Abstract
Objective
Subpopulations of highly tumorigenic cells, which have the unique capacity to self-renew and produce differentiated progeny, have been identified in multiple malignancies. In head and neck squamous cell carcinoma (HNSCC), this subpopulation of cells, termed cancer stem cells (CSCs) are contained within the population with high CD44 expression. It has been postulated that CSCs play a role in invasion and metastasis; however, there is little evidence to support this theory. We designed in vitro and in vivo models of metastasis to study the behavior of CSCs in HNSCC.
Design
Cells were sorted for CD44 expression using flow cytometry. Sorted cells were used in an in vitro invasion assay. For in vivo studies, CSCs and non-CSCs were injected into the tail veins of mice, and lungs were either harvested or imaged to evaluate for metastases.
Results
In vitro, CD44high cells were more motile but less invasive than CD44low cells. In vivo, 4/5 mice injected with CD44high cells and 0/5 mice injected with CD44low cells formed lung metastases. Two of the metastases arose from CSCs from a primary tumor and three from CSCs from HNSCC cell lines.
Conclusions
In vitro, CSCs do not have an increased ability to invade through basement membrane, but they do migrate more efficiently through a porous barrier. In contrast, CSCs formed metastases quite efficiently in vivo, whereas non-CSCs did not form metastases at all. This phenomenon could be due to enhanced migratory capacity of CSCs, which may be more important than basement membrane degradation in vivo.
Introduction
Worldwide, malignancies of the head and neck account for approximately 500,000 new cases of cancer annually.1 Despite an ever-expanding fund of knowledge regarding the etiology and pathophysiology of malignancies, squamous cell carcinoma of the head and neck (HNSCC) continues to be a disfiguring and deadly disease. For patients with squamous cell carcinoma of the oral cavity or oropharynx, the 5-year survival is a dismal 56%, which has remained relatively unchanged in recent years. 2 This poor prognosis reflects the fact that most patients present with advanced-stage disease, at times making complete cure a seemingly unattainable goal. In fact, only approximately 46% of oral cavity and 16% of oropharyngeal cancers are diagnosed when there is only local disease.2
Subpopulations of highly tumorigenic cells, or cancer stem cells (CSCs), have been identified in multiple tumor types, both solid and hematologic, using a variety of cell markers.3 These cells have the unique capacity to self-renew and produce differentiated progeny. These characteristics allow CSCs to maintain a pluripotent phenotype, while also producing a tumor composed of a heterogeneous cell population. In HNSCC, the CSC population is contained within the cell fraction that expresses high levels of the surface glycoprotein CD44.4 This transmembrane protein serves as a receptor for hyaluronan and, to a lesser extent, for other extracellular matrix components, and its function can be altered with alternative splicing and glycosylation.5 In HNSCC, cells expressing high levels of CD44 have a primitive morphology, express high levels of the stem cell marker BMI-1, and co-stain with cytokeratin 5/14, a basal cell marker. In vivo, CD44high cells are capable of regenerating a heterogeneous tumor, whereas their CD44low counterparts cannot.4
Although CSCs are known to exhibit increased tumorigenicity compared to the rest of the tumor population, it is largely unknown what role they play in local invasion and distant metastatic spread. In light of the fact that most cancer patients die of metastatic disease, it is crucial to elucidate the mechanisms by which cancers access and seed distant tissues. In breast cancer, it has been shown that approximately 30% of patients have occult disseminated tumor cells (DTCs) in their bone marrow. Using CD44+/CD24− co-staining, the majority of the DTCs were found to express a CSC phenotype.6 A link between metastasis and stem-like cells has also been shown in pancreatic cancer, in which co-staining with the CSC marker CD133 and CXCR4 co-staining has been used to identify a metastatic phenotype.7 To help elucidate the role of head and neck CSCs in the spread of cancer cells outside the primary tumor bed, we designed both in vitro and in vivo models of metastasis to study the behavior of this unique tumor cell subpopulation.
Materials & Methods
After obtaining informed consent, tumors were obtained from subjects at the University of Michigan hospital. Animal care and experimental protocols were performed in accordance with procedures and guidelines established by the University Committee on the Use and Care of Animals and the Unit for Laboratory Animal Medicine.
Cell Culture
The following HNSCC cells lines were used: UMSCC-6, a base of tongue (BOT) tumor from a male patient; UMSCC-10A, a tumor of the true vocal cord from a male patient; UMSCC-12, a laryngeal cancer from a male patient; UMSCC-14A, a floor of mouth (FOM) tumor from a female patient; UMSCC-14B, a recurrence of the same cancer; UMSCC-47, a lateral tongue cancer from a male patient; HN-111, a primary tumor of the lateral tongue from a female patient; and UMSCC-103, the cell line derived from HN-111 (Table 1). Cells were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 1% penicillin/streptomycin, and 1% non-essential amino acids (NEAAs).
Table 1.
Characteristics of HNSCC cell lines.
| Cell Line | Patient Gender | Specimen Site | TNM Stage |
|---|---|---|---|
| UMSCC-6 | M | BOT | T2N0M0 |
| UMSCC-10A | M | True vocal cord | T3N0M0 |
| UMSCC-12 | M | Larynx | T2N1M0 |
| UMSCC-14A | F | FOM | T1N0M0 |
| UMSCC-14B | F | FOM | T1N0M0 |
| UMSCC-47 | M | Lateral tongue | T3N1M0 |
| UMSCC-103a | F | Lateral tongue | T4N2bMX |
Abbreviations: BOT = base of tongue, FOM = floor of mouth, HNSCC = head and neck squamous cell carcinoma
UMSCC-103 was derived from primary tumor HN-111.
Luciferase Transduction
The cell line UMSCC-47 was transduced with HIV-Luc, a lentiviral vector containing a pLentilox backbone and a CMV promoter. Polybrane was added to increase efficiency of the transduction. Successful gene delivery was confirmed via GFP visualization in a side-by-side transduction of the HIV-GFP vector under identical conditions.
Primary Tumor Digestion
Tumors were cut into small fragments, further minced with a sterile scalpel, and then placed in a solution of Media 199 and 200 units/ml collagenase III. The mixture was incubated at 37°C for up to 3 h to allow complete digestion. Every 15 min, the solution was mixed through a 10-ml pipette to encourage dissociation. Cells were filtered through 40-μm nylon mesh and washed twice with HBSS/2% Heat Inactivated Calf Serum (HICS), then stained for flow cytometry.
Flow Cytometry
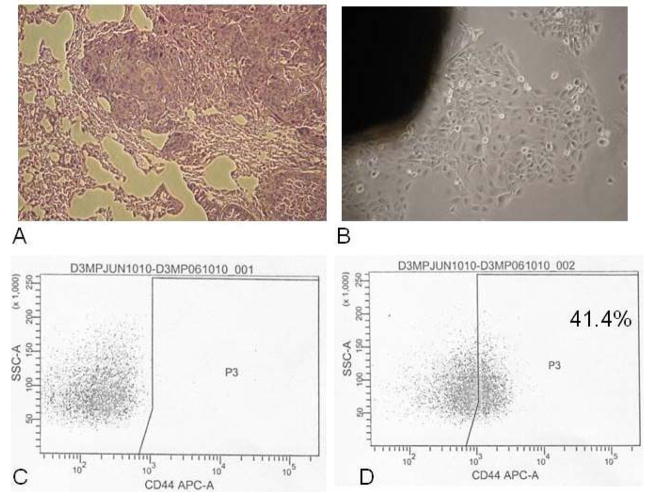
Cells were detached from tissue culture-treated flasks using a 0.125% trypsin solution with EDTA. The single-cell suspensions were washed in Hank’s Balanced Salt Solution containing 2% heat-inactivated calf serum (HBSS/2% HICS), counted, and then resuspended to a concentration of 106 cells per 1 mL HBSS/2% HICS). The suspensions were then incubated with either anti-CD44 antibody (allophycocyanin (APC)-conjugated, mouse anti-human, clone G44–26; BD Pharmingen) or mouse IgG2b κ isotype control antibody (allophycocyanin (APC)-conjugated, clone 27–35; BD Pharmigen), both used at a 1:50 dilution for 15–20 min. For the primary tumor, lineage markers anti-CD2, CD3, CD10, CD16, CD18, CD31, CD64, and CD140b (all diluted 1:50; BD Pharmingen) were used to allow identification of contaminating nontumor cells. Stained cells were washed and resuspended in HBSS/2%HICS containing the dead cell counterstain 4′,6-diamidino-2-phenylindole (DAPI; BD Pharmigen) at 0.5 ml per 106 cells, then immediately taken on ice for analysis and sorting. The 10–15% of cells with the highest and lowest CD44 expression was collected for use (Fig. 1).
Figure 1.
Lung lesion from UMSCC-47 CD44high cells. A. H& E stained section of lung (10x). B. Lung nodule cultured in vitro (20x). C. Fluorescence-activated cell sorting for lung lesion cells stained with CD44 isotype control antibody. D. Fluorescence-activated cell sorting plot for lung lesion cells stained with antihuman CD44 antibody. The CD44high cells reconstituted a heterogeneous population, with 41.4% of the lung lesion cells expressing CD44. APC-A indicates allophycocyanin; SSC-=A, side scatter.
Tail vein injections
CD44high and CD44low cells from HN-111, UMSCC-6, and UMSCC-47-Luc were resuspended in phosphate-buffered saline (PBS) to a concentration of 2.5×105 cells/mL (for cell lines) or 5×104 cells/mL (for primary tumor cells). A total volume of 200 μL of cell suspension was injected into the tail veins of four non-obese diabetic severe combined immunodeficient (NOD-SCID) mice per cell line. Two mice were lost due to a husbandry malfunction prior to bioluminescent imaging (BLI). After the injections with UMSCC-6 and HN-111, mice were sacrificed at 6 months to evaluate for the presence of metastases. Lungs were harvested, embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E). A pathologist confirmed the presence or absence of metastases (Table 2).
Table 2.
CD44 Expression and Lung Colonizationa
| Metastasis | ||||
|---|---|---|---|---|
| CD44high | CD44low | |||
| No. Mice Injected | No. Lung Lesions | No. Mice Injected | No. Lung Lesions | |
| HN-111b (1×104 cells) | 2 | 2 | 2 | 0 |
| UMSCC-6 (5×104 cells) | 2 | 1 | 2 | 0 |
| UMSCC-47 (5×104 cells) | 6 | 2 | 6 | 0 |
| UMSCC-14B (3 × 104 cells) | 2 | 1 | 2 | 0 |
| UMSCC-12 (5 × 104 cells) | 5 | 2 | 5 | 0 |
| Total | 17 | 8 | 17 | 0 |
CD44high and CD44low cells from a primary tumor (HN-111) and 4 cell lines were injected via the tail vein into NOD/SCID mice. Lungs were evaluated for metastasis via sectioning or bioluminescent imaging. CD44high cells formed lung metastases 8/17 times vs. 0/17 times by CD44low cells.
HN-111 later became the cell line UMSCC-103.
Bioluminescent Imaging
Two months after tail vein injections with UMSCC-47-Luc, mice were taken for bioluminescent imaging (BLI). Luciferin (100 μL of a 40 mg/mL suspension) was injected intraperitoneally into each mouse approximately 10 minutes prior to imaging. Mice were anesthetized with isoflurane and imaged in a Xenogen IVIS 200 (Fig. 5).
Figure 5.
Differences in invasion and migration for CD44high vs. CD44low cells. Differences were calculated as % change = [A560 nm for CD44high)−(A560 nm for CD44low)/(A560 nm for CD44low)] × 100%. Invasion was measured using matrigel coted chambers, while migration was calculated using control (uncoated) chambers. Negative values for % change reflect more efficient migration or invasion by CD44high cells.
Boyden Migration Chambers
Two hours prior to use, Matrigel-coated invasion chambers were rehydrated by incubation with DMEM at 37°C. CD44high and CD44low cells were resuspended in DMEM containing 1% FBS, 1% penicillin/streptomycin, and 1% NEAAs. Equal numbers of sorted cells were plated in the upper wells of Matrigel-coated and control chambers (BD BioCoat Matrigel Invasion Chamber; BD Biosciences), with DMEM containing 10% FBS and 30 ng/ml epidermal growth factor (EGF, human recombinant; Sigma-Aldrich) serving as the chemoattractant in the lower well. The chambers were incubated for 24–48 h at 37°C, with the duration dependent on the cell line. After incubation, cells remaining in the upper well were removed by scrubbing twice with a cotton-tipped swab, and cells that had migrated into the lower well were fixed and stained with crystal violet in 20% methanol for 30 min. The chambers were then washed twice in deionized water and allowed to dry. The dried stain was dissolved in 10% acetic acid, and the solution from each chamber was transferred to one well in a 96-well plate. Invasion was then quantified by measuring the absorbance at 560 nm. Control chambers were used to assess motility, while Matrigel chambers served as models for basement membrane invasion (Fig. 2–4).
Figure 2.
CD44 expression in head and neck squamous cell carcinoma lines. Each Bar represents the percentage of the cell population with CD44 expression, with the Threshold for expression defined by staining with an isotype control antibody. The 10–15% of cells with the highest expression were collected as CD44high, and the 10–15% with the lowest or no expression were collected as CD44low cells.
Figure 4.
Matrigel invasion and control migration chamber results. A. Matrigel invasion Chamber results. Invasion was quantified by staining cells in the lower well of the chamber and measuring absorbance from each chamber at 560nm. Absorbance’s for chambers containing CD44high vs. CD44low were compared. B. Control migration chamber results. Motility was quantified by staining cells in the lower well of the chamber and measuring absorbance from each chamber at 560nm. Absorbances for wells containing CD44high and CD44 low were compared. Error bars indicate standard deviations.
Results
A. In Vitro Model of Metastasis
Boyden migration chambers have served as a relatively simple and inexpensive in vitro model of invasion for over a decade. These chambers consist of an upper and a lower well. In the control chambers, the upper and lower wells are separated by a polycarbonate filter with 8-micron pores. Only cells with sufficient motility can migrate into the lower well of the chamber. In the Matrigel-coated chambers, there is a thin layer of Matrigel covering the upper surface of the polycarbonate filter8. Composed of extracellular matrix proteins, this gel layer serves as an analog for the basement membrane. Since the basement membrane serves as a barrier between epithelial or endothelial cells and the underlying stroma, the ability to invade through this line of defense is a key step in the metastatic process. Matrix metalloproteinases, integrins, and other matrix receptors are known to be essential in this pathologic step. CD44, a hyaluronan receptor, has been shown to mediate invasion in both melanoma and gliomas.9,10
Cells expressing the highest levels of CD44 and those expressing little or no CD44 were collected using fluorescence-activated cell sorting (FACS; Figure 1). Control chambers were used to quantify general motility of CD44high and CD44low populations, and Matrigel-coated chambers were used to quantify invasion. Interestingly, for all but one of the cell lines studied, the CD44low cells actually invaded through the Matrigel more efficiently than the CD44high cells. The only exception was UMSCC-12, derived from a laryngeal SCC, for which the CD44high cells were both more motile and more invasive (Fig. 2 & 4). In contrast, the CD44high cells migrated through the control chambers much more efficiently in almost all cell lines and, therefore, were more motile than their CD44high counterparts. UMSCC-14B, which arose from a recurrent SCC of the floor of mouth, was the only cell line in which low CD44 expression predicted better motility (Fig. 3 & 4).
Figure 3.
In vivo model of metastasis. Bioluminescence imaging of nonobese diabetic severe combined immunodeficient mice 8 weeks after tail vein injection with UMSCC-47 cells Transduced with luciferase. Left, injected with 5 × 104 cells with low CD44 expression; middle, Injected with high CD44 expression; and right, no injection. Min indicates minimum; Max, maximum; and sr, steradian
B. In Vivo Model of Metastasis
To study the effect of CD44 expression on metastasis in a more nuanced environment, an animal model was used. NOD-SCID mice were injected via the tail vein with either CD44high or CD44low cells. The presence of metastases was assessed either via necropsy and histologic examination six months post-injection (older method) or via luciferase-mediated bioluminescence two months post-injection (newer method). Using the older method, 3/4 mice injected with CD44high cells developed metastases, while only 0/4 injected with CD44low cells had lung lesions. Notably, the single cell suspension derived from a primary tumor formed metastases in 2/2 mice after injection with only 1×104 cells, while the established cell line UMSCC-6 grew a metastasis in 1/2 mice after injection of 5×104 cells (Table 2).
Bioluminescent imaging (BLI) was used to assess for the presence or absence of metastases after tail vein injection with UMSCC-47-Luc cells. These cells express luciferase, an enzyme that oxidizes luciferin in the presence of ATP to give off non-visible light, which is then captured with a coupled-charge device (CCD) camera. BLI is a well-established technique for studying tumor growth and metastasis, among many other applications.11 Four mice were injected with UMSCC-47-Luc, two with CD44high cells and two with CD44low cells. Unfortunately, two mice were not evaluable. Two months post-injection, the two remaining mice were imaged with BLI. The mouse injected with CD44high cells had bilateral lung metastases, while the injection with CD44low cells did not produce any signs of disease (Fig. 5). Overall, CSCs formed metastases in 4/5 mice, while the non-CSCs did not produce metastases.
Discussion
The finding that CSCs, as identified by high CD44 expression, were not more invasive than non-CSCs in vitro could be attributed to many factors. First, it may be due to shortcomings of the experimental model, which is a simplified representation of a complex system. Although Matrigel invasion chambers have been used in many experimental designs, they only represent the first steps in a long chain of events that must occur for a cell to successfully metastasize. As Paget described in the 19th century, the process of metastasis follows a seed-and-soil model. The cells (“seeds”) need to have the appropriate mechanisms in place to dissociate from the primary tumor, enter into the lymphatics or bloodstream, and escape the circulation to find a new home. In addition, the site of metastasis (“soil”) must be properly suited to signal to the circulating cells and allow a new tumor to form from them. This is often referred to as the tumor microenvironment, and its key attributes are still poorly understood.
While our model used EGF and serum as chemoattractants, both commonly described in the literature, perhaps these are not the signaling elements that entice a HNSCC cancer cell to metastasize in vivo. Matrigel is primarily composed of laminin, collagen IV, and heparan sulfate proteoglycans.12 Since CD44 is primarily a receptor for hyaluronan, it seems plausible that Matrigel is not an appropriate model for studying this surface protein. Draffin et al. studied in vitro invasion of two prostate cancer cell lines, one with and one without CD44 expression, using Matrigel invasion chambers. Although the CD44(+) cell line showed a significant increase in invasion when Matrigel chambers were supplemented with hyaluronan, the CD44(−) cell line invaded more efficiently when chambers were not supplemented.13
The lack of correlation between high CD44 expression and invasion may also be related to the limitations of using a single cell marker to identify CSCs. Previous studies of CSCs and metastasis in other tumor types have used two or three markers to identify the metastatic subset.6,7 It is likely that the same is true for head and neck cancer stem-like cells. Metastasis formation is a complex process and, although CD44 may mediate one or two pivotal steps in this series of events, it is plausible that there are additional crucial cell characteristics. For example, the relatively poor ability of CD44high cells to invade through the basement membrane may represent decreased expression of matrix metalloproteinases in these cells.
In contrast, the results from our animal model strongly suggest that HNSCC stem-like cells have enhanced metastatic potential. The somewhat contradictory results between our models are likely due to their significant differences in design and intricacies. Notably, the duration of the in vivo experiments was much longer than that of the in vitro assays. This time lapse could have allowed the CD44high cells to alter their expression profiles significantly, so that they expressed the factors necessary for invasion out of the bloodstream and into the tissues.
The epithelial-to-mesenchymal transition (EMT) is well described in the embryology literature; in addition, it is thought to mediate invasion of cancer cells into the surrounding stroma. EMT occurs when, in response to transforming growth factors (TGFs) or other signals, cells dissociate from one another, lose expression of epithelial markers and gain expression of mesenchymal ones, alter their polarization and cytoskeletal structure, and establish new cell-matrix interactions. A similar process is required of cancer cells that are destined to metastasize.14
The increased motility seen in CD44high cells is characteristic of cells undergoing EMT, and this may explain why head and neck CSCs metastasized in vivo while non-CSCs did not in our study. In fact, Takahashi et al. showed that, in EMT induced by tumor necrosis factor (TNF), the interaction between CD44 and hyaluronan mediated cell-cell dissociation, actin remodeling and, as a result, enhanced motility.15 These findings, in conjunction with our own, suggest that cell motility and ability to undergo EMT are some of the most important characteristics of a metastatic cell, and it appears that CSCs may have those capabilities.
Future studies focused on better understanding the role of CSCs in EMT as it relates to HNSCC are needed. In addition, further purification of the stem-like cell population in HNSCC is necessary to clarify what metastatic characteristics are indeed unique to these cells. Such knowledge would allow clinicians to exploit this particular set of attributes to target cancer cells that can keep a tumor growing and allow it to spread.
Acknowledgments
This project was made possible by funding from the University of Michigan Comprehensive Cancer Center, the American Academy of Otolaryngology-HNS Percy Memorial Award, the Kirschstein National Research Service Award (NRSA) in Advanced Research Training in Otolaryngology: 5 T32 DC005356, and a gift from Patricia Korican of Korican Real Estate. None of the funders had any role in the design, conduct, or interpretation of the above-mentioned experiments. The principal investigators in this study had full access to all data and take responsibility for the integrity of the data and the accuracy of the data analysis. Thank you to the Flow Cytometry Core, the Vector Core, the Center for Molecular Imaging, and the Department of Pathology at U-M for their help in acquiring this data. Thanks to Martin Graham for his assistance with the animal work, and to the Chinnyian lab for the use of their equipment.
Footnotes
Presented at the American Head and Neck Society 2010 Annual Meeting in Las Vegas, Nevada on April 29, 2010.
References
- 1.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Carvalho AL, Nishimoto IN, Califano JA, et al. Trends in incidence and prognosis for head and neck cancer in the United States: a site-specific analysis of the SEER database. Int J Cancer. 2005;114:806–16. doi: 10.1002/ijc.20740. [DOI] [PubMed] [Google Scholar]
- 3.Boman BM, Wicha MS. Cancer stem cells: a step toward the cure. J Clin Oncol. 2008;26:2795–9. doi: 10.1200/JCO.2008.17.7436. [DOI] [PubMed] [Google Scholar]
- 4.Prince ME, Sivanandan R, Kaczorowski A, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 2007;104:973–8. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rudzki Z, Jothy S. CD44 and the adhesion of neoplastic cells. Mol Pathol. 1997;50:57–71. doi: 10.1136/mp.50.2.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balic M, Lin H, Young L, et al. Most early disseminated cancer cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotype. Clin Cancer Res. 2006;12:5615–21. doi: 10.1158/1078-0432.CCR-06-0169. [DOI] [PubMed] [Google Scholar]
- 7.Hermann PC, Huber SL, Herrler T, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–23. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Kleinman HK, Jacob K. Invasion assays. Curr Protoc Cell Biol. 2001;Chapter 12(Unit 12):2. doi: 10.1002/0471143030.cb1202s00. [DOI] [PubMed] [Google Scholar]
- 9.Merzak A, Koocheckpour S, Pilkington GJ. CD44 mediates human glioma cell adhesion and invasion in vitro. Cancer Res. 1994;54:3988–92. [PubMed] [Google Scholar]
- 10.Knutson JR, Iida J, Fields GB, et al. CD44/chondroitin sulfate proteoglycan and alpha 2 beta 1 integrin mediate human melanoma cell migration on type IV collagen and invasion of basement membranes. Mol Biol Cell. 1996;7:383–96. doi: 10.1091/mbc.7.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Neill K, Lyons SK, Gallagher WM, et al. Bioluminescent imaging: a critical tool in pre-clinical oncology research. J Pathol. 220:317–27. doi: 10.1002/path.2656. [DOI] [PubMed] [Google Scholar]
- 12.Kleinman HK, McGarvey ML, Liotta LA, et al. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry. 1982;21:6188–93. doi: 10.1021/bi00267a025. [DOI] [PubMed] [Google Scholar]
- 13.Draffin JE, McFarlane S, Hill A, et al. CD44 potentiates the adherence of metastatic prostate and breast cancer cells to bone marrow endothelial cells. Cancer Res. 2004;64:5702–11. doi: 10.1158/0008-5472.CAN-04-0389. [DOI] [PubMed] [Google Scholar]
- 14.Iwatsuki M, Mimori K, Yokobori T, et al. Epithelial-mesenchymal transition in cancer development and its clinical significance. Cancer Sci. 101:293–9. doi: 10.1111/j.1349-7006.2009.01419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takahashi E, Nagano O, Ishimoto T, et al. Tumor necrosis factor-alpha regulates transforming growth factor-beta-dependent epithelial-mesenchymal transition by promoting hyaluronan-CD44-moesin interaction. J Biol Chem. 285:4060–73. doi: 10.1074/jbc.M109.056523. [DOI] [PMC free article] [PubMed] [Google Scholar]