Abstract
OBJECTIVES
To examine the association between the type and number of subjective memory complaints (SMCs) and performance on objective cognitive tests.
DESIGN
Cross-sectional.
SETTING
Nurses’ Health Study.
PARTICIPANTS
Sixteen thousand nine hundred sixty-four women (mean age 74) who provided information on SMCs.
MEASUREMENTS
Telephone cognitive assessments and seven questions regarding SMCs were administered. Cognitive impairment was defined as a score of less than 31 on the Telephone Interview for Cognitive Status (TICS) and below the 10th percentile on other cognitive measures. To assess associations with SMCs, multivariable logistic regression was used to calculate odds ratios for cognitive impairment and multivariable linear regression to calculate mean differences in cognitive test scores, adjusting for age and depressive symptoms.
RESULTS
Some SMCs, such as trouble following a group conversation or finding one’s way around familiar streets, were more highly associated than others with odds of cognitive impairment. The complaint of forgetting things from one second to the next, generally considered part of normal aging, was not associated with cognitive impairment. In addition, there were strong, linear trends of increasingly worse scores on cognitive tests with increasing numbers of memory complaints. For each additional SMC endorsed, the odds of cognitive impairment increased approximately 20% when each SMC was weighted equally.
CONCLUSION
SMCs are associated with objective cognitive status and may be considered by primary care physicians in determining whether follow-up is warranted.
Keywords: memory complaints, cognitive function, aging
Over the next 20 years, the number of U.S. adults aged 65 and older is projected to nearly double, resulting in approximately one-fifth of the population consisting of older persons.1 Along with this rise, a corresponding increase in Alzheimer’s disease (AD) can be expected. One estimate projects the number of new AD cases in the United States alone to increase from 454,000 in 2010 to 959,000 in 2050.2 In response to anticipated increases in AD incidence, the recently passed Affordable Care Act has incorporated cognitive screening as part of annual wellness visits for older persons. Thus, finding simple ways to identify older persons who may benefit from further cognitive assessment is of increasing importance and relevance.
Some data suggest that inquiring about subjective memory complaints (SMCs) may be a straightforward approach for approximating objective cognitive performance for primary care physicians. Nonetheless, prior studies considering reports of SMCs and current cognitive function have had several notable limitations that have made it difficult to draw clear inferences;3,4 for example, potential confounding by depression was not considered, and subjective report and cognitive testing were limited. The objective of the present study was to examine the cross-sectional relationships between types of complaints and specific cognitive difficulties and to determine whether the number of SMCs was associated with current cognitive status in older women, such that a more-extensive examination might be warranted. It was expected that specific complaints would vary in the strength of their relationship to specific cognitive domains (i.e., episodic memory and language functioning), and moreover, it was hypothesized that a greater number of complaints would be associated with poorer cognitive performance on objective testing.
METHODS
Study Population
This research was conducted within the Nurses’ Health Study, which began in 1976, when 121,700 female registered nurses aged 30 to 55 responded to a mailed questionnaire about their health and lifestyle. Information on health and lifestyle is updated using biennial questionnaires. Follow-up in the cohort is approximately 90%.
The analyses presented here were restricted to participants in the cognitive function subcohort, which includes 19,514 Nurses’ Health Study participants aged 70 to 81 and free of diagnosed stroke, who completed a telephone cognitive assessment including seven items regarding SMCs and a battery of cognitive tests. After excluding 2,550 women with missing data on any of the seven SMC questions, there were 16,964 women available for analysis. Women with missing data were identical to the population for analysis in important characteristics, such as mean age (74 in both groups) and highest attained education (6% with a master’s degree or higher in both groups). In addition, mean score on the Telephone Interview for Cognitive Status (TICS) was identical in those with and without SMC data, indicating that substantial bias due to missing data is highly unlikely.
Measurement of SMCs
At the beginning of the telephone cognitive assessment, trained interviewers, who were unaware of the study hypothesis, asked the participants seven questions about SMCs previously found to be useful in patients with possible cognitive impairment.5 Response options were yes or no for each question. The questions used are listed in Table 1 and are notated using a key name for ease of reporting findings.
Table 1.
Prevalence of Subjective Memory Complaints in Nurses’ Health Study Cognitive Substudy Participants
| Variable | % |
|---|---|
| Number of subjective memory complaints | |
| 0 | 27.3 |
| 1 | 29.5 |
| 2 | 23.5 |
| 3 | 12.6 |
| 4 | 5.0 |
| 5 | 1.5 |
| 6–7 | 0.6 |
| Specific subjective memory complaints | |
| Have you recently experienced any change in your ability to remember things? [CHANGE IN MEMORY] | 56.4 |
| Do you have more trouble than usual remembering a short list of items, such as a shopping list? [SHORT LIST] | 29.2 |
| Do you have trouble remembering things from one second to the next? [ONE SECOND TO THE NEXT] | 25.4 |
| Do you have much more trouble than usual remembering recent events? [RECENT EVENTS] | 18.5 |
| Do you have any difficulty in understanding or following spoken instructions? [UNDERSTANDING INSTRUCTIONS] | 8.6 |
| Do you have more trouble than usual following a group conversation or plot in a TV program due to your memory? [FOLLOWING A CONVERSATION] | 5.8 |
| Do you have trouble finding your way around familiar streets? [GETTING LOST] | 1.6 |
SMCs were evaluated using two approaches. First, each question was considered individually to examine the specific associations between individual SMCs and measures of different cognitive domains. In particular, the complaint of forgetting things from ONE SECOND TO THE NEXT is generally considered to be part of normal aging and thus would not be expected to be associated with any of the objective cognitive test scores. In contrast, the complaint of GETTING LOST in familiar streets, the most severe complaint of the seven, was expected to be strongly associated with all of the objective measures of cognition. Finally, it was thought that the SHORT LIST and RECENT EVENTS complaints would be more likely to be associated with impaired memory (delayed recall) than other aspects of cognitive functioning. In addition, a continuous variable was created for participants’ total number of complaints. Those with six or seven complaints were combined because of small numbers in these groups.
Objective Measurement of Cognition
After asking the participants about SMCs, the interviewers administered cognitive tests in the following order: the East Boston Memory Test (EBMT), the TICS (including the immediate recall of the TICS 10-word list), a test of semantic fluency in which animals are named during a 1-minute interval, a test of working memory in which increasingly long series of numbers are repeated backward, delayed recall of the EBMT, and delayed recall of the TICS 10-word list.
The analyses presented here focus on the TICS,6 a telephone adaptation of a cognitive screen similar to the Mini-Mental State Examination. The confrontation naming task in the TICS, a measure of expressive language, in which participants are given descriptions of four items (e.g., “What do people usually use to cut paper?”) and then are asked to name the described item (scissors) was also considered. Other specific tests examined for these analyses were the test of semantic fluency,7 delayed recall of the EBMT,8 and delayed recall of the TICS 10-word list.
To create a more-stable measure of delayed recall (episodic memory), a domain that is impaired in individuals with AD,9,10 z-scores from the delayed recall of the EBMT and TICS 10-word list were averaged in women who had completed both tests (n = 16,808). For each test, z-scores were calculated by dividing the difference between the participant’s score and the mean score in the population for analysis by the standard deviation. High reliability and validity of these tests when administered by telephone has been previously established.11
For these analyses, TICS score and delayed recall were analyzed as continuous outcome variables and binary variables (cognitive impairment vs no cognitive impairment). For the analyses using binary outcome variables, cognitive impairment was defined as a score of less than 31 on the TICS12 (equivalent to ≤26 on the MMSE) or delayed recall below the 10th percentile, a cutoff commonly used to define impairment in cognitive research if no standard cut points for impairment have been defined.13,14 In addition, impairment in semantic fluency was defined as a score below the 10th percentile, and impairment in confrontation naming was defined as correct naming of fewer than four of four items.
Statistical Analysis
To examine the association between SMCs and the likelihood of cognitive impairment, multivariable logistic regression was used to calculate odds ratios (ORs) and 95% confidence intervals (CIs), adjusted for age and depressive symptoms, which are probably the primary potential confounding variables. Depressive symptoms, defined as a score of 52 or lower on the mental health sub-scale of the Medical Outcomes Study 36-item Short-Form Survey (range 0–100)15 or self-reported use of an antidepressant, were measured at approximately the same time as the cognitive assessments. Additional adjustment for highest attained educational degree did not change the results, so education was not included as a covariate in the models. Analyses of individual SMCs were further adjusted for all other SMCs to isolate the associations with each specific complaint. In addition, linear trends in the association between greater number of SMCs and cognition were assessed by including number of SMCs in the regression models as a continuous variable.
For analyses of the cognitive tests as continuous outcome variables, multivariable linear regression was used to calculate mean differences (95% CIs) in cognitive test score, according to SMCs, adjusting for age and depressive symptoms.
In primary analyses of number of SMCs and cognitive impairment, all complaints were considered to be equally important, and thus a simple score tallying the number of SMCs was created, although the objective was also to examine associations with number of complaints while accounting for the varying strength of relations between each SMC and cognitive impairment. Thus, for secondary analyses, a more-complex score for number of SMCs was created by weighting each SMC by its log OR for cognitive impairment on the TICS. Details about the weighted score are given below.
Whether the association between number of SMCs and cognition varied according to several participant characteristics, including education, race, and depressive symptoms, was also explored. Finally, to explore whether associations were different between women with higher and lower levels of cognition, analyses were conducted within several strata of TICS score (≥31 points, < 31 points, and < 21 points).
For all analyses, two-tailed P <.05 was considered statistically significant. All data were analyzed using SAS 9.1 (SAS Institute, Inc, Cary, NC).
RESULTS
Study Population Characteristics
Ninety-eight percent of the participants were white, and the mean age was 74 (5th–95th percentile = 71–78). Highest attained educational level was registered nurse training after high school for the majority of women (78%); 17% had earned a bachelor’s degree and 6% a master’s or doctorate degree. Twelve percent reported depressive symptoms. Mean TICS score was 34 ± 3 points (interquartile range 32–35 points); 12% (n = 1,954) scored less than 31 points, the cutoff for cognitive impairment.
Prevalence of SMCs
Overall, 27.3% of women reported no SMCs, 53.0% reported one or two, and 7.1% reported four or more (Table 1). Of the seven SMCs included in the battery, CHANGE IN MEMORY was the most common complaint, reported by 56.4%. Approximately one-fourth of the women reported the SHORT LIST (29.2%) and ONE SECOND TO THE NEXT (25.4%) complaints. As expected in these generally healthy women, few reported GETTING LOST (1.6%).
Specific SMCs and Cognitive Test Scores
After adjusting for age and depressive symptoms and mutually adjusting each SMC for all of the others, suggestions of specific associations were found between several complaints and impaired performance on individual cognitive measures (Table 2). For example, CHANGE IN MEMORY was associated with 16% (95% CI = 5–29%) greater of impairment on the TICS and 35% (95% CI = 22–49%) greater odds of impairment in delayed recall, with no association for semantic fluency. Similar associations were observed for RECENT EVENTS and SHORT LIST, as expected, although as also expected, a complaint of GETTING LOST was strongly associated with greater odds of impairment on the TICS and impaired performance on cognitive tests that people with AD commonly have impairment on (delayed recall, semantic fluency, and confrontation naming). Also as expected, ONE SECOND TO THE NEXT, a complaint associated with normal aging, was not related to any cognitive impairment outcome.
Table 2.
Associations Between Specific Memory Complaints and Cognitive Test Scores
| Complaint* | Cases, Odds Ratio (95% Confidence Interval)† | |||
|---|---|---|---|---|
| Impairment on the TICS | Impairment in Delayed Recall | Impairment in Semantic Fluency | Impairment in Confrontation Naming | |
| Change in memory | 1,231, 1.16 (1.05–1.29) | 1,531, 1.35 (1.22–1.49) | 1,184, 1.05 (0.95–1.16) | 374, 1.29 (1.07–1.55) |
| Short list | 706, 1.23 (1.11–1.37) | 833, 1.18 (1.07–1.30) | 615, 0.98 (0.89–1.09) | 191, 1.01 (0.84–1.21) |
| One second to the next | 555, 1.00 (0.89–1.11) | 660, 0.96 (0.87–1.07) | 520, 0.94 (0.84–1.05) | 168, 1.01 (0.83–1.23) |
| Recent events | 456, 1.11 (0.99–1.26) | 581, 1.24 (1.11–1.39) | 423, 1.08 (0.96–1.22) | 136, 1.08 (0.87–1.33) |
| Understanding instructions | 250, 1.31 (1.13–1.53) | 276, 1.16 (1.00–1.34) | 230, 1.28 (1.09–1.50) | 74, 1.21 (0.93–1.58) |
| Following a conversation | 183, 1.37 (1.15–1.64) | 218, 1.42 (1.20–1.68) | 167, 1.35 (1.13–1.62) | 64, 1.62 (1.22–2.16) |
| Getting lost | 79, 2.22 (1.68–2.93) | 90, 2.15 (1.65–2.82) | 60, 1.71 (1.26–2.30) | 30, 2.55 (1.69–3.85) |
See Table 1 for full complaint description.
Adjusted for age, depressive symptoms, and all other memory complaints.
TICS = Telephone Interview for Cognitive Status.
Using the data from the analyses of individual SMCs and cognitive impairment on the TICS, a weighted score was created for number of complaints for use in the secondary analyses described below. Specifically, because ONE SECOND TO THE NEXT was not associated with impairment, this complaint was assigned a weight of 0. For the remaining six SMCs, weights were calculated using each SMC’s log OR while keeping the range in possible scores from 0 to 7 so that the scale remained comparable to the range of the unweighted scores. The specific weights were 0.56 for CHANGE IN MEMORY, 0.79 for SHORT LIST, 0.41 for RECENT EVENTS, 1.02 for UNDERSTANDING INSTRUCTIONS, 1.20 for FOLLOWING A CONVERSATION, and 3.01 for GETTING LOST. Number of complaints on the weighted scale was then calculated by summing the weights of all complaints that each participant endorsed.
Number of SMCs and Cognition
After adjusting for age and depressive symptoms, women with one or more SMCs (on the unweighted scale) had significantly worse mean TICS and delayed recall scores than those with no SMCs (Figure 1A and B). The relation was clearly linear, with strong and significant trends of steadily decreasing TICS or delayed recall scores with increasing number of SMCs (P for trend <.001 for both outcomes).
Figure 1.
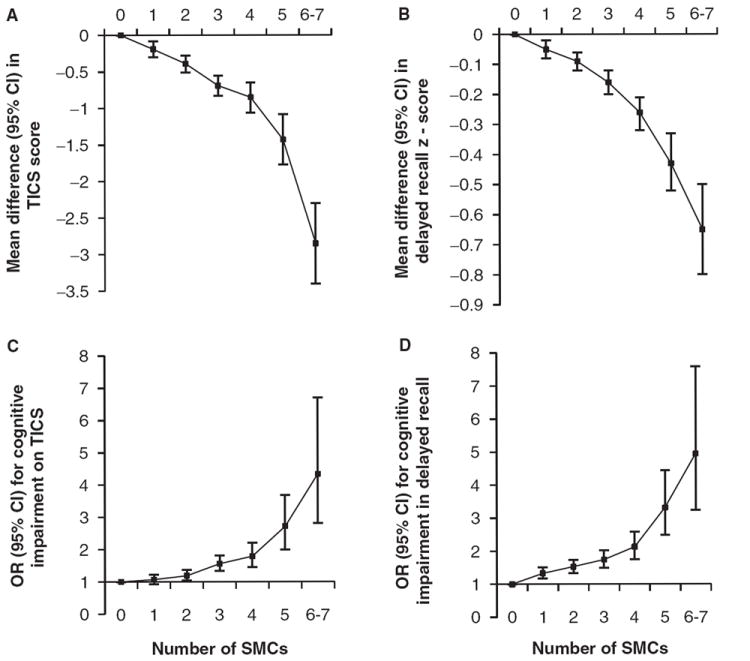
Relationship between total number of subjective memory complaints (SMCs) and cognitive test scores. All models were adjusted for age and depressive symptoms. P-values for linear trend were <.001 in all four models. CI = confidence interval; TICS = Telephone Interview for Cognitive Status.
In analyses of cognitive impairment, a more clinically relevant outcome, clear associations were also found with the simple score tallying number of SMCs. Overall, when cognitive impairment was defined using the TICS or delayed recall, there were approximately 20% greater odds of impairment for each additional SMC (OR = 1.19, 95% CI = 15–24%, P-trend <.001 for TICS; OR = 1.22, 95% CI = 18–26%, P-trend <.001 for delayed recall) (data not shown in tables or figure). For example, compared with women with no SMCs, the OR for cognitive impairment on the TICS ranged from 1.19 (95% CI = 1.04–1.37) in women with two complaints to 4.34 (95% CI = 2.81, 6.70) in women with six or seven complaints (Figure 1C and D). For delayed recall, there was a significant increase in impairment beginning in women with one SMC. Specifically, ORs ranged from 1.33 (95% CI = 1.17, 1.51) in women with one SMC to 4.95 (95% CI = 3.24, 7.59) in women with six or seven SMCs.
As described above, the relationship between number of complaints and odds of cognitive impairment on the TICS was further examined after weighting each SMC according to the strength of its relationship with impairment (data not shown in tables or figure). Using the weighted scale, the association with impairment strengthened; the odds of impairment increased 30% for each additional SMC endorsed (OR = 1.30, 95% CI = 1.25–1.36, P-trend <.001). In addition, ORs for impairment on the TICS were stronger in women with two to five SMCs on the weighted scale versus the unweighted scale used in the primary analysis, ranging from 1.96 (95% CI = 1.61–2.39) for a score of 2.0 to 2.9 SMCs to 4.18 (95% CI = 2.46, 7.10) for a score of 5.0 to 5.9 SMCs. Comparing women with a score of 6.0 to 7.0 SMCs on the weighted scale with those with no complaints, the OR for impairment on the TICS was similar to that in the primary analysis (OR = 4.37, 95% CI = 2.16–8.81).
There was no evidence of any difference in the association between total number of SMCs and cognitive test score in analyses stratified according to education, race, or depressive symptoms (data not shown in tables or figure). When whether the association between SMCs and objectively measured cognition varied according to cognitive performance was investigated, it was found that higher total numbers of SMCs appeared similarly related to lower TICS scores in women without cognitive impairment (mean difference in TICS score for each additional SMC −0.14 points, 95% CI = −0.16 to −0.11) an −0.12), although in the few women with very low scores on the TICS (<21 points, n = 20), the association between SMCs and TICS score appeared to attenuate (mean difference −0.07 points, 95% CI = −0.99–0.85), suggesting that SMCs might not be indicative of objectively measured cognition in women with more-severe cognitive impairment.
CONCLUSION
This large, population-based study found different associations between specific SMCs and odds of cognitive impairment. For example, GETTING LOST, a complaint indicative of dementia, was strongly associated with cognitive impairment as measured using the TICS, delayed recall, confrontation naming, and semantic fluency, as expected. By contrast, the complaint ONE SECOND TO THE NEXT was not associated with greater odds of cognitive impairment on any outcome, supporting its relationship to normal aging, rather than as an indication of cognitive impairment, as expected. The specificity of findings, along with the lack of association between a complaint considered to be part of normal aging and any objective measures of cognition, strongly indicate that certain subjective memory complaints should raise concern about the presence of cognitive impairment.
Moreover, in addition to the varying relationships between individual SMCs and specific cognitive domains, it was also found that worse performance on cognitive tests was related to more subjective complaints. For each additional SMC, there was an approximately 20% increase in odds of cognitive impairment, as defined by global TICS score or delayed memory recall score. A slightly more-complex SMC score, weighting each SMC by the strength of its relationship with cognitive impairment, resulted in stronger findings, with 30% greater odds of cognitive impairment on the TICS for each additional point on the SMC score. The association between total SMCs and objective cognitive functioning remained strong after adjusting for age and depressive symptoms and stratifying according to race and education, although in women with the most-significant cognitive impairment, the association between SMCs and TICS score appeared to attenuate, suggesting lack of awareness of cognitive deficits at this stage.
These findings help to inform the relevance of SMCs in indicating current cognitive functioning, because prior cross-sectional studies have been equivocal. Better understanding of the relationship between the number and types of older adults’ complaints and their current cognitive function is important, because this information could help ensure the delivery of appropriate medical care. Although several population-based studies have found significant associations between subjective and objective measures in individuals without dementia,3,4,16 others have not.17,18 In addition, methodological limitations have made findings of some of the existing studies more difficult to interpret than those of the current study. For example, in various studies, SMCs were measured using a single question, cognitive testing was limited, and the role of depression as a potential confounder was not considered.3,4 When more-comprehensive measures have been employed, as in the current study, results have been more promising.16,19,20 For example, when subjects were assigned to high and low memory groups based on self-ratings, the low memory group performed worse on multiple standardized memory measures than the high memory group.16
The sample and design of the current study also improved upon the previous studies in several ways. It had the benefit of a large study sample (n = 16,964). Participants enrolled in the parent cohort when they were younger than 55 and had no knowledge that a cognitive assessment would be administered in later years of follow-up; thus, unlike other study populations, which tend to be recruited for memory research, this one was not likely to be enriched with participants who were particularly concerned with their memory and potentially more aware of their cognitive status than typical participants. The age range of the sample, generally all women in their early 70s, coincided with the age at which risk for onset of neurodegenerative disease begins to climb;2 thus, the findings are particularly relevant in the context of increasing necessity to identify those with initial manifestations of cognitive concerns.21 Finally, cognitive testing included measures of global function, as well as language functioning and episodic memory, which allowed for analysis of SMCs and their relationship to different aspects of cognitive functioning.
Nonetheless, this study has several limitations. The sample was entirely women and largely white, so findings may not extend to samples with a different demographic composition. Although subjects were asked seven questions about their cognition, the scripted questions may not have fully captured the range of complaints that patients may experience and thus could have led to underestimation of the relationship between SMCs and objective cognition. Additionally, the cognitive battery was fairly brief, and a more-extensive cognitive examination may have quantified level of cognitive functioning more accurately and more broadly. Furthermore, the cognitive battery in this study is not specifically used in primary care settings. Future studies examining the relationship between SMCs and cognitive screens commonly used by primary care providers will be required to make specific statements about how whether SMCs might be more useful than other screening tools. Finally, the weighted score for number of complaints, which accounted for the strength of each SMC’s relationship with cognitive impairment, was derived using data from this cohort, and thus, the weights may not apply to other populations, although the different associations observed between individual SMCs and cognitive impairment suggest that weighting is important and may increase the utility of complaints in clinical practice.
In conclusion, the quantity and type of SMC appear to have robust and sensible relationships to cognitive measures. These results suggest that physicians should not discount the SMCs of older patients because they may help to target individuals who require further cognitive examination. These findings may be particularly relevant for primary care providers whose role in identifying individuals at early stages of cognitive impairment will become increasingly important as the incidence of AD increases and AD interventions become available.
Acknowledgments
We appreciate Dr. Marilyn Albert’s contribution of questions on subjective memory complaints to include in the telephone cognitive interview.
Sponsor’s Role: None.
Footnotes
Author Contributions: Rebecca E. Amariglio, Mary K. Townsend, Reisa A. Sperling, and Dorene M. Rentz: study concept and design, analysis and interpretation of data, and preparation of this manuscript. Francine Grodstein: study concept and design, acquisition of data, analysis and interpretation of data, and preparation of this manuscript.
Conflict of Interest: This research was supported by Grants CA87969 and AG15424 from the National Institutes of Health. Dr. Townsend was supported by the Yerby Postdoctoral Fellowship Program.
References
- 1.He W, Sengupta M, Velkoff VA, et al. 65+ in the United States: 2005. Washington, DC: U.S. Census Bureau; 2005. [Google Scholar]
- 2.Hebert LE, Beckett LA, Scherr PA, et al. Annual incidence of Alzheimer disease in the United States projected to the years 2000 through 2050. Alzheimer Dis Assoc Disord. 2001;15:169–173. doi: 10.1097/00002093-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Gagnon M, Dartigues JF, Mazaux JM, et al. Self-reported memory complaints and memory performance in elderly French community residents: Results of the PAQUID Research Program. Neuroepidemiology. 1994;13:145–154. doi: 10.1159/000110373. [DOI] [PubMed] [Google Scholar]
- 4.Bassett SS, Folstein MF. Memory complaint, memory performance, and psychiatric diagnosis: A community study. J Geriatr Psychiatry Neurol. 1993;6:105–111. doi: 10.1177/089198879300600207. [DOI] [PubMed] [Google Scholar]
- 5.Go RC, Duke LW, Harrell LE, et al. Development and validation of a Structured Telephone Interview for Dementia Assessment (STIDA): The NIMH Genetics Initiative. J Geriatr Psychiatry Neurol. 1997;10:161–167. doi: 10.1177/089198879701000407. [DOI] [PubMed] [Google Scholar]
- 6.Brandt J, Folstein MF. Telephone Interview for Cognitive Status: Professional Manual. Lutz: Psychological Assessment Resources, Inc; 2003. [Google Scholar]
- 7.Welsh KA, Butters N, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part V. A normative study of the neuropsychological battery. Neurology. 1994;44:609–614. doi: 10.1212/wnl.44.4.609. [DOI] [PubMed] [Google Scholar]
- 8.Albert M, Smith LA, Scherr PA, et al. Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer’s disease. Int J Neurosci. 1991;57:167–178. doi: 10.3109/00207459109150691. [DOI] [PubMed] [Google Scholar]
- 9.Elias MF, Beiser A, Wolf PA, et al. The preclinical phase of Alzheimer disease: A 22-year prospective study of the Framingham Cohort. Arch Neurol. 2000;57:808–813. doi: 10.1001/archneur.57.6.808. [DOI] [PubMed] [Google Scholar]
- 10.Small BJ, Fratiglioni L, Viitanen M, et al. The course of cognitive impairment in preclinical Alzheimer disease: Three- and 6-year follow-up of a population-based sample. Arch Neurol. 2000;57:839–844. doi: 10.1001/archneur.57.6.839. [DOI] [PubMed] [Google Scholar]
- 11.Lee S, Kawachi I, Berkman LF, et al. Education, other socioeconomic indicators, and cognitive function. Am J Epidemiol. 2003;157:712–720. doi: 10.1093/aje/kwg042. [DOI] [PubMed] [Google Scholar]
- 12.Brandt JSM, Folstein M. The telephone interview for cognitive status. Neuropsychiatry Neuropsychol Behav Neurol. 1988;1:111–117. [Google Scholar]
- 13.Yaffe K, Krueger K, Sarkar S, et al. Cognitive function in postmenopausal women treated with raloxifene. N Engl J Med. 2001;344:1207–1213. doi: 10.1056/NEJM200104193441604. [DOI] [PubMed] [Google Scholar]
- 14.Ganguli M, Belle S, Ratcliff G, et al. Sensitivity and specificity for dementia of population-based criteria for cognitive impairment: The MoVIES project. J Gerontol. 1993;48:M152–M161. doi: 10.1093/geronj/48.4.m152. [DOI] [PubMed] [Google Scholar]
- 15.Ware JE, Snow KK, Kosinski M, et al. SF-36 Health Survey: Manual and Interpretation Guide. Boston: The Health Institute, New England Medical Center; 1993. [Google Scholar]
- 16.Snitz BE, Morrow LA, Rodriguez EG, et al. Subjective memory complaints and concurrent memory performance in older patients of primary care providers. J Int Neuropsychol Soc. 2008;14:1004–1013. doi: 10.1017/S1355617708081332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clement F, Belleville S, Gauthier S. Cognitive complaint in mild cognitive impairment and Alzheimer’s disease. J Int Neuropsychol Soc. 2008;14:222–232. doi: 10.1017/S1355617708080260. [DOI] [PubMed] [Google Scholar]
- 18.Jungwirth S, Fischer P, Weissgram S, et al. Subjective memory complaints and objective memory impairment in the Vienna-Transdanube aging community. J Am Geriatr Soc. 2004;52:263–268. doi: 10.1111/j.1532-5415.2004.52066.x. [DOI] [PubMed] [Google Scholar]
- 19.Jonker C, Geerlings MI, Schmand B. Are memory complaints predictive for dementia? A review of clinical and population-based studies. Int J Geriatr Psychiatry. 2000;15:983–991. doi: 10.1002/1099-1166(200011)15:11<983::aid-gps238>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 20.Lam LC, Lui VW, Tam CW, et al. Subjective memory complaints in Chinese subjects with mild cognitive impairment and early Alzheimer’s disease. Int J Geriatr Psychiatry. 2005;20:876–882. doi: 10.1002/gps.1370. [DOI] [PubMed] [Google Scholar]
- 21.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


