Abstract
Synaptic transmission depends on the regulated surface expression of neurotransmitter receptors, but many of the cellular processes required to achieve this remain poorly understood. To better define specific mechanisms for the GABAB receptor (GABABR) trafficking, we screened for proteins that bind to the carboxy-terminus of the GABAB1 subunit. We report the identification and characterization of a novel 130-kDa protein, GPCR interacting scaffolding protein (GISP), that interacts directly with the GABAB1 subunit via a coiled-coil domain. GISP co-fractionates with GABABR and with the postsynaptic density and co-immunoprecipitates with GABAB1 and GABAB2 from rat brain. In cultured hippocampal neurons, GISP displays a punctate dendritic distribution and has an overlapping localization with GABABRs. When co-expressed with GABABRs in human embryonic kidney cells, GISP promotes GABABR surface expression and enhances both baclofen-evoked extracellular signal-regulated kinase (ERK) phosphorylation and G-protein inwardly rectifying potassium channel (GIRK) currents. These results suggest that GISP is involved in the forward trafficking and stabilization of functional GABABRs.
Keywords: A-kinase anchoring protein, cultured neurons, GABAB receptor, GPCR interacting scaffolding protein, hippocampus, receptor trafficking
GABA is the main inhibitory neurotransmitter in the mammalian brain. Metabotropic GABABRs are present at both presynaptic and postsynaptic membranes and mediate the late phase of GABAergic inhibitory transmission. Presynaptic GABABRs suppress neurotransmitter release by inhibiting voltage-sensitive P, N, and l-type Ca2+ channels, whereas postsynaptic GABABRs inhibit adenylate cyclase, leading to a decrease in Ca2+ and an increase in G-protein inwardly rectifying potassium channel (GIRK)-mediated K+ conductances (for reviews see Marshall et al. 1999; Couve et al. 2000; Bowery et al. 2002; Calver et al. 2002).
Functional GABABRs comprise heterodimers of GABAB1 and GABAB2 subunits (Marshall et al. 1999). GABAB1 contains the ligand-binding domain (Malitschek et al. 1999), whereas GABAB2 couples to the G-protein (Robbins et al. 2001). Studies using recombinant GABAB1 and GABAB2 showed that the individual subunits are functionally inert unless co-expressed. In addition to these in vitro data, knockout mice show no pre- or postsynaptic GABABR-mediated responses (Pagano et al. 2001; Prosser et al. 2001), demonstrating that GABAB1 is essential for functional GABABRs.
It is well established that GABAB2 is effectively expressed at the plasma membrane in the absence of GABAB1 (Couve et al. 1998; Marshall et al. 1999; Pagano et al. 2001). However, to form functional receptors, the two subunits associate, in part, via coiled-coil domains in their cytoplasmic C termini. This assembly is necessary to overcome GABAB1 retention in the endoplasmic reticulum via an RSRR motif, proximal to the coiled-coil domain of GABAB1 (Margeta-Mitrovic et al. 2000; Pagano et al. 2001). Intriguingly, in some tissues it has also been reported that GABAB1 can be surface expressed in the absence of GABAB2 (Calver et al. 2000), suggesting that other protein partner(s) can combine with GABAB1 to occlude the ER retention/retrieval motifs.
To determine what proteins interact with GABABRs, and to find possible additional subunits, chaperones and/or trafficking/scaffolding/anchoring proteins, several groups have performed yeast two-hybrid screens using the C-terminal domain of GABAB1 (Ige et al. 2000; Nehring et al. 2000; Couve et al. 2001; Vernon et al. 2001). Among these interactors, ATF4 and 14-3-3 proteins were shown to have inhibitory effects on the assembly of the GABABR subunits. However, none so far identified have positive effects on the forward trafficking of the GABABR. Here, we report a novel protein, GPCR interacting scaffolding protein (GISP), that binds to the intracellular C-terminal domain of GABAB1. We show that GISP interacts with both GABAB1 and the GABAB1/GABAB2 heterodimer complex in vivo. Furthermore, co-expression of GISP promotes the surface expression of both GABAB receptors in heterologous cells. We also show that co-expression of GISP with GABAB1 and GABAB2 in human embryonic kidney (HEK) cells increases both baclofen-evoked mitogen-activated protein (MAP) kinase activation and GIRK channels responses. These results suggest that GISP is involved in GABABR forward trafficking and may reduce receptor desensitization and/or degradation, resulting in increased levels of GABAB receptors and the stabilization of functional GABABR complexes.
Materials and methods
Yeast two-hybrid screening and analysis of GABAB1–GISP interaction
The cytosolic C-terminal domain of GABAB1 (residues Arg841– Lys944; Fig. 1a) was subcloned into the pBTM-116ADE vector and was used to screen an adult rat brain cDNA library (Clontech, Palo Alto, CA, USA) in Saccharomyces cerevisiae L-40 reporter strain as described previously (Nishimune et al. 1996, 1998).
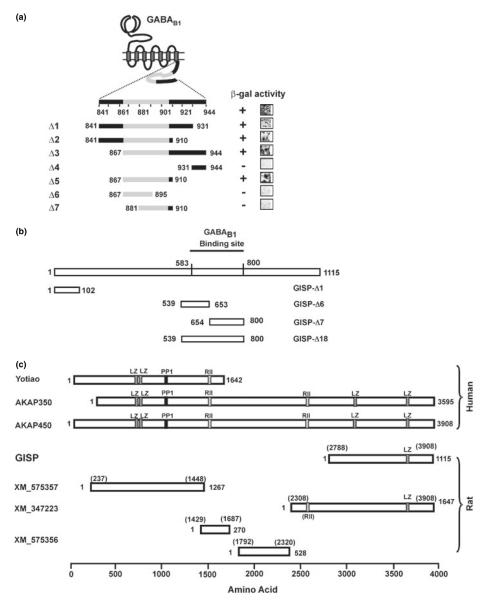
Fig. 1.
Isolation of GISP and characterization of binding domains. (a) Identification of GISP binding domain on GABAB1. Grey region denotes the coil-coiled domain. Truncation mutagenesis of GABAB1 defined residues Glu867–His910 as the minimal region required for GISP binding. Representative b-galactosidase stain of yeast colonies is illustrated. (b) Schematic diagram showing the truncated mutants of GISP used in this study. When used in the yeast two-hybrid assay, only Δ18 (539–800) and the original cDNA fragment from the screening activated the β-galactosidase reporter. (c) Comparison of GISP with other AKAPs transcribed from the same gene akap9. Human yotiao, AKAP350 and AKAP450 have been characterized at the protein level. With the exception of GISP, the rat proteins are hypothetical from expressed sequence tag (EST) sequences. LZ, leucine zipper domain; PP1, protein phosphatase 1 consensus binding motif, RII, PKA RII consensus binding motif.
GISP cDNA cloning
The original rat GISP cDNA fragment from two-hybrid screening was used as a hybridization probe to obtain full-length cDNA from adult rat hippocampal cDNA lZAPII phage library (Stratagene, La Jolla, CA, USA). The full-length GISP cDNA was cloned into pBluescriptII (Stratagene) by assembling two large overlapping fragments. This full-length nucleotide sequence was deposited in the database (DDBJ/EMBL/GenBank accession no. DQ228948).
GST pull-down
Truncated mutants of GISP shown in Fig. 1(b) were cloned into pGEX-4T-1 (Amersham Biosciences, Uppsala, Sweden). Glutathione S-transferase (GST) fusion proteins were purified and dialysed against phosphate-buffered saline (PBS) and then used for pull-down experiments. GST pull-downs were performed as previously described (Hirbec et al. 2002). Anti-GABAB1 antibody (Chemicon, Temecula, CA, USA) was used at 1 μg/mL for the immunoblotting.
Antibodies
Anti-GISP antisera were raised by immunizing rabbits with a hexahistidine (His6) tagged fusion protein incorporating residues 1–102 (as shown in Fig. 1b). GISP-specific antibodies were affinity-purified on a HiTrap NHS-activated SepharoseTM HP column (Amersham Biosciences) coupled to the original His6-tagged immunogen. The eluates containing specific antibodies were pooled, the buffer was exchanged to PBS and the purified antibodies were stored in PBS with 30% glycerol, 0.1% bovine serum albumin and 0.1% NaN3 at −20°C or −80°C. Other primary antibodies used were: anti-GABAB1a,b antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA), guinea pig anti-GABAB1a,b antibody and anti-GABAB2 (Chemicon), mouse anti-synaptotagmin (clone 41; Pharmingen BD Biosciences, Palo Alto, CA, USA), mouse monoclonal anti-extracellular signal-regulated kinase (ERK)1/2 (Sigma, St Louis, MO, USA), mouse monoclonal anti-postsynaptic density (PSD)95 (Upstate Biotechnology, Lake Placid, NY, USA), mouse monoclonal anti-β-actin (Sigma) and mouse monoclonal anti-calreticulin (BD Transduction Laboratories, Lexington, KY, USA). Horseradish peroxidase-conjugated secondary antibodies used were goat anti-rabbit IgG, goat anti-mouse IgG or goat anti-rabbit IgG (all from Sigma). Fluorochrome-conjugated secondary antibodies are anti-rabbit Alexa 488 (green) goat, anti-guinea pig Alexa 568 (red) goat and anti-mouse Alexa 568 (red) goat (Molecular Probes, Eugene, OR, USA).
Expression constructs and transfection
A GISP expression construct was created by subcloning the full-length GISP cDNA into the NheI site (5′) and the XhoI site (3′)of the mammalian expression vector pcDNA3.1(+) (Invitrogen, Carlsbad, CA, USA). HEK293 cells were transfected using Lipofectamine 2000 (Invitrogen). GABABR expression constructs pmyc-GABAB1a, pHA-GABAB2, were a gift from Steve Moss and Benny Bettler, respectively. The empty vector pcDNA3.1(+) (Invitrogen) was used to keep the amount of DNA for transfection constant at 5 mg per 6-cm dish. pEGFP-C1 (Clontech) was used to express non-interacting green fluorescent protein (GFP) as a negative control. Cells were harvested 48 h after transfection.
Primary hippocampal cultures
Primary hippocampal cultures were prepared from embryonic day-18 rats exactly as previously described (Perestenko and Henley 2003).
Immunocytochemistry and confocal microscopy
Immunocytochemistry was performed as previously described (Corrêa et al. 2004). For double staining, the concentration of the antibodies used were as follows; rabbit anti-GISP (15 μg/mL), guinea pig anti-GABAB1a,b (Chemicon; 10 μg/mL) or anti-GABAB2 (Chemicon, 30 μg/mL) and mouse anti-synaptotagmin (10 μg/mL). Secondary antibodies were used at 10 μg/mL. Negative control staining with pre-blocked primary antibody or staining only using the secondary antibodies was included for every experiment. Images were obtained on a Zeiss LSM510 Meta confocal microscope (Obelkochen, Germany). There was no bleach through between the channels under the conditions used and the same physical parameters were used for all images. Images were processed using Photoshop 5.0 (Adobe Systems, San Jose, CA, USA) and Corel-DRAW 11.0 (Corel, Ottawa, ON, Canada). Quantification of the co-localization of GISP and GABAB receptors or presynaptic markers was carried out by counting the number of the puncta per 100-μm length of dendrites for each antibodies within given fields.
Subcellular fractionation and immunoprecipitation
Fractions were obtained by differential centrifugation (Gray and Whittaker 1962). The PSD fractions were prepared as described previously (Carlin et al. 1980). For the microsomal fraction, S2 was centrifuged at 201 800 g for 1 h at 4°C to obtain S3 supernatant, the pellet was detergent extracted and the solubilized fractions recovered by centrifugation at 201 800 g for 1 h at 4°C. Protein concentrations were determined by the bicinchoninic acid method (Smith et al. 1985) to ensure that equal amounts of protein were used for immunoblotting. GABAB receptors were solubilized by incubating with extraction buffer (1% Triton X-100, 250 mM NaCl, protease inhibitors cocktail, 20 mM Tris-HCl) for 1 h and soluble fractions recovered as supernatant by centrifugation at 20 000 g for 15 min. The resulting supernatants from these extracts were diluted five times and used in subsequent experiments. For immunoprecipitation, 2.5 μg/mL anti-GISP was incubated by rotation with 250 μg of protein extracts at 4°C for 1 h and then with protein A–Sepharose beads (Sigma) overnight. Beads were washed three times with 5 × diluted solubilization buffer and proteins were eluted from beads using Laemmli’s sample buffer (Harlow and Lane 1988). Total proteins (input) were resolved in parallel as a control.
Cell-surface biotinylation
Living cells were biotinylated using the membrane impermeable and cleavable biotinylation reagent sulfosuccinimidyl-2-(biotinamido) ethyl-1,3-dithiopropionate (EZ-Link sulfo-NHS-SS-biotin; 0.1 mg/ mL in PBS; Pierce, Rockford, IL, USA) for 10 min on ice as described previously (Martin and Henley 2004). The integrity of the plasma membrane and cell surface specific biotinylation was confirmed using the intracellular protein β-actin as a control. Bands were quantified using ImageJ 1.30 software (Rasband 1997–2006) and normalized to the total receptor fraction. Unpaired Student’s t-tests were performed with a Newman–Keuls post-test for multiple comparison data sets.
Immunoblotting
Proteins were blotted onto Immobilon-P membrane (Millipore Corporation, Bedford, MA, USA) and probed with appropriate primary antibodies after blocking (Harlow and Lane 1988). For detection of the signal, the membrane was incubated with horseradish peroxidase-conjugated secondary antibodies (Sigma; 1 : 10 000 dilution) for 60 min followed by substrate incubation with BM Chemiluminescence Blotting Substrate (POD; Roche Molecular Biochemicals, Indianapolis, IN, USA) or SuperSignal West Femto (Pierce). The chemiluminescence signal was detected on the Hyperfilm HP (Amersham Biosciences).
Histoblots
Adult rat whole brain horizontal cryostat sections (10 μm; approximately bregma −4.60 mm) were transferred to nitrocellulose membrane (Tonnes et al. 1999) and probed with anti-GISP antibody (4 μg/mL) and secondary anti-rabbit alkaline phosphatase-conjugated secondary (0.25 μg/mL; Sigma).
Electrophysiology
HEK cells stably expressing Kir3.1/Kir3.2 channels (a gift from Trevor Smart) were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 2 mM glutamine, 1% penicillin/streptomycin and 0.5 mg/mL G418. Cells in 6-cm dishes were transiently transfected using Lipofectamine 2000 with 0.5 μg each of Myc-GABAB1a and HA-GABAB2, 0.25 μg GFP and 4 μg of either pcDNA-GISP or empty vector. Whole cell baclofen-activated potassium currents were obtained from fluorescent cells 40–48 h after transfection. Cells were perfused with HEPES-buffered solution containing 119 mM NaCl, 5mM KCl, 25 mM HEPES, 30 mM glucose, 2 mM CaCl2,2 mM MgCl2 and pipettes were filled with intracellular solution containing 110 mM potassium methanesulfonate, 10 mM NaCl, 40 mM HEPES, 0.6 mM EGTA, 4 mM Mg-ATP, 0.3 mM Na2-GTP, pH 7.2. Baclofen (100 μM; in HBS) was applied to the cell (held at −50 mV) via a computer-controlled sewer pipette for 4 s every 30 s for 10 min. The peak amplitude was recorded after each exposure and then normalized to the first peak. The time constants for the comparison of 50% inactivation were calculated by data fitted to single exponential.
ERK activation assay
Assays were performed essentially as described previously (Balasubramanian et al. 2004). Briefly, HEK293 cells were transfected using Lipofectamine 2000 (Invitrogen) with plasmids encoding for Myc-GABAB1a, HA-GABAB2, and GISP. To adjust the cDNA levels between the different conditions, cells were transfected with the pcDNA3.1(+) plasmid. Cells were plated in 6-well plates and transfected with 250 ng of Myc-GABAB1a, HA-GABAB2 and with 2.5 μg of plasmid coding for the GISP [or pcDNA3.1(+) as a control] in the combination indicated in the figure legends. Cells were used 48 h after transfection and serum starved overnight the day before the assay. The cells were stimulated with 100 μM baclofen at indicated times, rinsed with ice-cold PBS, and lysed in 250 μL of sample buffer. The cell lysates were subjected to sodium dodecyl sulfate – polyacrylamide gel electrophresis (SDS–PAGE) and then analysed via western blotting with anti-phospho-p44/42 MAP kinase antibody (Sigma).
Results
Isolation of GISP
We used the intracellular C-terminal domain (residues Arg841–Lys944) of the GABAB1 subunit as ‘bait’ in yeast two-hybrid (Y2H) screens of a rat brain cDNA library. The majority of positive interactors were ATF4, a cAMP-dependent transcription factor that we have reported previously (Vernon et al. 2001; and see also Nehring et al. 2000; White et al. 2000). In addition, we isolated partial cDNAs that, over the sequence isolated, had ~90% homology with human A-kinase anchoring protein (AKAP)450 (Witczak et al. 1999). A full-length clone, obtained by hybridization screening of the rat cDNA library, established that we had isolated a novel cDNA that encodes a protein we have named GISP (Fig. 2). We concluded that the GISP clone is full length because the GISP cDNA contains frame stop codons in its 5′ (upstream) untranslated region; the proposed amino acid sequence is the biggest protein that can be encoded in the cloned cDNA. We also detected a genomic sequence that is identical to this 5′ untranslated region sequence just upstream of the protein coding exons in the rat akap9 gene and it is extremely unlikely that this isolated 5′ untranslated region sequence is a cloning artifact.
Fig. 2.

Primary structure of GISP. The deduced primary structure of GISP is shown in single-letter amino acid code. The region of GISP prepared as His6-tagged immunogen is shown as highlighted in grey. The mapped epitope within this immunogen is shown as bold letters. Underlined regions represent predicted coiled-coil as predicted by the Lupas algorithm (Lupas et al. 1991). The minimum GABAB1 binding site is highlighted in black. Leucines in bold and larger font show the predicted leucine-zipper region. Methionine 47 is likely to be used as the start codon in vivo. The predicted calmodulin binding site (CRS motif; unclassified category) is shown by the dotted-underline (Yap et al. 2000).
GISP is not a degradation product because it is exclusively localized to brain, whereas other larger akap9 gene product proteins (i.e. AKAP450/AKAP350/AKAP9) are expressed in many other tissues. Furthermore, within the brain, GISP is localized to neurons while other akap9 gene products are also present in glia. GISP is also far more abundant than any other higher molecular weight species (which share their epitope with GISP) and we could not detect any smaller protein product than GISP. Finally, although GISP shares substantial sequence homology with AKAP9 (yotiao), as reported below, unlike AKAP9 (Lin et al. 1998), GISP does not bind to the NR1 subunit of NMDA receptors in the Y2H assay.
Subsequent Y2H assays using yeast transformed with GISP and plasmids encoding specific potential interacting proteins confirmed robust binding of GISP to GABAB1 but showed that it does not bind to GABAB2. In a series of Y2H controls to assess GISP specificity, we also established that GISP does not interact with AMPA (GluR1-4), kainate (GluR5 and 6), NMDA (NMDAR1) ionotropic glutamate receptor subunits, or metabotropic glutamate receptors (mGluR1–5, 7; data not shown). Truncation mutagenesis of GABAB1 defined residues Glu867–His910 as the minimal region required for GISP binding (Fig. 1a). This region comprises a coiled-coil domain that is also required for heterodimerization with the GABAB2 subunit (Kammerer et al. 1999). Corresponding truncation mutagenesis of GISP (Fig. 1b) revealed the minimal interaction domain as Ser583–Gln800. Of this region, only the N-terminal portion (Arg595–Ile650) contains a predicted coiled-coil domain. In an attempt to further define which part of this region constitutes the GABAB1 binding site, we constructed two truncations (Δ6 and Δ7) and tested for interaction with GABAB1 in the Y2H assay. Neither truncation activated the yeast reporter, indicating that the coiled-coil interaction is necessary but not sufficient for the interaction between GISP and GABAB1.
Primary structure of GISP
Using standard hybridization screening, we obtained the full-length protein coding sequence of GISP from the adult rat hippocampal cDNA library. The open reading frame we obtained is 3.3 kb in length and encodes a 1115 amino acid protein (Figs 1 and 2) with a molecular weight deduced from the primary structure as a simple polypeptide (128.5 kDa). There are several ATG codons in the sequence and we initially designated the methionine that yielded the largest open reading frame as the start codon, but subsequent analysis comparing the molecular masses of recombinant to native GISP revealed that the second ATG most likely corresponds to the actual start codon in vivo.
We envisage that GISP is transcribed from the same akap9 gene (AKAP) as AKAP450 (also known as CG-NAP, hyperion; Witczak et al. 1999), AKAP350 (Schmidt et al. 1999), AKAP9 (yotiao; Lin et al. 1998). However, GISP is distinct from these AKAP family members because it does not have a characteristic alpha-helical protein kinase A (PKA) regulatory (RII) subunit binding motif (Fig. 1c). Analysis by the Lupas algorithm (Lupas et al. 1991) predicts nine coiled-coil heptad repeats throughout the protein. GISP does not have any apparent hydrophobic clusters (average hydrophobicity was calculated as −0.803859) and is predicted as a soluble protein using the SOSUI algorithm (Hirokawa et al. 1998), The fact that GISP contains no signal peptide or hydrophobic cores is also consistent with it being an intracellular protein. Although it does not contain either protein phosphatase 1 or PKA binding sites, GISP shares several other binding motifs with AKAP450/350, including a calmodulin (Gillingham and Munro 2000; Takahashi et al. 2002) and a casein kinase-binding motif (Sillibourne et al. 2002). Examination of the database also revealed several additional hypothetical transcripts from the akap9 gene but none have been verified at the protein level. One of these hypothetical transcripts (XM_347223) has sequence identity to GISP although the hypothetical protein has a predicted molecular mass of 188 kDa, significantly larger than GISP, with an additional ~465 residues located at the N-terminal end. GISP is not a truncation of this larger protein for the reasons set out above and because expression of this 188 kDa protein, detected with our anti-GISP antibody (see below), was very low compared with GISP (Fig. S1).
GISP binds to GABAB1 in GST pull-down assays
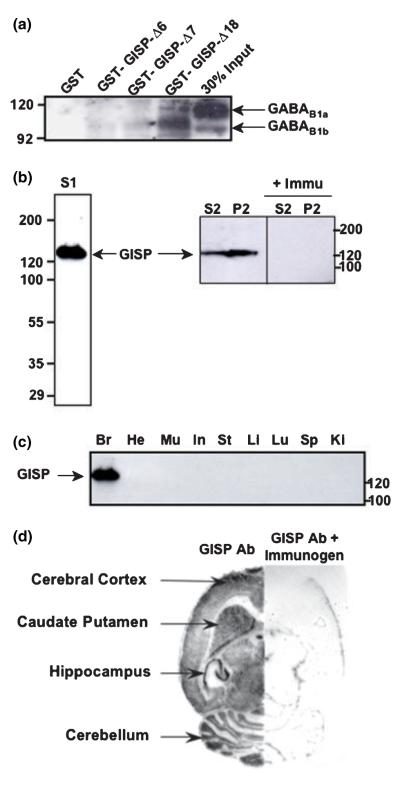
We used GST pull-down assays to confirm that the binding of GISP to GABAB1 occurs in vitro. The post-nuclear fraction of rat brain was incubated with GST-GISPΔ6, GST-GISPΔ7 and GST-GISPΔ18 truncations attached to glutathi-one–agarose beads. As expected from the Y2H data, only GST-GISPΔ18 retained both isoforms of GABAB1 and not GST alone (Fig. 3a). A faint GABAB1 immunoreactive band is visible in GST-GISPΔ6 and GST-GISPΔ7. GST-GISPΔ18 is the minimum region of GISP required for efficient binding of GABAB1 receptors and truncations of GST-GISPΔ18, GST-GISPΔ6 and GST-GISPΔ7 might retain some weak capacity of binding.
Fig. 3.
Interaction between GISP and GABAB1 in vitro and regional distribution of GISP. (a) Verification of the interaction between GISP and GABAB1 by GST-pull down: GST was used as negative control and GST-GISPΔ6, GST-GISPΔ7 and GST-GISPΔ18 (GABAB1 interacting region in GISP). GABAB1 binds to GST-GISPΔ18 but not to GST. The input was 30% obtained from post-nuclear fractions of P8 rats. Eight per cent gel was used and 10 μg of GST fusion protein was used for the pull-down. The blot is a representative of three different experiments. (b) Anti-GISP antibody recognizes a predominant 130-KDa band protein band in rat brain (S1 = post-nuclear supernatant = S2 + P2; S2 = crude cytosolic fraction; P2 = crude membrane fraction). No signal was present on pre-incubation with the immunogen. Representative of two different experiments. (c) Tissue distribution of GISP. All lanes were loaded with 100 μg protein and anti-GISP antibody was used at 1 μg/mL. Br, whole brain; He, heart; Mu, skeletal muscle; In, intestine; St, stomach; Li, liver; Lu, lung; Sp, spleen; Ki, kidney. Representative of three different experiments. (d) The distribution of GISP immunoreactivity in brain was assessed by histoblot technique. Representative of three different experiments.
Generation and characterization of a specific anti-GISP antibody
Because of the high degree of sequence identity between GISP and AKAPs, no available antibodies can specifically distinguish GISP. Furthermore, although there are commercially available anti-human AKAP450 antibodies, no antibodies directed against rat AKAP450 have been reported. We therefore made new polyclonal antibodies against the N-terminal sequence of GISP (residues 1–102; Fig. 2 and Fig. S2). The resultant affinity-purified anti-GISP antibody recognized the predominant ~130-kDa band in immunoblots of cytosolic (S2) and crude membrane fractions (P2) from rat brain and a single ~130 kDa band in cultured hippocampal neurons. When the antibody was pre-incubated with the immunogen, the specific signal disappeared completely (Fig. 3b).
Given the sequence identity between the antigenic peptide used to raise our anti-GISP antibody and human AKAP350 and AKAP450, we tested if it detected AKAP350/450 and related proteins expressed in brain and cultured neurons. The commercial anti-human AKAP450 antibodies did not detect any bands in rat tissue, although we did detect a 450-kDa band in HEK cells (data not shown). However, using high-sensitivity western blotting techniques with our GISP antibody, we did detect higher molecular weight protein bands consistent with AKAP450, AKAP350 and the protein encoded by XM_347223 in rat brain (Fig. S1). Significantly, none of these additional bands was present in primary cultured rat cortical/hippocampal neurons. Because (i) the roles of AKAP350 and AKAP450 are strongly associated with cell division, (ii) neither protein has not been reported in neurons, (iii) the immunoreactive band at the molecular mass predicted for GISP is far more intense than any other bands in western blots of brain and, (iv) only the GISP band was detected in cultured neurons grown in absence of glial cells, we conclude that the non-GISP bands from brain correspond to proteins that are far less abundant that GISP and that these are present only in glial cells. Thus, our results indicate that AKAP350 and AKAP450 are not present in mature neurons and, importantly for the immunocytochemistry, that our anti-GISP antibody recognizes only GISP in primary cultured hippocampal neurons.
It is also evident on close examination of western blots run in low percentage (5%) acrylamide gels that there is a small molecular weight difference between our recombinant and endogenous GISP. The molecular weight of the recombinant GISP is ~4 kDa larger than that of the endogenous GISP. This might be as a result of the translation starting at a different start codon. Therefore, we attempted to map the anti-GISP antibody epitope within the N-terminal of our recombinant antigen by splitting the antigen into two GST fusion proteins, GISP 1–46 and GISP 47–103. GISP antibody binding was not affected with pre-incubation of GST-GISP 1–46 recombinant protein, but completely blocked when pre-incubated with GST-GISP 47–103, suggesting that the GISP epitope is confined to this region (Fig. S2).
Regional distribution of GISP
Using this antibody, we first investigated the crude tissue distribution of GISP. Immunoblots of a variety of tissues revealed that GISP is present at detectable levels only in the brain (Fig. 3c). Histoblots were used to assess the pattern of GISP expression within the brain (Fig. 3d). GISP immunoreactivity was widespread throughout the structures of the brain with particularly high levels in the hippocampal formation.
Comparison of the developmental profiles of GABABR and GISP expression
We next determined the levels of expression of GISP, the two GABAB1 isoforms and GABAB2 in whole brain of rats at different age points (Fig. 4). GISP was present at all ages examined but expression increased with age, peaking around 2–3 weeks after birth. Consistent with a previous report (Fritschy et al. 1999), expression of GABAB1a and GABAB1b subunit isoforms showed an inverse relationship. While neither was abundant prior to birth, GABAB1a was most evident at P2 and levels decreased thereafter. Interestingly, the GABAB2 subunit showed a different profile. Very little GABAB2 immunoreactivity was present prior to birth, followed by increasing levels that peaked at age 2–3 weeks. The marked differences in the expression profiles of GISP and GABAB subunits suggests that GISP may have role(s) in addition to those involving interaction with GABABRs.
Fig. 4.
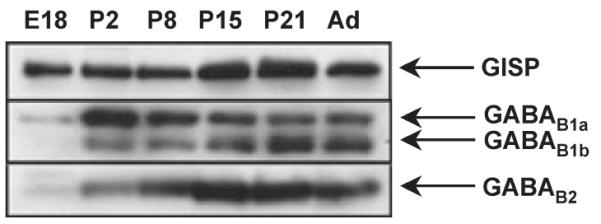
Developmental profiles of GISP and GABAB receptor subunits in rat brain. Membrane extracts were prepared from rat whole brain taken at the ages indicated in days (E, embryonic, P, post-natal, Ad, adult) and probed for GISP, GABAB1 and GABAB2 immunoreactivity. GISP was expressed at the every stage when either GABAB1 or GABAB2 is expressed. Note that the two GABAB1 isoforms show a markedly different profile. Only GISP was abundant prior to birth. The data are representative of three separate experiments. β-actin blots were performed as a loading controls (data not shown).
Subcellular localization of GISP and GABABR
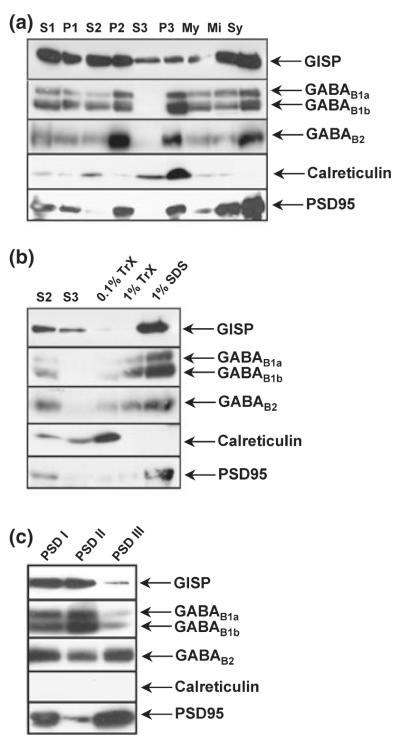
To determine the compartmentalization of GISP in neurons, we performed subcellular fractionations. GISP immunoreactivity was present in both cytosolic and membrane fractions including the S3 where no GABABRs were observed (Fig. 5a). We further investigated the compart-mentalization of GISP and GABABRs by detergent extraction of both microsomal and synaptic membrane fractions. GISP associated with microsomal membranes and showed a very similar extraction profile to both GABAB1 and GABAB2 subunits, with considerable resistance to the mild detergent conditions, only being efficiently extracted with 1% SDS (Fig. 5b). Similarly, in the synaptic membrane extracts, GISP and GABABRs were strongly associated with the mild detergent-resistant fraction (Fig. 5c). Calreticulin and PSD95 antibodies were used as controls for subcellular fractionation. As expected, calreticulin was enriched in the microsomal fraction and was not present in PSD fractions. PSD95 was predominantly enriched in the postsynaptic density fraction (Fig. 5), indicating that fractionation was efficient.
Fig. 5.
Subcellular compartmentalization of GISP and GABABRs in rat brain extracts. (a) Distribution of GISP in subcellular fractions from rat brain. Protein (50 μg) was loaded per lane. Purified anti-GISP antibody was used at 1 μg/mL. The fractions are; S1, cell contents minus nuclei and debris; P1, nuclei and debris; S2, crude microsomes and cytosol; P2, crude synaptosomes; S3, cytosol; P3, enriched microsomes; My, myelin; Mi, mitochondria; Sy, enriched synaptosomes. These data are representative of three separate experiments. (b) Detergent extraction of microsomal fraction. S2, crude microsomes and cytosol; S3, cytosol; 0.1%TrX, enriched microsomes (P3) solubilized with 0.1% Triton X-100; 1%TrX, enriched microsomes (P3) solubilized with 1% Triton X-100; 1% SDS, enriched microsomes (P3) solubilized in 1% SDS. These data are representative of three separate experiments. (c) Detergent extraction of PSD fraction. PSD I, insoluble PSD fraction after 0.5% Triton X-100 extraction; PSD II, PSD fraction insoluble after 1% Triton X-100 extraction; PSD III, insoluble PSD fraction after 3% sarcosyl extraction. These data are representative of three separate experiments.
Co-immunoprecipitation of GABAB1 and GABAB2 with anti-GISP antibody
Anti-GISP antibody immunoprecipitates from rat brain were probed with anti-GABAB1 or anti-GABAB2 antibody (Fig. 6). GISP binds to both GABAB1b and GABAB1a and our blots reflect the relative abundance of the GABAB1b and GABAB1a isoforms in mature rat brain (Fritschy et al. 1999). Furthermore, as shown in Fig. 4, the two isoforms are developmentally regulated, with GABAB1a more highly expressed in the developing brain. Both isoforms are present in the GISP immunoprecipitate from adult brain P2 fraction, although the GABAB1a isoform is comparatively weak. One of the reasons may be the reflection from differential compartmentalization of GABAB1 receptor variants (1a and 1b), where GABAB1a is preferentially localized at presynaptic sites and GABAB1b postsynaptically (Vigot et al. 2006). GABAB1 and GABAB2 subunits were detected in the co-immunopreciptiates, confirming that GISP binds GABAB1 in vivo and, because GISP does not directly interact with GABAB2, these results also demonstrate that GISP binds to the mature GABABR heterodimer.
Fig. 6.
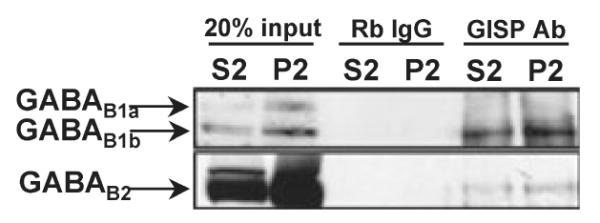
Co-immunoprecipitation of GABAB1 and GABAB2 subunits with anti-GISP antibody. Anti-GISP antibody was used to isolate GABAB receptor complex from rat brain. GISP antibody (2.5 lg) was used for immunoprecipitation of both GABAB1 and GABAB2 subunits from crude microsomal and crude membrane fractions. Rabbit IgG was used as a negative control. The data are representative of three separate experiments.
Immunolocalization of GISP
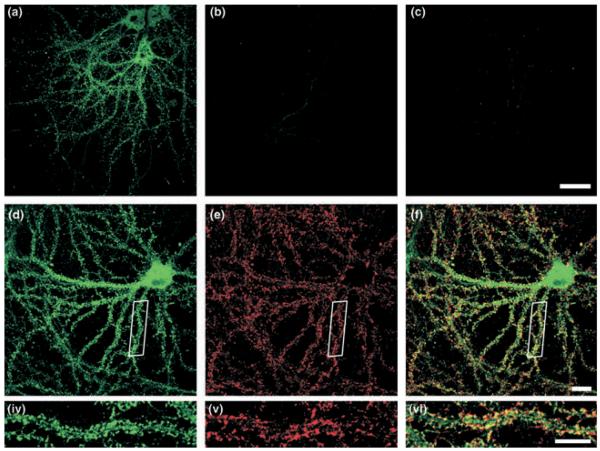
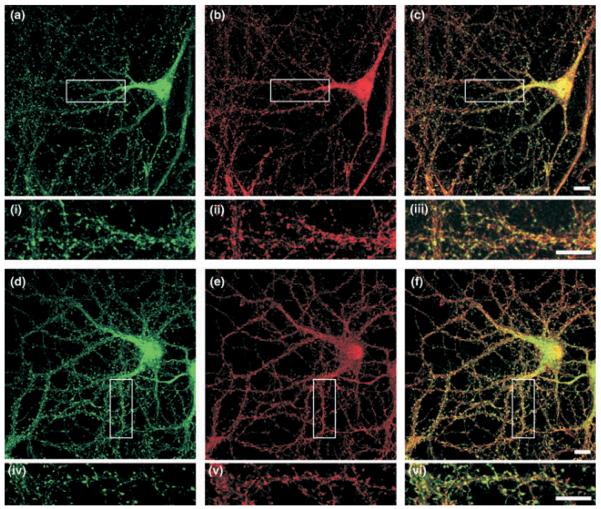
Our anti-GISP antibody recognizes a single ~130 kDa band in our glia-free hippocampal cultures. We therefore used this antibody to investigate the distribution of endogenous GISP in 21-days in vitro hippocampal cultures. Consistent with the immunoblot data, strong staining was detected which was completely abolished by pre-incubation of the antibody with the antigen peptide (Figs 7a–c). GISP expression was absent from the nucleus, but widespread throughout the cell soma. In dendrites, GISP was showed a defined punctate localization. To determine if the punctate dendritic staining corresponded to synapses, we investigated the extent of co-localization with the presynaptic protein synaptotagmin (Figs 7d–f and 4–6). There were 68.2 ± 4.8 synaptotagmin and 48.8 ± 5.1 GISP immunopositive puncta per 100 μm of dendrite. Quantification of co-localization revealed that 68.4 ± 7.5% GISP puncta were directly localized at synaptotagmin-labelled synapses.
Fig. 7.
Localization of GISP in neurons. Embryonic hippocampal neurons were permeabilized with 0.1% Triton X-100 and stained with anti-GISP antibody (green; 15 μg/mL). Anti-synaptotagmin antibody (red; 10 μg/mL) was used as a presynaptic marker. The images are representative of four different experiments. (a) GISP, (b) GISP antibody pre-incubated with immunogen, (c) secondary antibody only, (d) GISP, (e) synaptotagmin and (f) merged. Scale bar 10 μm.
Co-localization of GISP and GABABRs
The developmental profile of GABABR function and subunit localization in this culture system has been reported previously (Corrêa et al. 2004). In agreement with those data, highly defined punctate GABAB1 and GABAB2 immunostaining was present in 21-days in vitro hippocampal neurons (Fig. 8). In the cell body (excluding the nucleus), and in the proximal dendrites, there was a high level of overlapping distribution for GISP with both GABAB1 and GABAB2 subunits. We attribute this to the relatively high levels of endogenous expression and diffuse distribution of each of the proteins. In more distal dendrites, however, the co-localization of the proteins was more distinct. GISP labelled as puncta in the dendritic shaft and protuberances. In many cases, these structures were also labelled with GABAB1 staining, but less so by GABAB2 antibody. Quantitative analysis revealed that there were 38.2 ± 2.8 GABAB1 puncta and 31.6 ± 1.9 GABAB2 puncta per 100 μm of dendrite. Overall, there was 65.4 ± 4.4% co-localization of GABAB1 and 34.4 ± 5.1% co-localization of GABAB2 with GISP.
Fig. 8.
Comparison of the localization of GISP and GABABR subunits in cultured hippocampal neurons. Embryonic hippocampal neurons were permeabilized with 0.1% Triton X-100 and stained with anti-GISP antibody (green; 15 μg/mL). Anti-GABAB1 antibody (red; 10 μg/mL), or anti-GABAB2 antibody (red; 30 μg/mL) were used. The figure is representative of four different experiments. (a) GISP, (b) GABAB1, (c) merged; (d) GISP; (e) GABAB2, (f) merged. Scale bar 10 μm.
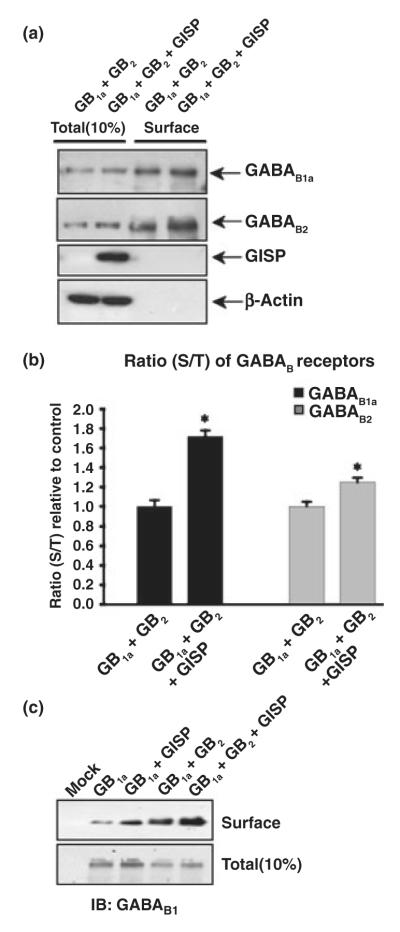
GISP promotes surface expression of GABAB receptors in HEK cells
As reported previously (Couve et al. 1998), co-expression of GABAB1 and GABAB2 subunits in HEK cells results in the efficient surface expression of both subunits. GABAB2 expressed alone also traffics to the plasma membrane, whereas GABAB1 alone shows very low levels of surface expression (Couve et al. 1998; Marshall et al. 1999; Pagano et al. 2001). As GISP interacts at the same coiled-coil domain required for heteromeric receptor assembly, we tested if expression of GISP would have an effect on surface expression of GABAB receptors. When GABAB1 and GABAB2 were co-expressed, efficient surface expression was observed, but this was further increased by GISP (Figs 9a and b). There was a more than 70% increase in GABAB1a expression ratio and a 25% increase in GABAB2 receptor expression ratio. As GISP interacts at the same coiled-coil domain required for heteromeric receptor assembly, we tested if expression of GISP would allow surface expression of GABAB1. There was an increase in surface myc-GABAB1 expression when co-expressed with GISP (Fig. 9c), although the surface expression was less than that achieved on co-expression with GABAB2. These results indicate that occupation of the coiled-coil domain of GABAB1 by GISP is required to promote the forward traffic of GABAB receptors from the ER/Golgi complexes to the plasma membrane.
Fig. 9.
GISP promotes surface expression of GABAB receptors in HEK cells. (a) Effect of GISP on GABAB1/GABAB2 complex surface expression assessed by surface biotinylation. HEK293 cells were transfected with myc-GABAB1a and HA-GABAB2 in combination with a control vector (pcDNA3.1) or vector encoding GISP. The data are representative of three separate experiments. Forty-eight hours posttransfection, the total and cell surface GABAB protein was determined as described in Materials and methods. Immunoblots were probed with anti-GABAB antibodies and re-probed with anti-β-actin antibody to ensure equal loading and the specificity of surface biotinylation. (b) Histogram of densitometric analysis of the effect of GISP on GABAB1a and GABAB2 protein surface expression ratio (surface to total) measured by biotinylation assays as illustrated in (a). The results shown are the ratios of three independent experiments. *p ≤ 0.001 compared with control (Students’s t-test). (c) Effect of GISP on GABAB1 surface expression assessed by surface biotinylation. HEK293 cells were transfected with myc-GABAB1a in combination with a control vector (pcDNA3.1) or the vector encoding GISP. Forty-eight hours post-transfection, the total and cell surface GABAB protein was determined as described in Materials and methods. The data are representative of three separate experiments. Immunoblots were probed with anti-GABAB1 antibody and re-probed with anti-β-actin antibody (data not shown) to ensure equal loading and the specificity of surface biotinylation.
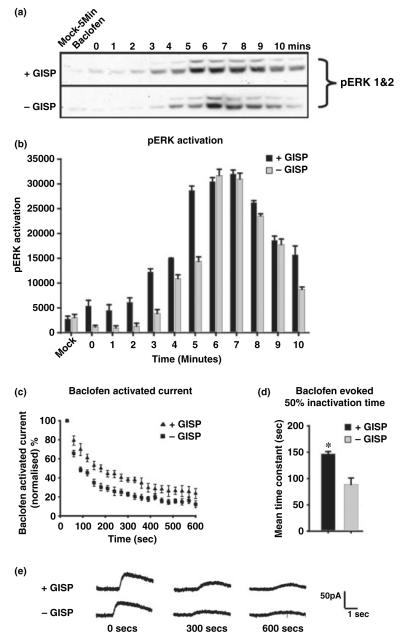
GISP enhances GABABR function
We first tested whether infusion of GISP in to CA1 hippocampal neurons altered synaptic GABABR-mediated responses. We did not detect any acute changes (monitored up to 40 min) in synaptic GABABR-mediated responses on infusion of purified recombinant GISPΔ18 from the patch pipette (data not shown). To assess the effects of GISP over a longer time course, we transfected HEK cells with GISP and GABABR subunits and used two independent functional assays, namely ERK MAP kinase (Balasubramanian et al. 2004) and GIRK channel activation (Couve et al. 2002). We detected robust responses in both physiological assay systems in cells co-expressing GABAB1 and GABAB2. As shown in the Fig. 10, in the phosphorylated ERK (pERK) assay, the time of onset and total ERK phosphorylation following baclofen application was significantly increased in cells expressing GABAB1/GABAB2 and GISP compared with cells expressing just GABAB1 and GABAB2. Specifically, there was an increase in pERK activation post-baclofen stimulation measured over 10 min. Interestingly, GISP did not affect the peak activation, rather it prolonged the pERK response. Similarly, in HEK cells stably expressing GIRK (Kir3.1 and Kir3.2) K+ channels and transfected with GABAB1 + GABAB2 ± GISP, we found that the peak depolarization response was unaltered by GISP. However, there was a significant difference in the profile and extent of rundown in the baclofen-evoked response. The time constants for inactivation were calculated by data fitted to single exponentials. The time constants were 88.8 ± 12.2 and 146.8 ± 4.36 s, respectively, for control and GISP expressing cells (p ≤ 0.01).
Fig. 10.
GISP increases GABABR function in HEK cells. (a) The effect of GISP on GABABR-evoked ERK phosphorylation assessed by MAP kinase assay. HEK293 cells were transfected with myc-GABAB1a and HA-GABAB2 in combination with a control vector (pcDNA3.1) or vector encoding GISP. Forty-eight hours post-transfection, the cells were stimulated with 100 μM baclofen at the times indicated and pERK was determined as described in Materials and methods. The blots were also probed with anti-β-actin antibody to ensure equal loading (data not shown). (b) Densitometric analysis of the effect of GISP on the GABABR activation pERK. Cummulative histogram of the time course and extent of pERK activation from three independent experiments. (c) Repeated application of baclofen (4 s every 30 s) results in desensitization of potassium currents in HEK cells stably expressing GIRK channels (KIR3.1/3.2) transfected with GABAB1 and GABAB2. In cells co-transfected with GISP (filled triangles; n = 4) the rate of desensitization is reduced compared with controls (filled squares; n = 7). (d) The time constants for the comparison of 50% inactivation were calculated from data fitted to a single exponential. In cells co-transfected with GISP, (black bars) the time of desensitization is longer compared with controls (grey bars). *p = 0.01 compared with control (without GISP; Student’s t-test). (e) Raw data from patch-clamp recordings of baclofen activated currents.
Discussion
We have identified GISP, a novel coiled-coil protein that interacts specifically with the GABAB1 subunit in a coiledcoil domain-dependent manner. Primary structure analysis revealed that GISP is transcribed form the akap9 gene that encodes at least three different AKAPs identified at the protein level; AKAP450 (CG-NAP), AKAP350, and AKAP9. AKAPs are a family of proteins that contain two classes of binding sites, a ‘targeting domain’ which directs the subcellular localization of the PKA-AKAP complex to membranes or cellular organelles, and an anchoring motif that binds the Arg subunit of PKA (Michel and Scott 2002). There are now known to be examples of AKAP family members that, in addition to anchoring PKA, also anchor a diverse range of other proteins, including different kinases and phosphatases (Wong and Scott 2004). Thus, AKAPs can be regarded as a class of scaffolding proteins that serve as focal reaction centres for PKA and other enzymes. We therefore propose that AKAP-like molecules such as a GISP are likely to function as scaffolding proteins for enzymes other than PKA. For example, GISP shares consensus of calmodulin and casein kinase binding sites (Gillingham and Munro 2000; Sillibourne et al. 2002; Takahashi et al. 2002) with AKAP450/CG-NAP. Moreover, the microtubule targeting domain of CG-NAP (AKAP450) is also conserved in GISP, suggesting that GISP may be involved in some microtubule-associated functions (Takahashi et al. 2002).
Using a novel GISP antibody, we demonstrated that GISP is a brain-specific protein. Interestingly, other proteins encoded by the akap9 gene are not expressed in a brain-specific manner and our data indicate that, of all these proteins so far characterized, only GISP is expressed in neurons. Within the brain, GISP is widely distributed with the highest levels present in the cerebellum and all regions of the hippocampus (Fig. 3d). GABAB receptors show a similar pattern of immunoreactivity, with particularly high levels in the hippocampus and cerebellum. These data are consistent with GABAB binding sites analysed by autoradiography (Bowery et al. 1987; Chu et al. 1990).
Although GISP is predicted as a soluble protein, it was detected as both a cytosolic protein and tightly membrane-attached forms. In particular, high levels of GISP were present in microsomal and synaptic membrane fractions. Furthermore, detergent extraction assays showed that GISP is enriched, together with GABAB1, in the 1% Triton X-100 insoluble fraction. This is likely because of the high content of the coiled-coil regions in GISP. Coiled-coil domains are known to be sites of homomultimeric and heteromultimeric interactions with other coiled-coil domain-containing proteins to form stable complexes. For example, it is known that many cytoskeletal and associated proteins, such as a myosin heavy chain (Adamson et al. 1993), contain coiled-coil domains that participate in the formation of filamentous structures.
The developmental profile of GISP showed that it is present at all stages of GABABR subunit expression. GISP was relatively abundant at embryonic day 18, the earliest age point measured, and expression increased to a peak at around the third week after birth. In contrast, very low levels of GABAB1 and GABAB2 were detected before birth. One explanation may be that, in addition to the role in GABABR trafficking reported here, GISP may have other as yet unknown functions.
A key test for the association of native proteins in vivo is co-immunoprecipitation. This approach does not necessarily establish if proteins bind directly to each other, but it does determine if they assemble in a complex. As expected from their direct binding, a strong GABAB1 band was present in the anti-GISP immunoprecipitate. In addition, a robust GABAB2 signal was also detected, indicating that both GISP and GABAB2 can simultaneously bind at the coiled-coil domain of GABAB1. These results suggest a role for GISP with the GABABR complex rather than specifically for the-non-heterodimerized GABAB1 subunit.
As GISP is a newly discovered protein, we set out to define its cellular localization in cultured hippocampal neurons. Immunocytochemical localization of GISP in cultured hippocampal neurons revealed a highly punctate distribution throughout the cell processes. Comparison with the synaptic marker synaptotagmin indicated that GISP was present in synapses and that the majority of puncta were synaptic. GISP was co-localized to varying extents with both GABAB1 and GABAB2 ( ~65 and ~34%, respectively). The fact that there were higher levels of GISP co-localization with GABAB1 than GABAB2 suggests that GABAB1-GISP complexes also exist. These data support the biochemical results by indicating that GISP is present in close proximity to GABABRs in physiologically relevant regions of the cells.
Coiled-coil interactions at the C-terminal of GABAB1 are known to play a role in the cell surface expression of GABABR subunits (Marshall et al. 1999). Coiled-coil domains are well-studied relatively complex structures that can specifically mediate the interaction of several protein partners at once (Lupas 1996). For example, our results for GISP differ from those obtained for ATF4, another protein that interacts with the coiled-coil domain of GABAB1. ATF4 binds specifically to only the free GABAB1 subunit and not to the GABABR complex (Vernon et al. 2001). This is relevant because the localization of the GABAB1 subunit when not associated with GABAB2, is restricted to the ER because of an RXR(R) retention motif immediately following the C-terminal coiled-coil domain (Margeta-Mitrovic et al. 2000). This retention motif is masked by the dimerization of GABAB1 with GABAB2, thus permitting the transport of the fully formed receptor to the cell surface.
As the GISP–GABAB1 interaction also accommodates the GABAB2 subunit, we wondered if GISP could have an effect on the forward traffic of GABAB receptors from the ER. We show that expression of the GISP efficiently enhances the GABAB receptors expression to the plasma membrane (Fig. 8). GISP is present in microsomal fraction from brain and, intriguingly, GISP enhances the surface expression of both subunits of the heteromeric receptor by promoting forward traffic of GABAB1 along with GABAB2 and allows the surface expression of the receptors. We postulate that GISP acts as a chaperone, releasing receptors from the ER to progress through the secretory pathway and therefore the GABAB receptors are less rapidly targeted for degradation. As the GISP–GABAB1 interaction also accommodates the GABAB2 subunit, we wondered if GISP could fulfil a similar role in allowing the forward traffic of GABAB1 from the ER in the absence of GABAB2. Expression of the GISP markedly enhanced GABAB1 expression at the plasma membrane, although the amount of GABAB1 surface expression is less on co-expression with GISP compared with GABAB2. In terms of trafficking, therefore, GISP can substitute to some extent for GABAB2. This is of particular interest because, in neurons in some brain areas (e.g. caudate putamen), the GABAB2 receptor mRNA message is virtually absent, and in some other regions, such as the septum, the preoptic area and the hypothalamus, its levels are significantly lower than that of GABAB1 mRNA (Jones et al. 1998; Margeta-Mitrovic et al. 1999; Clark et al. 2000). As shown in Fig. 3(d), GISP is very well expressed in the caudate putamen and we suggest that occupation of the coiled-coil domain on GABAB1 allows ER exit in the absence of GABAB2.
To determine the functional effects of GISP, we used two HEK cell-based assay systems. In both the pERK and GIRK channel activation assays, GISP increased the overall baclofen-evoked responses, but did not alter peak levels of activation. Taken together, these results are consistent with GISP acting to enhance GABABR function by promoting the forward trafficking of the GABABR to the surface (more receptors leading to larger second messenger signal and a quicker onset of ERK phosphorylation and a slower rundown of GIRK channel current). In addition, the more prolonged response may also have a component attributable to decreased desensitization of the functional receptors. Because GABABRs do not readily internalize, one possibility is that GISP may lead to conformational changes in the receptor complex, facilitating longer G-protein activation. The effects of GISP on GABAB receptor functional coupling and possible effects on membrane stabilization need further experimentation.
In conclusion, we have identified a new, brain-specific GABABR interacting protein that is involved in receptor forward traffic, receptor stability and possibly in slowing receptor desensitization. Further work will be directed to dissect the exact mechanisms for each of these roles.
Supplementary Material
Acknowledgements
We are grateful to the Wellcome Trust, the MRC and the EU (GRIPPANT) for financial support. We thank Abigail Woollard and Zafar Bashir for slice electrophysiology experiments and Stéphane Martin, Tristan Bouschet, David Holman, Claire Palmer, Simon Ball and Elek Molnar for their help and advice during the course of this work. We are also grateful to Steve Moss, GSK and Novartis for providing cDNA plasmids and Trevor Smart for the HEK cells stably expressing GIRKs.
Abbreviations used
- AKAP
A-kinase anchoring protein
- ERK
extracellular signal-regulated kinase
- GFP
green fluorescent protein
- GIRK
G-protein inwardly rectifying potassium channel
- GISP
GPCR interacting scaffolding protein
- GST
glutathione S-transferase
- HEK
human embryonic kidney
- MAP
mitogen-activated protein
- PBS
phosphate-buffered saline
- PKA
protein kinase A
- PSD
postsynaptic density
- SDS
sodium dodecyl sulfate
Footnotes
Supplementary Material The following material is available for this paper online.
Figure S1 Comparison of the molecular mass of recombinant (M1) and native GISP. HEK cell, cultured neuron and brain extracts were resolved on a 5% gel and blotted with GISP antibody. The M1 GISP construct migrates at a slightly larger Mr than native GISP. Note also that minor higher molecular mass bands are detected in brain extract that correspond to the expected sizes of rat AKAP450, AKAP350 and the hypothetical protein product of transcript XM_347223. See text for details.
Figure S2 Determination of the GISP start codon. A, Met 1 indicates the first methionine of predicted open reading frame. Met 2, Met 3 and Met 4 indicate the successive methionines in the GISP sequence. Immunogen is the protein used to raise the anti-GISP antibody. B, Deletion mutant GISP constructs starting from the different potential start codons indicated in A were transfected into HEK cells resolved on 5% gel and analysed by immunoblotting with anti-GISP antibody. M1 to M4, methionine 1 to 4; BE, rat brain extract. C, Mapping the GISP antibody recognition site. GST fusion proteins of GISP peptides encompassing the antigen sequence used to raise the GISP antibody. D, The purified peptides shown in C were tested for blockade of GISP antibody binding to the M1 protein illustrated in A and brain extract (BE). The preincubation with the 47-103 peptide prevented GISP antibody binding.
This material is available as part of the online article from: http://www.blackwell-synergy.com/doi/abs/10.1111/j.1471-4159.2006.04271.x.
Please note: Blackwell Publishing are not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Adamson JG, Zhou NE, Hodges RS. Structure, function and application of the coiled-coil protein folding motif. Curr. Opin. Biotechnol. 1993;4:428–337. doi: 10.1016/0958-1669(93)90008-k. [DOI] [PubMed] [Google Scholar]
- Balasubramanian S, Teissere JA, Raju DV, Hall RA. Hetero-oligomerization between GABAA and GABAB receptors regulates GABAB receptor trafficking. J. Biol. Chem. 2004;279:18 840–18 850. doi: 10.1074/jbc.M313470200. [DOI] [PubMed] [Google Scholar]
- Bowery NG, Hudson AL, Price GW. GABAA and GABAB receptor site distribution in the rat central nervous system. Neuroscience. 1987;20:365–383. doi: 10.1016/0306-4522(87)90098-4. [DOI] [PubMed] [Google Scholar]
- Bowery NG, Bettler B, Froestl W, Gallagher JP, Marshall F, Raiteri M, Bonner TI, Enna SJ. International Union of Pharmacology. XXXIII. Mammalian γ-aminobutyric acid B receptors: structure and function. Pharmacol. Rev. 2002;54:247–264. doi: 10.1124/pr.54.2.247. [DOI] [PubMed] [Google Scholar]
- Calver AR, Medhurst AD, Robbins MJ, et al. The expression of GABAB1 and GABAB2 receptor subunits in the CNS differs from that in peripheral tissues. Neuroscience. 2000;100:155–170. doi: 10.1016/s0306-4522(00)00262-1. [DOI] [PubMed] [Google Scholar]
- Calver AR, Davies CH, Pangalos M. GABAB receptors: from monogamy to promiscuity. Neurosignals. 2002;11:299–314. doi: 10.1159/000068257. [DOI] [PubMed] [Google Scholar]
- Carlin RK, Grab DJ, Cohen RS, Siekevitz P. Isolation and characterization of postsynaptic densities from various brain regions: enrichment of different types of postsynaptic densities. J. Cell Biol. 1980;86:831–845. doi: 10.1083/jcb.86.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu DC, Albin RL, Young AB, Penney JB. Distribution and kinetics of GABAB binding sites in rat central nervous system: a quantitative autoradiographic study. Neuroscience. 1990;34:341–357. doi: 10.1016/0306-4522(90)90144-s. [DOI] [PubMed] [Google Scholar]
- Clark JA, Mezey E, Lam AS, Bonner TI. Distribution of the GABAB receptor subunit gb2 in at CNS. Brain Res. 2000;860:41–52. doi: 10.1016/s0006-8993(00)01958-2. [DOI] [PubMed] [Google Scholar]
- Corrêa SA, Munton R, Nishimune A, Fitzjohn S, Henley JM. Development of GABAB subunits and functional GABAB receptors in rat cultured hippocampal neurons. Neuropharmacology. 2004;47:475–484. doi: 10.1016/j.neuropharm.2004.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couve A, Filippov AK, Connolly CN, Bettler B, Brown DA, Moss SJ. Intracellular retention of recombinant GABAB receptors. J. Biol. Chem. 1998;273:26 361–26 367. doi: 10.1074/jbc.273.41.26361. [DOI] [PubMed] [Google Scholar]
- Couve A, Moss SJ, Pangalos MN. GABAB receptors: a new paradigm in G protein signaling. Mol. Cell. Neurosci. 2000;16:296–312. doi: 10.1006/mcne.2000.0908. [DOI] [PubMed] [Google Scholar]
- Couve A, Kittler JT, Uren JM, Calver AR, Pangalos MN, Walsh FS, Moss SJ. Association of GABAB receptors and members of the 14-3-3 family of signaling proteins. Mol. Cell. Neurosci. 2001;17:317–328. doi: 10.1006/mcne.2000.0938. [DOI] [PubMed] [Google Scholar]
- Couve A, Thomas P, Calver AR, Hirst WD, Pangalos MN, Walsh FS, Smart TG, Moss SJ. Cyclic AMP-dependent protein kinase phosphorylation facilitates GABAB receptor-effector coupling. Nat. Neurosci. 2002;5:415–424. doi: 10.1038/nn833. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Meskenaite V, Weinmann O, Honer M, Benke D, Mohler H. GABAB-receptor splice variants GB1a and GB1b in rat brain: developmental regulation, cellular distribution and extrasynaptic localization. Eur. J. Neurosci. 1999;11:761–768. doi: 10.1046/j.1460-9568.1999.00481.x. [DOI] [PubMed] [Google Scholar]
- Gillingham AK, Munro S. The PACT domain, a conserved centrosomal targeting motif in the coiled-coil proteins AKAP450 and pericentrin. EMBO Rep. 2000;1:524–529. doi: 10.1093/embo-reports/kvd105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray EG, Whittaker VP. The isolation of nerve endings from brain: an electron-microscopic study of cell fragments derived by homogenisation and centrifugation. J. Anat. 1962;96:79–88. [PMC free article] [PubMed] [Google Scholar]
- Harlow E, Lane D. Antibodies, A Laboratory Manual. Cold Spring Harbour Laboratory; Cold Spring Harbour, NY: 1988. pp. 471–510. [Google Scholar]
- Hirbec H, Perestenko O, Nishimune A, Meyer G, Nakanishi S, Henley JM, Dev KK. The PDZ proteins PICK1, GRIP, and syntenin bind multiple glutamate receptor subtypes: analysis of PDZ binding motifs. J. Biol. Chem. 2002;277:15 221–15 224. doi: 10.1074/jbc.C200112200. [DOI] [PubMed] [Google Scholar]
- Hirokawa T, Boon-Chieng S, Mitaku S. SOSUI: classification and secondary structure prediction system for membrane proteins. Bioinformatics. 1998;14:378–379. doi: 10.1093/bioinformatics/14.4.378. [DOI] [PubMed] [Google Scholar]
- Ige AO, Bolam JP, Billinton A, White JH, Marshall FH, Emson PC. Cellular and sub-cellular localisation of GABAB1 and GABAB2 receptor proteins in the rat cerebellum. Brain Res. Mol. Brain Res. 2000;83:72–80. doi: 10.1016/s0169-328x(00)00199-6. [DOI] [PubMed] [Google Scholar]
- Jones KA, Borowsky B, Tamm JA, et al. GABAB receptors function as a heteromeric assembly of the subunits GABAB R1 and GABAB R2. Nature. 1998;396:674–679. doi: 10.1038/25348. [DOI] [PubMed] [Google Scholar]
- Kammerer RA, Frank S, Schulthess T, Landwehr R, Lustig A, Engel J. Heterodimerization of a functional GABAB receptor is mediated by parallel coiled-coil α-helices. Biochemistry. 1999;38:13 263–13 269. doi: 10.1021/bi991018t. [DOI] [PubMed] [Google Scholar]
- Lin JW, Wyszynski M, Madhavan R, Sealock R, Kim JU, Sheng M. Yotiao, a novel protein of neuromuscular junction and brain that interacts with specific splice variants of NMDA receptor subunit NR1. J. Neurosci. 1998;18:2017–2027. doi: 10.1523/JNEUROSCI.18-06-02017.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupas A. Coiled coils: new structures and new functions. Trends Biochem. Sci. 1996;21:375–382. [PubMed] [Google Scholar]
- Lupas A, Van Dyke M, Stock J. Predicting coiled coils from protein sequences. Science. 1991;252:1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- Malitschek B, Schweizer C, Keir M, et al. The N-terminal domain of c-aminobutyric acid B receptors is sufficient to specify agonist and antagonist binding. Mol. Pharmacol. 1999;56:448–454. doi: 10.1124/mol.56.2.448. [DOI] [PubMed] [Google Scholar]
- Margeta-Mitrovic M, Mitrovic I, Riley RC, Jan LY, Basbaum AI. Immunohistochemical localization of GABAB receptors in the rat central nervous system. J. Comp. Neurol. 1999;405:299–321. doi: 10.1002/(sici)1096-9861(19990315)405:3<299::aid-cne2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Margeta-Mitrovic M, Jan YN, Jan LY. A trafficking checkpoint controls GABAB receptor heterodimerization. Neuron. 2000;27:97–106. doi: 10.1016/s0896-6273(00)00012-x. [DOI] [PubMed] [Google Scholar]
- Marshall FH, Jones KA, Kaupmann K, Bettler B. GABAB receptors – the first 7TM heterodimers. Tips. 1999;20:396–399. doi: 10.1016/s0165-6147(99)01383-8. [DOI] [PubMed] [Google Scholar]
- Martin S, Henley JM. Activity-dependent endocytic sorting of kainate receptors to recycling or degradation pathways. EMBO J. 2004;23:4749–4759. doi: 10.1038/sj.emboj.7600483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel JJ, Scott JD. AKAP mediated signal transduction. Annu. Rev. Pharmacol. Toxicol. 2002;42:235–257. doi: 10.1146/annurev.pharmtox.42.083101.135801. [DOI] [PubMed] [Google Scholar]
- Nehring RB, Horikawa HP, El Far O, Kneussel M, Brandstatter JH, Stamm S, Wischmeyer E, Betz H, Karschin A. The metabotropic GABAB receptor directly interacts with the activating transcription factor 4 (ATF-4) J. Biol. Chem. 2000;45:35 185–35 191. doi: 10.1074/jbc.M002727200. [DOI] [PubMed] [Google Scholar]
- Nishimune A, Nash SR, Nakanishi S, Henley JM. Detection of protein–protein interactions in the nervous system using the two-hybrid system. Trends Neurosci. 1996;19:261–266. doi: 10.1016/S0166-2236(96)40003-0. [DOI] [PubMed] [Google Scholar]
- Nishimune A, Isaac JT, Molnar E, Noel J, Nash SR, Tagaya M, Collingridge GL, Nakanishi S, Henley JM. NSF binding to GluR2 regulates synaptic transmission. Neuron. 1998;21:87–97. doi: 10.1016/s0896-6273(00)80517-6. [DOI] [PubMed] [Google Scholar]
- Pagano A, Rovelli G, Mosbacher J, et al. C–terminal interaction is essential for surface trafficking but not for heteromeric assembly of GABAB receptors. J. Neurosci. 2001;21:1189–1202. doi: 10.1523/JNEUROSCI.21-04-01189.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perestenko PV, Henley JM. Characterization of the intracellular transport of GluR1 and GluR2 α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor subunits in hippocampal neurons. J. Biol. Chem. 2003;278:43 525–43 532. doi: 10.1074/jbc.M306206200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser HM, Gill CH, Hirst WD, et al. Epileptogenesis and enhanced prepulse inhibition in GABAB1-deficient mice. Mol. Cell. Neurosci. 2001;17:1059–1070. doi: 10.1006/mcne.2001.0995. [DOI] [PubMed] [Google Scholar]
- Rasband W. ImageJ 1.30 software. US National Institutes of Health; Bethesda, Maryland, USA: 1997–2006. http://rsb.info.nih.gov/ij. [Google Scholar]
- Robbins MJ, Calver AR, Filippov AK, et al. GABAB2 is essential for G-protein coupling of the GABAB receptor heterodimer. J. Neurosci. 2001;21:8043–8052. doi: 10.1523/JNEUROSCI.21-20-08043.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt PH, Dransfield DT, Claudio JO, Hawley RG, Trotter KW, Milgram SL, Goldenring JR. AKAP350, a multiply spliced protein kinase A-anchoring protein associated with centrosomes. J. Biol. Chem. 1999;274:3055–3066. doi: 10.1074/jbc.274.5.3055. [DOI] [PubMed] [Google Scholar]
- Sillibourne JE, Milne DM, Takahashi M, Ono Y, Meek DW. Centrosomal anchoring of the protein kinase CK1d mediated by attachment to the large, coiled-coil scaffolding protein CG-NAP/AKAP450. J. Mol. Biol. 2002;322:785–797. doi: 10.1016/s0022-2836(02)00857-4. [DOI] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Yamagiwa A, Nishimura T, Mukai H, Ono Y. Centrosomal proteins CG-NAP and kendrin provide microtubule nucleation sites by anchoring c-tubulin ring complex. Mol. Biol. Cell. 2002;13:3235–3245. doi: 10.1091/mbc.E02-02-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonnes J, Stierli B, Cerletti C, Behrmann JT, Molnar E, Streit P. Regional distribution and developmental changes of GluR1-flop protein revealed by monoclonal antibody in rat brain. J. Neurochem. 1999;73:2195–2205. [PubMed] [Google Scholar]
- Vernon E, Meyer G, Pickard L, Dev K, Molnar E, Collingridge GL, Henley JM. GABAB receptors couple directly to the transcription factor ATF4. Mol Cell. Neurosci. 2001;17:637–645. doi: 10.1006/mcne.2000.0960. [DOI] [PubMed] [Google Scholar]
- Vigot R, Barbieri S, Brauner-Osborne H, et al. Differential compartmentalization and distinct functions of GABAB receptor variants. Neuron. 2006;50:589–401. doi: 10.1016/j.neuron.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JH, McIllhinney RA, Wise A, Ciruela F, Chan WY, Emson PC, Billinton A, Marshall FH. The GABAB receptor interacts directly with the related transcription factors CREB2 and ATFx. Proc. Natl Acad. Sci. USA. 2000;97:13 967–13 972. doi: 10.1073/pnas.240452197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witczak O, Skalhegg BS, Keryer G, Bornens M, Tasken K, Jahnsen T, Orstavik S. Cloning and characterisation of a cDNA encoding an A-kinase anchoring protein located in the centrosome, AKAP450. EMBO J. 1999;18:1858–1868. doi: 10.1093/emboj/18.7.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong W, Scott JD. AKAP signalling complexes: focal points in space and time. Nat. Rev. Mol. Cell Biol. 2004;5:959–970. doi: 10.1038/nrm1527. [DOI] [PubMed] [Google Scholar]
- Yap KL, Kim J, Truong K, Sherman M, Yuan T, Ikura M. Calmodulin target database. J. Struct. Funct. Genomics. 2000;1:8–14. doi: 10.1023/a:1011320027914. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.