based controls |
18–45 (Pre and post- menopausal) |
No data | No data | Age. |
| Chang et al., 2011 (43) |
Taiwan | Parity | Cohort | 1,090/ ~1,291,372 (33,686,828 p-yr) |
Participants in a cohort of women with a record of a first and singleton childbirth in the Birth Registry between 1978 to 1987 |
(Pre and post- menopausal) |
No data | No data | Age, marital status, years of schooling and birthplace. |
| Green et al., 2011 (44) |
UK | HRT use. | Nested case- control |
750/3,722 | Participants in the GPRD |
50–84 (all post- menopausal) |
No data | No data | Age, calendar time and length of observation in GPRD, tobacco smoking, alcohol consumption, and BMI. |
Abbreviations: JAAC, Japan Collaborative Cohort Study; JPHC, Japan Public Health Center-based Prospective Study; EPIC, European Prospective Investigation into Cancer and Nutrition; BMI, body mass index; GPRD, UK General Research Database.
Statistically significant result.
Literature search for tamoxifen exposure
The two independent literature searches identified 115 citations that were potentially relevant to this re-analysis (Figure 1B). Based on the information provided in the title and abstract, we retrieved for further evaluation 13 articles in which drug therapy in the treatment arm differed from that in the control arm solely by the use of tamoxifen. References of these articles and of two previous meta-analyses led to identification of 12 additional studies. Besides irrelevant and duplicate citations, we excluded articles that had either no cases of gastric cancer or did not distinguish them within larger categories (e.g., digestive tract). There were overlapping results from the Danish Breast Cancer Cooperative Group (45, 46), the Christ Hospital Adjuvant Tamoxifen Trial (47, 48), the B-14 trial (49, 50), and the Stockholm Breast Cancer Study Group (51, 52), so data from the more recent articles were extracted (46, 48, 50, 52). In addition, two reports based on US cancer registrations overlapped (53, 54), so data from the longer study period were extracted (53). Thus, a total of 14 independent studies, including nine randomized trials (48, 50, 52, 55–60) and five cohorts (46, 53, 61–63), were included in the meta-analysis (Table 2).
Table 2.
Characteristics of randomized trials and observational studies addressing the association of gastric cancer with tamoxifen therapy.
| Authors, year (reference) |
Country where conducted |
Study name or target population |
Study Type |
Median follow-up time in years |
Age range or menopausal status |
Treatment regimen (daily dose and duration) | Treatment regimen | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment regimen | Gastric cancer cases |
n/women-years at risk |
Treatment regimen (daily dose and duration) |
Gastric cancer cases |
n/women-years at risk |
||||||
| Ribeiro et al., 1992 (48) | UK | The Christie Hospital Adjuvant Tamoxifen trial |
RCT | 10 | Postmenopausal | TAM (20 mg/1 yr) | 2 | 282/2,820 | no treatment | 0 | 306/3,060 |
| Ryden et al., 1992 (55) | Sweden | The Southern Sweden Breast Cancer Trial |
RCT | 9 | Postmenopausal | RT + TAM (30 mg/1 yr) |
2 | 239/1,847 a | RT | 0 | 236/1,812 a |
| Cummings et al., 1993 (56) |
US | Eastern Cooperative Oncology Group |
RCT | TAM: 7.4 placebo: 4.4 |
65–84 | TAM (20 mg/1 yr) | 1 | 85/629 | placebo | 1 | 83/365 |
| Rivkin et al., 1994 (57) | US | The Southern Oncology Group Study |
RCT | 6.5 | Postmenopausal | CHEMO + TAM (20 mg/1 yr) |
1 | 303/1,970 | CHEMO | 0 | 300/1,950 |
| Rutquvist et al., 1995 (52) |
Sweden | The Stockholm Breast Cancer Study Group |
RCT | 9 | Postmenopausal | TAM (40 mg /2 or 5 yrs) |
5 | 1372/9,610 | no treatment | 2 | 1357/9,378 |
| Fisher et al.,1996 (50) | US and Canada |
(NSABP) B-14 | RCT | 10.4 (mean) | Mean age: 55 yrs | TAM (20 mg/5 or 10 yrs) |
4 | 1404/14,602 | placebo | 2 | 1414/14,706 |
| Cuzick et al., 2002 (58) | UK | The International Breast Cancer Intervention Study (IBIS-I) |
RCT | 4.2 | 35 – 70 | TAM (20 mg /5 yrs) |
1 | 3578/15,028 | placebo | 3 | 3574/15,011 |
| Fisher et al., 2005 (59) | US | The National Surgical Adjuvant Breast and Bowel Project Breast Cancer Prevention trial (P-1) |
RCT | 6.2 (mean) | ≥35 | TAM (20 mg /5 yrs) |
2 | 6,681/40,844 | placebo (including crossovers after unblinding) |
2 | 6,707/40,648 |
| Veronesi et al., 2007 (60) |
Italy | Italian Randomized Tamoxifen Prevention Trial |
RCT | 9.1 (mean) | 35 – 70 | TAM (20 mg /5 yrs) |
1 | 2700/30,303 | placebo | 4 | 2708/30,310 |
| Curtis et al., 1996 (53) | US | Cancer registration data from 1980 to 1992 |
Cohort | 12 (maximum) |
≥50 yrs | hormone therapy for their first course of therapy |
15 | 14,358 /39,736 | no hormonal therapy/unknown |
118 | 72,965/348,393 |
| Matsuyama et al., 2000 (61) |
Japan | 9 medical institutions | Cohort | TAM: 7.64 Non-TAM: 8.1 |
Adult women | TAM (20 mg /≤2 yrs mainly) |
32 | 3,497/26717 | CHEMO or no further treatment |
19 | 2,529/20,485 |
| Ursic-Vrscaj et al., 2001 (62) |
Slovenia | Population-based registry from 1987 to 1994 |
Cohort | 8.5(mean); 5–12 (range) |
≥55 yrs | TAM (20 mg /3.33 yrs, median) |
2 | 440/3,740 | no TAM | 0 | 190/1,615 |
| Fowble et al., 2001 (63) | US | University of Pennsylvania or Fox Chase Cancer Center |
Cohort | TAM: 7.4 Non-TAM: 9.6 |
Pre and postmenopausal b |
TAM (−/1 yr) | 0 | 234/1,732 | no TAM | 1 | 681/6,538 |
| Andersson et al., 2008 (46) |
Denmark | Patients registered by the population based Danish Breast Cancer Cooperative Group (DBCG) |
Cohort | 7.8 | 19 – 89 | TAM (−/1 yr) | 19 | 7,204/47,465 | other treatment | 38 | 24,614/209,098 |
Abbreviations: RCT, randomized clinical trial; RT, radiotherapy; TAM, tamoxifen; CHEMO, chemotherapy.
Person-time estimates taken from Rutquvist et al., 1995 (52).
All women received radiotherapy.
Italicized numbers were approximated based on reported data.
Years of fertility
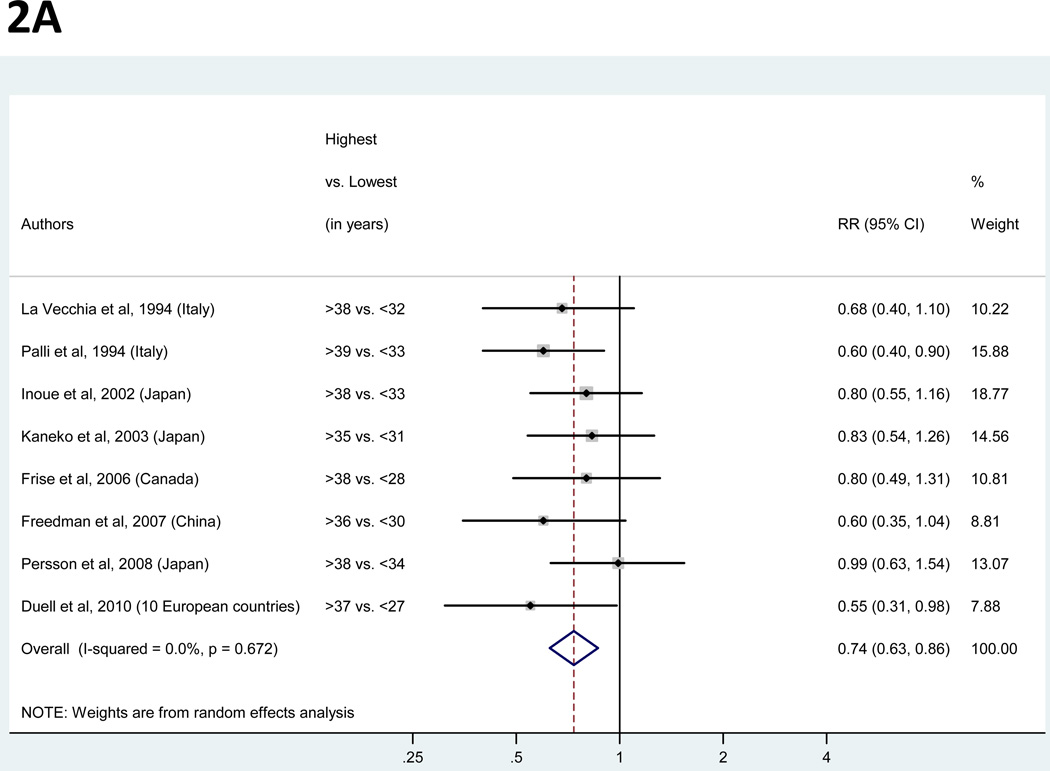
For the analysis of years of fertility, a total of eight studies were identified (11, 26, 27, 30, 31, 35, 36, 38; Figure 2A). Study-specific RRs for the longest versus the shortest duration of fertility ranged from 0.55 to 0.99. The pooled RR suggested a significant inverse association with a 26% decreased risk of gastric cancer (Table 3) and low between-study heterogeneity. The pooled RR was robust to the exclusion of any individual study.
Figure 2.
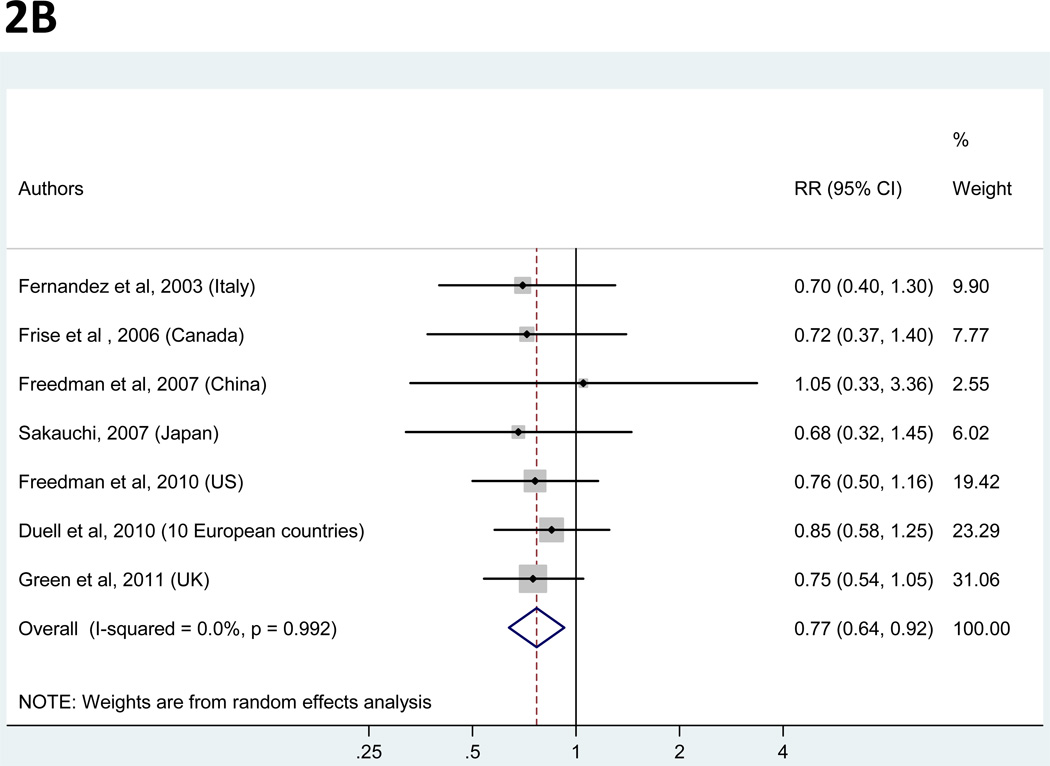
Panels A and B. Random-effects estimates and 95% confidence intervals (CI) of gastric cancer relative risk (RR) associated with (A) years of fertility (highest vs. lowest category) and (B) hormone replacement therapy (ever vs. never). Study-specific RRs are shown as squares, with the size of the symbol inversely proportional to the study-specific variance. Pooled RRs are shown as diamonds, with the middle corresponding to the point estimate and the width representing the 95% CI.
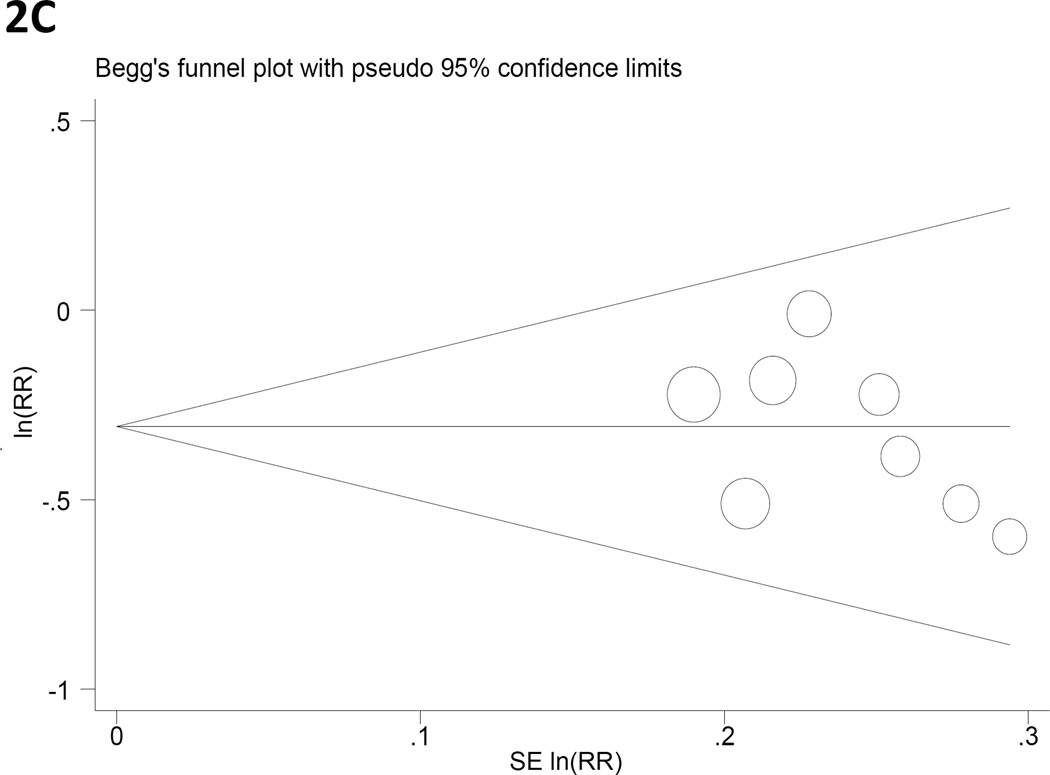
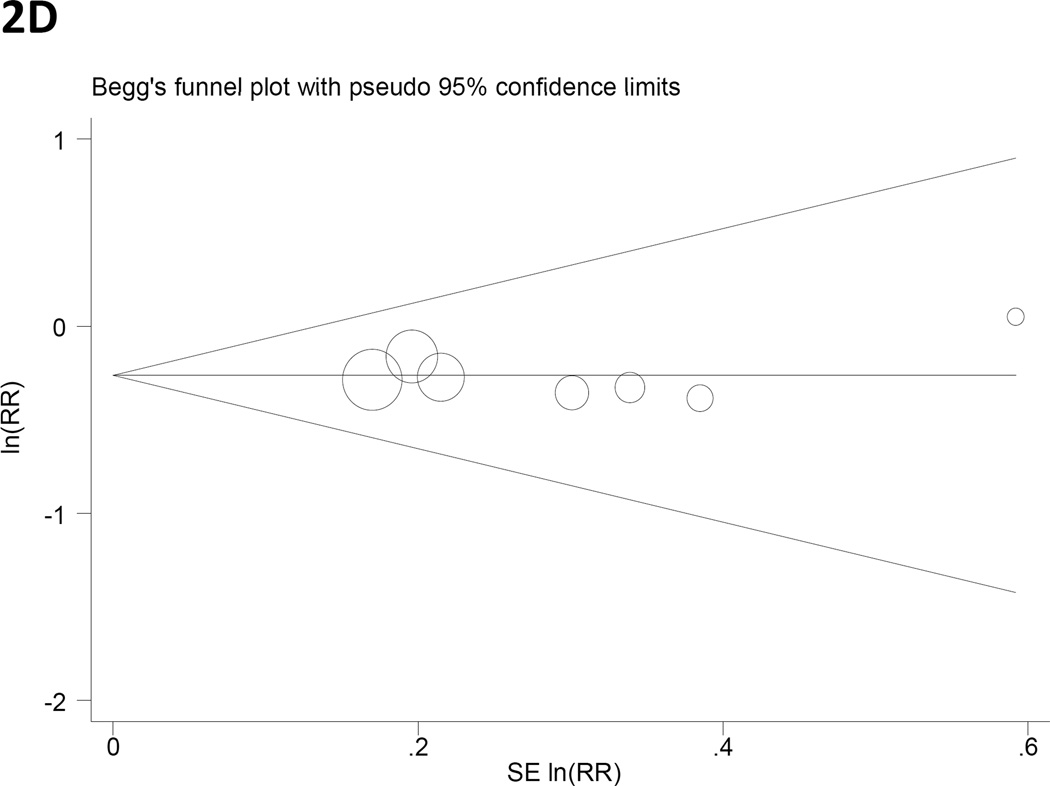
Panels C and D. Begg’s funnel plots with pseudo 95% CIs for gastric cancer RRs associated with (C) years of fertility and (D) hormone replacement therapy.
Table 3.
Summary of meta-analytic results.
| Exposure | Exposure categories | Study design | Pooled RR for gastric cancer (95% CI) |
PQ | I2 (%) | Outlier studies b | PEgger’s | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Highest (min to max) |
Lowest a (min to max) |
Cohorts | Case-control studies |
RCT | ||||||
| Years of fertility | >35 to >39 | <27 to <34 | 4 | 4 | 0 | 0.74 (0.63 – 0.86) | 0.67 | 0 | none | 0.29 |
| Age at menarche (years) | >14 to >16 | <13 to <16 | 6 | 5 | 0 | 1.0 (0.85 – 1.19) | 0.03 | 50.4 | Frise et al. (35) and Persson et al. (38). | 0.25 |
| Age at menopause (years) | >50 to >55 | <45 to 50–54 | 6 | 4 | 0 | 0.84 (0.67 –1.05) | 0.18 | 28.9 | none | 0.41 |
| Parity (number of children) | >2 to >4 | 0 to 1–2 | 7 | 5 c | 0 | 0.94 (0.74 – 1.19) | <0.001 | 83.4 | La Vecchia et al. (26), Chung et al. (42), and Chang et al. (43). | 0.19 |
| Age at first birth (years) | >25 to >34 | <20 to 20–30 | 6 | 4 | 0 | 0.99 (0.85 – 1.15) | 0.23 | 23.1 | none | 0.92 |
| OC use | ever | never | 3 | 3 | 0 | 1.15 (0.71 – 1.88) | <0.001 | 89.7 | Dorjgochoo et al. (40), Freedman et al. (41), and Chung et al. (42). | 0.10 |
| HRT | ever | never | 4 | 3 | 0 | 0.77 (0.64 – 0.92) | 0.99 | 0 | none | 0.95 |
| Tamoxifen treatment | treated | untreated | 5 | 0 | 9 | 1.82 d (1.39 – 2.38) | 0.41 | 0 | N/A | N/A |
Abbreviations: PQ, p from Q statistics; PEgger’s, p from Egger’s test; CI, confidence interval; RCT, randomized clinical trials; OC, oral contraceptives; HRT, hormone replacement therapy; N/A: non-applicable.
Reference category.
Studies identified based on visual inspection of Galbraith plots.
Excludes the study by Inoue et al. (30) which reported a linear trend without stratum-specific estimates.
Based on the marginal RR estimates for cohort studies and RCT.
Age at menarche
Associations of gastric cancer with age at menarche were reported in 11 studies (11, 26, 27, 29, 30, 35–38, 41, 42). Study-specific RRs for the oldest age at menarche as compared to the youngest age ranged from 0.70 to 1.93, and the pooled RR was 1.0 (Table 3). Between-study heterogeneity was high, but meta-regression analysis of potential explanatory factors failed to explain the variability. A Galbraith plot (not shown) indicated the studies by Frise et al. (35) and Persson et al. (38) as outliers contributing to this heterogeneity. The pooled RR derived with exclusion of those studies was 0.89 (95% CI= 0.80 to 1.0). Notably, analysis restricted to the same set of studies (n=8) included in the meta-analysis of years of fertility (including data from Kaneko et al. (31) instead of Sakauchi et al. (37)), had a pooled RR of 1.08 (95% CI= 0.86 to 1.35) for oldest age at menarche as compared to the youngest age, similar to the effect based on all 11 studies.
Age at menopause
Ten studies examined the association of gastric cancer and age at menopause (11, 26, 27, 29, 30, 35–38, 41). Study-specific RRs for the oldest age at menopause as compared to the youngest ranged from 0.52 to 1.44. The pooled RR was 0.84 (95% CI= 0.67 to 1.05), with low heterogeneity across studies. This estimate was robust to the exclusion of any individual study. The pooled RR was 0.81 (95% CI= 0.62 to 1.06) for the eight studies (including data from Kaneko et al. (31) instead of Sakauchi et al. (37)) that also reported on years of fertility.
Parity
Twelve studies provided information on parity (11, 26, 27, 29, 35–39, 41–43), with study-specific RRs for highest number of full-term pregnancies in comparison to the lowest ranging from 0.52 to 1.90. The summary RR was 0.94 (95% CI= 0.74 to 1.19). For the five studies (11, 26, 35, 38, 41) that used nulliparous women as the reference group the pooled RR was 0.95 (95% CI= 0.66 to 1.38; I2=56.3%), whereas for the other seven that used a parous comparison group (either 1 child or 1–2 children), the pooled RR was 0.93 (95% CI= 0.68 to 1.27; I2=89.3%). High heterogeneity was detected among all 12 studies, but there were no significant explanatory variables in meta-regression analysis. A Galbraith plot (not shown) identified the studies by La Vecchia et al. (26), Chung et al. (42), and Chang et al. (43) as outliers contributing to between-study heterogeneity. In an analysis excluding those three studies, the pooled RR was essentially unchanged at 0.96 (95% CI= 0.85 to 1.07).
Age at first birth
Risk estimates for oldest versus youngest age at first birth were reported in 10 studies (11, 26, 27, 29, 30, 36–39, 41) and ranged from 0.43 to 1.45. The pooled RR was 0.99 (95% CI= 0.85 to 1.15), with low heterogeneity among the studies (Table 3). This estimate was robust to the exclusion of any individual study.
Oral contraceptive use
Risk estimates for ever vs. never OC use were reported in four studies (26, 35, 40, 42) and ranged from 0.79 to 2.50. In addition, the studies by Duell et al. (11) and Freedman et al. (41) reported two to three RRs depending on duration of use, which we pooled to obtain overall RRs for ever use of 1.18 (95% CI= 0.89 to 1.56) for Duell et al. (11) and 0.85 (95% CI= 0.54 to 1.34) for Freedman et al. (41). The proportion of OC users ranged from 3% in a study from Italy using data collected before 1993 (26) to 55% in the European Prospective Investigation into Cancer and Nutrition Study which included women enrolled from 1992 to 1998 (11). The overall pooled RR of gastric cancer for ever users versus never users of OC was 1.15 (95% CI=0.71 to 1.88). We found high heterogeneity among the studies, but none of the available variables significantly explained this variation. A Galbraith plot (not shown) indicated the studies by Dorjgochoo et al. (40), Freedman et al. (41), and Chung et al. (42) as outliers contributing to this heterogeneity. The pooled RR derived with exclusion of those studies was 1.11 (95% CI= 0.87 to 1.42).
Hormone replacement therapy
Figure 2B represents a forest plot of the effect size distribution for the seven studies that reported on post-menopausal HRT (11, 32, 35–37, 41, 44). The proportion of HRT users ranged from 4% in China (36) to 55% in the US (41). The pooled RR of gastric cancer for ever users of HRT as compared to never users was 0.77 (95% CI=0.64 to 0.92), and there was low heterogeneity among all studies. The average pooled RR was robust to the exclusion of any one study from the overall meta-analysis. In a sensitivity analysis restricted to the four studies that adjusted for a proxy variable of SES (11, 32, 35, 36), the point pooled RR was minimally changed but statistical significance was lost (RR=0.80; 95% CI= 0.60 to 1.06).
Tamoxifen therapy
Table 2 summarizes studies with data for comparison of primary gastric cancer incidence among women treated or untreated with tamoxifen. Nine randomized controlled trials including 33,329 patients reported a total of 19 gastric cancer cases in the tamoxifen arms and 14 in the control arms. Five separate observational cohort studies reported combined incidence rates of 0.57 and 0.30 gastric cancers per 1,000 patient-years in the tamoxifen-treated and -untreated groups, respectively. Thus, tamoxifen treatment was associated with a non-significantly increased risk in the randomized trials (RR= 1.35; 95% CI= 0.64 to 2.92) and a significantly increased risk in the observational studies (RR= 1.90; 95% CI= 1.41 to 2.52). A meta-analysis of these two marginal RRs (with inverse variance weights of 13% and 87%, respectively) found a significantly increased gastric cancer risk among women treated with tamoxifen (RR= 1.82; 95% CI=1.39 to 2.38).
Publication bias
The p-values for Egger’s test of publication bias were greater than 0.1 for all exposure variables with the exception of OC use (P= 0.10; Table 3). Figure 2 presents Begg’s funnel plots for years of fertility (2C) and HRT (2D), the two variables found to be significantly associated with gastric cancer risk.
DISCUSSION
Although much has been learned about the epidemiology of gastric cancer, it is still unclear why males have higher risk than females. Our meta-analysis identified decreased gastric cancer risks among women with longer duration of fertility or exposure to HRT, and increased risk with exposure to the antiestrogenic agent tamoxifen. However, we found no significant associations with age at menarche, age at menopause, parity, age at first birth or OC use. On balance, these findings support the notion that estrogen exposure influences the risk of gastric cancer in women.
Given the narrow range of age at menarche, variation of years of fertility is mainly determined by age at menopause. Accordingly, we expected similar associations of gastric cancer with these latter two variables, at least with restriction to the common set of eight studies in which both variables were reported. A potential explanation for the discrepancy in our results, not addressable with aggregated data, may be inconsistency between these variables in categorizing individuals as having high (or low) exposure within a given study.
The other null associations of our analysis may also be understood in context. OC use is not a strong risk factor for breast cancer, an estrogen-driven malignancy (64, 65). Hence, it may not be surprising that OC use does not appear to be associated with gastric cancer risk, for which estrogen exposure presumably has a smaller role. Furthermore, parity and age at first birth do not have clear interpretations regarding quantitative exposure to estrogen, so the failure to find significant associations with these variables is less relevant to the estrogen hypothesis.
The studies we included vary with respect to the factors controlled in the original analyses. Although we used the reported multivariable adjusted RRs where available, there may have been residual confounding. In the case of HRT, it is possible that postmenopausal women who used hormone therapy may have differed from never users in ways that influence their risk of gastric cancer. Nevertheless, the four studies that adjusted for these differences with a proxy variable for SES had a similar pooled RR as all seven studies of HRT. Thus, confounding by SES would not explain the association between HRT use and gastric cancer, to the extent that these proxies adequately controlled for SES differences without residual confounding.
As a selective estrogen receptor modulator, tamoxifen has both anti-estrogenic (e.g., breast tissue) and estrogenic (e.g., bone) effects (66). In a mouse model of gastric cancer, tamoxifen upregulated estrogen-responsive pathways and prevented gastric cancer development (67). We found an opposite effect on risk of gastric cancer in humans, which may speculatively reflect species differences in gastric epithelial susceptibility to the dual tamoxifen effects. Additional potential explanations for the inconsistency between animal and epidemiological studies include differences in relative age, dose and duration of treatment as well as drug metabolism.
Chronic infection with H. pylori is the primary cause of gastric cancer, and this bacterium is designated a Class I carcinogen by the World Health Organization (68). Sex differences in age at acquisition and infection prevalence have been proposed as potential explanations for differences in gastric cancer incidence between males and females (3, 69, 70). Indeed, a meta-analysis of international population-based surveys (71) found that males had slightly higher infection prevalence among adults (adjusted OR= 1.16), but not among children. Studies measuring spontaneous clearance of H. pylori infection by sex have varied, with some studies indicating slightly higher seroreversion rates for women than men (72–74) while others found similar rates (75–77). Regarding therapeutic eradication, no significant variation by sex has been reported. In sum, the small magnitude of these sex differences in H. pylori acquisition and clearance cannot fully explain the 2:1 incidence gap.
Steroid-based molecules are incorporated by H. pylori into its membrane lipids and differ in their potential effects on bacterial survival (78, 79). Free-cholesterol, for example, is glucosylated after incorporation into H. pylori and acts to inhibit specific T cell responses (80). In vitro, estradiol is bacteriostatic while progesterone and androstendione are bactericidal (81). In H. pylori infected insulin-gastrin transgenic (INS-GAS) male mice, estradiol supplementation results in decreased expression of IFNG, TNFA, and IL1B, and increased expression of IL10 in the epithelial mucosa. Interestingly, these effects are associated with attenuation of gastric lesions, and in some models protect against the development of cancer (67, 82, 83). In addition, infected mice treated with estradiol have reduced gastric mRNA expression and serum levels of the neutrophil chemo-attractant CXCL1 (67), suggesting that estradiol may limit mucosal injury caused by activated netrophils. Another study based on a chemically induced model of gastric cancer found that estrogen-treated male rats, as well as female rats, have a lower risk than non-treated male controls (84).
There are several lines of evidence that estrogens may protect against gastric cancer: 1) estrogens interact with receptors in normal, precancerous and cancerous gastric cells (85, 86), which could regulate the growth and clonal expansion of these cells; 2) CpG islands in the estrogen receptor gene promoters become hypermethylated with aging, leading to reduced expression with effects on tumor suppressor activity (87); 3) estrogens increase expression of trefoil factor family genes, which encode products that protect gastric mucosa from endogenous and exogenous insults (88); 4) estrogens increase apoptosis in human gastric cancer cells in vitro (89); 5) estrogens increase the strength of the immune response to bacterial pathogens by directly blocking expression of caspase-12 (90); 6) estrogens retard cell migration after simulated “epithelial wounding” in primary cultured cells and particularly in cancer cell lines (91); 7) high concentrations of plasma isoflavones from phytoestrogens are associated with decreased risk of gastric cancer (92); 8) polymorphisms in genes involved in estrogen inactivation and hormone bioavailability have been associated with gastric cancer risk (93); and 9) men with prostate cancer potentially exposed to therapeutic exogenous estrogens had a reduced incidence of gastric cancer as compared to an age-matched reference population (94).
Smoking may facilitate persistence of H. pylori infection (95), increases risk of eradication failure (96), and is considered to have a causal role in the development of gastric cancer (97). Thus, sex differences in smoking patterns may contribute to the male predominance of gastric cancer incidence. However, Freedman et al. (5) found roughly similar male/female ratios for cancer incidence among smokers and nonsmokers, suggesting that the difference in smoking does not entirely explain the marked sex difference in gastric cancer risk.
About 9% of gastric cancers harbor Epstein-Barr virus (EBV) infection (98, 99). Furthermore, tumors in males are more than twice as likely to be EBV-positive than tumors in females. Given this sex difference in incidence rates overall, the 2-fold sex difference in EBV positivity implies that the incidence of EBV-positive gastric cancer is four times higher in males than females.
Differences in diet between men and women might also be related to sex differences in gastric cancer incidence. In particular, low consumption of fresh fruits and vegetables may increase the risk of noncardia tumors (100), and some studies have suggested that women eat fruits and vegetables both more frequently and in greater quantities than men (101, 102). Other factors that may potentially explain the higher risk of gastric cancer among males as compared to females are differences in medication and occupational exposures.
Our analysis was limited by the inconsistent categorization of the exposure variables, particularly those with more than two strata. As with any meta-analysis, we cannot exclude the possibility that other studies may have been missed during our literature search, or that studies that observed null effects were absent from the literature altogether. Nevertheless, we found little evidence of publication bias. A greater potential concern regarding data completeness is that some of the published studies on HRT or tamoxifen did not specifically report incidence of gastric cancer, and many registered tamoxifen trials are still unpublished (7).
Our inability to detect significant between-study heterogeneity may be due to the insensitivity of the Q statistic and/or limited sample sizes. Furthermore, insufficient data precluded analyses for histologic and anatomic subtypes, which might have varying associations with the reviewed exposures. We were also unable to evaluate HRT formulation (unopposed estrogen versus estrogen plus progesterone compounds) and duration of therapy.
Our finding about tamoxifen primarily reflects observational studies with unmeasured confounding of treatment assignment. Nevertheless, limited data from randomized controlled trials was consistent. The analyses of both the randomized trial and the observational cohort data were hampered by inclusion of groups with zero events, which we addressed by marginal analyses. While this analytic approach has recognized limitations (103), alternative approaches such as continuity corrections also have drawbacks (104). Furthermore, we could not account for the differences in dose and duration of tamoxifen treatment among studies. Thus, these results should be interpreted with caution.
We restricted our meta-analysis to associations with overt gastric cancer. However, given the recognized multistep process of gastric carcinogenesis (105), it is necessary to consider how estrogens might influence earlier stages such as intestinal metaplasia and dysplasia. Direct assessment of estrogens would be additionally informative, as studies to date are almost exclusively based on surrogate measures. Furthermore, the effect of other selective estrogen receptor modulating drugs on gastric carcinogenesis could be usefully examined.
In conclusion, our results are consistent with the hypothesis that estrogen’s effects may lower the risk of gastric cancer in women. Further studies are needed to extend these observations and identify the biologic bases of this epidemiologic association. Better understanding of how sex differences influence carcinogenesis would provide important insights into gastric cancer etiology.
ACKNOWLEDGEMENTS
The authors thank Nancy Terry, Biomedical Librarian at the U.S. National Institutes of Health Library for her help with reviewing the search strategies.
GRANT SUPPORT
This study was funded by the Intramural Research Program of the U.S. National Cancer Institute, National Institutes of Health.
Footnotes
All authors declare they have no conflicts of interest regarding this study.
REFERENCES
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Griffith GW. The sex ratio in gastric cancer and hypothetical considerations relative to aetiology. Br J Cancer. 1968;22:163–172. doi: 10.1038/bjc.1968.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sipponen P, Correa P. Delayed rise in incidence of gastric cancer in females results in unique sex ratio (M/F) pattern: etiologic hypothesis. Gastric Cancer. 2002;5:213–219. doi: 10.1007/s101200200037. [DOI] [PubMed] [Google Scholar]
- 4.Lindblad M, Rodríguez LA, Lagergren J. Body mass, tobacco and alcohol and risk of esophageal, gastric cardia, and gastric non-cardia adenocarcinoma among men and women in a nested case-control study. Cancer Causes Control. 2005;16:285–294. doi: 10.1007/s10552-004-3485-7. [DOI] [PubMed] [Google Scholar]
- 5.Freedman ND, Derakhshan MH, Abnet CC, Schatzkin A, Hollenbeck AR, McColl KE. Male predominance of upper gastrointestinal adenocarcinoma cannot be explained by differences in tobacco smoking in men versus women. Eur J Cancer. 2010;46:2473–2478. doi: 10.1016/j.ejca.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandanos E, Lagergren J. Oestrogen and the enigmatic male predominance of gastric cancer. Eur J Cancer. 2008;44:2397–2403. doi: 10.1016/j.ejca.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 7.Early Breast Cancer Trialists' Collaborative Group. Tamoxifen for early breast cancer. Cochrane Database Syst Rev. 2001;1 CD000486. [Google Scholar]
- 8.Braithwaite RS, Chlebowski RT, Lau J, George S, Hess R, Col NF. Meta-analysis of vascular and neoplastic events associated with tamoxifen. J Gen Intern Med. 2003;18:937–947. doi: 10.1046/j.1525-1497.2003.20724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chandanos E, Lindblad M, Jia C, Rubio CA, Ye W, Lagergren J. Tamoxifen exposure and risk of oesophageal and gastric adenocarcinoma: a population-based cohort study of breast cancer patients in Sweden. Br J Cancer. 2006;95:118–122. doi: 10.1038/sj.bjc.6603214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mellemkjaer L, Friis S, Olsen JH, Scélo G, Hemminki K, Tracey E, et al. Risk of second cancer among women with breast cancer. Int J Cancer. 2006;118:2285–2292. doi: 10.1002/ijc.21651. [DOI] [PubMed] [Google Scholar]
- 11.Duell EJ, Travier N, Lujan-Barroso L, Boutron-Ruault MC, Clavel-Chapelon F, Palli D, et al. Menstrual and reproductive factors, exogenous hormone use, and gastric cancer risk in a cohort of women from the European Prospective Investigation Into Cancer and Nutrition. Am J Epidemiol. 2010;172:1384–1393. doi: 10.1093/aje/kwq321. [DOI] [PubMed] [Google Scholar]
- 12.Bradburn MJ. Updated and New Commands for Meta-Analysis in STATA. 2004. Cancer Research UK Medical Statistics Group. Oxford: Centre for Statistics in Medicine; [accessed 20 May 2011]. http://www.medepi.net/meta/software/Bradburn_metan_updates.pdf. [Google Scholar]
- 13.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galbraith RF. Graphical Display of Estimates Having Differing Standard Errors. Technometrics. 1988;30:271–281. [Google Scholar]
- 17.Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354–362. doi: 10.3748/wjg.v12.i3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finley C, Gregg EW, Solomon LJ, Gay E. Disparities in hormone replacement therapy use by socioeconomic status in a primary care population. J Community Health. 2001;26:39–50. doi: 10.1023/a:1026537114638. [DOI] [PubMed] [Google Scholar]
- 19.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 20.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sterne JAC, editor. Meta-Analysis in Stata: An Updated Collection from the Stata Journal. Stata Press; 2009. [Google Scholar]
- 22.Miller AB, Barclay TH, Choi NW, Grace MG, Wall C, Plante M, et al. A study of cancer, parity and age at first pregnancy. J Chronic Dis. 1980;33:595–605. doi: 10.1016/0021-9681(80)90002-8. [DOI] [PubMed] [Google Scholar]
- 23.Plesko I, Preston-Martin S, Day NE, Tzonou A, Dimitrova E, Somogyi J. Parity and cancer risk in Slovakia. Int J Cancer. 1985;36:529–533. doi: 10.1002/ijc.2910360502. [DOI] [PubMed] [Google Scholar]
- 24.Tsukuma H, Fujimoto I, Furukawa H, Iwanaga T, Okuda S, Tatsuta M, et al. A case-control study of stomach cancer in young females, with special reference to the effects of pregnancy and delivery. Gan No Rinsho. 1989;35:35–40. [Article in Japanese] [PubMed] [Google Scholar]
- 25.La Vecchia C, Negri E, Franceschi S, Parazzini F. Long-term impact of reproductive factors on cancer risk. Int J Cancer. 1993;53:215–219. doi: 10.1002/ijc.2910530207. [DOI] [PubMed] [Google Scholar]
- 26.La Vecchia C, D'Avanzo B, Franceschi S, Negri E, Parazzini F, Decarli A. Menstrual and reproductive factors and gastric-cancer risk in women. Int J Cancer. 1994;59:761–764. doi: 10.1002/ijc.2910590609. [DOI] [PubMed] [Google Scholar]
- 27.Palli D, Cipriani F, Decarli A, Galli M, Saieva C, Fraumeni JF, Jr, et al. Reproductive history and gastric cancer among post-menopausal women. Int J Cancer. 1994;56:812–815. doi: 10.1002/ijc.2910560609. [DOI] [PubMed] [Google Scholar]
- 28.Kvåle G, Heuch I, Nilssen S. Parity in relation to mortality and cancer incidence: a prospective study of Norwegian women. Int J Epidemiol. 1994;23:691–699. doi: 10.1093/ije/23.4.691. [DOI] [PubMed] [Google Scholar]
- 29.Heuch I, Kvåle G. Menstrual and reproductive factors and risk of gastric cancer: a Norwegian cohort study. Cancer Causes Control. 2000;11:869–874. doi: 10.1023/a:1008975817061. [DOI] [PubMed] [Google Scholar]
- 30.Inoue M, Ito LS, Tajima K, Yamamura Y, Kodera Y, Takezaki T, et al. Height, weight, menstrual and reproductive factors and risk of gastric cancer among Japanese postmenopausal women: analysis by subsite and histologic subtype. Int J Cancer. 2002;97:833–838. doi: 10.1002/ijc.10149. [DOI] [PubMed] [Google Scholar]
- 31.Kaneko S, Tamakoshi A, Ohno Y, Mizoue T, Yoshimura T JACC Study Group. Menstrual and reproductive factors and the mortality risk of gastric cancer in Japanese menopausal females. Cancer Causes Control. 2003;14:53–59. doi: 10.1023/a:1022596104796. [DOI] [PubMed] [Google Scholar]
- 32.Fernandez E, Gallus S, Bosetti C, Franceschi S, Negri E, La Vecchia C. Hormone replacement therapy and cancer risk: a systematic analysis from a network of case-control studies. Int J Cancer. 2003;105:408–412. doi: 10.1002/ijc.11083. [DOI] [PubMed] [Google Scholar]
- 33.Heuch I, Kvåle G. Does breastfeeding affect the risk of gastric cancer? Int J Cancer. 2003;106:982–983. doi: 10.1002/ijc.11291. [DOI] [PubMed] [Google Scholar]
- 34.Lindblad M, García Rodríguez LA, Chandanos E, Lagergren J. Hormone replacement therapy and risks of oesophageal and gastric adenocarcinomas. Br J Cancer. 2006;94:136–141. doi: 10.1038/sj.bjc.6602906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frise S, Kreiger N, Gallinger S, Tomlinson G, Cotterchio M. Menstrual and reproductive risk factors and risk for gastric adenocarcinoma in women: findings from the canadian national enhanced cancer surveillance system. Ann Epidemiol. 2006;16:908–916. doi: 10.1016/j.annepidem.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 36.Freedman ND, Chow WH, Gao YT, Shu XO, Ji BT, Yang G, et al. Menstrual and reproductive factors and gastric cancer risk in a large prospective study of women. Gut. 2007;56:1671–1677. doi: 10.1136/gut.2007.129411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakauchi F. Japan Collaborative Cohort Study for Evaluation of Cancer. Reproductive history and health screening for women and mortality in the Japan Collaborative Cohort Study for Evaluation of Cancer (JACC) Asian Pac J Cancer Prev. 2007;8(Suppl):129–134. [PubMed] [Google Scholar]
- 38.Persson C, Inoue M, Sasazuki S, Kurahashi N, Iwasaki M, Ye W, et al. Female reproductive factors and the risk of gastric cancer in a large-scale population-based cohort study in Japan (JPHC study) Eur J Cancer Prev. 2008;17:345–353. doi: 10.1097/CEJ.0b013e3282f521e4. [DOI] [PubMed] [Google Scholar]
- 39.Bahmanyar S, Lambe M, Zendehdel K, Nyrén O, Boffetta P, Ye W. Parity and risk of stomach cancer by sub-site: a national Swedish study. Br J Cancer. 2008;98:1295–1300. doi: 10.1038/sj.bjc.6604283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dorjgochoo T, Shu XO, Li HL, Qian HZ, Yang G, Cai H, et al. Use of oral contraceptives, intrauterine devices and tubal sterilization and cancer risk in a large prospective study, from 1996 to 2006. Int J Cancer. 2009;124:2442–2449. doi: 10.1002/ijc.24232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freedman ND, Lacey JV, Jr, Hollenbeck AR, Leitzmann MF, Schatzkin A, Abnet CC. The association of menstrual and reproductive factors with upper gastrointestinal tract cancers in the NIH-AARP cohort. Cancer. 2010;116:1572–1581. doi: 10.1002/cncr.24880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chung HW, Noh SH, Lim JB. Analysis of demographic characteristics in 3242 young age gastric cancer patients in Korea. World J Gastroenterol. 2010;16:256–263. doi: 10.3748/wjg.v16.i2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chang CC, Chen CC, Chiu HF, Yang CY. Higher parity associated with higher risk of death from gastric cancer. World J Gastroenterol. 2011;17:784–788. doi: 10.3748/wjg.v17.i6.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Green J, Czanner G, Reeves G, Watson J, Wise L, Roddam A, et al. Menopausal hormone therapy and risk of gastrointestinal cancer: Nested case-control study within a prospective cohort, and meta-analysis. Int J Cancer. 2011 Jun 13; doi: 10.1002/ijc.26236. [DOI] [PubMed] [Google Scholar]
- 45.Andersson M, Storm HH, Mouridsen HT. Incidence of new primary cancers after adjuvant tamoxifen therapy and radiotherapy for early breast cancer. J Natl Cancer Inst. 1991;83:1013–1017. doi: 10.1093/jnci/83.14.1013. [DOI] [PubMed] [Google Scholar]
- 46.Andersson M, Jensen MB, Engholm G, Henrik Storm H. Risk of second primary cancer among patients with early operable breast cancer registered or randomised in Danish Breast Cancer cooperative Group (DBCG) protocols of the 77, 82 and 89 programmes during 1977–2001. Acta Oncol. 2008;47:755–764. doi: 10.1080/02841860801978921. [DOI] [PubMed] [Google Scholar]
- 47.Ribeiro G, Swindell R. The Christie hospital adjuvant tamoxifen trial--status at 10 years. Br J Cancer. 1988;57:601–603. doi: 10.1038/bjc.1988.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ribeiro G, Swindell R. The Christie Hospital adjuvant tamoxifen trial. J Natl Cancer Inst Monogr. 1992;11:121–125. [PubMed] [Google Scholar]
- 49.Fisher B, Costantino JP, Redmond CK, Fisher ER, Wickerham DL, Cronin WM. Endometrial cancer in tamoxifen-treated breast cancer patients: findings from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14. J Natl Cancer Inst. 1994;86:527–537. doi: 10.1093/jnci/86.7.527. [DOI] [PubMed] [Google Scholar]
- 50.Fisher B, Dignam J, Bryant J, DeCillis A, Wickerham DL, Wolmark N, et al. Five versus more than five years of tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen receptor-positive tumors. J Natl Cancer Inst. 1996;88:1529–1542. doi: 10.1093/jnci/88.21.1529. [DOI] [PubMed] [Google Scholar]
- 51.Fornander T, Rutqvist LE, Cedermark B, Glas U, Mattsson A, Skoog L, et al. Adjuvant tamoxifen in early-stage breast cancer: effects on intercurrent morbidity and mortality. J Clin Oncol. 1991;9:1740–1748. doi: 10.1200/JCO.1991.9.10.1740. [DOI] [PubMed] [Google Scholar]
- 52.Rutqvist LE, Johansson H, Signomklao T, Johansson U, Fornander T, Wilking N. Adjuvant tamoxifen therapy for early stage breast cancer and second primary malignancies. Stockholm Breast Cancer Study Group. J Natl Cancer Inst. 1995;87:645–651. doi: 10.1093/jnci/87.9.645. [DOI] [PubMed] [Google Scholar]
- 53.Curtis RE, Boice JD, Jr, Shriner DA, Hankey BF, Fraumeni JF., Jr Second cancers after adjuvant tamoxifen therapy for breast cancer. J Natl Cancer Inst. 1996;88:832–834. doi: 10.1093/jnci/88.12.832. [DOI] [PubMed] [Google Scholar]
- 54.Newcomb PA, Solomon C, White E. Tamoxifen and risk of large bowel cancer in women with breast cancer. Breast Cancer Res Treat. 1999;53:271–277. doi: 10.1023/a:1006117220284. [DOI] [PubMed] [Google Scholar]
- 55.Rydén S, Fernö M, Möller T, Aspegren K, Bergljung L, Killander D, et al. Long-term effects of adjuvant tamoxifen and/or radiotherapy. The South Sweden Breast Cancer Trial. Acta Oncol. 1992;31:271–274. doi: 10.3109/02841869209088914. [DOI] [PubMed] [Google Scholar]
- 56.Cummings FJ, Gray R, Tormey DC, Davis TE, Volk H, Harris J, et al. Adjuvant tamoxifen versus placebo in elderly women with node-positive breast cancer: long-term follow-up and causes of death. J Clin Oncol. 1993;11:29–35. doi: 10.1200/JCO.1993.11.1.29. [DOI] [PubMed] [Google Scholar]
- 57.Rivkin SE, Green S, Metch B, Cruz AB, Abeloff MD, Jewell WR, et al. Adjuvant CMFVP versus tamoxifen versus concurrent CMFVP and tamoxifen for postmenopausal, node-positive, and estrogen receptor-positive breast cancer patients: a Southwest Oncology Group study. J Clin Oncol. 1994;12:2078–2085. doi: 10.1200/JCO.1994.12.10.2078. [DOI] [PubMed] [Google Scholar]
- 58.Cuzick J, Forbes J, Edwards R, Baum M, Cawthorn S, Coates A, et al. First results from the International Breast Cancer Intervention Study (IBIS-I): a randomised prevention trial. Lancet. 2002;360:817–824. doi: 10.1016/s0140-6736(02)09962-2. [DOI] [PubMed] [Google Scholar]
- 59.Fisher B, Costantino JP, Wickerham DL, Cecchini RS, Cronin WM, Robidoux A, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652–1662. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 60.Veronesi U, Maisonneuve P, Rotmensz N, Bonanni B, Boyle P, Viale G, et al. Tamoxifen for the prevention of breast cancer: late results of the Italian Randomized Tamoxifen Prevention Trial among women with hysterectomy. J Natl Cancer Inst. 2007;99:727–737. doi: 10.1093/jnci/djk154. [DOI] [PubMed] [Google Scholar]
- 61.Matsuyama Y, Tominaga T, Nomura Y, Koyama H, Kimura M, Sano M, et al. Second cancers after adjuvant tamoxifen therapy for breast cancer in Japan. Ann Oncol. 2000;11:1537–1543. doi: 10.1093/oxfordjournals.annonc.a010406. [DOI] [PubMed] [Google Scholar]
- 62.Ursic-Vrscaj M, Kovacic J, Bebar S, Djurisic A, Fras PA, Robic V. Endometrial and other primary cancers after tamoxifen treatment of breast cancer -- results of retrospective cohort study. Eur J Obstet Gynecol Reprod Biol. 2001;95:105–110. doi: 10.1016/s0301-2115(00)00376-6. [DOI] [PubMed] [Google Scholar]
- 63.Fowble B, Hanlon A, Freedman G, Nicolaou N, Anderson P. Second cancers after conservative surgery and radiation for stages I-II breast cancer: identifying a subset of women at increased risk. Int J Radiat Oncol Biol Phys. 2001;51:679–690. doi: 10.1016/s0360-3016(01)01665-0. [DOI] [PubMed] [Google Scholar]
- 64.Romieu I, Berlin JA, Colditz G. Oral contraceptives and breast cancer. Review and meta-analysis. Cancer. 1990;66:2253–2263. doi: 10.1002/1097-0142(19901201)66:11<2253::aid-cncr2820661102>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 65.Kahlenborn C, Modugno F, Potter DM, Severs WB. Oral contraceptive use as a risk factor for premenopausal breast cancer: a meta-analysis. Mayo Clin Proc. 2006;81:1290–1302. doi: 10.4065/81.10.1290. [DOI] [PubMed] [Google Scholar]
- 66.Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med. 1998;339:1609–1618. doi: 10.1056/NEJM199811263392207. [DOI] [PubMed] [Google Scholar]
- 67.Sheh A, Ge Z, Parry NM, Muthupalani S, Rager JE, Raczynski AR, et al. 17(lowercase beta)-estradiol and Tamoxifen prevent gastric cancer by modulating leukocyte recruitment and oncogenic pathways in Helicobacter pylori-infected INS-GAS male mice. Cancer Prev Res (Phila) 2011 Jun 16; doi: 10.1158/1940-6207.CAPR-11-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.IARC Monographs on the evaluation of carcinogenic risks to humans, Schistosomes, liver flukes and Helicobacter pylori. Vol. 61. IARC Press; 1994. pp. 218–220. [PMC free article] [PubMed] [Google Scholar]
- 69.Goodman KJ, Correa P, Tenganá Aux HJ, Ramírez H, DeLany JP, Guerrero Pepinosa O, et al. Helicobacter pylori infection in the Colombian Andes: a population-based study of transmission pathways. Am J Epidemiol. 1996;144:290–299. doi: 10.1093/oxfordjournals.aje.a008924. [DOI] [PubMed] [Google Scholar]
- 70.Replogle ML, Glaser SL, Hiatt RA, Parsonnet J. Biologic sex as a risk factor for Helicobacter pylori infection in healthy young adults. Am J Epidemiol. 1995;142:856–863. doi: 10.1093/oxfordjournals.aje.a117725. [DOI] [PubMed] [Google Scholar]
- 71.de Martel C, Parsonnet J. Helicobacter pylori infection and gender: a meta-analysis of population-based prevalence surveys. Dig Dis Sci. 2006;51:2292–2301. doi: 10.1007/s10620-006-9210-5. [DOI] [PubMed] [Google Scholar]
- 72.Klein PD, Gilman RH, Leon-Barua R, Diaz F, Smith EO, Graham DY. The epidemiology of Helicobacter pylori in Peruvian children between 6 and 30 months of age. Am J Gastroenterol. 1994;89:2196–2200. [PubMed] [Google Scholar]
- 73.Kikuchi S, Ohgihara A, Hasegawa A, Miki K, Kaneko E, Mizukoshi H. Seroconversion and seroreversion of Helicobacter pylori antibodies over a 9-year period and related factors in Japanese adults. Helicobacter. 2004;9:335–341. doi: 10.1111/j.1083-4389.2004.00233.x. [DOI] [PubMed] [Google Scholar]
- 74.Fawcett JP, Barbezat GO, Poulton R, Milne BJ, Xia HH, Talley NJ. Helicobacter pylori serology in a birth cohort of New Zealanders from age 11 to 26. World J Gastroenterol. 2005;11:3273–3276. doi: 10.3748/wjg.v11.i21.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ashorn M, Mäki M, Hällström M, Uhari M, Akerblom HK, Viikari J, et al. Helicobacter pylori infection in Finnish children and adolescents. A serologic cross-sectional and follow-up study. Scand J Gastroenterol. 1995;30:876–879. doi: 10.3109/00365529509101594. [DOI] [PubMed] [Google Scholar]
- 76.Rehnberg-Laiho L, Rautelin H, Valle M, Kosunen TU. Persisting Helicobacter antibodies in Finnish children and adolescents between two and twenty years of age. Pediatr Infect Dis J. 1998;17:796–799. doi: 10.1097/00006454-199809000-00009. [DOI] [PubMed] [Google Scholar]
- 77.Rosenstock S, Jørgensen T, Andersen L, Bonnevie O. Seroconversion and seroreversion in IgG antibodies to Helicobacter pylori: a serology based prospective cohort study. J Epidemiol Community Health. 2000;54:444–450. doi: 10.1136/jech.54.6.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hosoda K, Shimomura H, Hayashi S, Yokota K, Oguma K, Hirai Y. Anabolic utilization of steroid hormones in Helicobacter pylori. FEMS Microbiol Lett. 2009;297:173–179. doi: 10.1111/j.1574-6968.2009.01685.x. [DOI] [PubMed] [Google Scholar]
- 79.Shimomura H, Hosoda K, Hayashi S, Yokota K, Oguma K, Hirai Y. Steroids mediate resistance to the bactericidal effect of phosphatidylcholines against Helicobacter pylori. FEMS Microbiol Lett. 2009;301:84–94. doi: 10.1111/j.1574-6968.2009.01807.x. [DOI] [PubMed] [Google Scholar]
- 80.Wunder C, Churin Y, Winau F, Warnecke D, Vieth M, Lindner B, et al. Cholesterol glucosylation promotes immune evasion by Helicobacter pylori. Nat Med. 2006;12:1030–1038. doi: 10.1038/nm1480. [DOI] [PubMed] [Google Scholar]
- 81.Hosoda K, Shimomura H, Hayashi S, Yokota K, Hirai Y. Steroid hormones as bactericidal agents to Helicobacter pylori. FEMS Microbiol Lett. 2011;318:68–75. doi: 10.1111/j.1574-6968.2011.02239.x. [DOI] [PubMed] [Google Scholar]
- 82.Ohtani M, García A, Rogers AB, Ge Z, Taylor NS, Xu S, et al. Protective role of 17 beta -estradiol against the development of Helicobacter pylori-induced gastric cancer in INS-GAS mice. Carcinogenesis. 2007;28:2597–2604. doi: 10.1093/carcin/bgm150. [DOI] [PubMed] [Google Scholar]
- 83.Ohtani M, Ge Z, García A, Rogers AB, Muthupalani S, Taylor NS, et al. 17{beta}-Estradiol suppresses Helicobacter pylori-induced gastric pathology in male hypergastrinemic INS-GAS mice. Carcinogenesis. 2011 Jul 4; doi: 10.1093/carcin/bgr072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Furukawa H, Iwanaga T, Koyama H, Taniguchi H. Effect of sex hormones on the experimental induction of cancer in rat stomach - a preliminary study. Digestion. 1982;23:151–155. doi: 10.1159/000198722. [DOI] [PubMed] [Google Scholar]
- 85.Tokunaga A, Kojima N, Andoh T, Matsukura N, Yoshiyasu M, Tanaka N, et al. Hormone receptors in gastric cancer. Eur J Cancer Clin Oncol. 1983;19:687–689. doi: 10.1016/0277-5379(83)90186-4. [DOI] [PubMed] [Google Scholar]
- 86.Matsuyama S, Ohkura Y, Eguchi H, Kobayashi Y, Akagi K, Uchida K, et al. Estrogen receptor beta is expressed in human stomach adenocarcinoma. J Cancer Res Clin Oncol. 2002;128:319–324. doi: 10.1007/s00432-002-0336-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhao YF, Zhang YG, Tian XX, Juan Du, Jie Zheng. Aberrant methylation of multiple genes in gastric carcinomas. Int J Surg Pathol. 2007;15:242–251. doi: 10.1177/1066896907302117. [DOI] [PubMed] [Google Scholar]
- 88.Taupin D, Podolsky DK. Trefoil factors: initiators of mucosal healing. Nat Rev Mol Cell Biol. 2003;4:721–732. doi: 10.1038/nrm1203. [DOI] [PubMed] [Google Scholar]
- 89.Pricci M, Linsalata M, Russo F, Messa C, Amati L, Caradonna L, et al. Effects of 17beta-estradiol administration on apoptosis and polyamine content in AGS cell line. Anticancer Res. 2001;21:3215–3220. [PubMed] [Google Scholar]
- 90.Yeretssian G, Doiron K, Shao W, Leavitt BR, Hayden MR, Nicholson DW, et al. Gender differences in expression of the human caspase-12 long variant determines susceptibility to Listeria monocytogenes infection. Proc Natl Acad Sci U S A. 2009;106:9016–9020. doi: 10.1073/pnas.0813362106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nylander-Koski O, Kiviluoto T, Puolakkainen P, Kivilaakso E, Mustonen H. The effect of nitric oxide, growth factors, and estrogen on gastric cell migration. J Surg Res. 2007;143:230–237. doi: 10.1016/j.jss.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 92.Ko KP, Park SK, Park B, Yang JJ, Cho LY, Kang C, et al. Isoflavones from phytoestrogens and gastric cancer risk: a nested case-control study within the Korean Multicenter Cancer Cohort. Cancer Epidemiol Biomarkers Prev. 2010;19:1292–1300. doi: 10.1158/1055-9965.EPI-09-1004. [DOI] [PubMed] [Google Scholar]
- 93.Freedman ND, Ahn J, Hou L, Lissowska J, Zatonski W, Yeager M, et al. Polymorphisms in estrogen- and androgen-metabolizing genes and the risk of gastric cancer. Carcinogenesis. 2009;30:71–77. doi: 10.1093/carcin/bgn258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lindblad M, Ye W, Rubio C, Lagergren J. Estrogen and risk of gastric cancer: a protective effect in a nationwide cohort study of patients with prostate cancer in Sweden. Cancer Epidemiol Biomarkers Prev. 2004;13:2203–2207. [PubMed] [Google Scholar]
- 95.Cardenas VM, Graham DY. Smoking and Helicobacter pylori infection in a sample of U.S. adults. Epidemiology. 2005;16:586–590. doi: 10.1097/01.ede.0000165365.52904.4a. [DOI] [PubMed] [Google Scholar]
- 96.Suzuki T, Matsuo K, Ito H, Sawaki A, Hirose K, Wakai K, et al. Smoking increases the treatment failure for Helicobacter pylori eradication. Am J Med. 2006;119:217–224. doi: 10.1016/j.amjmed.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 97.IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Tobacco Smoke and Involuntary Smoking Lyon. Vol. 83. IARC Press; 2004. pp. 557–613. [PMC free article] [PubMed] [Google Scholar]
- 98.Murphy G, Pfeiffer R, Camargo MC, Rabkin CS. Meta-analysis shows that prevalence of Epstein-Barr virus-positive gastric cancer differs based on sex and anatomic location. Gastroenterology. 2009;137:824–833. doi: 10.1053/j.gastro.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Camargo MC, Murphy G, Koriyama C, Pfeiffer RM, Kim WH, Herrera-Goepfert R, et al. Determinants of Epstein-Barr virus-positive gastric cancer: an international pooled analysis. Br J Cancer. 2011;105:38–43. doi: 10.1038/bjc.2011.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lunet N, Valbuena C, Vieira AL, Lopes C, Lopes C, David L, et al. Fruit and vegetable consumption and gastric cancer by location and histological type: case-control and meta-analysis. Eur J Cancer Prev. 2007;16:312–327. doi: 10.1097/01.cej.0000236255.95769.22. [DOI] [PubMed] [Google Scholar]
- 101.Baker AH, Wardle J. Sex differences in fruit and vegetable intake in older adults. Appetite. 2003;40:269–275. doi: 10.1016/s0195-6663(03)00014-x. [DOI] [PubMed] [Google Scholar]
- 102.Schätzer M, Rust P, Elmadfa I. Fruit and vegetable intake in Austrian adults: intake frequency, serving sizes, reasons for and barriers to consumption, and potential for increasing consumption. Public Health Nutr. 2010;13:480–487. doi: 10.1017/S136898000999142X. [DOI] [PubMed] [Google Scholar]
- 103.Altman DG, Deeks JJ. Meta-analysis, Simpson’s paradox, and the number needed to treat. BMC Medical Research Methodology. 2002;2 doi: 10.1186/1471-2288-2-3. (available at: http://www.biomedcentral.com/1471-2288/2/3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med. 2004;23:1351–1375. doi: 10.1002/sim.1761. [DOI] [PubMed] [Google Scholar]
- 105.Correa P, Haenszel W, Cuello C, Tannenbaum S, Archer M. A model for gastric cancer epidemiology. Lancet. 1975;2:58–60. doi: 10.1016/s0140-6736(75)90498-5. [DOI] [PubMed] [Google Scholar]