Abstract
Introduction and hypothesis
The objective of the study was to compare anterior compartment compliance between women with and without pelvic organ prolapse and to explore factors determining the extent of anterior compartment prolapse.
Methods
Ten women with normal pelvic support and nine with anterior compartment prolapse were analyzed. Abdominal pressure was measured during Valsalva and simultaneous midsagittal dynamic MR imaging. The distance between the most dependent anterior vaginal wall point and a previously determined average nulliparous anterior vaginal wall point was measured. A best-fit line was determined when anterior vaginal wall displacement was plotted relative to abdominal pressure. The slope of this line is a measure of anterior compartment compliance. Multivariate analyses and t tests were performed.
Results
Mean compliance (centimeters per centimeter of water) was higher for cases [0.05±0.006 standard error of the mean (SEM)] than controls (0.03±.007, p=0.039). Degree of anterior compartment prolapse correlated best with compliance (R2=0.75, p<0.01) and also with resting anterior vaginal wall point (R2=0.55, p<0.01).
Conclusions
Women with anterior compartment prolapse have a 67% more compliant support system compared with those with normal support. Both compliance and resting anterior vaginal wall location are predictors of the degree of anterior compartment prolapse.
Keywords: Anterior compartment prolapse, Biomechanics, In vivo material property testing, Pelvic organ support
Introduction
The anterior compartment is the most common site of both primary pelvic organ prolapse [1] and also operative failure [2]. Understanding the mechanism of anterior wall support and failure is, therefore, of primary importance. Anterior vaginal wall support is influenced by several structural elements including: apical connective tissue (cardinal and uterosacral ligaments), paravaginal attachments, and the connective tissues forming the anterior vaginal wall and the levator ani muscles. Abnormal anterior vaginal wall support has been associated with loss of apical support [3], increased vaginal wall length [4], and levator ani muscle disruptions [5, 6]. Previous biomechanical modeling studies from our group have demonstrated how the interaction between muscle and connective tissue both contribute to anterior compartment support [7].
Engineers characterize the stiffness of a biological structure by measuring the pattern of deformations that occur under a given pressure or force; the compliance of the structure can be found from the inverse of the stiffness value. The in vivo testing of the lateral vaginal wall compliance [8] has been quantified by measuring the stiffness of vesical neck support using ultrasound [9, 10], but there are no such in vivo data for the anterior compartment support. Dynamic MR imaging allows superior visualization of slow soft tissue movements which makes it ideal for looking at deformation or displacement of the anterior compartment during abdominal loading such as the Valsalva maneuver. This study was undertaken to test the null hypothesis that anterior compartment compliance in vivo does not differ between women with and without prolapse and to explore factors predicting the variation in the degree of anterior compartment prolapse or cystocele size.
Methods
Nineteen parous women with normal support (controls, N=10) and varying degrees of anterior compartment prolapse (cases, N=9) were taken from an ongoing Institutional Review Board approved study (1999-0395) of pelvic organ support. The controls had all vaginal points higher or equal to 1 cm above the hymen while the cases had anterior compartment prolapse lower than or equal to 1 cm below the hymen. Selected subjects had cystocele-predominant prolapse; women in whom the cervix was the leading point of prolapse were excluded. Demographic characteristics of the subjects is as follows: age (mean ± SD) for controls=62±10 years and cases=53±12 (p=0.101), and for parity: controls=3±2 and cases=3±1, (p=.565). All women with uterus in situ who were consecutively enrolled between February 2007 and March 2008 who agreed to catheter placement during the MR exam were included for analysis. Women with normal support were recruited through newspaper and online advertisements. Women with prolapse were recruited through the urogynecologic clinic at the University of Michigan. Patients were excluded if they had previous surgery for prolapse or pelvic floor dysfunction, had any findings which may distort pelvic anatomy such as a pelvic mass or history of pelvic radiation, were unable to complete the clinic exam or MR imaging study, or had medical conditions that would place them at increased risk for the urodynamic portion of this study (not reported here).
MR imaging technique
Prior to the MR examination, a 12 French Foley catheter was placed in the bladder and connected to an MR-compatible pressure monitoring device (Datascope, Montvale, NJ) to measure abdominal pressure. The use of a Foley catheter to measure intra-abdominal pressure has been validated against other intra-abdominal measurement devices [11]. MR imaging was performed on a 3-T system (Philips Medical Systems, Best, The Netherlands) using a 6-channel torso phased array coil with the subject in the supine position. Before starting the examination, the patient was instructed how to properly perform straining maneuvers to be used during the examination starting from minimal to maximal straining. For dynamic imaging, a multiphase, single-level image of the pelvis in the midsagittal plane was obtained approximately every 1.4 s over 30 s using a single-shot turbo spin-echo sequence (TR, approximately 1,300 ms; TE, 105 ms; slice thickness, 6 mm; field of view 34 cm; matrix, 256×90; and 1 NSA). A set of 20 successive images were acquired during rest and graded Valsalva effort as follows: after imaging during rest, the operator instructed the patient to strain minimally, moderately, and maximally, then to breathe normally and relax before ending the acquisition. This was done so that we could obtain a graded Valsalva response as opposed to an “all or none” effect. Women were asked to relax their pelvic floor muscles to allow maximal prolapse development as is usual during pelvic examination for prolapse.
The Valsalva attempt which resulted in the greatest descent of the anterior vaginal wall was selected for analysis. On each MR image, the most dependent anterior vaginal wall point was plotted (black dot, Fig. 1). When there was no anterior compartment prolapse, the point was placed at the urethral–vesicle junction. The average location of the normal resting anterior vaginal wall point has been previously determined in 20 nulliparous women [3] (black triangle, Fig. 1). This point was imported into the MR images using the sacro-coccygeal inferior pubic line as previously described [3]. Anterior vaginal wall displacement or the distance between the most dependent vaginal wall point to the average nulliparous vaginal wall point was determined using MATLAB v7.0 (MathWorks, Natick, MA). This distance was assessed rather than the distance between the resting and Valsalva vaginal wall points because in some women with large prolapse, the resting vaginal wall point can be quite abnormal. When this is true, little movement occurs during Valsalva, underestimating the size of the prolapse.
Fig. 1.
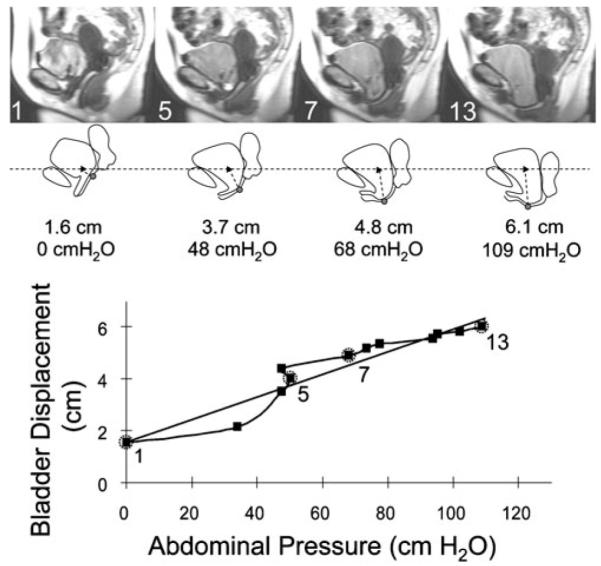
Obtaining system compliance for a subject. Dynamic MR images during Valsalva are shown in the top row. Image 1 is at rest. The bladder, anterior vaginal wall and uterus tracings are shown below the MR image. The black dot represents the most dependent anterior vaginal wall point and the black triangle the previously determined average nulliparous anterior vaginal wall point. The measured displacement between these points and the abdominal pressures corresponding to the image are also shown. Images 5, 7, and 13 show how displacement and abdominal pressures increase during Valsalva attempt. The anterior vaginal wall displacement was plotted against the abdominal pressure for each image (the data points corresponding to the example images are labeled and circled with dashed gray line). The loading portion of the curve is shown as a solid dark line. A best-fit line for all the points on the loading curve (solid gray line) was plotted. The slope of this line is a measure of system compliance
The strategy for assessing compliance is shown in Fig. 1. Abdominal pressure and anterior vaginal wall displacement was plotted for each subject. Different curve fitting models, linear, quadratic, and exponential were performed for each data set. Using the correlation coefficient as a criterion, a linear equation was found to provide the best fit across all subjects and so was used to analyze the results. The best-fit line for the loading portion of the curve (from rest to the maximum vaginal wall displacement) was obtained using linear regression, where the line slope is a measure of compliance and the y intercept a measure of resting position. Maximum anterior vaginal wall displacement was used as a proxy for cystocele size or the degree of anterior compartment prolapse. Non-parametric Mann–Whitney U-test was performed to compare cases and controls and multivariate analyses of all subjects (correlation coefficients and linear regression modeling) were performed with p<0.05 being considered significant.
Results
The mean (±SEM) compliance for cases was 67% higher than for controls (0.05±0.006 vs. 0.03±.007 cm/cmH2O, respectively; p=0.043). The strength of the linear relationship for each data set was assessed by Pearson’s correlation coefficient and showed coefficients of determination (R2) ranging from 0.21 to 0.98 with a mean of 0.85 and median of 0.92.
Compliance ranged from 0.03 to 0.097 cm/cmH2O with median of 0.049 cm/cmH2O for cases and from 0.002 to 0.074 with median of 0.029 cm/cmH2O for controls (Fig. 2). The mean (SEM) resting anterior vaginal wall point in cases (2.4±0.5 cm) was lower than controls (0.9± 0.1 cm, p=0.024). The mean (SEM) maximum pressure reached during Valsalva for controls was not significantly different than that for cases (83.0±8.1 vs. 87.0±9.0 cmH2O, respectively; p=.154).
Fig. 2.

Best-fit lines of loading curves for subjects. Cases are women with prolapse shown as solid lines, controls as dotted lines. More vertical slopes reflect increased compliance. The y intercept is the resting anterior vaginal wall point (larger numbers represent lower resting anterior vaginal wall point)
When subjects were grouped for multivariate analysis, we found that maximal anterior vaginal wall descent (a measure of cystocele size) correlated with compliance with a Pearson’s correlation coefficient of 0.86 (R2=0.74, p< 0.001). The degree of anterior vaginal descent was also associated with resting anterior vaginal wall point with correlation of 0.74 (R2=0.55, p<0.001). There was a weak but statistically significant correlation between resting anterior vaginal wall point and compliance R=0.49. (R2= 0.24, p=0.0411). There was not a significant correlation between the degree of anterior vaginal wall descent and maximal abdominal pressure R=0.39 (R2=0.15, p=0.102).
Linear regression modeling that included case and control data showed that the location of the resting anterior vaginal wall point alone helped to explain 55% of the variation of maximum anterior vaginal wall displacement (p<0.001). Adding the compliance of anterior compartment support increased this percentage to 86% (p<0.001), while adding maximum abdominal pressure increased it slightly to 93% (p=0.001).
Discussion
We report a novel approach to measure the compliance and behavior of the anterior compartment support system under physiologic load in women with and without prolapse. Using this dynamic MR-based strategy, it was possible to show that patients with prolapse have more compliant anterior compartment support systems than controls. Since compliance has an inverse relationship to stiffness, these results suggest that women with prolapse have less stiff vaginal supportive systems.
The linear regression analyses undertaken to determine the contribution of biomechanical factors showed, as expected, that compliance correlated strongly with descent of the anterior vaginal wall, but weakly with abdominal pressure. However, as is shown in Fig. 2, there is considerable overlap in the compliance (line slopes) between women with and without prolapse which led us to examine other factors that contributed to anterior compartment descent. We were surprised to find that the resting anterior vaginal wall point alone explained 55% of the variation in anterior compartment descent. This finding that, prior to loading, the starting state of the anterior vaginal wall in women with prolapse differs from those with normal support is intriguing. This may represent longterm adaptation by the anterior support system to repetitive loading which could have resulted in stretching and elongation of the connective tissue supports of the anterior compartment over time.
The technique used in this study makes two important contributions to the existing literature on the biomechanics of pelvic organ prolapse. It uses both an in vivo test method as well as a design where the entire anterior support system rather than a part is measured. Epstein et al. used a vaginal suction device in vivo and found women with prolapse to have more extensible lateral vaginal skin and a lower stiffness index compared with age-matched controls [8]. While this overcomes some of the difficulties with in vitro testing, only a small area of the vagina was tested; at present, measurements have been made of the lateral vaginal wall, but not the anterior vaginal wall. Testing the entire support system takes into account the complicated interactions between the various connective tissue components such as the ligaments and the vaginal wall properties. Several investigators have used in vitro testing methods such as uniaxial tensiometer to test the properties of vaginal biopsy specimens. For example, Cosson et al. found large variations in the rupture strength and elongation prior to rupture both in cadaver pelvic ligaments and in vaginal tissue from prolapse patients [12, 13]. While these data are important to the understanding of tissue properties, frank tissue rupture is likely beyond the range of stress or strain experienced during everyday activities. Lei et al. simulated more physiologic conditions in performing uniaxial tensiometer testing and, by assuming linear elastic behavior, found that subjects with prolapse had less elastic or stiffer vaginal tissues [14]. Rubod et al. who compared vaginal tissue from prolapse patients to that of cadaveric controls found prolapse tissues exhibit larger deformations and behave in a hyperelastic manner [15]. Although we are aware that biologic tissue usually exhibits hyperelastic behavior, a linear relationship was found to be the best fit between load and displacement in the present study. This linear behavior differs from the well-known hyperelastic behavior of isolated passive tissues because our outcome measure reflects the complex interactions between the active and passive systems that support the anterior vaginal wall. These interactions include changes in the recruitment of the pelvic floor muscles, the mechanical interaction between pelvic organs as they prolapse, the behavior of the apical supports, geometric non-linearities associated with tissue unfolding, as well as the hyperelastic behavior of the passive tissues. This complexity helps explain the variability in the shapes of the pressure-displacement curves as well as the interindividual differences in slope (Figs. 1 and 2). The differences between our findings and the existing literature is itself provocative and suggests how our in vivo technique can complement existing methods in better understanding the material properties of the vaginal support system.
Several factors must be kept in mind in interpreting the results of this study. The ability to test the overall behavior of the anterior vaginal wall support system is a strength of this study, but it must be kept in mind that it is not possible to make specific inferences about which anatomic structure is responsible for differences between cases and controls. Specifically, these data should not be interpreted as representing the status of the connective tissues alone, and we cannot quantify the separate contribution of the levator ani muscles. In reviewing the dynamic MR images, there are cases where one can see the levator ani muscles resist the downward movements of the abdominal contents generated by the Valsalva. Eventually, a threshold is reached, and the pelvic contents descend past the levator hiatus. In our current method, we did not measure levator ani muscle activity during dynamic MR imaging. Furthermore, it should be noted that we are only measuring displacement in the midsagittal plane; therefore, we are not able to infer the 3-D behavior of the system such as distinguishing between central and lateral defects. In addition, these measurements are made from a single Valsalva maneuver. A trained research assistant knowing the subject’s POP-Q measures was, however, present to ensure that the maximal extent of the prolapse was achieved in the MR scanner, but factors such as the state of bladder or bowel filling may also influence these measures and further investigations will be required to fully understand these relationships.
This pilot study of anterior compartment compliance suggests many research questions for the future. How does in vivo compliance correlate with in vitro material properties from harvested tissue in the same individual? A woman with prolapse and higher compliance may have normal vaginal wall material properties but altered material properties of her apical ligaments. What accounts for the variation in compliance among women with prolapse? Do these variations represent different etiologies of pelvic organ prolapse? For example, do some women have connective tissue failure while others have pelvic floor muscle failure while others have both? Answering these questions would help in understanding the pathophysiology of prolapse.
Acknowledgments
This study is funded by: RO1 HD 38665, K12RR17607, and K12RR0249-87.
Footnotes
Conflicts of interest None.
This study was presented at Society of Gynecologic Surgeons meeting, New Orleans, LA, on April 1, 2009.
Contributor Information
Yvonne Hsu, Department of Ob-Gyn, University of Michigan, Ann Arbor, Michigan, USA; Department of Obstetrics & Gynecology, University of Utah Health Sciences Center, 30 North 1900 East, Suite 2B200, Salt Lake City, Utah 84132-2209, USA.
Luyun Chen, Department of Biomechanical Engineering, University of Michigan, Ann Arbor, Michigan, USA; Department of Obstetrics & Gynecology, University of Utah Health Sciences Center, 30 North 1900 East, Suite 2B200, Salt Lake City, Utah 84132-2209, USA.
Julie Tumbarello, Department of Obstetrics & Gynecology, University of Utah Health Sciences Center, 30 North 1900 East, Suite 2B200, Salt Lake City, Utah 84132-2209, USA.
James A. Ashton-Miller, Department of Biomechanical Engineering, University of Michigan, Ann Arbor, Michigan, USA
John O. L. DeLancey, Department of Obstetrics & Gynecology, University of Utah Health Sciences Center, 30 North 1900 East, Suite 2B200, Salt Lake City, Utah 84132-2209, USA
References
- 1.Hendrix SL, Clark A, Nygaard I, Aragaki A, Barnabei V, McTiernan A. Pelvic organ prolapse in the women’s health initiative: gravity and gravidity. Am J Obstet Gynecol. 2002;186(6):1160–1166. doi: 10.1067/mob.2002.123819. [DOI] [PubMed] [Google Scholar]
- 2.Miedel A, Tegerstedt G, Mörlin B, Hammarström M. A 5-year prospective follow-up study of vaginal surgery for pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(12):1593–1601. doi: 10.1007/s00192-008-0702-z. [DOI] [PubMed] [Google Scholar]
- 3.Summers A, Winkel LA, Hussain HK, DeLancey JO. The relationship between anterior and apical compartment support. Am J Obstet Gynecol. 2006;194(5):1438–1443. doi: 10.1016/j.ajog.2006.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu Y, Chen L, Summers A, Ashton-Miller JA, DeLancey JO. Anterior vaginal wall length and degree of anterior compartment prolapse seen on dynamic MRI. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(1):137–142. doi: 10.1007/s00192-007-0405-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoyte L, Jakab M, Warfield SK, Shott S, Flesh G, Fielding JR. Levator ani thickness variations in symptomatic and asymptomatic women using magnetic resonance-based 3-dimensional color mapping. Am J Obstet Gyneco. 2004;191(3):856–861. doi: 10.1016/j.ajog.2004.06.067. [DOI] [PubMed] [Google Scholar]
- 6.DeLancey JO, Morgan DM, Fenner DE, Kearney R, Guire K, Miller JM, Hussain H, Umek W, Hsu Y, Ashton-Miller JA. Comparison of levator ani muscle defects and function in women with and without pelvic organ prolapse. Obstet Gynecol. 2007;109(2):295–302. doi: 10.1097/01.AOG.0000250901.57095.ba. [DOI] [PubMed] [Google Scholar]
- 7.Chen L, Ashton-Miller JA, Hsu Y, DeLancey JO. Interaction among apical support, levator ani impairment, and anterior vaginal wall prolapse. Obstet Gynecol. 2006;108(2):324–332. doi: 10.1097/01.AOG.0000227786.69257.a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Epstein LB, Graham CA, Heit MH. Systemic and vaginal biomechanical properties of women with normal vaginal support and pelvic organ prolapse. Am J Obstet Gynecol. 2007;197(2):165.e1–165.e6. doi: 10.1016/j.ajog.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 9.Howard D, Miller JM, Delancey JO, Ashton-Miller JA. Differential effects of cough, Valsalva, and continence status on vesical neck movement. Obstet Gynecol. 2000;95(4):535–540. doi: 10.1016/s0029-7844(99)00618-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng Q, Jones R, Shishido K, Constantinou CE. Ultrasound evaluation of dynamic responses of female pelvic floor muscles. Ultrasound Med Biol. 2007;33(3):342–352. doi: 10.1016/j.ultrasmedbio.2006.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malbrain ML, De Laet I, Viaene D, Schoonheydt K, Dits H. In vitro validation of a novel method for continuous intra-abdominal pressure monitoring. Intensive Care Med. 2008;34:740–745. doi: 10.1007/s00134-007-0952-0. [DOI] [PubMed] [Google Scholar]
- 12.Cosson M, Lambaudie E, Boukerrou M, Lobry P, Crépin G, Ego A. A biomechanical study of the strength of vaginal tissues. Results on 16 post-menopausal patients presenting with genital prolapse. Eur J Obstet Gynecol Reprod Biol. 2004;112(2):201–205. doi: 10.1016/s0301-2115(03)00333-6. [DOI] [PubMed] [Google Scholar]
- 13.Cosson M, Boukerrou M, Lacaze S, Lambaudie E, Fasel J, Mesdagh H, Lobry P, Ego A. A study of pelvic ligament strength. Eur J Obstet Gynecol Reprod Biol. 2007;109(1):80–87. doi: 10.1016/s0301-2115(02)00487-6. [DOI] [PubMed] [Google Scholar]
- 14.Lei L, Song Y, Chen R. Biomechanical properties of prolapsed vaginal tissue in pre- and postmenopausal women. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(6):603–607. doi: 10.1007/s00192-006-0214-7. [DOI] [PubMed] [Google Scholar]
- 15.Rubod C, Boukerrou M, Brieu M, Jean-Charles C, Dubois P, Cosson M. Biomechanical properties of vaginal tissue: preliminary results. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(6):811–816. doi: 10.1007/s00192-007-0533-3. [DOI] [PubMed] [Google Scholar]


