Abstract
Bladder cancer is approximately three times more common in men as compared to women. We and others have previously investigated the contribution of androgens and the androgen receptor (AR) to bladder cancer. JMJD2A and LSD1 are recently discovered AR coregulator proteins that mediate AR-dependent transcription via recently described histone-lysine demethylation (KDM) mechanisms. We used immunohistochemistry to examine JMJD2A, LSD1 and AR expression in 72 radical cystectomy specimens, resulting in evaluation of 129 tissue samples (59 urothelial carcinoma, 70 benign). We tested levels of these proteins for statistical association with clinicopathologic variables and patient survival. Expression of these markers was also assessed in human bladder cancer cell lines. The effects of pharmacological inhibition of LSD1 on the proliferation of these bladder cancer cells was determined.
JMJD2A and AR levels were significantly lower in malignant versus benign urothelium, while increased LSD1 levels were observed in malignant urothelium relative to benign. A significant reduction in all three proteins occurred with cancer stage progression, including muscle invasion (JMJD2A/LSD1/AR), extravesical extension (JMJD2A/LSD1) and lymph node metastasis (JMJD2A/AR). Lower JMJD2A intensity correlated with additional poor prognostic features, including lymphovascular invasion, concomitant carcinoma in situ and tobacco usage, and predicted significantly worse overall survival. Pharmacological inhibition of LSD1 suppressed bladder cancer cell proliferation and androgen induced transcription. Our results support a novel role for the AR-KDM complex in bladder cancer initiation and progression, identify JMJD2A as a promising prognostic biomarker, and demonstrate targeting of the KDM activity as an effective potential approach for bladder cancer growth inhibition.
BACKGROUND
Urothelial cell carcinoma of the bladder is the fourth most common solid tumor malignancy among men and ninth most common solid tumor malignancy among women in the U.S. [1]. Risk factors include smoking, male gender, advanced age, Caucasian race and prior pelvic irradiation [2,3]. While most tumors are superficial, recurrence after endoscopic resection with or without intravesical pharmacotherapy is frequent and carries a lifelong risk. Radical cystectomy achieves a durable cure, however relapse can be expected in approximately one third of patients, with worse outcomes associated with advanced tumor stage, lymphovascular invasion, lymph-node metastasis, concomitant carcinoma in situ and surgical margin involvement [4–7]. Worse outcomes also correlate with certain clinical traits, including tobacco use, female gender and preoperative hydronephrosis [2,8–10]. Relapse after definitive surgery is typically fatal, with current cytotoxic chemotherapies providing no definite survival benefit in the adjuvant or salvage setting. Thus an urgent need exists to identify key molecular signaling pathways governing bladder carcinogenesis. Such knowledge may improve patient outcomes by enabling development of better biomarkers for patient risk stratification and novel targeted therapies.
In this study we investigate the role of the androgen receptor (AR, NR3C4) signaling complex in human bladder carcinogenesis. Embryologically, the bladder derives from the urogenital sinus, where AR is expressed [11]. A role for AR signaling in bladder carcinogenesis is suggested epidemiologically by a male predominance (~3:1) in bladder cancer diagnoses in the U.S. independent of tobacco usage or occupational carcinogen exposure [12]. Interestingly, although males more frequently develop bladder cancer, they have a better prognosis than females, suggesting a complex role for androgens in bladder carcinogenesis [2,8,9]. In rodent models employing the T-antigen oncogene or the chemical carcinogen, N-butyl-N-(4-hydroxybutyl)nitrosamine (BBN), bladder tumors are induced preferentially in wild-type males relative to females or castrate males [13,14]. Formation of these tumors is increased by androgen supplementation and decreased by AR blockade or experimental downregulation, with androgens and AR exerting independent effects [13–17]. However, as neither BBN nor T-antigen plays a known role in human bladder carcinogenesis, the clinical relevance of these findings remains unknown.
In human tissue, current understanding of AR signaling in normal and malignant bladder urothelium is limited. Independent recent reports by Miyamoto et al and Boorjian et al. in human bladder cancer cell lines suggest a growth-promoting role for AR, consistent with the BBN and T-antigen rodent models [13,18]. In contrast, AR immunostaining in clinical bladder cancers indicate reduced AR levels with progression to muscularis propria (detrusor muscle) invasion [19,20]. Thus, while AR may be implicated in early events of bladder carcinogenesis, it may not be required for progression of disease, and the prognostic significance of AR loss during clinical bladder cancer progression remains unclear.
AR function is dependent upon the direct interaction of the AR with multiple coregulator proteins with diverse enzymatic actions that can serve to either activate (coactivators) or repress (corepressors) AR-mediated transcription [21]. Aberrant AR signaling could therefore arise from changes in the expression of AR coregulators, independent of levels of AR or endogenous androgen hormone. This phenomenon is well described in prostate cancer, where AR-coregulators have been implicated in prostate carcinogenesis and the progression to androgen-independent disease [22–25]. Epigenetic coregulators mediate AR transcription via chemical modifications of histone proteins [21,26]. Lysine-Specific Demethylase 1 (LSD1, also referred to as KDM1A/AOF2/BHC110) and members of the Jumoni-domain containing (JMJD2) coregulator family (JMJD2A, JMJD2C/GASC1; JMJD2D) are recently indentified nuclear receptor epigenetic coregulators that cooperatively mediate sex steroid hormone-induced transcription by removal of methyl groups from specific histone lysine residues, causing chromatin reconfiguration [27–29]. Until recently, it was believed that histone lysine methylation was a biochemically irreversible state. However it is now established that in the presence of hormone-activated AR, the LSD1-JMJD2 complex cooperate to remove the repressive histone H3-lysine-9 (H3K9) tri-methylation mark that facilitates transcriptional activation [21,27,30]. JMJD2 family members are required to initiate the demethylation of tri-methylated lysine residues [30]. LSD1 completes the demethylation of mono- and di-methylated lysine residues via an FAD-monoamine oxidase mechanism [27,30]. LSD1 also regulates other transcription factors, including the estrogen receptor (ER) [29] and p53 [31] and can function as a transcriptional corepressor [32]. The recent description of their KDM-enzymatic activity has drawn considerable attention to JMJD2A and LSD1 as novel potential therapeutic targets for the reversal of aberrant epigenetic expression/silencing. Investigation of selective and non-selective agents are underway, the latter including the monoamine oxidase inhibitor (MAOI) class of antidepressants [27,33–38]. Novel inhibitors of the JMJD2-family of KDMs are also in development [35].
Consistent with their roles in sex steroid hormone receptor regulation, JMJD2A and LSD1 are both implicated clinically in the regulation of hormonally responsive malignancies [28,39,40]. Specifically, JMJD2A is expressed at higher levels during prostate tumorigenesis [28], while higher LSD1 levels are observed in advanced prostate and breast cancers, predicting recurrent disease in the former [39,40]. In vitro, JMJD2A and LSD1 expression drives AR-mediated prostate cancer cell growth, while siRNA-mediated downregulation or pharmacologic inhibition of LSD1 suppresses this effect [27,28]. However, whether these two proteins play a similar role in bladder carcinogenesis has yet to be studied.
In this study we tested the hypothesis that levels of the AR coregulators LSD1 and JMJD2A are altered in clinical bladder cancer. Protein expression of AR and these coregulators was measured by immunohistochemical staining in 129 bladder tissue specimens and assessed for statistical association with the presence of malignancy, adverse cancer histopathology, and multiple clinical variables, including known risk factors for bladder carcinogenesis and/or bladder cancer-specific survival. Association between protein levels and patient outcomes after radical cystectomy, including recurrence and survival, was also investigated. Finally, RNA expression of AR, LSD1 and JMJD2A was measured in four bladder cancer cell lines, and the effect of LSD1 pharmaco-inhibition on bladder cancer cell proliferation and androgen induced transcription was determined using MAOI drugs, specifically pargyline and tranylcypromine.
MATERIALS AND METHODS
Patients and tissue specimens
Institutional Review Board approval was acquired for all parts of this study. Seventy-two radical cystectomy specimens accessioned from May 2002 to December 2007 were retrieved from the surgical pathology archives of the Department of Pathology and Laboratory Medicine at New York-Presbyterian Hospital/Weill Cornell Medical College. Although all samples were acquired by cystectomy, the cohort reflects the clinical spectrum of localized to advanced bladder cancers. One hundred twenty-nine formalin-fixed, paraffin-embedded tissue samples, encompassing 59 urothelial cell carcinomas and 70 sections of benign urothelium, were subjected to immunohistochemistry for the purposes of this study. All patients had preoperatively diagnosed urothelial carcinoma of the bladder and underwent pelvic lymphadenectomy at the time of cystectomy. Thirteen patients had no detectable cancer at the time of surgery (stage pT0), and only benign urothelium was acquired in these cases. For 57 of the remaining 59 patients, both benign and malignant tissue samples were obtained; the remaining two patients had large, advanced primary tumors such that only malignant tissue was available for immunohistochemistry. Clinicopathologic and survival data were collected for all patients in a prospectively updated cystectomy database. Clinical variables included age, gender race, body mass index, smoking history, preoperative hydronephrosis, pelvic radiation history and history of intravesical or neoadjuvant systemic chemotherapy. Pathologic variables included primary tumor stage, lymphovascualar invasion, perineural invasion, concomitant CIS, surgical margin positivity and lymph node metastasis. Tumor stage was defined according to the American Joint Committee on Cancer guidelines. Cancer recurrence after cystectomy was defined as radiographic suggestion of disease on postoperative CT scan or MRI obtained during routine clinical surveillance. Death certificates and/or autopsy reports were reviewed to determine whether cause of death was cancer-related or from another cause. Patient characteristics are summarized in Table 1.
Table 1. Cystectomy patient characteristics.
| TOTAL PATIENTS, n= (%) | 72 (100) |
| AGE, years mean +/− s. d. median range |
66.4 +/− 10.0 66.6 43.3 – 88.8 |
| GENDER, n= (%) Female Male |
21 (29) 51 (71) |
| RACE, n= (%) Caucasian Other |
66 (92%) 6 (8%) |
| BMI, kg/m2 mean +/− s. d. median |
26. 8 +/− 4. 8 26. 5 |
| SMOKING HISTORY, n= (%) | 56 (74) |
| ACTIVE SMOKER, n= (%) | 15 (21) |
| PRIOR PELVIC IRRADIATION, n= (%) | 8 (11) |
| PRIOR INTRAVESICAL CHEMOTHERAPY, n= (%) | 21 (29) |
| PRIOR NEOADJUVANT SYSTEMIC CHEMOTHERAPY, n= (%) | 11 (15) |
| PRE-OPERATIVE HYDRONEPHROSIS, n= (%) | 20 (28) |
| PATHOLOGIC STAGE, n= (%) pT0 pTa pTis pT1 pT2 pT3 pT4 |
13 (18) 9 (13) 11 (15) 7 (10) 10 (15) 11 (15) 11 (15) |
| LYMPH NODE METASTASES, n= (%) | 18 (25) |
| N-STAGE N1 N2 N3 |
10 (14) 5 (7) 3 (4) |
| LYMPHOVASCULAR INVASION, n= (%) | 15 (21) |
| PERINEURAL INVASION, n= (%) | 6 (8) |
| CONCOMITANT CARCINOMA IN SITU (CIS), n= (%) | 34 (47) |
| POST-OPERATIVE FOLLOW UP INTERVAL, months mean +/− s. d. median |
26. 3 +/− 21. 2 21. 2 |
| POST-OPERTATIVE RECURRENCE, n= (%) | 19 (26) |
| POST-OPERATIVE DEATH FROM BLADDER CANCER, n= (%) | 15 (21) |
Immunohistochemistry
Immunohistochemical staining was performed using the Leica BOND-MAX Autostainer and supplied reagents (Leica Microsystems), except where otherwise specified. Each antibody was evaluated individually on serial, 5-micron sections of representative whole tissue blocks from each cystectomy specimen. Sections were deparaffinized, and endogenous peroxidase was inactivated followed by Heat-Induced Epitope Retrieval (HIER)-1 (for LSD1 and JMJD2A antibodies) or HIER-2 (for AR antibody) according to the manufacturer's instructions (Leica Microsystems). Primary antibodies for LSD1 (1B2E5, #NB-100-1762, Novus Biologicals), JMJD2A (# NB100-57563, Novus Biologicals) and AR (AM256, BioGenex) were detected using the Leica Microsystems Refine Detection Kit. Negative controls were also performed by using normal serum from the same species and at the same concentration as the primary antibody. Benign and malignant prostate tissue was used as a positive control, and negative control staining without primary antibody was also performed. All stained tissue sections were then evaluated by a urologic pathologist (BDR), and separate scores were given to each sample based on: a) tissue positivity, i.e., the percentage of tissue staining positive (0–100%), and b) the intensity of positively staining cells (0– none, 1– weak, 2– moderate, and 3– strong). Only nuclear staining was considered positive, and a minimum of 100 tumor nuclei were evaluated for each case.
Bladder cancer cell lines and culture conditions
Four bladder cancer cell lines (HTB1, HTB3, HTB5 and HT1376) were purchased from the American Type Culture Collection (Rockville, MD). The HTB1 (J82), HTB5 (TCCSUP), and HT1376 cell lines are derived from high-grade, invasive transitional cell carcinomas, while HTB3 (SCaBER) cell line originated from an invasive squamous cell carcinoma of the bladder. HTB5 expresses AR, while HTB1 is reported to be AR-negative [13,18]. Bladder cancer cells were cultured as described (ATCC) and maintained at 37°C, 10% CO2 in MEM supplemented with 100 µg/mL streptomycin, 100 units/mL penicillin, and 10% FCS. Cell lines were verified according to ATCC guidelines (Technical Bulletin 8).
RNA extraction and RT-PCR
Total cellular RNA was extracted using the TRIzol reagent (Invitrogen, Carlsbad, CA). RNA-extraction experiments were repeated in triplicate. First-strand cDNA was synthesized from 1 µg of total RNA by reverse transcription with Superscript II (Invitrogen) or qScript (Quanta) reverse transcriptase at 42°C for 60 minutes. PCR amplification of cDNA for AR (GeneID: 376), LSD1(GeneID: 23028), and JMJD2A(GeneID: 9682), was performed using iQ Supermix (BioRad) and transcript-specific oligonucleotide PCR primers. Primer sequences spanned intron-exon boundaries, preventing amplification of any contaminating genomic DNA. PCR amplification was internally controlled using primers for GAPDH to confirm the integrity of cDNA. Primers and annealing temperatures were as follows: GAPDH – F:agccacatcgctcagacac, R:gaggcattgctgatgatcttg, 63°C; LSD1 - F: ggaagaaaatgaaagtgagcctgaag, R:cttctagcaaccggttaaactcttgc, 59°C; JMJD2A- F: gtggtcttcattacctgctttcgg, R:ggaccaaactggagacagtcctgg, 59°C; AR - F: caacccagaagctgacagtgtcac, R: ccatctggtcgtccacgtgta. Negative control PCR using reverse osmosis–grade water in the place of template were included in all experiments. PCR products were separated on 1.5% agarose gels and stained with ethidium bromide. The identity of the cDNA product was confirmed by comparison of the PCR-amplified DNA to the predicted fragment size and DNA sequencing. PCR experiments were repeated on three independent RNA preparations.
Real time PCR was performed using Perfecta SYBR Green Super Mix for iQ (Quanta Bioscience) in a BioRad MyIQ real time system. The oligonucleotides employed were F: tggaggtcttggtcaagcatacag, R-caaggctgttagagagataattgga for NEP (GeneID: 4311), and HPRT (GeneID: 3251) was as described previously [41]. The threshold cycle (Ct) was determined by the MyIQ software (BioRad). The Ct values showed a linear correlation with relative cDNA input for both the NEP and HPRT genes. Reaction conditions were template denaturation at 95°C for three minutes followed by 45 cycles of 94°C, 10s; primer annealing at 60°C or 55°C for NEP and HPRT respectively; extension at 72°C for 30s and fluorescence was read after each cycle at 80°C. Real time analysis was performed in triplicate on two independent cell treatments. Negative control PCRs using water in the place as template were incorporated into each experiment. Expression of NEP was normalized by HPRT and compared to the levels detected in the vehicle treated control cells using the ΔΔCt method [42].
Functional analysis of the effects of pargyline and tranylcypromine on bladder cancer cells
Growth analyses were performed on bladder cancer cell lines plated at 1 × 106 cells/well in six-well culture dishes. Dose response comparison of the effects on cellular proliferation of 3 mM and 1 mM concentrations of the non-selective monoamine oxidase/LSD1 inhibitors, pargyline and tranylcypromine (Sigma-Aldrich) were performed on HTB-1, HTB-3, HTB-5 and HT-1376 bladder cancer cell lines. Cells were treated with drug for 48 hours and counted using a Coulter Counter (Beckman). Each treatment condition represents five data points accrued over two separate experiments.
The effects of tranylcypromine on androgen induced transcription were measured in cultured HTB1 and HTB5 bladder cancer cells. Tranylcypromine (50M) is an effective irreversible inhibitor of LSD1 enzymatic activity [43]. HTB1 and HTB-5 bladder cells were treated for 48 hours with vehicle control or tranylcypromine (50µM). Following this 48 hour treatment, androgen regulated transcription was stimulated in cells by treatment with the synthetic androgen, R1881, (1nM) for three hours. In addition cells treated with tranylcypromine for 48 hours but not stimulated with R1881 were used as controls. Quantitative PCR was performed in triplicate on two independent experiments, starting from drug treatment. Relative expression of NEP normalized to HPRT in bladder cancer cell lines was determined using RT-qPCR with vehicle control treated cells set as the calibrator sample.
Statistical analysis
Fisher’s Exact Test was used to test for statistical association between AR, JMJD2A and LSD1 immunostaining scores and the clinicopathologic variables shown in Table 1. Separate analyses were performed for tissue positivity and intensity scores. Tissue positivity was tested as a categorical variable using a 4-tier scale (0–10%, 11–40%, 41–70%, 71–100%). Immunostaining intensity score was tested using the 0–3 scale described above. Patients with final pathology of urothelial carcinoma in situ (pTis) were censored from analyses testing for association with concomitant CIS. Staining scores were tested for association with each other using a two-tailed Spearman Rank Correlation Test. Spearman coefficients >0.8 were considered to represent strong correlations, while coefficients between 0.4 and 0.8 were considered to have slight correlation. The Kaplan-Meier method was used to generate survival curves stratified by immunostaining scores for each protein. Univariate testing of immunostaining scores and each clinicopathologic variable shown in Table 1 for association with disease recurrence, cancer-specific mortality or overall mortality (yes/no) was performed using a Fisher’s Exact Test. Multivariate analysis using exact logistic regression was used to determine the ability of immunostaining scores to predict overall mortality after adjusting for other clinicopathologic variables demonstrating significant association on univariate analysis. For in vitro growth assays an ANOVA test were utilized to determine statistical significance between mean cell counts. RT-qPCR data were for analyzed for statistical significance using student’s t test. Statistical analyses were conducted using StataSE 10® (StataCorp College Station, TX, USA) or Prism4 (GraphPad). In all cases, p≤ 0.05 was considered significant.
RESULTS
JMJD2A/LSD1/AR immunostaining: association with cancer histopathology
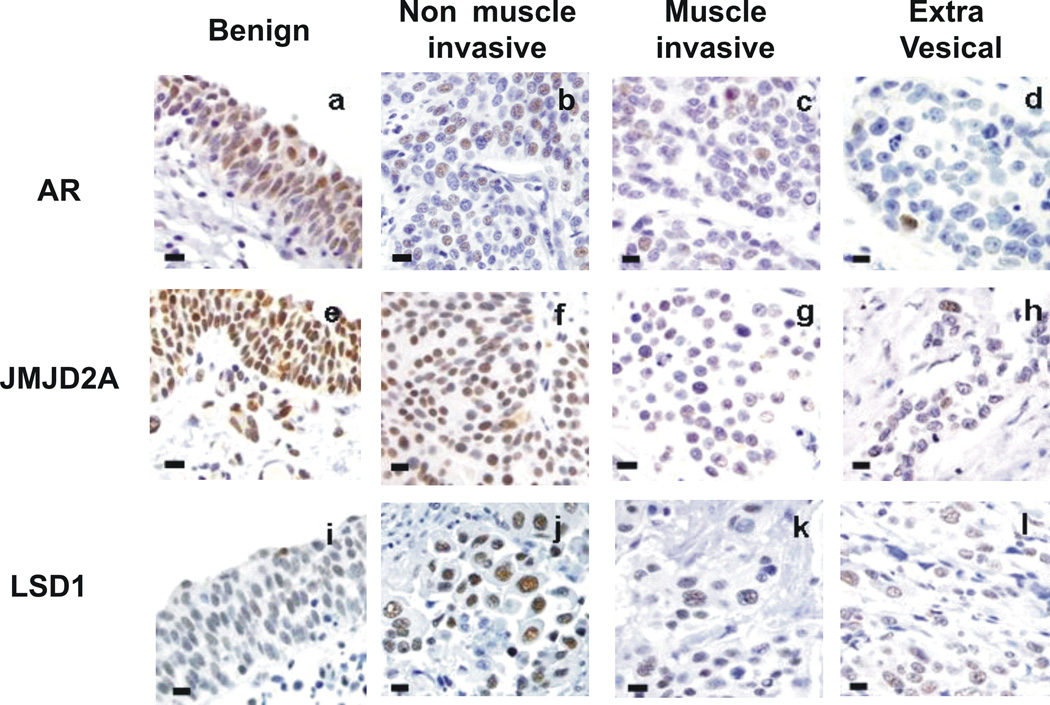
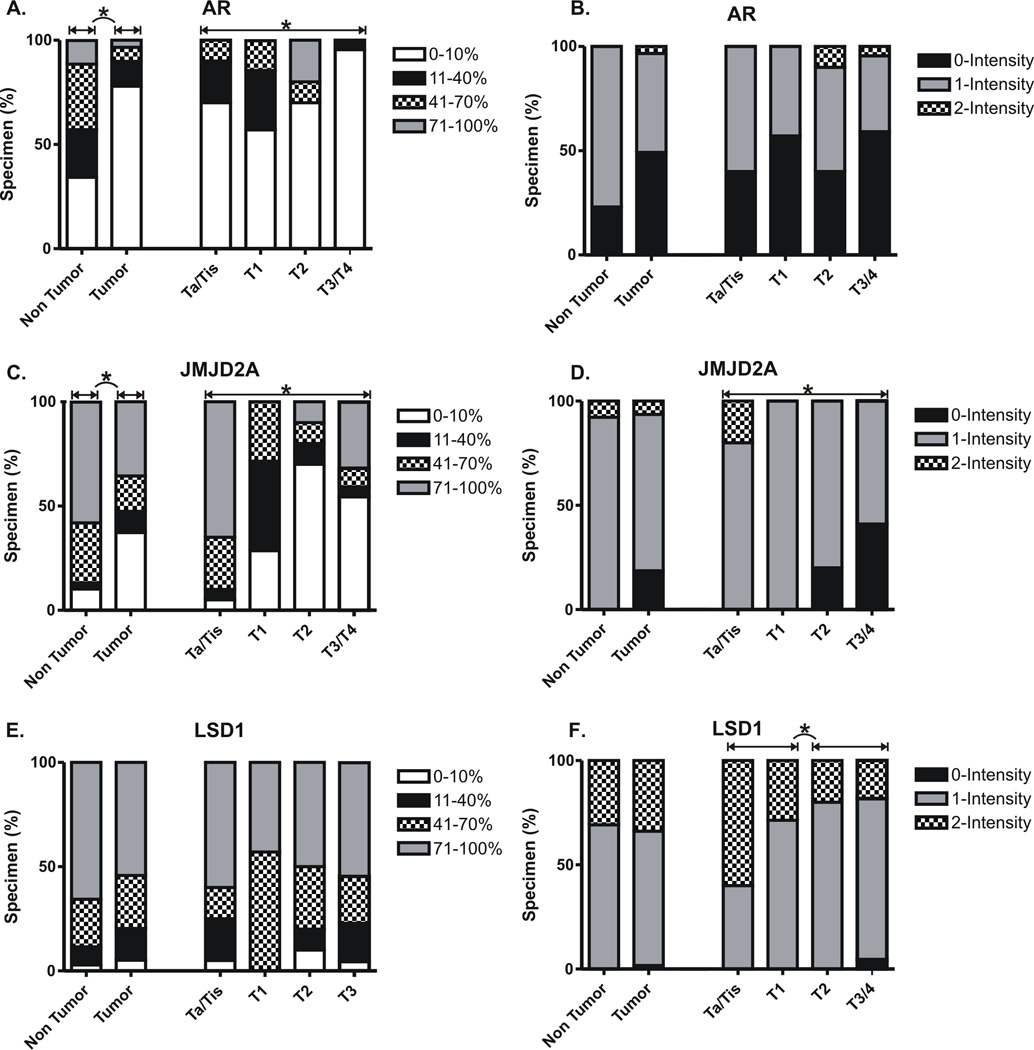
Significantly lower levels of JMJD2A immunostaining were detected in malignant versus benign urothelium, based on both %-tissue positivity and intensity scores (Table 2, Figures 1, 2). For example, >10% tissue positivity was observed in the vast majority (90%) of benign specimens compared to around only one third of malignant specimens (Figures 1, 2). Among JMJD2A positive cases, staining intensity was typically low or moderate (1–2+). However, patients with 2+ or 3+ staining in benign urothelium generally had only 0+ or 1+ staining in matching cancer specimens (11/14, 79%). By both %-tissue positivity and intensity scoring, lower JMJD2A levels correlated significantly with the acquisition of an invasive phenotype, including detrusor muscle invasion (Table 2, Figures 1, 2). Lower JMJD2A intensity but not %-tissue positivity also correlated significantly with the presence of extravesical tumor extension (Table 2, Figures 1, 2). This association was most pronounced for pT4 disease, with complete JMJD2A loss being present in 64% of these specimens compared to only 8% of all lower staged cancers (0% pTa, 0% pTis, 0% pT1, 20% pT2, 18% pT3). As detailed in Table 2, reduced JMJD2A levels were also significantly associated with other adverse histopathologic descriptors, including lymphovascular invasion (JMJD2A intensity), concomitant carcinoma in situ (JMJD2A %-tissue positivity), lymph node positivity (JMJD2A %-tissue positivity) and lymph node stage (JMJD2A %-tissue positivity). There was also a trend towards association between JMJD2A intensity and perineural invasion, but this finding did not reach statistical significance (Table 2).
Table 2. Association of JMJD2A, LSD1 and AR levels with patient clinicopathologic traits, disease recurrence and cancer-specific mortality in tumor specimens.
Fishers exact p-values are shown for each statistical association. Dark shading = significant (p<0.05). Superficial (Ta, T1, CIS) and muscle invasive (T2, T3, T4) bladder cancers. NMI:non muscle invasive; MI= muscle invasive ; BC: bladder confined, EVE; extravsical.
| Marker | JMJD2A | LSD1 | AR | |||
|---|---|---|---|---|---|---|
| % Positivity | Intensity | % Positivity | Intensity | % Positivity | Intensity | |
| p value | p value | p value | ||||
| CLINICAL | ||||||
| Age | 0.101 | 0.817 | 0.560 | 0.294 | 0.638 | 0.821 |
| Gender | 0.557 | 0.06 | 0.200 | 0.582 | 0.961 | 0.900 |
| Race | 0.121 | 0.911 | 0.565 | 0.821 | 0.686 | 0.926 |
| Smoking: Any History | 0.344 | 1 | 0.533 | 0.669 | 0.705 | 1 |
| Active | 0.027 | 0.001 | 0.562 | 0.755 | 0.606 | 0.317 |
| Body Mass Index | 0.535 | 0.114 | 0.273 | 0.595 | 0.998 | 0.37 |
| Prior Pelvic Radiation | 0.241 | 0.787 | 0.831 | 1 | 1 | 0.428 |
| Hydronephrosis | 0.736 | 0.065 | 0.856 | 0.573 | 0.619 | 0.537 |
| Intravesical Chemotherapy | 0.813 | 0.298 | 0.969 | 0.289 | 0.009 | 0.189 |
| Neoadjuvant Chemotherapy | 0.664 | 0.465 | 0.09 | 0.141 | 0.637 | 0.666 |
| HISTOPATHOLOGIC | ||||||
| Malignant vs. Benign | <0.001 | 0.012 | 0.493 | 0.096 | <0.001 | <0.001 |
| Stage: | ||||||
| Overall | <0.001 | <0.001 | 0.639 | 0.121 | 0.014 | 0.443 |
| Superficial vs Invasive | <0.001 | <0.001 | 0.574 | 0.006 | 0.358 | 0.35 |
| NMI vs MU | 0.001 | <0.001 | 1 | 0.012 | 0.028 | 0.382 |
| BC vs EVE | 0.177 | 0.001 | 0.939 | 0.046 | 0.092 | 0.376 |
| Lymphovascular Invasion | 0.062 | 0.016 | 1 | 0.129 | 0.762 | 0.532 |
| Perineural Invasion | 0.417 | 0.097 | 0.777 | 0.165 | 0.496 | 0.496 |
| Presence of CIS | 0.033 | 1 | 0.649 | 0.485 | 0.894 | 0.191 |
| Positive Bladder Margin | 0.687 | 0.125 | 0.426 | 0.565 | 1 | 0.619 |
| Lymph Node: Positivity | 0.034 | 0.141 | 0.225 | 0.084 | 0.02 | 0.166 |
| Density | 0.088 | 0.309 | 0.204 | 0.112 | 0.256 | 0.082 |
| Stage | 0.022 | 0.028 | 0.306 | 0.155 | 0.675 | 0.276 |
| SURVIVAL | ||||||
| Disease-free survival | 0.799 | 0.409 | 0.372 | 0.494 | 0.284 | 0.409 |
| Cancer Specific Survival | 0.896 | 0.761 | 0.885 | 0.810 | 0.665 | 0.456 |
| Overall Survival | 0.064 | 0.033 | 0.740 | 0.402 | 0.766 | 0.745 |
Figure 1. Immunohistochemical staining.
Representative immunohistochemical staining for AR (a-d), LSD1 (d-f) and JMJD2A (g-i)in benign urothelium, non-muscle invasive urothelial carcinoma (pTis, pTa, pT1), muscle invasive carcinoma (pT2) and extravesical (pT3) carcinoma. (All images taken at 400X total magnification; bar – 20 microns)
Figure 2. Distribution of staining positivity and intensity by pathological tumor stage.
Separate scores were given to the stained tissue sections based on: a) tissue positivity, i.e., the percentage of tissue staining positive (0–100%), and b) the intensity of positively staining cells (0– none, 1– weak, 2– moderate, and 3– strong). Only nuclear staining was considered positive, and a minimum of 100 tumor nuclei were counted for each case. A summary of statistically significant differences in staining positivity and intensity are indicated (*). Please refer to Table 2 for detailed statistical analysis.
In contrast to JMJD2A, LSD1 stained with at least >10% tissue positivity in nearly all (96%) bladder specimens, with no differences observed between malignant and benign urothelium (Table 2, Figures 1, 2). Greater than 70% tissue positivity was detected in the majority of these cases. However, intensity of staining tended to be higher among malignant specimens (p=0.096) (Table 2, Figures 1, 2). This finding reached statistical significance when comparing only those patients with both benign and cancer specimens available (N=57, p=0.05). In contrast, LSD1 intensity decreased significantly with acquisition of the invasive phenotype, including detrusor muscle invasion and extravesical extension (Table 2, Figures 1, 2). LSD1 levels were not significantly associated with other adverse histopathologic parameters, including lymphovascular invasion, perineural invasion and concomitant carcinoma in situ, although we observed a nonsignificant trend towards association of lower LSD1 intensity with the presence of lymph node metastasis (Table 2).
As with JMJD2A, significantly lower AR staining intensity and %-tissue positivity were observed in malignant urothelium relative to benign urothelium (Table 2, Figures 1, 2). For example, >10% tissue positivity was found in two thirds of benign specimens compared to less than one quarter of cancer specimens, and total absence of AR was observed in roughly half of all cancer specimens compared to just 16% of benign specimens. As with JMJD2A and LSD1, lower AR %-tissue positivity correlated significantly with the acquisition of an invasive phenotype, including detrusor muscle invasion, and also correlated significantly with the presence of lymph node metastasis (Table 2, Figures 1, 2). A trend towards association of AR %-tissue positivity with extravesical extension did not reach statistical significance (Table 2, Figures 1, 2). No association was detected between AR levels and other adverse histopathologic descriptors, including lymphovascular invasion, perineural invasion and concomitant carcinoma in situ (Table 2).
JMJD2A/LSD1/AR immunostaining: association with clinical risk factors
Immunostaining levels of AR, JMJD2A and LSD1 were tested for association with several clinical variables, including known risk factors for bladder cancer diagnosis or cancer-specific mortality (Table 2). Lower JMJ2DA staining intensity was found to be strongly associated with active patient smoking (p=0.001). There was also a nonsignificant trend towards association of JMJD2A staining intensity with preoperative hydronephrosis (p=0.065) and female gender (p=0.062), as 30% of female patients had JMJD2A-negative tumors compared to just 9% of males. AR %-tissue positivity was significantly associated with preoperative intravesical chemotherapy (p=0.009), and there was a nonsignificant trend towards association between LSD1 %-tissue positivity and neoadjuvant systemic chemotherapy (p=0.09). No association was detected between JMJD2A/LSD1/AR levels and patient age, race, body mass index or pelvic radiation history (Table 2).
JMJD2A/LSD1/AR immunostaining: association with patient survival
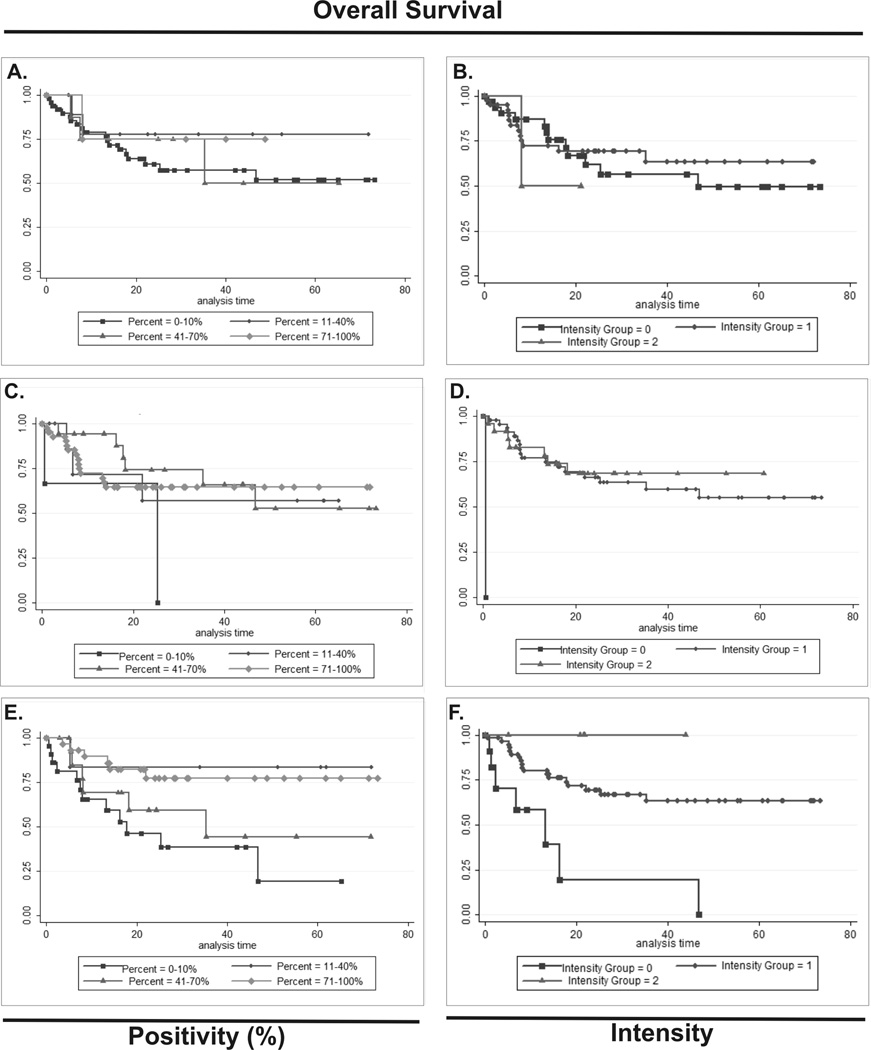
Immunostaining scores for AR, JMJD2A and LSD1 were tested alongside other clinicopathologic variables for association with bladder cancer recurrence, cancer-specific mortality and overall mortality by univariate analysis. Disease recurrence was predicted by tumor stage (p>0.001) and lymph node stage/positivity/density (p=0.003/0.005/0.006), while cancer-specific mortality was predicted by tumor stage (p=0.007), lymph node stage/positivity/density (p=0.02/0.04/0.01) and lymphovascular invasion (p=0.01). AR, JMJD2A or LSD1 levels were not associated disease recurrence or cancer-specific survival (Table 2). In contrast, lower JMJD2A staining intensity was significantly associated with overall mortality (p=0.033), as was tumor stage (p<0.001), lymph node stage/positivity/density (p=0.001/0.007/0.001), lymphovascular invasion (p<0.001), neoadjuvant chemotherapy (p=0.041) and active tobacco usage (p=0.012), but not levels of LSD1 or AR (Table 2). We performed a multivariate analysis to determine whether the association of JMJD2A intensity with overall mortality was independent of other clinicopathologic variables, however no variable carrying significance on univariate analysis reached significance on multivariate analysis likely due to the limited number of death events (data not shown). Kaplan-Meier curves for overall survival were generated for each of the three AR-complex proteins and are displayed in Figure 3. While 100% of patients with moderate or high (2–3+) JMJD2A intensity demonstrated durable overall survival, patients with JMJD2A negative (0+) tumors experienced had accelerated death, with a 20% survival rate at just 2 years after cystectomy. Patients with low (1+) JMJD2A staining intensity had intermediate survival outcomes, with a >75% survival rate at 2 years after cystectomy.
Figure 3. Kaplan Meier survival estimates.
Kaplan-Meier overall survival estimates for 72 cystectomy patients, stratified by immunohistochemical staining positivity and intensity for AR (A-B), LSD1(C-D) and JMJD2A (E-F) in bladder tumors.
JMJD2A/LSD1 immunostaining: association with AR expression
Levels of JMJD2A and LSD1 immunostaining were tested for association with levels of AR and each other using a Spearman correlation test (Table 3). Weak, but statistically significant correlations were observed between LSD1 tissue positivity and AR tissue positivity and intensity, and between JMJD2A intensity and AR intensity (Table 3). LSD1 tissue positivity also weakly but significantly correlated with JMJD2 tissue positivity and intensity (Table 3).
Table 3. Correlation of levels of AR coregulators to levels of AR and to each other.
Expression of each marker was assessed for correlation using the Spearman rank correlation test. Spearman correlation coefficient and p values are shown for each correlation.
| JMJD2A Positivity (%) |
JMJD2A Intensity |
AR Positivity (%) |
AR Intensity |
LSD1 Positivity (%) |
LSD1 Intensity |
||
|---|---|---|---|---|---|---|---|
| JMJD2A Positivity (%) |
Correlation coefficient |
1 | 0.511 | 0.171 | 0.161 | 0.201 | 0.117 |
| p-value | <0.001 | 0.053 | 0.070 | 0.023 | 0.187 | ||
| JMJD2A Intensity |
Correlation coefficient |
1 | 0.168 | 0.254 | 0.177 | 0.066 | |
| p-value | 0.057 | 0.004 | 0.046 | 0.461 | |||
| AR Positivity (%) |
Correlation coefficient |
1 | 0.569 | 0.193 | 0.077 | ||
| p-value | <0.001 | 0.029 | 0.387 | ||||
| AR Intensity |
Correlation coefficient |
1 | 0.209 | 0.080 | |||
| p-value | 0.018 | 0.369 | |||||
| LSD1 Positivity (%) |
Correlation coefficient |
1 | 0.440 | ||||
| p-value | <0.001 | ||||||
Bladder cancer cell growth response to LSD1 inhibition
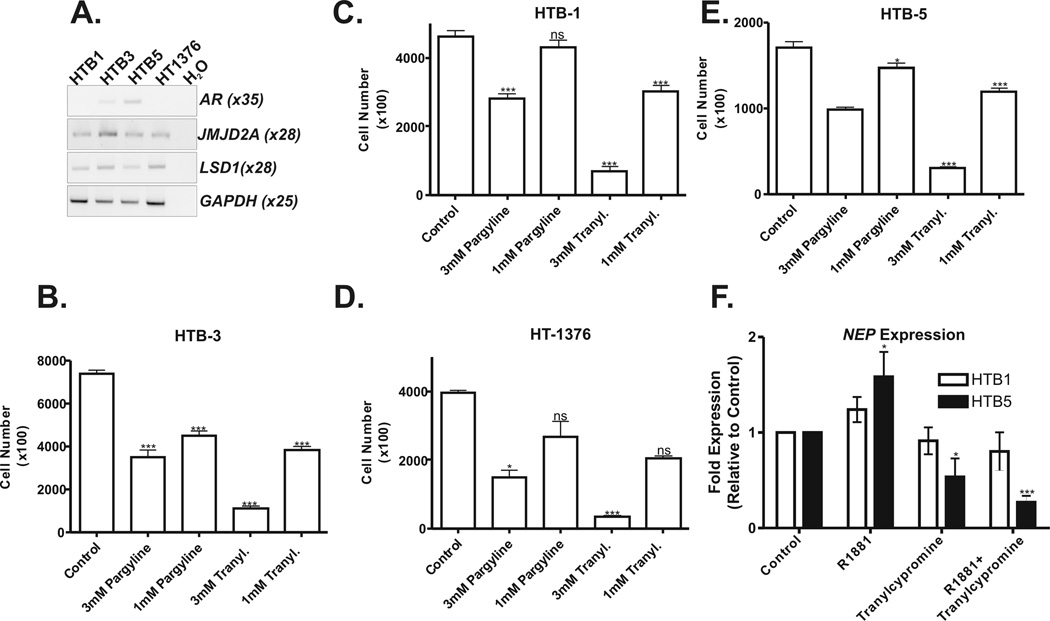
Levels of AR, JMJD2A and LSD1 mRNA transcripts were assessed by RT-PCR in four human bladder cancer cell lines derived from advanced stage tumors. Consistent with previous reports, the AR transcript was detected at low levels in the HTB-5 and HTB-3 cell lines and was not detected in the HTB-1 and HT1376 cell lines (Figure 4A) [13,18]. In contrast, mRNA expression of AR coregulators, JMJD2A and LSD1, were detectable in all four cell lines (Figure 4A). Each cell line was treated with the non-specific LSD1 inhibitors pargyline and tranylcypromine (1 mM and 3 mM) to determine the dose response effects on proliferation (Figure 4C-F). Both drugs showed dose-dependent suppression of cancer cell proliferation in the AR-positive cell lines, HTB3 and HTB5. In the AR-negative cell lines, HTB1 and HT1376, higher dose (3 mM) pargyline similarly inhibited cell proliferation, whereas the lower dose (1 mM) had no significant effect. Identical results were observed for tranylcypromine in the AR-negative cell line, HT1376. (Figure 4C-F)
Figure 4. RT-PCR expression detection of AR and AR coregulators in human bladder cancer cell lines and effects of monoamine oxidase inhibition on bladder cancer cell proliferation and transcription.
Confirmation of mRNA expression of AR, JMJD2A, LSD1 in bladder cancer cell lines (A). Full length gels are presented in supplemental figure 1. The number of PCR cycles employed for each gene is indicated. GAPDH served as an internal control for cDNA integrity. Comparison of effects of 3mM and 1mM pargline and tranylcypromine (Tranyl) on proliferation of HTB-1 (B), HTB-5 (C), HTB-3 (D) and HT-1376 (E) bladder cancer cells. Quantitative RT-PCR was used to assess the effects of tranylcypromine on androgen regulated transcription (F). Neutral endopeptidase (NEP) is a representative AR-regulated gene expressed in both HTB1 and HTB5 bladder cancer cells. Bladder cells were treated with tranylcypromine (50µM) for 48 hours prior to transcriptional activation with the R1881 (1nM) synthetic androgen for 30 minutes. R1881 induces NEP expression in AR-positive HTB5 cells. This effect of R1881 on NEP expression in HTB5 cells is impaired by tranylcypromine. Neither R1881 nor tranylcypromine alter NEP expression in AR-negative HTB1 cells, suggesting the effects of tranylcypromine are dependent upon the presence of a functional AR-KDM complex.(*:p<0.05, ***p<0.01, ns: not significant).
Effect of tranylcypromine on AR-regulated gene expression in human bladder cancer cells
We utilized RT-PCR to identify a representative AR-regulated test gene expressed in bladder cancer cells. This analysis confirmed expression of the androgen receptor regulated neutral endopeptidase (NEP) gene [44] in both HTB-1(AR negative) and HTB-5 (AR positive) bladder cell lines (data not shown). We next examined the effects of tranylcypromine on androgen induced transcription in cultured HTB-1 and HTB-5 bladder cancer cells. R1881 induced a statistically significant increase in NEP expression in AR-positive HTB-5 cells, but not in HTB-1 AR-negative bladder cells (Figure 4F). Tranylcypromine effected a modest repression of basal NEP expression in HTB-5 cells and blocked R1881 induced expression of NEP in HTB-5 cells (Figure 4F). Tranylcypromine and R1881 had no detectable effect on NEP expression in HTB-1 cells (Figure 4F).
DISCUSSION
While recent studies implicate AR signaling in bladder carcinogenesis [13], the underlying mechanisms are unknown. AR coregulators represent a enzymatically diverse set of proteins whose direct interaction with AR is required for assembly of a functional transcriptional complex [21]. Alteration in coregulator levels and/or activity was shown to dysregulate epigenetic transcriptional regulation independent of AR and circulating androgen levels [22]. Indeed there is mounting evidence for key roles of AR coregulators in the development of androgen-independent disease [22–25]. These findings highlight the dependence of AR transcriptional activity on AR-coregulator complex composition and underscore the signaling complexity that can result from differences in coregulator expression levels.
The current study investigated the role of recently identified AR coregulators JMJD2A and LSD1, in human bladder cancer. JMJD2A and LSD1 employ a recently described lysine demethylase mechanism to induce chromatin structural alterations required for AR-mediated transcription [27,28,30,32]. In hormonally driven malignancies such as prostate cancer, levels of JMJD2A and LSD1 are altered in patient tumors, and a functional role for each in mediating AR-dependent prostate cancer cell proliferation has been described [28,39,40]. Recent studies implicate human bladder carcinogenesis as a hormonally driven disease.[13,18,19] Here, we performed immunohistochemical analysis in 129 clinical bladder specimens to test whether protein levels of either JMJD2A or LSD1 are also altered during bladder carcinogenesis. We observed significant differences in JMJD2A and LSD1 coregulator levels between malignant and benign urothelium. Lower levels of both proteins were detected with primary tumor progression, including detrusor muscle invasion and extravesical extension. JMJD2A reduction also correlated with lymph node metastasis, lymphovascular invasion, and presence of carcinoma in situ, all independent predictors of worse patient outcomes after radical cystectomy [5–7]. Together, our data support reduced expression of these lysine demethylating AR coregulators during bladder cancer progression. Interestingly, the expression patterns observed in this study are different from those reported for prostate tissue, where JMJD2A and LSD1 are upregulated during tumorigenesis and cancer progression, respectively [28,40]. Thus, LSD1 and JMJD2A appear to play different roles in bladder versus prostate carcinogenesis.
We also investigated AR protein levels in bladder tissue. Similar to JMJD2A and LSD1, lower AR levels were observed during primary tumor progression to invasion. Consistent with prior reports from our group and Tuygun et al, we found AR reduction to be associated with the acquisition of the muscle-invasive phenotype [19,20]. In contrast to these reports, Mir and colleagues recently failed to detect any association between stage and loss of AR expression [45]. However AR was not detected in the majority of bladder cancer specimens examined regardless of stage in this study though the authors acknowledge a limitation to their study was that the majority of samples examined were muscle invasive tumors [45]. Of note, we observed reduced AR levels in malignant versus benign urothelium, suggesting downregulation during tumorigenesis (Table 2). While other studies have noted little or no AR expression in benign urothelium [18,20], we [19] and Mir et al [45] detected AR expression in benign urothelium. Finally, we detected a novel association between lower AR levels and lymph node metastasis. As with the coregulators, these findings are in contrast to the persistent AR expression observed during prostate cancer progression/metastasis and further suggest a novel function for the AR signaling complex in bladder carcinogenesis [46].
In prostate cancer cells, expression of several AR coregulators is described as being under AR regulation [47–50]. In this study, we observed only very weak correlations between levels of AR and levels of JMD2A or LSD1 in bladder tissue. Similarly, we detected JMJD2A and LSD1 transcripts in bladder cancer cell lines regardless of whether or not the AR transcript was present. We thus conclude that JMJD2A and LSD1 expression in bladder is likely not regulated by the AR in bladder cells.
The expression patterns observed in this study support a complex function for AR and its associated coregulator protein complexes in bladder tissue. In prostate cancer, conventional understanding is that AR activity promotes proliferation, however recent studies suggest a greater complexity of AR signaling, including the ability to suppress cancer growth cell proliferation under specific circumstances [24,51]. The frequent loss of AR and JMJD2A in early and advanced bladder cancers suggests these proteins may not be required for initiation and/or progression of disease and may indicate a potential role in impairing or delaying carcinogenesis. However, the functional significance of AR and JMJD2A loss with regards to effects on androgen signaling remains to be determined. With LSD1, the dynamic expression pattern consistent with early stage upregulation and advanced stage downregulation suggests a more complex role for LSD1 in bladder carcinogenesis. This complexity may relate to the fact that LSD1 also regulates additional signaling pathways implicated in human cancers, most notably those of p53 [31] and estrogen receptor, both of which are implicated in bladder carcinogenesis [29,39,52]. Thus, LSD1 represents a point of convergence of multiple, distinct signaling pathways implicated in human cancer. Regardless, it is possible that higher LSD1 levels during early bladder carcinogenesis may be important in altering the function of AR transcriptional complexes and compensating in part for lower AR levels.
To further elucidate the contribution of the AR-lysine demethylase complex to bladder carcinogenesis we have described the association of AR and coregulator protein levels with multiple clinical variables, including known risk factors for diagnosis and/or cancer-specific death. We report a novel association between decreased JMJD2A and active patient smoking, the most common risk factor for bladder tumorigenesis and also a poor prognostic trait [2]. A link between AR signaling and smoking has been suggested by various clinical observations, such as lower testosterone levels or worse oncologic responses to androgen-deprivation therapy among active tobacco users [53,54]. A specific mechanism is unknown but may relate to the ability of AR complexes to regulate N-acetyltransferases, enzymes necessary for bioactivation of tobacco carcinogens [55]. Lower levels of JMJD2A in this study also trended towards association with female gender [2,8–10], an additional poor prognostic features among bladder cancer patients, although this finding did not reach statistical significance (p=0.06). Nevertheless, this observation is provocative and raises the intriguing possibility that alterations in levels of sex-steroid hormone receptor coregulators may contribute to the worse female prognosis, given levels of the sex-steroid hormone receptors themselves appear similar between the genders [20]. It remains to be determined whether JMJD2A also interacts with the estrogen receptor, as already demonstrated for other AR-coactivators including LSD1 [27]. Finally, this study also uncovers a novel association between AR-complex signaling and preoperative chemotherapy. Specifically, higher AR protein levels were associated with the receipt of preoperative intravesical chemotherapy with mitomycin-C. The efficacy of chemotherapeutic drugs is well known to be negatively affected by cell cycle arrest, and the function of AR as key regulator of G1-S cell-cycle transition could therefore be important in modifying drug response.
The prognostic significance of reduced AR protein during clinical bladder cancer progression has not been well characterized. Tuygun et al found no association between AR reduction and disease recurrence. However nearly half of the patients in that study were excluded from analysis due to poor follow-up, and an association with patient survival was not investigated [20]. While we observed no prognostic value for lower AR or LSD1 levels with regards to disease recurrence or patient survival, we found JMJD2A staining intensity to be predictive of overall patient survival, as 100% of patients with at least moderate JMJD2A staining intensity remained alive at the time of the study, while over 80% of patients with JMJD2A-negative tumors had died within less than 2 years of surgery. Thus, consistent with its association with numerous adverse clinicopathologic parameters, JMJD2A loss appears to be an accurate marker of particularly aggressive favorable bladder disease. JMJD2A immunostaining in clinical bladder specimens may thus have utility in facilitating the identification of high-risk patients who may benefit from more aggressive therapeutic approaches, including immediate radical cystectomy, extended lymphadectomy, and neoadjuvant or adjuvant systemic therapies.
An additional promising clinical application for JMJD2A and LSD1 in bladder cancer is their ability to serve as novel targets for epigenetic pharmocotherapies [27,33–38]. The recent groundbreaking discovery that histone lysine demethylation could be biochemically achieved under physiologic conditions has ignited efforts to develop drugs that target this activity for reversal of aberrant epigenetic modifications associated with carcinogenesis [33,37]. Crystal structures of LSD1 reveal the protein to belong to the amine oxidase enzyme superfamily, and the ability of the MAOI class of clinical drugs to inhibit LSD1 has been confirmed in prostate cancer models [27]. Here we show that an MAOI, tranylcypromine, effectively suppresses AR-dependent transcription and growth of bladder cancer cell lines. Furthermore, tranylcypromine demonstrated higher efficacy in the AR-positive cell lines, consistent with growth inhibition being attributable to disruption of AR-KDM complex signaling. Thus the potential future application of KDM-targeting therapeutics will require a more detailed understanding of the role of the AR-lysine demethylase complex in bladder carcinogenesis, as well as knowledge of the AR-status of individual bladder tumours.
We have found increased tumor LSD1 levels relative to normal urothelium. These findings support AR lysine-demethylase coregulators as potential therapeutic targets to delay or suppress bladder carcinogenesis. Efforts to develop drugs targeting LSD1 as well as JMJD2 members with greater specificity are currently underway [33–38]. Huang and associates recently exploited the structural similarity of LSD1 and polyamine oxidases to identify several inhibitors of LSD1, which effectively reversed aberrant genetic silencing implicated in tumorigenesis, consistent with an oncogenic role for LSD1 [33,34]. Similarly, identification of small molecule inhibitors with antiproliferative activity and selectivity for LSD1 was recently described by Ueda and colleagues [38]. Our findings suggest that agents such as these may hold promise in the future treatment of bladder cancer patients.
CONCLUSION
Limited knowledge is available regarding endocrine signaling pathways in bladder carcinogenesis compared to other hormonally regulated solid tumors such as prostate and breast cancers. This study provides the first investigation to our knowledge on the combined roles of the LSD1-JMJD2 histone lysine-demethylase coregulators and AR in bladder cancer. We show that alterations in JMJD2A and LSD1 occur during clinical bladder carcinogenesis, with reduction in both occurring during cancer stage progression in an AR-independent manner. JMJD2A reduction correlates with numerous pathologic and clinical variables conferring a poor patient prognosis, and JMJD2A itself lends important prognostic value with regards to patient survival, providing a novel biomarker for bladder cancer patient risk stratification. Increased LSD1 levels in malignant versus benign urothelium suggest a complex role for this protein in bladder carcinogenesis that may include an oncogenic function. Accordingly, we show that pharmacologic inhibition of LSD1 effectively suppresses androgen induced transcription and bladder cancer cell growth, supporting the biologic efficacy of targeting the AR-complex in this tissue type. Altogether, this study supports a role for AR signaling in human bladder carcinogenesis and has identified a novel contribution of lysine-demethylase AR coregulators in this process. The ability to target lysine-demethylase coregulators with specificity and thus modulate aberrant epigenetic gene regulation may hold promise for future intravesical and systemic pharmacotherapies.
Supplementary Material
Uncropped gel images as presented in Figure 4A.
ACKNOWLEDGEMENTS
The authors are grateful to members of the Gudas and Scherr laboratories for helpful discussions. This research was supported in part by R01CA043796 (LJG), by WCMC Department of Urology funds and the Irish government Science Challenge Initiative (MD and LGP).
Abbreviations
- AR
Androgen receptor
- FAD
flavin adenine dinucleotide
- JMJD2A
jumanji-domain-2A
- KDM
lysine demethylases
- LSD1
lysine specific demethylases
- MAOI
monoamine oxidase inhibitor
Footnotes
Disclosures of potential conflicts of interest
No conflicts to declare (EK, BDRMD, LGP, MML, DS, LJG, NPM,)
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA: a cancer journal for clinicians. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Messing EM. Urothelial Tumors of the Bladder. In: Wein AJ, editor. Campbell-Walsh Urology. 9th ed. Volume 2. Philadelphia: Saunders Elsevier; 2007. pp. 2407–2446. [Google Scholar]
- 3.Shirodkar SP, Kishore TA, Soloway MS. The risk and prophylactic management of bladder cancer after various forms of radiotherapy. Current opinion in urology. 2009;19(5):500–503. doi: 10.1097/MOU.0b013e32832eb3b3. [DOI] [PubMed] [Google Scholar]
- 4.Herr HW, Faulkner JR, Grossman HB, et al. Surgical factors influence bladder cancer outcomes: a cooperative group report. J Clin Oncol. 2004;22(14):2781–2789. doi: 10.1200/JCO.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 5.Shariat SF, Karakiewicz PI, Palapattu GS, et al. Outcomes of radical cystectomy for transitional cell carcinoma of the bladder: a contemporary series from the Bladder Cancer Research Consortium. The Journal of urology. 2006;176(6 Pt 1):2414–2422. doi: 10.1016/j.juro.2006.08.004. discussion 2422. [DOI] [PubMed] [Google Scholar]
- 6.Shariat SF, Palapattu GS, Karakiewicz PI, et al. Concomitant carcinoma in situ is a feature of aggressive disease in patients with organ-confined TCC at radical cystectomy. European urology. 2007;51(1):152–160. doi: 10.1016/j.eururo.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 7.Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19(3):666–675. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 8.Madeb R, Messing EM. Gender, racial and age differences in bladder cancer incidence and mortality. Urologic oncology. 2004;22(2):86–92. doi: 10.1016/S1078-1439(03)00139-X. [DOI] [PubMed] [Google Scholar]
- 9.Tilki D, Svatek RS, Karakiewicz PI, et al. Characteristics and outcomes of patients with pT4 urothelial carcinoma at radical cystectomy: a retrospective international study of 583 patients. The Journal of urology. 2010;183(1):87–93. doi: 10.1016/j.juro.2009.08.145. [DOI] [PubMed] [Google Scholar]
- 10.Bartsch GC, Kuefer R, Gschwend JE, de Petriconi R, Hautmann RE, Volkmer BG. Hydronephrosis as a prognostic marker in bladder cancer in a cystectomy-only series. European urology. 2007;51(3):690–697. doi: 10.1016/j.eururo.2006.07.009. discussion 697–698. [DOI] [PubMed] [Google Scholar]
- 11.Sajjad Y, Quenby S, Nickson P, Lewis-Jones DI, Vince G. Immunohistochemical localization of androgen receptors in the urogenital tracts of human embryos. Reproduction (Cambridge, England) 2004;128(3):331–339. doi: 10.1530/rep.1.00227. [DOI] [PubMed] [Google Scholar]
- 12.Hartge P, Harvey EB, Linehan WM, et al. Unexplained excess risk of bladder cancer in men. Journal of the National Cancer Institute. 1990;82(20):1636–1640. doi: 10.1093/jnci/82.20.1636. [DOI] [PubMed] [Google Scholar]
- 13.Miyamoto H, Yang Z, Chen YT, et al. Promotion of bladder cancer development and progression by androgen receptor signals. Journal of the National Cancer Institute. 2007;99(7):558–568. doi: 10.1093/jnci/djk113. [DOI] [PubMed] [Google Scholar]
- 14.Johnson AM, O'Connell MJ, Miyamoto H, et al. Androgenic dependence of exophytic tumor growth in a transgenic mouse model of bladder cancer: a role for thrombospondin-1. BMC urology. 2008;8:7. doi: 10.1186/1471-2490-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imada S, Akaza H, Ami Y, Koiso K, Ideyama Y, Takenaka T. Promoting effects and mechanisms of action of androgen in bladder carcinogenesis in male rats. European urology. 1997;31(3):360–364. doi: 10.1159/000474484. [DOI] [PubMed] [Google Scholar]
- 16.Okajima E, Hiramatsu T, Iriya K, Ijuin M, Matsushima S. Effects of sex hormones on development of urinary bladder tumours in rats induced by N-butyl-N-(4-hydroxybutyl) nitrosamine. Urological research. 1975;3(2):73–79. doi: 10.1007/BF00256185. [DOI] [PubMed] [Google Scholar]
- 17.Tanahashi NK, Suzawa N, Azuma C. Effects of sex hormones on oncogenesis in rat urinary bladder by N-butyl-N-(4-hydroxybutyl)-nitrosamine. International journal of clinical pharmacology and biopharmacy. 1977;15(3):101–105. [PubMed] [Google Scholar]
- 18.Boorjian SA, Heemers HV, Frank I, et al. Expression and significance of androgen receptor coactivators in urothelial carcinoma of the bladder. Endocrine-related cancer. 2009;16(1):123–137. doi: 10.1677/ERC-08-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boorjian S, Ugras S, Mongan NP, et al. Androgen receptor expression is inversely correlated with pathologic tumor stage in bladder cancer. Urology. 2004;64(2):383–388. doi: 10.1016/j.urology.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 20.Tuygun C, Kankaya D, Imamoglu A, et al. Sex-specific hormone receptors in urothelial carcinomas of the human urinary bladder: A comparative analysis of clinicopathological features and survival outcomes according to receptor expression. Urologic oncology. 2009 doi: 10.1016/j.urolonc.2009.01.033. [DOI] [PubMed] [Google Scholar]
- 21.Heemers HV, Tindall DJ. Androgen receptor (AR) coregulators: a diversity of functions converging on and regulating the AR transcriptional complex. Endocrine reviews. 2007;28(7):778–808. doi: 10.1210/er.2007-0019. [DOI] [PubMed] [Google Scholar]
- 22.Debes JD, Comuzzi B, Schmidt LJ, Dehm SM, Culig Z, Tindall DJ. p300 regulates androgen receptor-independent expression of prostate-specific antigen in prostate cancer cells treated chronically with interleukin-6. Cancer research. 2005;65(13):5965–5973. doi: 10.1158/0008-5472.CAN-04-2837. [DOI] [PubMed] [Google Scholar]
- 23.Shi XB, Xue L, Zou JX, Gandour-Edwards R, Chen H, deVere White RW. Prolonged androgen receptor loading onto chromatin and the efficient recruitment of p160 coactivators contribute to androgen-independent growth of prostate cancer cells. The Prostate. 2008;68(16):1816–1826. doi: 10.1002/pros.20849. [DOI] [PubMed] [Google Scholar]
- 24.Ligr M, Li Y, Zou X, et al. Tumor suppressor function of androgen receptor coactivator ARA70alpha in prostate cancer. The American journal of pathology. 2010;176(4):1891–1900. doi: 10.2353/ajpath.2010.090293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rahman MM, Miyamoto H, Takatera H, Yeh S, Altuwaijri S, Chang C. Reducing the agonist activity of antiandrogens by a dominant-negative androgen receptor coregulator ARA70 in prostate cancer cells. The Journal of biological chemistry. 2003;278(22):19619–19626. doi: 10.1074/jbc.M210941200. [DOI] [PubMed] [Google Scholar]
- 26.Marmorstein R, Trievel RC. Histone modifying enzymes: structures, mechanisms, and specificities. Biochimica et biophysica acta. 2009;1789(1):58–68. doi: 10.1016/j.bbagrm.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Metzger E, Wissmann M, Yin N, et al. LSD1 demethylates repressive histone marks to promote androgen-receptor-dependent transcription. Nature. 2005;437(7057):436–439. doi: 10.1038/nature04020. [DOI] [PubMed] [Google Scholar]
- 28.Shin S, Janknecht R. Activation of androgen receptor by histone demethylases JMJD2A and JMJD2D. Biochemical and biophysical research communications. 2007;359(3):742–746. doi: 10.1016/j.bbrc.2007.05.179. [DOI] [PubMed] [Google Scholar]
- 29.Perillo B, Ombra MN, Bertoni A, et al. DNA oxidation as triggered by H3K9me2 demethylation drives estrogen-induced gene expression. Science (New York, NY. 2008;319(5860):202–206. doi: 10.1126/science.1147674. [DOI] [PubMed] [Google Scholar]
- 30.Wissmann M, Yin N, Muller JM, et al. Cooperative demethylation by JMJD2C and LSD1 promotes androgen receptor-dependent gene expression. Nature cell biology. 2007;9(3):347–353. doi: 10.1038/ncb1546. [DOI] [PubMed] [Google Scholar]
- 31.Huang J, Sengupta R, Espejo AB, et al. p53 is regulated by the lysine demethylase LSD1. Nature. 2007;449(7158):105–108. doi: 10.1038/nature06092. [DOI] [PubMed] [Google Scholar]
- 32.Lee MG, Wynder C, Cooch N, Shiekhattar R. An essential role for CoREST in nucleosomal histone 3 lysine 4 demethylation. Nature. 2005;437(7057):432–435. doi: 10.1038/nature04021. [DOI] [PubMed] [Google Scholar]
- 33.Huang Y, Greene E, Murray Stewart T, et al. Inhibition of lysine-specific demethylase 1 by polyamine analogues results in reexpression of aberrantly silenced genes. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(19):8023–8028. doi: 10.1073/pnas.0700720104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang Y, Stewart TM, Wu Y, et al. Novel oligoamine analogues inhibit lysine-specific demethylase 1 and induce reexpression of epigenetically silenced genes. Clin Cancer Res. 2009;15(23):7217–7228. doi: 10.1158/1078-0432.CCR-09-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rose NR, Woon EC, Kingham GL, et al. Selective inhibitors of the JMJD2 histone demethylases: combined nondenaturing mass spectrometric screening and crystallographic approaches. Journal of medicinal chemistry. 2010;53(4):1810–1818. doi: 10.1021/jm901680b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson JR. Targeting the JMJD2A histone lysine demethylase. Nature structural & molecular biology. 2007;14(8):682–684. doi: 10.1038/nsmb0807-682. [DOI] [PubMed] [Google Scholar]
- 37.Yang M, Culhane JC, Szewczuk LM, et al. Structural basis for the inhibition of the LSD1 histone demethylase by the antidepressant trans-2-phenylcyclopropylamine. Biochemistry. 2007;46(27):8058–8065. doi: 10.1021/bi700664y. [DOI] [PubMed] [Google Scholar]
- 38.Ueda R, Suzuki T, Mino K, et al. Identification of cell-active lysine specific demethylase 1-selective inhibitors. Journal of the American Chemical Society. 2009;131(48):17536–17537. doi: 10.1021/ja907055q. [DOI] [PubMed] [Google Scholar]
- 39.Lim S, Janzer A, Becker A, et al. Lysine-specific demethylase 1 (LSD1) is highly expressed in ER-negative breast cancers and a biomarker predicting aggressive biology. Carcinogenesis. 2010;31(3):512–520. doi: 10.1093/carcin/bgp324. [DOI] [PubMed] [Google Scholar]
- 40.Kahl P, Gullotti L, Heukamp LC, et al. Androgen receptor coactivators lysine-specific histone demethylase 1 and four and a half LIM domain protein 2 predict risk of prostate cancer recurrence. Cancer research. 2006;66(23):11341–11347. doi: 10.1158/0008-5472.CAN-06-1570. [DOI] [PubMed] [Google Scholar]
- 41.Raman JD, Mongan NP, Liu L, et al. Decreased expression of the human stem cell marker, Rex-1 (zfp-42), in renal cell carcinoma. Carcinogenesis. 2006;27(3):499–507. doi: 10.1093/carcin/bgi299. [DOI] [PubMed] [Google Scholar]
- 42.Livak K, Schmittgen T. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 43.Lee MG, Wynder C, Schmidt DM, McCafferty DG, Shiekhattar R. Histone H3 lysine 4 demethylation is a target of nonselective antidepressive medications. Chem Biol. 2006;13(6):563–567. doi: 10.1016/j.chembiol.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 44.Zheng R, Shen R, Goodman OB, Jr, Nanus DM. Multiple androgen response elements cooperate in androgen regulated activity of the type 1 neutral endopeptidase promoter. Mol Cell Endocrinol. 2006;259(1–2):10–21. doi: 10.1016/j.mce.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 45.Mir C, Shariat SF, Van Der Kwast TH, et al. Loss of androgen receptor expression is not associated with pathological stage, grade, gender or outcome in bladder cancer: a large multi-institutional study. BJU international. 2010 doi: 10.1111/j.1464-410X.2010.09834.x. [DOI] [PubMed] [Google Scholar]
- 46.Hobisch A, Culig Z, Radmayr C, Bartsch G, Klocker H, Hittmair A. Androgen receptor status of lymph node metastases from prostate cancer. The Prostate. 1996;28(2):129–135. doi: 10.1002/(SICI)1097-0045(199602)28:2<129::AID-PROS9>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 47.Agoulnik IU, Weigel NL. Androgen receptor action in hormone-dependent and recurrent prostate cancer. Journal of cellular biochemistry. 2006;99(2):362–372. doi: 10.1002/jcb.20811. [DOI] [PubMed] [Google Scholar]
- 48.Comuzzi B, Nemes C, Schmidt S, et al. The androgen receptor co-activator CBP is up-regulated following androgen withdrawal and is highly expressed in advanced prostate cancer. The Journal of pathology. 2004;204(2):159–166. doi: 10.1002/path.1609. [DOI] [PubMed] [Google Scholar]
- 49.Heemers HV, Sebo TJ, Debes JD, et al. Androgen deprivation increases p300 expression in prostate cancer cells. Cancer research. 2007;67(7):3422–3430. doi: 10.1158/0008-5472.CAN-06-2836. [DOI] [PubMed] [Google Scholar]
- 50.Urbanucci A, Waltering KK, Suikki HE, Helenius MA, Visakorpi T. Androgen regulation of the androgen receptor coregulators. BMC cancer. 2008;8:219. doi: 10.1186/1471-2407-8-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Niu Y, Altuwaijri S, Lai KP, et al. Androgen receptor is a tumor suppressor and proliferator in prostate cancer. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(34):12182–12187. doi: 10.1073/pnas.0804700105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shen SS, Smith CL, Hsieh JT, et al. Expression of estrogen receptors-alpha and -beta in bladder cancer cell lines and human bladder tumor tissue. Cancer. 2006;106(12):2610–2616. doi: 10.1002/cncr.21945. [DOI] [PubMed] [Google Scholar]
- 53.Isidori AM, Lenzi A. Risk factors for androgen decline in older males: lifestyle, chronic diseases and drugs. Journal of endocrinological investigation. 2005;28(3 Suppl):14–22. [PubMed] [Google Scholar]
- 54.Oefelein MG, Resnick MI. Association of tobacco use with hormone refractory disease and survival of patients with prostate cancer. The Journal of urology. 2004;171(6 Pt 1):2281–2284. doi: 10.1097/01.ju.0000125123.46733.93. [DOI] [PubMed] [Google Scholar]
- 55.Butcher NJ, Tetlow NL, Cheung C, Broadhurst GM, Minchin RF. Induction of human arylamine N-acetyltransferase type I by androgens in human prostate cancer cells. Cancer research. 2007;67(1):85–92. doi: 10.1158/0008-5472.CAN-06-2635. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Uncropped gel images as presented in Figure 4A.