Abstract
Retinoids are ubiquitous signaling molecules that influence nearly every cell type, exert profound effects on development, and complement cancer chemotherapeutic regimens. All-trans retinoic acid (RA) and other active retinoids are generated from vitamin A (retinol), but key aspects of the signaling pathways required to produce active retinoids remain unclear. Retinoids generated by one cell type can affect nearby cells, so retinoids also function in intercellular communication. RA induces differentiation primarily by binding to RARs, transcription factors that associate with RXRs and bind RAREs in the nucleus. Binding of RA: (1) initiates changes in interactions of RAR/RXRs with co-repressor and co-activator proteins, activating transcription of primary target genes; (2) alters interactions with proteins that induce epigenetic changes; (3) induces transcription of genes encoding transcription factors and signaling proteins that further modify gene expression (e.g., FOX03A, Hoxa1, Sox9, TRAIL, UBE2D3); and (4) results in alterations in estrogen receptorα signaling. Proteins that bind at or near RAREs include Sin3a, N-CoR1, PRAME, Trim24, NRIP1, Ajuba, Zfp423, and MN1/TEL. Interactions among retinoids, RARs/RXRs, and these proteins explain in part the powerful effects of retinoids on stem cell differentiation. Studies of this retinol signaling cascade enhance our ability to understand and regulate stem cell differentiation for therapeutic and scientific purposes. In cancer chemotherapeutic regimens retinoids can promote tumor cell differentiation and/or induce proteins that sensitize tumors to drug combinations. Mechanistic studies of retinoid signaling continue to suggest novel drug targets and will improve therapeutic strategies for cancer and other diseases, such as immune-mediated inflammatory diseases.
Keywords: cancer, epigenetics, receptor, review, transcription
Vitamin A (all-trans retinol) and its active metabolites, collectively called retinoids, regulate many events in patterning during vertebrate development and control many aspects of cell proliferation, differentiation, and apoptosis (Gudas, 1994; Dolle, 2009; Mark et al., 2009). Two types of transcription factors, the retinoic acid receptors (multiple isoforms of RARα, β, and γ) and the retinoid X receptors (multiple isoforms of RXRα, β, and γ), mediate the majority of the actions of the biologically active retinoid all-trans retinoic acid (RA) and specific metabolites of RA. This review focuses on the molecular actions of retinoids in the regulation of cell differentiation. The questions that will be addressed are: (1) How are biologically active retinoids generated? (2) Why is all-trans retinoic acid (RA) such a powerful inducer of stem cell differentiation? (3) What are the early events in the differentiation process? (4) How can additional research exploit the unusual ability of RA and related retinoids to regulate stem cell differentiation? (5) How can the recent molecular discoveries concerning retinoid induction of cell differentiation be used to improve differentiation therapy for cancer? Other areas of retinoid biology and pharmacology have recently been reviewed, including retinoic acid receptor (RAR) actions during development (Dolle, 2009; Mark et al., 2009), genomic and non-genomic activities of the RARs (Rochette-Egly and Germain, 2009), retinoid related orphan receptors (Jetten, 2009), RA regulation of apoptosis (Noy, 2010), retinoids and cancer prevention and treatment (Fields et al., 2007; Mongan and Gudas, 2007; Tang and Gudas, 2011), RA effects on embryonal carcinoma and embryonic stem cells (Soprano et al., 2007), and synthetic RAR and RXR ligands (Dawson and Zhang, 2002).
How is the Biologically Active Signaling Molecule, RA, Generated?
To understand the roles of retinoids in cell differentiation it is essential to define the roles of biologically active retinoids in terms of their regulation of transcription and their retinoid precursors stored in cells and tissues. First, all retinoids are obtained from the diet as dietary vitamin A (retinol) itself, as a retinyl ester, or as the proretinoid carotenoid β-carotene (Blaner and Olson, 1994). Because the biologically active retinoids that promote differentiation in vivo are generally synthesized locally and act locally, it is important to learn more about how the metabolism of vitamin A to bioactive retinoids is regulated and how the biologically active retinoids are further metabolized.
Retinol from the diet is transported in the blood throughout the body. It is transported bound to a protein called serum retinol binding protein (sRBP or RBP4; Mouse GENE ID: 19662). The recently identified, multi-transmembrane protein Stra6 (GENE ID: 20897) binds vitamin A in a complex with RBP4 (Kawaguchi et al., 2007), (Fig. 1). The enzyme LRAT (lecithin:retinol acyl transferase), which esterifies vitamin A within the cell (Liu and Gudas, 2005; Kawaguchi et al., 2007; Kim et al., 2008; Wu and Ross, 2010), is also required for robust uptake of vitamin A via Stra6 (Kawaguchi et al., 2007) (Fig. 1). This allows for delivery of vitamin A to defined cell types in the body with high specificity. Stra6 is expressed at high levels at blood/organ barriers and in various different cell types during development and in the adult (Bouillet et al., 1997), and high Stra6 expression is suggestive of a requirement for the actions of retinol.
Fig 1.
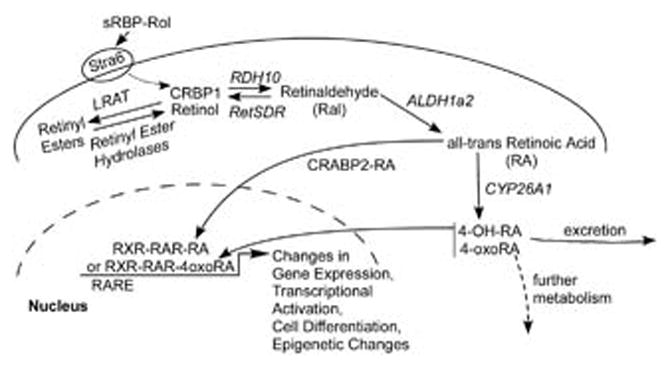
Uptake and intracellular metabolism of retinol (modified from Mongan and Gudas, [2007]).
Since retinaldehyde (Ral) can also be generated enzymatically from the dietary precursor β-carotene, this process must be regulated to generate appropriate amounts of retinaldehyde. Retinaldehyde is then metabolized to retinol or to all-trans retinoic acid (RA). The transcription factor Isx (GENE ID: 71597) regulates the maintenance of appropriate vitamin A levels via regulation of β-carotene 15,15′-monooxygenase (Bcmo1; GENE ID: 63857), the enzyme that cleaves β-carotene to form retinaldehyde in the intestine. The intestine is the organ involved in the initial uptake of retinoids and carotenoids from the diet (Seino et al., 2008). Bcmo1 is also highly expressed in hepatic stellate cells, which are an important cellular site in the liver where dietary β-carotene metabolites (i.e. retinaldehyde, retinyl esters) accumulate (Shmarakov et al., 2010). Indeed, hepatic stellate cells store about 90–95% of the vitamin A present in the liver as retinyl esters (Shmarakov et al., 2010).
Once inside the cell, vitamin A can be metabolized by several different enzymes; however, only some cell types have the ability to metabolize vitamin A to RA during development and in the adult. In numerous cell types RDH10 (short-chain dehydrogenase/reductase, retinol dehydrogenase 10; GENE ID: 98711) is the primary enzyme that metabolizes vitamin A to retinaldehyde in a NAD+ dependent manner. Subsequently, RA is formed by oxidation of retinaldehyde, primarily via ALDH1a2 (RALDH2) (GENE ID:19378) (Sandell et al., 2007; Cammas et al., 2007; Belyaeva et al., 2008) (Fig. 1). Thus, biologically active retinoids are generally synthesized locally. How this metabolism of vitamin A to RA is regulated is an important topic that requires further research.
Another important question in the retinoid research field is whether RA can also be transported from one cell to another, and if so, how this is accomplished. There are data from the developing embryo that suggest that RA synthesized in one cell type can act on another adjacent cell type (Matt et al., 2005; Stafford et al., 2006; Duester, 2008; Siegenthaler et al., 2009; Rosselot et al., 2010). For example, RAR signaling in developing ureteric bud cells requires RA generated in nearby stromal cells by the enzyme ALDH1a2 (Rosselot et al., 2010), an enzyme needed for most fetal RA synthesis (Fig. 1). While paracrine RA signaling is a common theme in development, there is not enough information available about whether RA itself moves between cells or whether signaling molecules produced in response to RA also play a role in such paracrine signaling. The mechanisms by which RA might move from cell to cell and whether RA itself or more polar forms of RA, such as all-trans 4-oxo-RA, are capable of moving from cell to cell must still be explored in depth.
The more polar metabolites of RA, such as 4-hydroxy-retinoic acid (4-OH-RA), 18-hydroxy-retinoic acid (18-OH-RA), 4-oxo-retinoic acid (4-oxo-RA), and 5,6-epoxy-retinoic acid (5,6-epoxy-RA), are generally viewed as biologically inactive products of RA catabolism (e.g., Niederreither et al., 2002). They are produced from all-trans retinoic acid by the cytochrome P450 family of enzymes Cyp26a, b, and c (Fig. 1) (Abu-Abed et al., 2001). However, these polar RA metabolites have biological activity in some systems (Pijnappel et al., 1993; Sonneveld et al., 1999; Idres et al., 2001; Baron et al., 2005; Langton and Gudas, 2008), and they can bind to the RARs with high affinity (Idres et al., 2002). The specific functions of the more polar RA metabolites remain unclear.
Retinol itself is also enzymatically converted to polar metabolites with biological activity (Achkar et al., 1996; Blumberg et al., 1996; Lane et al., 2008; Liu et al., 2009) and retinol can also induce the differentiation of cultured ES cells, though it is not as potent as RA (Lane et al, 2008). In fact, the major bioactive retinoid stored in the Xenopus egg and early embryo is all-trans 4-oxoretinaldehyde, which is capable of binding to and transactivating RARs. 4-Oxoretinaldehyde is also a metabolic precursor of two RAR ligands, 4-oxoretinoic acid and 4-oxoretinol (Achkar et al., 1996; Blumberg et al., 1996). The regulated metabolism of retinoids in various cell types and the ability of various cell types to store retinol or retinol metabolites in the ester form are undoubtedly some of the properties that create specificity of this signaling pathway, but it is equally important to decipher the mechanisms by which each retinoid signals to the transcription machinery.
How does RA Activate Transcription in Stem Cells?
Inside the cell, RA is transported to the nucleus bound to CRABP2 (cellular retinoic acid binding protein 2; GENE ID: 12904) (Delva et al., 1999; Schug et al., 2007). Once in the nucleus, RA binds to RARα, β, or γ. These RARs can bind to one of the RXRs (RXRα, β, or γ). When the agonist RA is present, this RXR/RAR heterodimer complex, bound to DNA, activates transcription of RA primary response genes (Fig. 2). Activation of transcription is one of the first steps in the RA associated differentiation process. It occurs rapidly, within minutes to a few hours, after RA addition in cell culture experiments. Numerous “immediate early” genes, such as the transcription factor homeobox gene Hoxa1 (LaRosa and Gudas, 1988; Langston and Gudas, 1992), are direct, “primary response” targets of RA and possess enhancers (a DNA sequence to which transcription factors bind) containing an RARE (retinoic acid DNA response elements, e.g. Direct Repeat5 [GGTTCA(N5)AGTTCA]) to which the RXR/RAR heterodimer can bind (Fig. 1). Other genes have more complex RAREs (e.g. Das et al., 2007). At later times after RA addition, many genes are transcriptionally regulated indirectly and don’t possess RAREs. This indirect regulation occurs because the direct target genes of RA include many different transcription factor genes; these transcription factors then transcriptionally activate their target genes to generate secondary responses (Fig. 2). Thus, a gene can be transcriptionally activated in response to RA and yet not possess an enhancer with an RARE if it is a “secondary” target gene.
Fig 2.
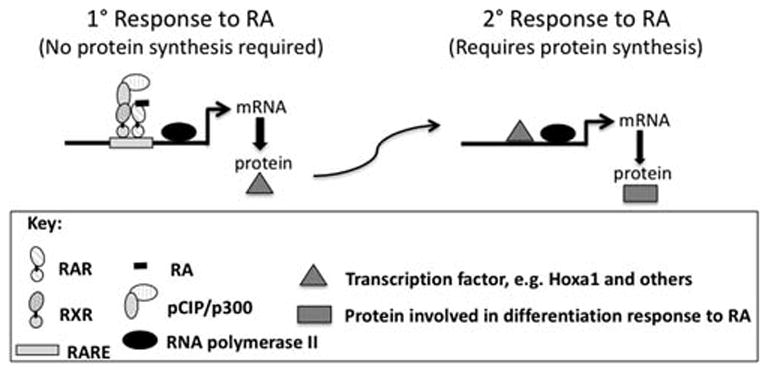
Primary versus secondary transcriptional responses to RA.
At certain primary, RA-responsive genes, the RXR/RAR complex binds to highly compacted, higher order chromatin, leading to recruitment of the ATP-dependent chromatin remodeling SWI/SNF complex and the binding of the protein NF1 to nucleosomal DNA (Li et al., 2010). NF1 (nuclear factor-1) then stabilizes the open nucleosome structure of the RA-responsive gene, allowing RNA polymerase II and other general transcription factors to activate transcription (e.g. phosphoenolpyruvate carboxykinase (PEPCK), see Li et al., 2010). This ability of the RA agonist to alter the structure of the RXR/RAR protein complex and facilitate binding of this heterodimer to compacted, higher order chromatin near RA primary target genes leads to cell lineage-specific, epigenetic modifications (i.e., inherited changes in gene expression that don’t involve changes in the DNA sequence but rather involve changes in the modifications, such as methylation or acetylation, on histones) at these RA primary target genes. While it has not yet been demonstrated for RA-regulated genes, we speculate that RA addition leads to major intra- and interchromosomal transcription “interactomes” so that active, RA co-regulated genes and their regulatory factors cooperate to generate specialized nuclear areas for coordinated transcriptional control. For example, such “transcription factories” have been shown to occur during erythroid differentiation (Schoenfelder et al., 2010) (Fig. 3). These RA-associated, epigenetic modifications of histones ultimately result in the modification of transcriptional circuits involving multiple, RA-regulated genes and ultimately, the fixation of differentiated cell lineages (Mongan and Gudas, 2007).
Fig 3.
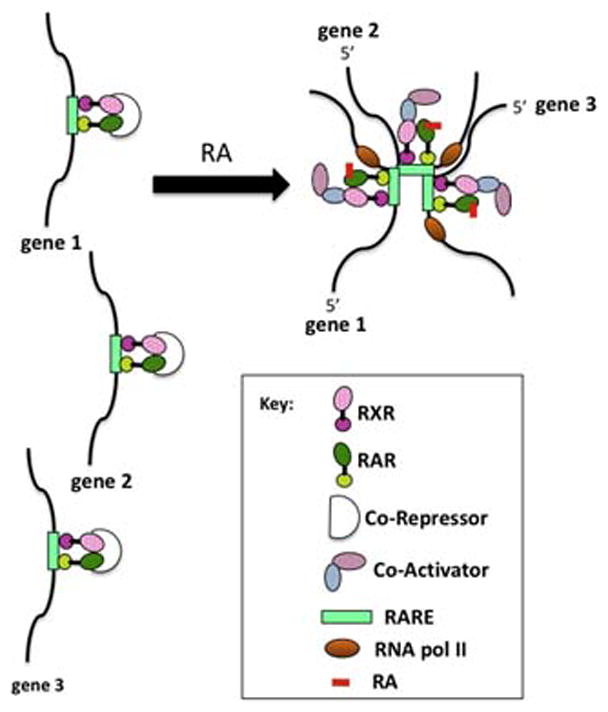
Hypothetical depiction of transcriptional activation at “transcription” factories in response to RA. Genes 1, 2, and 3 may be on different chromosomes.
Additional proteins, including co-activators and co-repressors, bind to the RXR/RAR complex and regulate the sensitivity of cells to RA’s differentiation-inducing effects and the effects of other signaling pathways. The RXR/RAR heterodimer binds co-activator proteins, such as pCip (GENE ID: 17979, Ncoa3, also known as Actr; Aib1; Rac3; Src3; p160; Tram1), and recruits these proteins to RA “primary target“ gene enhancers (Fig. 4). Co-activator recruitment is dependent on the appropriate RAR being bound at the RARE of the target gene (Gillespie and Gudas, 2007a; Gillespie and Gudas, 2007b). Specific RARs (α, β, or γ) are required for specific actions of RA in various cell types (e.g., Boylan et al., 1993; Chen et al., 2004; Glasow et al., 2005; Purton et al., 2006; Yasuhara et al., 2010; Shimono et al., 2010).
Fig 4.
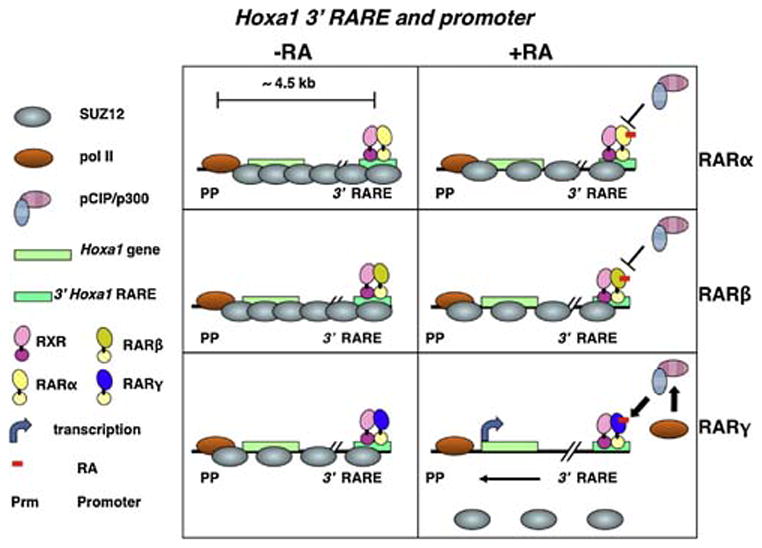
RA activates transcription by recruiting co-activators (e.g., pCIP/p300) and influencing chromatin modifying proteins (e.g., Suz12, a Polycomb group protein) if the appropriate RAR/RXR complex is bound to the RARE (Gillespie and Gudas, [2007a], [2007b]).
The RAR/RXR complex is itself a target of other signaling pathways; e.g., the phosphorylation status of the RARs is also a key factor in their ability to function as transcription factors in controlling cell differentiation (Gaillard et al., 2006; Nasr et al., 2008; Bruck et al., 2009; Wang et al., 2010; Santos and Kim, 2010). Thus, retinoids provide an essential, early signal that initiates a cascade of events leading to the differentiation of both totipotent stem cells and many types of lineage restricted stem cells.
Despite its importance, much less research has focused on how RA signaling leads to transcriptional repression. The Rex1 (Zfp42, GENE ID: 22702) gene is expressed at high levels in embryonic stem cells (Hosler et al., 1989; Hosler et al., 1993; Ben-Shushan et al., 1998), and is transcriptionally inhibited by RA. The mechanism of inhibition involves loss of binding of the positively acting transcription factor Oct4 (Pou5F1; GENE ID: 18999) to an Oct4 site in the Rex1 promoter in response to RA (Hosler et al., 1993). Interestingly, transcriptional repression by RA in embryonic stem cells is often mediated by a different mechanism, an increase in the expression of the orphan nuclear receptor GCNF (germ cell nuclear receptor) (Nr6a1 GENE ID: 14536), which then represses pluripotency genes such as Sox2, Nanog, or Oct4 (Gu et al., 2005; Akamatsu et al., 2009). GCNF mRNA and protein expression is induced in ES cells upon RA treatment, consistent with such a role for this nuclear receptor (Gu et al., 2005) (Fig. 5).
Fig 5.
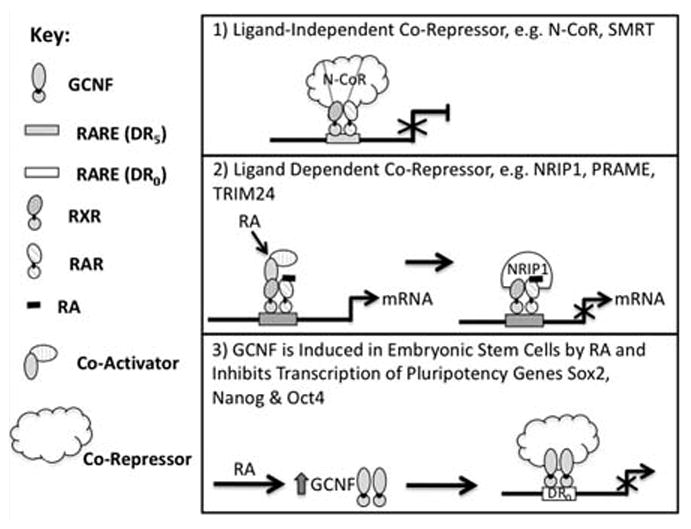
Repression of transcription: various mechanisms 1) and 2) co-repressors act at RAREs to block transcriptional activation. 3) Co-repressor/GCNF complex acts to inhibit gene transcription in response to RA.
RA Signaling Activates Transcription and Antagonizes the Actions of Polycomb Genes in Stem Cells and in Cancer Cells
Both transcriptional repression in the absence of RA and transcriptional activation in the presence of RA require a large number of proteins that form multi-protein complexes that bind specific regions of DNA/chromatin (Xu et al., 1999; Perissi et al., 2010; le Maire et al., 2010), so RAR/RXRs must interact with other proteins to change the transcription status of genes (Mongan and Gudas, 2007). For example, retinoid regulation of gene expression often involves interaction with polycomb group (PcG) proteins (Gillespie and Gudas, 2007a; Gillespie and Gudas, 2007b; Lee et al., 2007; Sessa et al., 2007; Amat and Gudas, 2010) (Fig. 4). PcG proteins (e.g. Suz12, EZH2) can form large complexes of gene-silencing proteins that are widely distributed during embryogenesis and play a major role in patterning and differentiation (Simon and Kingston, 2009). PcG proteins act as global cellular regulators for maintenance of epigenetically repressed states. In embryonic stem (ES) cells PcG proteins actively repress genes important to embryonic development and cell fate decisions. The mechanism by which these PcG proteins are recruited to specific regions of mammalian DNA is not known. In untreated stem cells, RA primary target genes, such as the homeobox gene Hoxa1, Cyp26a1, and retinoic acid receptor β2 (RARβ2), are coated with PcG proteins. Following RA addition to the stem cells, there is a rapid dissociation of the PcG proteins from these RA target genes by a mechanism that is not fully understood (Fig. 4) (Gillespie and Gudas, 2007a; Gillespie and Gudas, 2007b; Lee et al., 2007; Kashyap and Gudas, 2010; Kashyap et al, (2010)). Clearly, there is a functional and antagonistic relationship between retinoid signaling and the presence of these PcG proteins that helps to maintain stem cell characteristics.
In fact, sustained, ectopic expression of the PcG protein CBX7 in teratocarcinoma stem cells confers both resistance to retinoic acid-induced differentiation and a growth advantage. This resistance to RA-induced differentiation is associated with increased promoter methylation of many genes (Mohammad et al., 2009). Polycomb group proteins are also involved in the establishment and maintenance of the silencing of tumor suppressor genes during neoplastic transformation induced by the PML-RARα fusion protein in acute promyelocytic leukemia (Martens et al., 2010; Villa et al., 2007). Similarly, the fusion protein TMPRSS2-ERG promotes prostate cancer progression by disrupting lineage-specific differentiation and potentiating the PcG protein EZH2-mediated dedifferentiation program (Yu et al., 2010), indicating that dysregulation of PcG proteins is a common theme in cancer.
Moreover, targeted, pharmacologic disruption of the PcG protein, EZH2, by the S-adenosylhomocysteine hydrolase inhibitor 3-deazaneplanocin A or specific reduction of EZH2 by short hairpin RNA (shRNA) limits glioblastoma multiforme cancer stem cell (CSC) self-renewal and tumor-initiating capacity (Suva et al., 2009). It would, therefore, be of great interest to apply RA in combination with a drug, such as 3-deazaneplanocin A, which reduces the activity of the PcG protein EZH2, for differentiation therapy to treat glioblastoma multiforme.
Transcription Regulatory Proteins Enhance the Actions of the Retinoic Acid Receptors: Implications for the Use of RA in Differentiation Therapy for Cancer
Additional transcription factors modulate RA signaling and induction of differentiation in a positive manner. For example, Rere (atrophin2, Gene ID: 68703), which is present in a complex with Nr2f2 (Gene ID: 7026, COUP-TF2), p300 (Ep300, Gene ID: 328572) and RXR/RAR at the RAREs, enhances RA’s actions on target genes during development (Vilhais-Neto et al., 2010).
In another example, neuroblastoma cells differentiate in response to RA (Sidell et al., 1983; Turano et al., 2006; Muley et al., 2008); and, after completion of chemoradiotherapy, differentiation therapy with RA significantly improves survival in high risk neuroblastoma patients (Reynolds et al., 2003). More recently, differentiation therapy with RA plus histone deacetylase inhibitors, drugs which modify the epigenetic state of cells, was shown to be a powerful combination for neuroblastoma treatment (Hahn et al., 2008). An interaction of the zinc-finger protein Zfp423 (Gene ID: 94187) with the RXRα/RARα complex at RAREs was recently shown to be required for RA to induce cell proliferation arrest and differentiation of neuroblastoma cells (Huang et al., 2009). Thus, Zfp423 is a key co-activator of RXR/RAR signaling. Reduced levels of Zfp423 result in resistance of the neuroblastoma cells to differentiation induced by RA and are associated with a poor clinical prognosis (Huang et al., 2009). Repression of ZNF423 (the human homolog of murine Zfp423) expression in neuroblastoma can result from activation of the RAS-MEK pathway. Recently, an RNAi genetic screen identified NF1 (neurofibromatosis 1, GENE ID: 18015) as a tumor suppressor gene in neuroblastoma. Loss of NF1 expression or mutation of NF1 causes increased activation of the RAS-MEK pathway and subsequent repression of ZNF423 expression with loss of RA-associated differentiation (Holzel et al., 2010). Holzel et al. (2010) suggest the use of a MEK inhibitor to overcome RA resistance in NF1 deficient human neuroblastomas. Zfp423 is also a transcriptional regulator of preadipocyte determination (Gupta et al., 2010), and its activity is required for normal cerebellar development (Warming et al., 2006).
RA has antitumor effects in nestin+ and CD133+ human stem-like glioma cells via its ability to induce differentiation of these cells (Campos et al., 2010). Thus, it will be of interest to determine if the activity of Zfp423 is required for RA to induce differentiation of these glioma cells as it is in neuroblastoma cells.
Transcription Regulatory Proteins Reduce the Activity of RA in the Induction of Cell Differentiation
Alterations in retinoid signaling occur during the development of many types of cancer, and retinoid-dependent signaling pathways provide attractive targets for therapy (Mongan and Gudas, 2007; Tang and Gudas, 2011). Indeed, retinoids are a key component of some types of cancer therapy. However, there are many transcription factors and co-repressors that block or reduce the ability of RA to signal and induce cell differentiation, and some of these proteins may limit the effectiveness of RA in cancer treatment regimens (Fig. 5). For instance, overexpression of the “zinc finger” protein Zfp42 (Rex1, Gene ID: 22702) reduces RA-induced ES cell differentiation (Scotland et al., 2009). ZNF536 (Gene ID: 243937), a novel zinc finger protein specifically expressed in the brain, inhibits neuronal differentiation by repressing RA-induced gene transcription (Qin et al., 2009). Forced overexpression of ZNF536 also inhibits RA-induced P19 teratocarcinoma stem cell differentiation (Qin et al., 2009).
The multiple LIM domain protein, Ajuba (Jub, Gene ID: 16475), selectively interacts with the RXR/RAR complex in stem cells in the absence of ligand at RAREs of primary target genes to repress them (Hou et al., 2010). Addition of the agonist RA leads to the dissociation of Ajuba from these RAREs (Hou et al., 2010). Thus, Ajuba, though structurally different, behaves in a manner similar to the well known “ligand independent” co-repressors N-CoR (NCoR1) and SMRT (NCoR2) (Perissi et al., 2010) (Fig. 5). Strikingly, N-CoR is overexpressed in human glioblastoma multiforme specimens, and treatment of glioma cells with RA plus a low-dose of the phosphatase inhibitor okadaic acid resulted in inhibition of cell proliferation and increased cell differentiation (Park et al., 2007).
RA-induced recruitment of NRIP1 (RIP140; Gene ID: 268903) to endogenous RA target genes after short term RA treatment correlates with reduced induction of gene expression (Hu et al., 2004; Heim et al., 2007) (Fig. 5). Thus, NRIP1 acts as a ligand-dependent co-repressor in P19 murine embryonal carcinoma cells and in human embryonal carcinoma cells.
The protein PRAME (RP23, Gene ID: 75829) is a dominant repressor of RAR actions, and overexpression of PRAME blocks RA-induced ES cell differentiation (Epping et al., 2005). Zfp206 (Zscan10, GENE ID: 332221) is a transcription factor that is expressed at high levels in ES cells and its expression level decreases rapidly in response to RA. Engineered overexpression of Zfp206 in ES cells blocks RA-induced ES cell differentiation (Wang et al., 2007) (Fig. 5). NRIP, PRAME, and TRIM24 (GENE ID: 21848) are recruited to the RA bound RXR/RAR heterodimer via LxxLL motifs, but these proteins also contain repressor domains (Gurevich et al., 2007). Importantly, the ligand-dependent co-repressor TRIM24 (Tif1α) acts as a tumor suppressor in the liver; without TRIM24, RARα shows oncogenic activity in the liver (Khetchoumian et al., 2007).
The “orphan” nuclear receptor Nr2F1 (COUP-TF1) can inhibit the induction of the homeobox gene cdx1 by RA (Beland and Lohnes, 2005). The MN1/TEL fusion protein, found in acute myeloid leukemia patients, prevents RXR/RAR mediated transcription by not allowing co-activator complexes to be recruited to the RXR/RAR complex (van Wely et al., 2007).
In most of these examples, solid evidence is presented that these inhibitory proteins are in a complex with the RXR/RAR heterodimer at RAREs of target genes, indicative of inhibitory actions on primary target genes positively regulated by RA; but, in other examples, less mechanistic information is available. From these examples it is clear that normal stem cells require mechanisms to regulate the intensity and duration of RXR/RAR transcriptional activity and that this is accomplished in part by signal modulation via proteins that interact with RXR/RAR complexes. It is also evident that in various types of cancer cells these inhibitory proteins, when aberrantly expressed, can lead to aberrant RA signaling and/or reduce the effectiveness of RA as a therapeutic agent.
Key Transcription Factors are Induced by the RARs
RA is a potent and effective inducer of embryonic stem cell differentiation (e.g., Tighe and Gudas, 2004). Several transcription factors and other proteins act “downstream” of RA and these transcription factors are often essential for mediating RA’s differentiation inducing effects in stem cells and other cell types. These transcription factors mediate the transcriptional activation of some of the RA secondary response genes (Fig. 2).
RA promotes the differentiation of natural regulatory T cells (nTregs), and the transcription factor Foxp3 plays a critical role in this differentiation process (Zhou et al., 2010). Zhou et al. show that RA plays a key role in sustaining the stability and function of nTregs when the inflammatory cytokine IL-6 is present, and Zhou et al. and Bai et al. (Bai et al., 2009) suggest that nTreg cells treated with RA could be a new “differentiation therapy” for chronic, immune-mediated inflammatory diseases.
A mixed ester of hyaluronan with butyric and retinoic acid induces the differentiation of human mesenchymal stem cells isolated from fetal membranes of term placenta, and this treatment increases transcription of the key cardiac lineage transcription factors GATA-4 and Nkx-2.5 (Ventura et al., 2007). Furthermore, when these stem cells treated with hyaluronan esters of butyric and retinoic acid were transplanted into infarcted rat hearts, the infarct size was decreased (Ventura et al., 2007).
The transcription factor FOX03A is activated by RA and translocates into the nucleus in acute promyelocytic leukemia derived NB4 cells, leading to granulocytic differentiation. When the activation of FOX03A is blocked by an shRNA against FOX03A, RA-induced differentiation is blocked. Thus, activation of FOX03A is a requirement for RA-induced differentiation of these cells and a potential therapeutic target (Sakoe et al., 2010). In addition, transcription of the gene for TRAIL (tumor necrosis factor-related apoptosis inducing ligand), a target of FOX03A, was increased in response to RA (see below for additional discussion of TRAIL). Another gene, ubiquitin conjugating enzyme (UBE2D3), was identified through an RNAi screen performed to find novel genes involved in differentiation/growth arrest of NB4 cells after RA (Hattori et al., 2007). UBE2D3 is also a potential therapeutic target.
In ES (embryonic stem) cells, RA-associated induction of the homeodomain containing protein Hoxa1 is required for neuronal differentiation (Martinez-Ceballos and Gudas, 2008). Appropriately regulated Wnt signaling is also necessary for RA to induce neuronal differentiation (Engberg et al., 2010) and an inhibitor of the enzyme GSK3β activity prevented RA from inducing neural lineage differentiation (Tonge and Andrews, 2010). Cdc42-mTOR signaling is required for P19 embryonal carcinoma cells to differentiate to neural cells in response to RA (Endo et al., 2009). The suppression of transcription of the stem cell transcription factor Oct4 gene by GCNF in response to RA is important for the transition from primitive to definitive neural stem cells (Akamatsu et al., 2009).
Sox9 mediates the actions of RA in terms of inhibiting the proliferation of breast cancer cells (Afonja et al., 2002), and the increase in Sox9 expression in response to RA mediates the inhibition of cell proliferation in MCF7 breast cancer cells (Muller et al., 2010). Recently, Sox9 was shown to inhibit proliferation by activating the transcription factor Hes1 (Muller et al, 2010). Sox9 transcriptional activation by RA is also required for inhibition of melanoma cell proliferation in response to RA (Passeron et al., 2009). One action of Sox9 in melanoma cells is to down-regulate the levels of PRAME, a negative regulator of RA action (a ligand-dependent co-repressor, see above, Fig. 5). These recent data suggest that small molecule activators of Sox9 transcription could be beneficial in combination cancer therapy by improving the therapeutic effects of RA.
RA acts upstream of the transcription factor STAT3 (GENE ID: 20848) to prevent its phosphorylation in response to phorbol ester, resulting in a reduction in the incidence of skin cancer. Since RA can’t prevent carcinogenesis in mice expressing a constitutively active STAT3 (Syed et al., 2009), phosphorylated-STAT3 must be a critical mediator of carcinogenesis in this mouse model. The mechanism by which RA prevents STAT3 phosphorylation is not clear, but may involve the actions of the transcription factor Rex1 (Xu et al., 2008).
While the examples above highlight the actions of RA in promoting stem cell and cancer cell differentiation and proliferation arrest, conversely RA can inhibit adipogenesis. In mesenchymal stem cells, treatment with RA increases expression of the transcription factor SMAD3; SMAD3 then interacts with the transcription factor C/EBPβ and interferes with the binding of C/EBPβ to DNA. Since C/EBPβ activity is necessary for stimulation of the later stages of adipogenesis, without C/EBPβ activity adipogenesis doesn’t occur (Marchildon et al., 2010). Furthermore, RA is not effective in inhibiting adipogenesis in the absence of SMAD3 expression (Marchildon et al., 2010).
How does RA Influence Signaling From Other Nuclear Receptors with Respect to Cell Differentiation?
When RARα and RARγ gene targets were identified in MCF7 human breast cancer cells by chromatin immunoprecipitation, many of the same genomic regions bound RARα/RARγ and estrogen receptorα (ERα). Data from gene expression profiling indicated that RA and estrogen antagonistically regulated breast cancer target genes (Hua et al., 2009), suggesting that RA has potential as a therapeutic agent in breast cancer. However, other researchers reported results that differed from those of Hua et al (Hua et al., 2009). Ross-Innes et al. (Ross-Innes et al., 2010) showed that RARα is required for efficient ERα mediated transcription and cell proliferation and that RARα can interact with ER-binding sites in an ERα-dependent manner in MCF7 cells (Ross-Innes et al., 2010). It is clear that RA can inhibit the proliferation of ERα+ human breast cancer cells (Seewaldt et al., 1995; Liu et al., 1996; Farias et al., 2002), suggesting that the RAR and the ERα signaling pathways interact in an antagonistic manner in terms of cell proliferation, with ERα promoting cell proliferation and RARα promoting cell proliferation inhibition and cell differentiation. The RA ligand-dependent corepressor protein RIP140 (NRIP1, see Fig. 5) has been reported to mediate the RA-associated repression of ERα activity, and a knockdown of RIP140 levels in human breast cancer cells resulted in a proliferation advantage for these estrogen dependent cells (White et al., 2005).
The actions of ERβ (estrogen receptor β) sensitize breast cancer cells to retinoids (Rousseau et al., 2004). Retinoids altered ERβ-mediated transcriptional activity from an estrogen response element and conversely, the expression of ERβ caused a greater induction of RARβ2 gene expression during RA treatment without altering the expression of RARα (Rousseau et al., 2004).
Sin3 (Sin3a, GENE ID: 20466) is a co-repressor protein that can silence gene transcription via associated histone deacetylases (HDACs) (Grzenda et al., 2009). In cancer cells, aberrant recruitment of Sin3 to genes results in abnormal gene silencing. A recent, exciting development is that interference with Sin3 function causes epigenetic reprogramming and restores sensitivity of even “triple negative” (i.e., no ERα, PR (progesterone receptor), or HER2 expression) human breast cancer cells to both tamoxifen and retinoids (Farias et al., 2010). Interference with the actions of Sin3 reversed the silencing of genes involved in the differentiation of breast epithelial cells and restored the RA sensitivity of these cells (Farias et al., 2010). Because a small molecule, a SID (Sin3 interaction domain) decoy, was employed in these studies, these results have major implications for human breast cancer treatment. These recent results indicate that a combination of tamoxifen and retinoids may be useful in the treatment of “triple negative” human breast cancer if administered along with a SID to block the repressive activity of Sin3a. Furthermore, RA plus tamoxifen inhibited cell proliferation and altered cell differentiation in HER2-overexpressing, ERα positive BT474 human breast cancer cells (Koay et al., 2010). In a related study a RARα agonist, AM580, was employed in the MMTV-Wnt1 murine transgenic breast cancer model. This drug induced differentiation, increased RARβ expression, inhibited the Wnt signaling pathway, and increased tumor-free survival in the mice (Lu et al., 2010).
β-Catenin, TRAIL, and Retinoic Acid Signaling Pathways
TRAIL (tumor necrosis factor-related apoptosis inducing ligand; TNFSF10, GENE ID: 22035) is a membrane-bound tumor necrosis factor ligand that causes apoptosis in cancer, but not normal cells (Koschny et al., 2007). Retinoids can increase the expression of TRAIL receptors and decrease expression of TRAIL decoy receptors in cancer cells (e.g., Sun et al., 2000; Altucci et al., 2001). Recently, it was shown that vitamin A (retinyl acetate) plus TRAIL can induce apoptosis in premalignant cells in the intestine, without affecting normal cells, in a murine colon cancer model (Zhang et al., 2010). Vitamin A, presumably via metabolism to RA, induces the TRAIL receptors TNFRSF10A and TNFRSF10B (DR4, DR5) in this model (Zhang et al, 2010). The induction of the receptors TNFRSF10A and TNFRSF10B by vitamin A in these cells is associated with the differentiation of these intestinal epithelial cells. In contrast to the normal epithelial cells, these pre-malignant cells exhibit activated β-catenin, which leads to apoptosis only when these cells are in the presence of both TRAIL and retinoids. Moreover, TRAIL plus vitamin A treatment, in an intermittent or in a short term dosing schedule, causes apoptosis in intestinal polyps in the ApcMin mouse model of intestinal carcinogenesis (Zhang et al. 2010). Therefore, vitamin A plus TRAIL “differentiation” therapy might be very useful in the prevention of colon cancer in humans.
Conclusions
When biologically active retinoids are produced, they bind to RAR/RXR receptors, initiating a cascade of changes in chromatin structure. These changes can promote differentiation, initiate stable epigenetic changes, and change the sensitivity of cells to other signaling pathways. In cancer cells, these changes have the potential to promote differentiation to a less neoplastically-transformed state. Retinoids are effective components of some current cancer therapies. Because they can act both by promoting stem cell differentiation and changing the pattern of gene expression in tumor cells to make them more sensitive to other therapies, retinoids are likely to be a component in many future cancer therapies. Our emerging knowledge of these molecular mechanisms is providing a rational basis both for understanding the role of retinoids in normal differentiation and for developing additional retinoid-based differentiation cancer chemotherapies, especially for use in combination with other drugs that modulate the epigenetic state of cells.
Acknowledgments
Contract grant sponsor: NIH/NCI; Contract grant number: R01CA043796.
Contract grant sponsor: NIH/NIDCR; Contract grant number: R01DE10389.
We would like to thank members of the Gudas lab, Tamara Weissman for editorial assistance, and Katarzyna Marcinkiewicz for critically reading this review.
Abbreviations used
- CRABP1
2, cellular retinoic acid binding protein 1, 2
- CRBP1
cellular retinol binding protein
- ES
embryonic stem
- ERα
estrogen receptor α
- GCNF
germ cell nuclear receptor
- LRAT
lecithin:retinol acyl transferase
- PcG
Polycomb group
- RA
retinoic acid
- RAR
retinoic acid receptor
- RARE
retinoic acid responsive element
- RDH
retinol dehydrogenase
- shRNA
short hairpin RNA
- TRAIL
tumor necrosis factor-related apoptosis inducing ligand
References
- Abu-Abed S, Dolle P, Metzger D, Beckett B, Chambon P, Petkovich M. The retinoic acid-metabolizing enzyme, CYP26A1, is essential for normal hindbrain patterning, vertebral identity, and development of posterior structures. Genes Dev. 2001;15(2):226–240. doi: 10.1101/gad.855001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achkar CC, Derguini F, Blumberg B, Langston A, Levin AA, Speck J, Evans RM, Bolado J, Jr, Nakanishi K, Buck J, Gudas LJ. 4-Oxoretinol, a new natural ligand and transactivator of the retinoic acid receptors. Proc Natl Acad Sci U S A. 1996;93(10):4879–4884. doi: 10.1073/pnas.93.10.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afonja O, Raaka BM, Huang A, Das S, Zhao X, Helmer E, Juste D, Samuels HH. RAR agonists stimulate SOX9 gene expression in breast cancer cell lines: evidence for a role in retinoid-mediated growth inhibition. Oncogene. 2002;21(51):7850–7860. doi: 10.1038/sj.onc.1205985. [DOI] [PubMed] [Google Scholar]
- Akamatsu W, DeVeale B, Okano H, Cooney AJ, van der Kooy D. Suppression of Oct4 by germ cell nuclear factor restricts pluripotency and promotes neural stem cell development in the early neural lineage. J Neurosci. 2009;29(7):2113–2124. doi: 10.1523/JNEUROSCI.4527-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altucci L, Rossin A, Raffelsberger W, Reitmair A, Chomienne C, Gronemeyer H. Retinoic acid-induced apoptosis in leukemia cells is mediated by paracrine action of tumor-selective death ligand TRAIL. Nat Med. 2001;7(6):680–686. doi: 10.1038/89050. [DOI] [PubMed] [Google Scholar]
- Amat R, Gudas LJ. RARγ is required for correct deposition of Suz12 and H2A.Z in embryonic stem cells. J Cell Physiol. 2010 doi: 10.1002/jcp.22420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai A, Lu N, Guo Y, Liu Z, Chen J, Peng Z. All-trans retinoic acid down-regulates inflammatory responses by shifting the Treg/Th17 profile in human ulcerative and murine colitis. J Leukoc Biol. 2009;86(4):959–969. doi: 10.1189/jlb.0109006. [DOI] [PubMed] [Google Scholar]
- Baron JM, Heise R, Blaner WS, Neis M, Joussen S, Dreuw A, Marquardt Y, Saurat JH, Merk HF, Bickers DR, Jugert FK. Retinoic acid and its 4-oxo metabolites are functionally active in human skin cells in vitro. J Invest Dermatol. 2005;125(1):143–153. doi: 10.1111/j.0022-202X.2005.23791.x. [DOI] [PubMed] [Google Scholar]
- Beland M, Lohnes D. Chicken ovalbumin upstream promoter-transcription factor members repress retinoic acid-induced Cdx1 expression. J Biol Chem. 2005;280(14):13858–13862. doi: 10.1074/jbc.M412981200. [DOI] [PubMed] [Google Scholar]
- Belyaeva OV, Johnson MP, Kedishvili NY. Kinetic analysis of human enzyme RDH10 defines the characteristics of a physiologically relevant retinol dehydrogenase. J Biol Chem. 2008;283(29):20299–20308. doi: 10.1074/jbc.M800019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shushan E, Thompson JR, Gudas LJ, Bergman Y. Rex-1, a gene encoding a transcription factor expressed in the early embryo, is regulated via Oct-3/4 and Oct-6 binding to an octamer site and a novel protein, Rox-1, binding to an adjacent site. Mol Cell Biol. 1998;18(4):1866–1878. doi: 10.1128/mcb.18.4.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaner WS, Olson JA. In: The Retinoids, Biology, Chemistry and Medicine. 2. Sporn MB, Roberts AB, Goodman DS, editors. New York: Raven Press; 1994. pp. 229–256. [Google Scholar]
- Blumberg B, Bolado J, Jr, Derguini F, Craig AG, Moreno TA, Chakravarti D, Heyman RA, Buck J, Evans RM. Novel retinoic acid receptor ligands in Xenopus embryos. Proc Natl Acad Sci U S A. 1996;93(10):4873–4878. doi: 10.1073/pnas.93.10.4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouillet P, Sapin V, Chazaud C, Messaddeq N, Decimo D, Dolle P, Chambon P. Developmental expression pattern of Stra6, a retinoic acid-responsive gene encoding a new type of membrane protein. Mech Dev. 1997;63(2):173–186. doi: 10.1016/s0925-4773(97)00039-7. [DOI] [PubMed] [Google Scholar]
- Boylan JF, Lohnes D, Taneja R, Chambon P, Gudas LJ. Loss of retinoic acid receptor gamma function in F9 cells by gene disruption results in aberrant Hoxa-1 expression and differentiation upon retinoic acid treatment. Proc Natl Acad Sci U S A. 1993;90(20):9601–9605. doi: 10.1073/pnas.90.20.9601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruck N, Vitoux D, Ferry C, Duong V, Bauer A, de The H, Rochette-Egly C. A coordinated phosphorylation cascade initiated by p38MAPK/MSK1 directs RARalpha to target promoters. EMBO J. 2009;28(1):34–47. doi: 10.1038/emboj.2008.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammas L, Romand R, Fraulob V, Mura C, Dolle P. Expression of the murine retinol dehydrogenase 10 (Rdh10) gene correlates with many sites of retinoid signalling during embryogenesis and organ differentiation. Dev Dyn. 2007;236(10):2899–2908. doi: 10.1002/dvdy.21312. [DOI] [PubMed] [Google Scholar]
- Campos B, Wan F, Farhadi M, Ernst A, Zeppernick F, Tagscherer KE, Ahmadi R, Lohr J, Dictus C, Gdynia G, Combs SE, Goidts V, Helmke BM, Eckstein V, Roth W, Beckhove P, Lichter P, Unterberg A, Radlwimmer B, Herold-Mende C. Differentiation therapy exerts antitumor effects on stem-like glioma cells. Clin Cancer Res. 2010;16(10):2715–2728. doi: 10.1158/1078-0432.CCR-09-1800. [DOI] [PubMed] [Google Scholar]
- Chen CF, Goyette P, Lohnes D. RARgamma acts as a tumor suppressor in mouse keratinocytes. Oncogene. 2004;23(31):5350–5359. doi: 10.1038/sj.onc.1207682. [DOI] [PubMed] [Google Scholar]
- Das P, Doyle TJ, Liu D, Kochar J, Kim KH, Rogers MB. Retinoic acid regulation of eye and testis-specific transcripts within a complex locus. Mech Dev. 2007;124(2):137–145. doi: 10.1016/j.mod.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson MI, Zhang XK. Discovery and design of retinoic acid receptor and retinoid X receptor class- and subtype-selective synthetic analogs of all-trans-retinoic acid and 9-cis-retinoic acid. Curr Med Chem. 2002;9(6):623–637. doi: 10.2174/0929867023370789. [DOI] [PubMed] [Google Scholar]
- Delva L, Bastie JN, Rochette-Egly C, Kraiba R, Balitrand N, Despouy G, Chambon P, Chomienne C. Physical and functional interactions between cellular retinoic acid binding protein II and the retinoic acid-dependent nuclear complex. Mol Cell Biol. 1999;19(10):7158–7167. doi: 10.1128/mcb.19.10.7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolle P. Developmental expression of retinoic acid receptors (RARs) Nucl Recept Signal. 2009;7:e006. doi: 10.1621/nrs.07006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duester G. Retinoic acid synthesis and signaling during early organogenesis. Cell. 2008;134(6):921–931. doi: 10.1016/j.cell.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M, Antonyak MA, Cerione RA. Cdc42-mTOR signaling pathway controls Hes5 and Pax6 expression in retinoic acid-dependent neural differentiation. J Biol Chem. 2009;284(8):5107–5118. doi: 10.1074/jbc.M807745200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epping MT, Wang L, Edel MJ, Carlee L, Hernandez M, Bernards R. The human tumor antigen PRAME is a dominant repressor of retinoic acid receptor signaling. Cell. 2005;122(6):835–847. doi: 10.1016/j.cell.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Farias EF, Arapshian A, Bleiweiss IJ, Waxman S, Zelent A, Mira YLR. Retinoic acid receptor alpha2 is a growth suppressor epigenetically silenced in MCF-7 human breast cancer cells. Cell Growth Differ. 2002;13(8):335–341. [PubMed] [Google Scholar]
- Farias EF, Petrie K, Leibovitch B, Murtagh J, Chornet MB, Schenk T, Zelent A, Waxman S. Interference with Sin3 function induces epigenetic reprogramming and differentiation in breast cancer cells. Proc Natl Acad Sci U S A. 2010;107(26):11811–11816. doi: 10.1073/pnas.1006737107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields AL, Soprano DR, Soprano KJ. Retinoids in biological control and cancer. J Cell Biochem. 2007;102(4):886–898. doi: 10.1002/jcb.21530. [DOI] [PubMed] [Google Scholar]
- Gaillard E, Bruck N, Brelivet Y, Bour G, Lalevee S, Bauer A, Poch O, Moras D, Rochette-Egly C. Phosphorylation by PKA potentiates retinoic acid receptor alpha activity by means of increasing interaction with and phosphorylation by cyclin H/cdk7. Proc Natl Acad Sci U S A. 2006;103(25):9548–9553. doi: 10.1073/pnas.0509717103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie RF, Gudas LJ. Retinoic acid receptor isotype specificity in F9 teratocarcinoma stem cells results from the differential recruitment of coregulators to retinoic response elements. J Biol Chem. 2007a;282(46):33421–33434. doi: 10.1074/jbc.M704845200. [DOI] [PubMed] [Google Scholar]
- Gillespie RF, Gudas LJ. Retinoid regulated association of transcriptional co-regulators and the polycomb group protein SUZ12 with the retinoic acid response elements of Hoxa1, RARbeta(2), and Cyp26A1 in F9 embryonal carcinoma cells. J Mol Biol. 2007b;372(2):298–316. doi: 10.1016/j.jmb.2007.06.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasow A, Prodromou N, Xu K, von Lindern M, Zelent A. Retinoids and myelomonocytic growth factors cooperatively activate RARA and induce human myeloid leukemia cell differentiation via MAP kinase pathways. Blood. 2005;105(1):341–349. doi: 10.1182/blood-2004-03-1074. [DOI] [PubMed] [Google Scholar]
- Grzenda A, Lomberk G, Zhang JS, Urrutia R. Sin3: master scaffold and transcriptional corepressor. Biochim Biophys Acta. 2009;1789(6–8):443–450. doi: 10.1016/j.bbagrm.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu P, LeMenuet D, Chung AC, Mancini M, Wheeler DA, Cooney AJ. Orphan nuclear receptor GCNF is required for the repression of pluripotency genes during retinoic acid-induced embryonic stem cell differentiation. Mol Cell Biol. 2005;25(19):8507–8519. doi: 10.1128/MCB.25.19.8507-8519.2005. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Gudas LJ. Retinoids and vertebrate development. J Biol Chem. 1994;269(22):15399–15402. [PubMed] [Google Scholar]
- Gupta RK, Arany Z, Seale P, Mepani RJ, Ye L, Conroe HM, Roby YA, Kulaga H, Reed RR, Spiegelman BM. Transcriptional control of preadipocyte determination by Zfp423. Nature. 2010;464(7288):619–623. doi: 10.1038/nature08816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich I, Flores AM, Aneskievich BJ. Corepressors of agonist-bound nuclear receptors. Toxicol Appl Pharmacol. 2007;223(3):288–298. doi: 10.1016/j.taap.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn CK, Ross KN, Warrington IM, Mazitschek R, Kanegai CM, Wright RD, Kung AL, Golub TR, Stegmaier K. Expression-based screening identifies the combination of histone deacetylase inhibitors and retinoids for neuroblastoma differentiation. Proc Natl Acad Sci U S A. 2008;105(28):9751–9756. doi: 10.1073/pnas.0710413105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori H, Zhang X, Jia Y, Subramanian KK, Jo H, Loison F, Newburger PE, Luo HR. RNAi screen identifies UBE2D3 as a mediator of all-trans retinoic acid-induced cell growth arrest in human acute promyelocytic NB4 cells. Blood. 2007;110(2):640–650. doi: 10.1182/blood-2006-11-059048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim KC, White KA, Deng D, Tomlinson CR, Moore JH, Freemantle SJ, Spinella MJ. Selective repression of retinoic acid target genes by RIP140 during induced tumor cell differentiation of pluripotent human embryonal carcinoma cells. Mol Cancer. 2007;6:57. doi: 10.1186/1476-4598-6-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzel M, Huang S, Koster J, Ora I, Lakeman A, Caron H, Nijkamp W, Xie J, Callens T, Asgharzadeh S, Seeger RC, Messiaen L, Versteeg R, Bernards R. NF1 is a tumor suppressor in neuroblastoma that determines retinoic acid response and disease outcome. Cell. 2010;142(2):218–229. doi: 10.1016/j.cell.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosler BA, LaRosa GJ, Grippo JF, Gudas LJ. Expression of REX-1, a gene containing zinc finger motifs, is rapidly reduced by retinoic acid in F9 teratocarcinoma cells. Mol Cell Biol. 1989;9(12):5623–5629. doi: 10.1128/mcb.9.12.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosler BA, Rogers MB, Kozak CA, Gudas LJ. An octamer motif contributes to the expression of the retinoic acid-regulated zinc finger gene Rex-1 (Zfp-42) in F9 teratocarcinoma cells. Mol Cell Biol. 1993;13(5):2919–2928. doi: 10.1128/mcb.13.5.2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Z, Peng H, White DE, Negorev DG, Maul GG, Feng Y, Longmore GD, Waxman S, Zelent A, Rauscher FJ., 3rd LIM protein Ajuba functions as a nuclear receptor corepressor and negatively regulates retinoic acid signaling. Proc Natl Acad Sci U S A. 2010;107(7):2938–2943. doi: 10.1073/pnas.0908656107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Chen Y, Farooqui M, Thomas MC, Chiang CM, Wei LN. Suppressive effect of receptor-interacting protein 140 on coregulator binding to retinoic acid receptor complexes, histone-modifying enzyme activity, and gene activation. J Biol Chem. 2004;279(1):319–325. doi: 10.1074/jbc.M307621200. [DOI] [PubMed] [Google Scholar]
- Hua S, Kittler R, White KP. Genomic antagonism between retinoic acid and estrogen signaling in breast cancer. Cell. 2009;137(7):1259–1271. doi: 10.1016/j.cell.2009.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Laoukili J, Epping MT, Koster J, Holzel M, Westerman BA, Nijkamp W, Hata A, Asgharzadeh S, Seeger RC, Versteeg R, Beijersbergen RL, Bernards R. ZNF423 is critically required for retinoic acid-induced differentiation and is a marker of neuroblastoma outcome. Cancer Cell. 2009;15(4):328–340. doi: 10.1016/j.ccr.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idres N, Benoit G, Flexor MA, Lanotte M, Chabot GG. Granulocytic differentiation of human NB4 promyelocytic leukemia cells induced by all-trans retinoic acid metabolites. Cancer Res. 2001;61(2):700–705. [PubMed] [Google Scholar]
- Idres N, Marill J, Flexor MA, Chabot GG. Activation of retinoic acid receptor-dependent transcription by all-trans-retinoic acid metabolites and isomers. J Biol Chem. 2002;277(35):31491–31498. doi: 10.1074/jbc.M205016200. [DOI] [PubMed] [Google Scholar]
- Jetten AM. Retinoid-related orphan receptors (RORs): critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nucl Recept Signal. 2009;7:e003. doi: 10.1621/nrs.07003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashyap V, Gudas LJ. Epigenetic regulatory mechanisms distinguish retinoic acid-mediated transcriptional responses in stem cells and fibroblasts. J Biol Chem. 2010;285(19):14534–14548. doi: 10.1074/jbc.M110.115345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi R, Yu J, Honda J, Hu J, Whitelegge J, Ping P, Wiita P, Bok D, Sun H. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science. 2007;315(5813):820–825. doi: 10.1126/science.1136244. [DOI] [PubMed] [Google Scholar]
- Khetchoumian K, Teletin M, Tisserand J, Mark M, Herquel B, Ignat M, Zucman-Rossi J, Cammas F, Lerouge T, Thibault C, Metzger D, Chambon P, Losson R. Loss of Trim24 (Tif1alpha) gene function confers oncogenic activity to retinoic acid receptor alpha. Nat Genet. 2007;39(12):1500–1506. doi: 10.1038/ng.2007.15. [DOI] [PubMed] [Google Scholar]
- Kim YK, Wassef L, Hamberger L, Piantedosi R, Palczewski K, Blaner WS, Quadro L. Retinyl ester formation by lecithin: retinol acyltransferase is a key regulator of retinoid homeostasis in mouse embryogenesis. J Biol Chem. 2008;283(9):5611–5621. doi: 10.1074/jbc.M708885200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koay DC, Zerillo C, Narayan M, Harris LN, Digiovanna MP. Anti-tumor effects of retinoids combined with trastuzumab or tamoxifen in breast cancer cells: induction of apoptosis by retinoid/trastuzumab combinations. Breast Cancer Res. 2010 doi: 10.1186/bcr2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koschny R, Walczak H, Ganten TM. The promise of TRAIL--potential and risks of a novel anticancer therapy. J Mol Med. 2007;85(9):923–935. doi: 10.1007/s00109-007-0194-1. [DOI] [PubMed] [Google Scholar]
- Lane MA, Xu J, Wilen EW, Sylvester R, Derguini F, Gudas LJ. LIF removal increases CRABPI and CRABPII transcripts in embryonic stem cells cultured in retinol or 4-oxoretinol. Mol Cell Endocrinol. 2008;280(1–2):63–74. doi: 10.1016/j.mce.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston AW, Gudas LJ. Identification of a retinoic acid responsive enhancer 3′ of the murine homeobox gene Hox-1.6. Mech Dev. 1992;38(3):217–227. doi: 10.1016/0925-4773(92)90055-o. [DOI] [PubMed] [Google Scholar]
- Langton S, Gudas LJ. CYP26A1 knockout embryonic stem cells exhibit reduced differentiation and growth arrest in response to retinoic acid. Dev Biol. 2008;315(2):331–354. doi: 10.1016/j.ydbio.2007.12.021. [DOI] [PubMed] [Google Scholar]
- LaRosa GJ, Gudas LJ. An early effect of retinoic acid: cloning of an mRNA (Era-1) exhibiting rapid and protein synthesis-independent induction during teratocarcinoma stem cell differentiation. Proc Natl Acad Sci U S A. 1988;85(2):329–333. doi: 10.1073/pnas.85.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- le Maire A, Teyssier C, Erb C, Grimaldi M, Alvarez S, de Lera AR, Balaguer P, Gronemeyer H, Royer CA, Germain P, Bourguet W. A unique secondary-structure switch controls constitutive gene repression by retinoic acid receptor. Nat Struct Mol Biol. 2010;17(7):801–807. doi: 10.1038/nsmb.1855. [DOI] [PubMed] [Google Scholar]
- Lee ER, Murdoch FE, Fritsch MK. High histone acetylation and decreased polycomb repressive complex 2 member levels regulate gene specific transcriptional changes during early embryonic stem cell differentiation induced by retinoic acid. Stem Cells. 2007;25(9):2191–2199. doi: 10.1634/stemcells.2007-0203. [DOI] [PubMed] [Google Scholar]
- Li G, Margueron R, Hu G, Stokes D, Wang YH, Reinberg D. Highly compacted chromatin formed in vitro reflects the dynamics of transcription activation in vivo. Mol Cell. 2010;38(1):41–53. doi: 10.1016/j.molcel.2010.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Derguini F, Gudas LJ. Metabolism and regulation of gene expression by 4-oxoretinol versus all-trans retinoic acid in normal human mammary epithelial cells. J Cell Physiol. 2009;220(3):771–779. doi: 10.1002/jcp.21824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Gudas LJ. Disruption of the lecithin:retinol acyltransferase gene makes mice more susceptible to vitamin A deficiency. J Biol Chem. 2005;280(48):40226–40234. doi: 10.1074/jbc.M509643200. [DOI] [PubMed] [Google Scholar]
- Liu Y, Lee MO, Wang HG, Li Y, Hashimoto Y, Klaus M, Reed JC, Zhang X. Retinoic acid receptor beta mediates the growth-inhibitory effect of retinoic acid by promoting apoptosis in human breast cancer cells. Mol Cell Biol. 1996;16(3):1138–1149. doi: 10.1128/mcb.16.3.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Bertran S, Samuels TA, Mira-y-Lopez R, Farias EF. Mechanism of inhibition of MMTV-neu and MMTV-wnt1 induced mammary oncogenesis by RARalpha agonist AM580. Oncogene. 2010;29(25):3665–3676. doi: 10.1038/onc.2010.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchildon F, St-Louis C, Akter R, Roodman V, Wiper-Bergeron NL. Transcription factor Smad3 is required for the inhibition of adipogenesis by retinoic acid. J Biol Chem. 2010;285(17):13274–13284. doi: 10.1074/jbc.M109.054536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark M, Ghyselinck NB, Chambon P. Function of retinoic acid receptors during embryonic development. Nucl Recept Signal. 2009;7:e002. doi: 10.1621/nrs.07002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens JH, Brinkman AB, Simmer F, Francoijs KJ, Nebbioso A, Ferrara F, Altucci L, Stunnenberg HG. PML-RARalpha/RXR Alters the Epigenetic Landscape in Acute Promyelocytic Leukemia. Cancer Cell. 2010;17(2):173–185. doi: 10.1016/j.ccr.2009.12.042. [DOI] [PubMed] [Google Scholar]
- Martinez-Ceballos E, Gudas LJ. Hoxa1 is required for the retinoic acid-induced differentiation of embryonic stem cells into neurons. J Neurosci Res. 2008;86(13):2809–2819. doi: 10.1002/jnr.21729. [DOI] [PubMed] [Google Scholar]
- Matt N, Dupe V, Garnier JM, Dennefeld C, Chambon P, Mark M, Ghyselinck NB. Retinoic acid-dependent eye morphogenesis is orchestrated by neural crest cells. Development. 2005;132(21):4789–4800. doi: 10.1242/dev.02031. [DOI] [PubMed] [Google Scholar]
- Mohammad HP, Cai Y, McGarvey KM, Easwaran H, Van Neste L, Ohm JE, O’Hagan HM, Baylin SB. Polycomb CBX7 promotes initiation of heritable repression of genes frequently silenced with cancer-specific DNA hypermethylation. Cancer Res. 2009;69(15):6322–6330. doi: 10.1158/0008-5472.CAN-09-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongan NP, Gudas LJ. Diverse actions of retinoid receptors in cancer prevention and treatment. Differentiation. 2007;75(9):853–870. doi: 10.1111/j.1432-0436.2007.00206.x. [DOI] [PubMed] [Google Scholar]
- Muley PD, McNeill EM, Marzinke MA, Knobel KM, Barr MM, Clagett-Dame M. The atRA-responsive gene neuron navigator 2 functions in neurite outgrowth and axonal elongation. Dev Neurobiol. 2008;68(13):1441–1453. doi: 10.1002/dneu.20670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller P, Crofts JD, Newman BS, Bridgewater LC, Lin CY, Gustafsson JA, Strom A. SOX9 mediates the retinoic acid-induced HES-1 gene expression in human breast cancer cells. Breast Cancer Res Treat. 2010;120(2):317–326. doi: 10.1007/s10549-009-0381-6. [DOI] [PubMed] [Google Scholar]
- Nasr R, Guillemin MC, Ferhi O, Soilihi H, Peres L, Berthier C, Rousselot P, Robledo-Sarmiento M, Lallemand-Breitenbach V, Gourmel B, Vitoux D, Pandolfi PP, Rochette-Egly C, Zhu J, de The H. Eradication of acute promyelocytic leukemia-initiating cells through PML-RARA degradation. Nat Med. 2008;14(12):1333–1342. doi: 10.1038/nm.1891. [DOI] [PubMed] [Google Scholar]
- Niederreither K, Abu-Abed S, Schuhbaur B, Petkovich M, Chambon P, Dolle P. Genetic evidence that oxidative derivatives of retinoic acid are not involved in retinoid signaling during mouse development. Nat Genet. 2002;31(1):84–88. doi: 10.1038/ng876. [DOI] [PubMed] [Google Scholar]
- Noy N. Between death and survival: retinoic acid in regulation of apoptosis. Annu Rev Nutr. 2010;30:201–217. doi: 10.1146/annurev.nutr.28.061807.155509. [DOI] [PubMed] [Google Scholar]
- Park DM, Li J, Okamoto H, Akeju O, Kim SH, Lubensky I, Vortmeyer A, Dambrosia J, Weil RJ, Oldfield EH, Park JK, Zhuang Z. N-CoR pathway targeting induces glioblastoma derived cancer stem cell differentiation. Cell Cycle. 2007;6(4):467–470. doi: 10.4161/cc.6.4.3856. [DOI] [PubMed] [Google Scholar]
- Passeron T, Valencia JC, Namiki T, Vieira WD, Passeron H, Miyamura Y, Hearing VJ. Upregulation of SOX9 inhibits the growth of human and mouse melanomas and restores their sensitivity to retinoic acid. J Clin Invest. 2009;119(4):954–963. doi: 10.1172/JCI34015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perissi V, Jepsen K, Glass CK, Rosenfeld MG. Deconstructing repression: evolving models of co-repressor action. Nat Rev Genet. 2010;11(2):109–123. doi: 10.1038/nrg2736. [DOI] [PubMed] [Google Scholar]
- Pijnappel WW, Hendriks HF, Folkers GE, van den Brink CE, Dekker EJ, Edelenbosch C, van der Saag PT, Durston AJ. The retinoid ligand 4-oxo-retinoic acid is a highly active modulator of positional specification. Nature. 1993;366(6453):340–344. doi: 10.1038/366340a0. [DOI] [PubMed] [Google Scholar]
- Purton LE, Dworkin S, Olsen GH, Walkley CR, Fabb SA, Collins SJ, Chambon P. RARgamma is critical for maintaining a balance between hematopoietic stem cell self-renewal and differentiation. J Exp Med. 2006;203(5):1283–1293. doi: 10.1084/jem.20052105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Z, Ren F, Xu X, Ren Y, Li H, Wang Y, Zhai Y, Chang Z. ZNF536, a novel zinc finger protein specifically expressed in the brain, negatively regulates neuron differentiation by repressing retinoic acid-induced gene transcription. Mol Cell Biol. 2009;29(13):3633–3643. doi: 10.1128/MCB.00362-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CP, Matthay KK, Villablanca JG, Maurer BJ. Retinoid therapy of high-risk neuroblastoma. Cancer Lett. 2003;197(1–2):185–192. doi: 10.1016/s0304-3835(03)00108-3. [DOI] [PubMed] [Google Scholar]
- Rochette-Egly C, Germain P. Dynamic and combinatorial control of gene expression by nuclear retinoic acid receptors (RARs) Nucl Recept Signal. 2009;7:e005. doi: 10.1621/nrs.07005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross-Innes CS, Stark R, Holmes KA, Schmidt D, Spyrou C, Russell R, Massie CE, Vowler SL, Eldridge M, Carroll JS. Cooperative interaction between retinoic acid receptor-alpha and estrogen receptor in breast cancer. Genes Dev. 2010;24(2):171–182. doi: 10.1101/gad.552910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosselot C, Spraggon L, Chia I, Batourina E, Riccio P, Lu B, Niederreither K, Dolle P, Duester G, Chambon P, Costantini F, Gilbert T, Molotkov A, Mendelsohn C. Non-cell-autonomous retinoid signaling is crucial for renal development. Development. 2010;137(2):283–292. doi: 10.1242/dev.040287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau C, Nichol JN, Pettersson F, Couture MC, Miller WH., Jr ERbeta sensitizes breast cancer cells to retinoic acid: evidence of transcriptional crosstalk. Mol Cancer Res. 2004;2(9):523–531. [PubMed] [Google Scholar]
- Sakoe Y, Sakoe K, Kirito K, Ozawa K, Komatsu N. FOXO3A as a key molecule for all-trans retinoic acid-induced granulocytic differentiation and apoptosis in acute promyelocytic leukemia. Blood. 2010;115(18):3787–3795. doi: 10.1182/blood-2009-05-222976. [DOI] [PubMed] [Google Scholar]
- Sandell LL, Sanderson BW, Moiseyev G, Johnson T, Mushegian A, Young K, Rey JP, Ma JX, Staehling-Hampton K, Trainor PA. RDH10 is essential for synthesis of embryonic retinoic acid and is required for limb, craniofacial, and organ development. Genes Dev. 2007;21(9):1113–1124. doi: 10.1101/gad.1533407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos NC, Kim KH. Activity of retinoic acid receptor-alpha is directly regulated at its protein kinase A sites in response to follicle-stimulating hormone signaling. Endocrinology. 2010;151(5):2361–2372. doi: 10.1210/en.2009-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfelder S, Sexton T, Chakalova L, Cope NF, Horton A, Andrews S, Kurukuti S, Mitchell JA, Umlauf D, Dimitrova DS, Eskiw CH, Luo Y, Wei CL, Ruan Y, Bieker JJ, Fraser P. Preferential associations between co-regulated genes reveal a transcriptional interactome in erythroid cells. Nat Genet. 2010;42(1):53–61. doi: 10.1038/ng.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schug TT, Berry DC, Shaw NS, Travis SN, Noy N. Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell. 2007;129(4):723–733. doi: 10.1016/j.cell.2007.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotland KB, Chen S, Sylvester R, Gudas LJ. Analysis of Rex1 (zfp42) function in embryonic stem cell differentiation. Dev Dyn. 2009;238(8):1863–1877. doi: 10.1002/dvdy.22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seewaldt VL, Johnson BS, Parker MB, Collins SJ, Swisshelm K. Expression of retinoic acid receptor beta mediates retinoic acid-induced growth arrest and apoptosis in breast cancer cells. Cell Growth Differ. 1995;6(9):1077–1088. [PubMed] [Google Scholar]
- Seino Y, Miki T, Kiyonari H, Abe T, Fujimoto W, Kimura K, Takeuchi A, Takahashi Y, Oiso Y, Iwanaga T, Seino S. Isx participates in the maintenance of vitamin A metabolism by regulation of beta-carotene 15,15′-monooxygenase (Bcmo1) expression. J Biol Chem. 2008;283(8):4905–4911. doi: 10.1074/jbc.M707928200. [DOI] [PubMed] [Google Scholar]
- Sessa L, Breiling A, Lavorgna G, Silvestri L, Casari G, Orlando V. Noncoding RNA synthesis and loss of Polycomb group repression accompanies the colinear activation of the human HOXA cluster. RNA. 2007;13(2):223–239. doi: 10.1261/rna.266707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimono K, Morrison TN, Tung WE, Chandraratna RA, Williams JA, Iwamoto M, Pacifici M. Inhibition of ectopic bone formation by a selective retinoic acid receptor alpha-agonist: a new therapy for heterotopic ossification? J Orthop Res. 2010;28(2):271–277. doi: 10.1002/jor.20985. [DOI] [PubMed] [Google Scholar]
- Shmarakov I, Fleshman MK, D’Ambrosio DN, Piantedosi R, Riedl KM, Schwartz SJ, Curley RW, Jr, von Lintig J, Rubin LP, Harrison EH, Blaner WS. Arch Biochem Biophys. 2010. Hepatic stellate cells are an important cellular site for beta-carotene conversion to retinoid. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidell N, Altman A, Haussler MR, Seeger RC. Effects of retinoic acid (RA) on the growth and phenotypic expression of several human neuroblastoma cell lines. Exp Cell Res. 1983;148(1):21–30. doi: 10.1016/0014-4827(83)90184-2. [DOI] [PubMed] [Google Scholar]
- Siegenthaler JA, Ashique AM, Zarbalis K, Patterson KP, Hecht JH, Kane MA, Folias AE, Choe Y, May SR, Kume T, Napoli JL, Peterson AS, Pleasure SJ. Retinoic acid from the meninges regulates cortical neuron generation. Cell. 2009;139(3):597–609. doi: 10.1016/j.cell.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JA, Kingston RE. Mechanisms of polycomb gene silencing: knowns and unknowns. Nat Rev Mol Cell Biol. 2009;10(10):697–708. doi: 10.1038/nrm2763. [DOI] [PubMed] [Google Scholar]
- Sonneveld E, van den Brink CE, Tertoolen LG, van der Burg B, van der Saag PT. Retinoic acid hydroxylase (CYP26) is a key enzyme in neuronal differentiation of embryonal carcinoma cells. Dev Biol. 1999;213(2):390–404. doi: 10.1006/dbio.1999.9381. [DOI] [PubMed] [Google Scholar]
- Soprano DR, Teets BW, Soprano KJ. Role of retinoic acid in the differentiation of embryonal carcinoma and embryonic stem cells. Vitam Horm. 2007;75:69–95. doi: 10.1016/S0083-6729(06)75003-8. [DOI] [PubMed] [Google Scholar]
- Stafford D, White RJ, Kinkel MD, Linville A, Schilling TF, Prince VE. Retinoids signal directly to zebrafish endoderm to specify insulin-expressing beta-cells. Development. 2006;133(5):949–956. doi: 10.1242/dev.02263. [DOI] [PubMed] [Google Scholar]
- Sun SY, Yue P, Hong WK, Lotan R. Augmentation of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis by the synthetic retinoid 6-[3-(1-adamantyl)-4-hydroxyphenyl]-2-naphthalene carboxylic acid (CD437) through up-regulation of TRAIL receptors in human lung cancer cells. Cancer Res. 2000;60(24):7149–7155. [PubMed] [Google Scholar]
- Suva ML, Riggi N, Janiszewska M, Radovanovic I, Provero P, Stehle JC, Baumer K, Le Bitoux MA, Marino D, Cironi L, Marquez VE, Clement V, Stamenkovic I. EZH2 is essential for glioblastoma cancer stem cell maintenance. Cancer Res. 2009;69(24):9211–9218. doi: 10.1158/0008-5472.CAN-09-1622. [DOI] [PubMed] [Google Scholar]
- Syed Z, Cheepala SB, Gill JN, Stein J, Nathan CA, Digiovanni J, Batra V, Adegboyega P, Kleiner HE, Clifford JL. All-trans retinoic acid suppresses Stat3 signaling during skin carcinogenesis. Cancer Prev Res (Phila Pa) 2009;2(10):903–911. doi: 10.1158/1940-6207.CAPR-09-0041. [DOI] [PubMed] [Google Scholar]
- Tang XH, Gudas LJ. Retinoids, Retinoic Acid Receptors, and Cancer. Annual Review of Pathology. 2011;6 doi: 10.1146/annurev-pathol-011110-130303. [DOI] [PubMed] [Google Scholar]
- Tighe AP, Gudas LJ. Retinoic acid inhibits leukemia inhibitory factor signaling pathways in mouse embryonic stem cells. J Cell Physiol. 2004;198(2):223–229. doi: 10.1002/jcp.10424. [DOI] [PubMed] [Google Scholar]
- Tonge PD, Andrews PW. Retinoic acid directs neuronal differentiation of human pluripotent stem cell lines in a non-cell-autonomous manner. Differentiation. 2010;80(1):20–30. doi: 10.1016/j.diff.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Turano M, Napolitano G, Dulac C, Majello B, Bensaude O, Lania L. Increased HEXIM1 expression during erythroleukemia and neuroblastoma cell differentiation. J Cell Physiol. 2006;206(3):603–610. doi: 10.1002/jcp.20502. [DOI] [PubMed] [Google Scholar]
- van Wely KH, Meester-Smoor MA, Janssen MJ, Aarnoudse AJ, Grosveld GC, Zwarthoff EC. The MN1-TEL myeloid leukemia-associated fusion protein has a dominant-negative effect on RAR-RXR-mediated transcription. Oncogene. 2007;26(39):5733–5740. doi: 10.1038/sj.onc.1210382. [DOI] [PubMed] [Google Scholar]
- Ventura C, Cantoni S, Bianchi F, Lionetti V, Cavallini C, Scarlata I, Foroni L, Maioli M, Bonsi L, Alviano F, Fossati V, Bagnara GP, Pasquinelli G, Recchia FA, Perbellini A. Hyaluronan mixed esters of butyric and retinoic Acid drive cardiac and endothelial fate in term placenta human mesenchymal stem cells and enhance cardiac repair in infarcted rat hearts. J Biol Chem. 2007;282(19):14243–14252. doi: 10.1074/jbc.M609350200. [DOI] [PubMed] [Google Scholar]
- Vilhais-Neto GC, Maruhashi M, Smith KT, Vasseur-Cognet M, Peterson AS, Workman JL, Pourquie O. Rere controls retinoic acid signalling and somite bilateral symmetry. Nature. 2010;463(7283):953–957. doi: 10.1038/nature08763. [DOI] [PubMed] [Google Scholar]
- Villa R, Pasini D, Gutierrez A, Morey L, Occhionorelli M, Vire E, Nomdedeu JF, Jenuwein T, Pelicci PG, Minucci S, Fuks F, Helin K, Di Croce L. Role of the polycomb repressive complex 2 in acute promyelocytic leukemia. Cancer Cell. 2007;11(6):513–525. doi: 10.1016/j.ccr.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Wang A, Alimova IN, Luo P, Jong A, Triche TJ, Wu L. Loss of CAK phosphorylation of RAR{alpha} mediates transcriptional control of retinoid-induced cancer cell differentiation. FASEB J. 2010;24(3):833–843. doi: 10.1096/fj.09-142976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZX, Kueh JL, Teh CH, Rossbach M, Lim L, Li P, Wong KY, Lufkin T, Robson P, Stanton LW. Zfp206 is a transcription factor that controls pluripotency of embryonic stem cells. Stem Cells. 2007;25(9):2173–2182. doi: 10.1634/stemcells.2007-0085. [DOI] [PubMed] [Google Scholar]
- Warming S, Rachel RA, Jenkins NA, Copeland NG. Zfp423 is required for normal cerebellar development. Mol Cell Biol. 2006;26(18):6913–6922. doi: 10.1128/MCB.02255-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White KA, Yore MM, Deng D, Spinella MJ. Limiting effects of RIP140 in estrogen signaling: potential mediation of anti-estrogenic effects of retinoic acid. J Biol Chem. 2005;280(9):7829–7835. doi: 10.1074/jbc.M412707200. [DOI] [PubMed] [Google Scholar]
- Wu L, Ross AC. Acidic retinoids synergize with vitamin A to enhance retinol uptake and STRA6, LRAT, and CYP26B1 expression in neonatal lung. J Lipid Res. 2010;51(2):378–387. doi: 10.1194/jlr.M001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Sylvester R, Tighe AP, Chen S, Gudas LJ. Transcriptional activation of the suppressor of cytokine signaling-3 (SOCS-3) gene via STAT3 is increased in F9 REX1 (ZFP-42) knockout teratocarcinoma stem cells relative to wild-type cells. J Mol Biol. 2008;377(1):28–46. doi: 10.1016/j.jmb.2007.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Glass CK, Rosenfeld MG. Coactivator and corepressor complexes in nuclear receptor function. Curr Opin Genet Dev. 1999;9(2):140–147. doi: 10.1016/S0959-437X(99)80021-5. [DOI] [PubMed] [Google Scholar]
- Yasuhara R, Yuasa T, Williams JA, Byers SW, Shah S, Pacifici M, Iwamoto M, Enomoto-Iwamoto M. Wnt/beta-catenin and retinoic acid receptor signaling pathways interact to regulate chondrocyte function and matrix turnover. J Biol Chem. 2010;285(1):317–327. doi: 10.1074/jbc.M109.053926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Mani RS, Cao Q, Brenner CJ, Cao X, Wang X, Wu L, Li J, Hu M, Gong Y, Cheng H, Laxman B, Vellaichamy A, Shankar S, Li Y, Dhanasekaran SM, Morey R, Barrette T, Lonigro RJ, Tomlins SA, Varambally S, Qin ZS, Chinnaiyan AM. An integrated network of androgen receptor, polycomb, and TMPRSS2-ERG gene fusions in prostate cancer progression. Cancer Cell. 2010;17(5):443–454. doi: 10.1016/j.ccr.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Ren X, Alt E, Bai X, Huang S, Xu Z, Lynch PM, Moyer MP, Wen XF, Wu X. Chemoprevention of colorectal cancer by targeting APC-deficient cells for apoptosis. Nature. 2010;464(7291):1058–1061. doi: 10.1038/nature08871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Kong N, Wang J, Fan H, Zou H, Horwitz D, Brand D, Liu Z, Zheng SG. Cutting Edge: All-Trans Retinoic Acid Sustains the Stability and Function of Natural Regulatory T Cells in an Inflammatory Milieu. J Immunol. 2010;185(5):2675–9. doi: 10.4049/jimmunol.1000598. [DOI] [PMC free article] [PubMed] [Google Scholar]


