Abstract
Increased de novo fatty acid (FA) synthesis is one hallmark of tumor cells, including prostate cancer (PC). We present here our most recent results showing that lipids composition in human prostate cancer (PC) is characterized by an increased ratio of monounsaturated FA (MUFA) to saturated FA (SFA), compared to normal prostate, and evidence the overexpression of the lipogenic enzyme stearoyl CoA desaturase 1 (SCD1) in human PC. As a new therapeutic strategy, we show that pharmacological inhibition of SCD1 activity impairs lipid synthesis and results in decreased proliferation of both androgen-sensitive and androgen-resistant PC cells, abrogates the growth of prostate tumor xenografts in nude mice, and confers therapeutic benefit on animal survival. We show that these changes in lipid synthesis are translated into the inhibition of the AKT pathway, and that the decrease in concentration of phosphatidyl inositol tri-phosphate (PI (3,4,5)P3) might at least partially mediates this effect.
Inhibition of SCD1 also promotes the activation of AMP-activated kinase (AMPK) and GSK3α/β, the latter on being consistent with a decrease in β-catenin activity and mRNA levels of various β-catenin growth promoting trancriptional targets. Furthermore, we show that activity is required for cell transformation by Ras oncogene. Together, our data support for the first time the concept of targeting the lipogenic enzyme SCD1 as a new promising therapeutic approach to block oncogenesis and PC progression.
Keywords: Animals; Cell Line, Transformed; Cell Line, Tumor; Cell Proliferation; drug effects; Cell Survival; drug effects; Disease Progression; Enzyme Inhibitors; pharmacology; Fatty Acids, Monounsaturated; metabolism; Fibroblasts; drug effects; pathology; Humans; Lipogenesis; drug effects; Male; Mice; Prostatic Neoplasms; enzymology; pathology; Signal Transduction; drug effects; Stearoyl-CoA Desaturase; antagonists & inhibitors; metabolism; Tumor Suppressor Protein p53; metabolism; Xenograft Model Antitumor Assays
Keywords: Metabolism, desaturase, monounsaturated fatty acids, AKT, small molecule
Introduction
Prostate cancer (PC) is the most commonly diagnosed cancer and the second leading cause of cancer death in western men after middle age and remained a major research and public health priority. Tumor growth is originally dependent on androgens which exert their effects on prostate cancer cells by activating the androgen receptor (AR), a member of the hormone nuclear receptor superfamily. In the mature prostatic gland, the AR regulates the expression of genes involved in diverse cellular function, including survival and proliferation of the epithelial cells and lipid metabolism (1). In early stage tumors, therapy based on androgen deprivation is only temporary effective (2), and many men develop recurrent androgen-independent prostate cancer, which has a very poor prognosis (3). In view of this, new therapeutic options are wanted.
One of the first identified biochemical hallmark of cancer cells was an alteration in metabolism. Early in the last century, Otto Warburg (1928) observed that tumors have a higher rate of glucose metabolism than normal tissues (4–6). Over the past decades, accumulating evidence of a metabolic reorganization has emerged from studies on various proliferating cells. Indeed, most tumor cells are characterized by higher rates of glycolysis, lactate production, and biosynthesis of lipids (7). Cells use two major sources of fatty acids (FA): exogenously derived (dietary) FA and de-novo endogenously synthesized FA. De novo FA synthesis is very active during embryogenesis, whereas most adult normal cells and tissues, even those with high cellular turnover, preferentially use circulating FA for the synthesis of new structural, and signaling lipids. In contrast, it is now well documented that various tumors and their precursor lesions, including prostate cancer, undergo exacerbated endogenous FA biosynthesis irrespective of the levels of extracellular lipids (8, 9). The increased lipogenesis in cancer is reflected in overexpression and hyperactivity of lipogenic enzymes such as ATP citrate lyase (ACL), acetyl-CoA carboxylase (ACC), or the fatty acid synthase (FAS) (10). Moreover, studies with chemical inhibitors have revealed that inhibition of FAS activity results in decreased proliferation and increased apoptosis of cancer cells (11). De novo fatty acid biosynthesis is required for cancer cells to synthesize new membranes, which have a particular lipidic composition that facilitates the formation of lipid rafts for increased signaling of cell growth receptors, such as erbB2 (12). Lipogenesis also participates, in cancer cells to generate signaling molecules, such as phosphatidyl inositol, phosphatidyl serine, or phosphatidyl choline, which are important factors to activate proliferative and survival pathways. This is consistent with the observation that in most tumor cells examined, the majority of newly synthesized lipids are phospholipids (13, 14). We therefore hypothetized that targeting de novo lipid synthesis might abrogate tumor progression.
An important rate-limiting enzyme in lipogenesis is stearoyl CoA desaturase 1 (SCD1), also commonly known as Δ9-desaturase. SCD1 is a microsomal enzyme that catalyses the committed step in the biosynthesis of the mono-unsaturated fatty acids (MUFA) from saturated fatty acids (SFA), by introducing a cis-double bond to a fatty acyl-CoAs (15). The preferred substrates are palmitate (16:0) and stearate (18:0), which yeld palmitoleate (16:1n-7) and oleate (18:1n-9) respectively. These represent the major MUFA of membrane phospholipids, triglycerides, wax esters, and cholesterol esters. Of note, most cancer cells contain higher levels of MUFA that tend to partition into detergent-resistant lipid rafts (reviewed in (16)). Since the ratio of monounsaturated to saturated fatty acids (desaturation index) affects phospholipids composition, and alteration in this ratio has been observed in several cancers (16), our main hypothesis in this study is that targeting SCD1 and associated lipid synthesis in prostate cancer cells might strongly impact on their proliferation and/or survival, and represent a potent new therapeutic strategy to block prostate cancer progression. As a proof of concept, we have characterized in this study the lipid composition and SCD1 expression in human prostate cancer tissue, and analyzed the effects of SCD1 targeting in prostate cancer cells and prostate tumors in mice.
Materials and Methods
Chemicals
All solvents were HPLC grade and were purchased from various suppliers. Methanol, chloroform, hexane, isooctane were purchased from Carlo Erba (val de Reuil, France). Potassium chloride and standards cholesterol esters (CE), triacylglycerides (TAG) and phospholipids (PL), and fatty methyl ester standarts were purchased from Sigma-Aldrich (Saint Quentin Fallavier, France). Phosphatidylinositol-3,4,5-trisphosphate (PI(3,4,5)-P3) was purchased from Cayman chemical (Interchim, Montluçon, France).
BZ36 synthesis
6-[4-(2-Bromo-5-methoxy-benzoyl)-piperazin-1-yl]-N-phenylpropyl-nicotinamide compound, was previously characterized as a specific competitive inhibitor of SCD1 with a IC50 of 100nM (17). We have synthezised it in our laboratory as BZ36 compound, according the method described in the patent. Structure of the molecule is described in Supplemental Figure 1.
Human prostate tissue samples
Human prostate tissue was collected from consenting patients, after protocol approval by the local ethics committee (CHU Henry Mondor, Creteil, France). Localized prostate cancer specimens from the peripheral zone of the prostate were obtained from men who had radical prostatectomy as treatment for their prostate cancer, gleason ≥7 (n=10). Non tumoral prostate specimens were obtained from men with benign hyperplasia of the prostate (HBP) who had radical prostatectomy (n=10). The patients did not received any hormonal or chemical treatment before obtaining tissue specimens. Concerning the methods of tissue handling, the cancer specimens were examined on hematein eosin-stained cryosections. As the cancer cells could be admixed with benign epithelial and stromal cells, areas with more than 70 % of cancers cells were selected and macro-dissected. For each selected area, ten sections of 50 μm thickness (overall 10 mg tissue) were prepared and used for ratios of MUFA to SFA assessment, as well as for transcripts analysis. For the benign prostatic hyperplasia specimens (including admixed benign epithelial and stromal cells), absence of malignant cells was verified on cryosections. The samples were then sectionned and processed as for the cancer specimens.
Cell culture, transient transfections and RNA interference experiments
The benign PNT2 prostate epithelial cell line was obtained from the European Collection of Cell Cultures (ECACC) (Sigma Aldrich). The androgen-sensitive LNCaP and the androgen-independent C4-2 human prostate carcinoma cell lines were purchased from Viromed Laboratory Inc (Minnetonka, MN, USA). Monolayer cell cultures were maintained in a RPMI 1640 media (Invitrogen, Cergy, France) supplemented with 10% fetal calf serum (FCS), 100 U/ml penicillin, 100 μg/ml streptomycin, 10mM HEPES and 1.0 mM sodium pyruvate (Invitrogen) at 37°C in 5% CO2. Primary MEFs were obtained from embryos at embryonic day 13.5 by standard methods. Monolayer cell cultures were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 25mM glucose and 10% FCS. The transcriptional activity of the β-catenin–Tcf4 complex was analyzed by performing transient transfections with 0.25 μg TCF/LEF-1 reporter (pTOP-FLASH) or control vector (pFOPFLASH), which were kindly provided by Dr Philippe Blache (Institut de Génomique Fonctionnelle-CNRS UMR5203-Inserm U661-Université Montpellier 1, France). Luciferase activities in cell lysates were normalized relative to the β-galactosidase activity to correct for differences in transfection efficiency. Figure shows representative results of at least two independent experiments performed in triplicate. For small interfering (si) RNA experiments, transfection of LNCaP or C4-2 cells was carried out with pre-designed ON-TARGET plus siRNA oligonucleotides control or targeting the human SCD1 sequence GCACAUCAACUUCACCACA (Dharmacon, Lafayette, Co, USA). Nucleofaction of cells with 2.5 μg siRNA were performed on 2×106 cells using Amaxa nucleofactor R kit (Lonza, Cologne, Germany). Twenty-four hours and 48 hours after transfection, cells were processed for cell proliferation by BrdU staining and harvested for RNA and protein analysis. For SCD1 overexpression experiments, nucleofaction of LNCaP or C4-2 cells was performed with 2 μg hSCD1 cDNA (Cliniscience, Montrouge, France) or control pcdna3 (mock) vector using Amaxa nucleofactor R kit (Lonza) and cells were processed for cell proliferation by BrdU staining and harvested for RNA and protein analysis 72 hours after.
Animal experiments
Male athymic nude Mice (Foxn1 nu/nu) (Harlan, Grannat, France) were used at the age of 7 weeks (weight 25–30g). All procedures were performed in compliance with the European Convention for the Protection of Vertebrate Animals Used for Experimentation (animal house agreement # B-34-172-27, authorization for animal experimentation # 34.324). Experiments were done at least twice for each tested condition. Animals were sacrificed before they became compromised. Xenografts were established by subcutaneously injecting 2×106 LNCaP cells, 2×106 C4-2 cells or 5×105 MEFs SV40 in 100 μl of a Matrigel solution. For curative experiments, tumors were allowed to growth until they were measurable with a caliper. In each group, the mice were randomized according to their established tumor volume and given SCD1 inhibitor BZ36 at 80 mg/kg in 100 μl of a labrafil-DMA-tween80 solution (89:10:1) (treated group) or vehicle alone (control group), by daily intra-peritoneal (i.p.) injection 5 days of week. For preventive experiments, the mice were treated daily for 7 days before the day of xenograft until the end of the experiment. Tumor volume measurements were taken twice to tree times a week and calculated according to the formula: lenght × widht × height × 0.5236. Data are expressed as the mean tumor volume or as fold of the start point tumor volume. For survival analysis, animals bearing pre-established C4-2 tumors were treated with BZ36 at 80 mg/kg or 160 mg/kg or with vehicle by daily i.p. injection 5 days of week. All mice were monitored for survival until tumor volume had reached 2000 mm3 or until death. Analysis of survival was conducted by a log-rank test based on the Kaplan-Meier method. At euthanasia, tumors were excised and either fixed in 4% formalin for immunohistological analysis or prepared for fatty acids analysis. Blood was harvested by cardiac puncture and sera were prepared for further biochemical analysis.
RNA isolation, Reverse transcription and quantitative real-time PCR
RNA was extracted with the use of TRI-Reagent (Euromedex, Mundolsheim, France) according to the manufacturers’ recommendations. Reverse transcription of total RNA was performed at 37°C using the M-MLV reverse transcriptase (Invitrogen) and random hexanucleotide primers (Promega, Madison, WI), followed by a 15 min inactivation at 70°C. Quantitative PCR was conducted using the primers specific for human SCD1 and SYBR Green Light Cycler Master Mix (Eurofins MWG Operon, Roissy CDG). Measurement and analysis of gene expression were performed using the ABI Prism 7300 Sequence detection System software (Applied Biosystems), under the following conditions: 2 minutes at 50°C and 10 minutes at 95°C; and then 40 cycles of 15 seconds at 95°C and 1 minute at 60°C. The relative content of cDNA samples was normalized by substracting the threshold cycle (Ct) of the endogenous 18S reference gene to the target gene (ΔCt = Ct of target gene − Ct of 18S). Values are expressed as the relative mRNA level of specific gene expression as obtained using the formula 2−(ΔCt). C4-2 cells treated with BZ36 at 25 μM were analyzed for the expression profiles of 84 genes related to Wnt/β-catenin mediated signal transduction by using Human Wnt Signaling Pathway PCR Array according to the manufacturers’ recommendations (Tebu-Bio, Le Perray en Yvelines, France). The relative content of cDNA samples was normalized with the endogenous β2M, HPRT1, RPL13 and GAPDH reference genes, and the relative mRNA level was calculated according to the formula 2−(ΔCt). Values are expressed as the fold change in relative mRNA level (2−(ΔCt)) following treatment of cells with BZ36 at 25 μM, as compared to control.
Proliferation assay
LNCaP, C4-2, PNT2 or MEFs cells were seeded in triplicate 24-wells dishes at a density of 2.5 × 105 cells/well. At 24 h, 48 h, 72 h and 96 h following addition of increasing concentrations of BZ36 inhibitor of SCD1 in DMSO or DMSO alone, cells were trypsinized, pelleted by centrifugation at 1200 rpm for 5 min, resuspended in 500 μl of culture medium and counted in an hemocytometer. Figures show representative results of at least two independently performed experiments.
MTT assay
LNCaP, C4-2 or PNT2 cells were seeded in triplicate 24-wells dishes at a density of 2.5×105 cells/well and cells viability was tested after treatment with increasing concentrations of the BZ36 inhibitor of SCD1 for 48 h. For rescue experiment, increasing concentrations of PIP3 from 1 to 20 μM were added. After 48 h, medium was removed and 250 μl of a 5mg/ml Thiazolyl Blue Tetrazolium Bromide (MTT) (Sigma) solution in PBS was added to each well. After 4 h incubation at 37°C, the MTT solution was removed, 200 μl of DMSO (Sigma) was added and cells were incubated for 5 min. Two hundred microliters of each samples was distributed in 96-well plates and the reduction of yellow MTT to purple formazan product was measured by optical density reading at 540 nm. Figures show representative results of at least two independently performed experiments.
Flow cytometry analysis of cell cycle and apoptosis
For cell cycle analysis, LNCaP, C4-2 or PNT2 cells were plated at a density of 2.5×106 cells/well in 6-wells dishes and treated with increasing concentrations of the BZ36 inhibitor of SCD1 for 24 h or 48 h. Cells were then rinsed in PBS, pelleted at 400g for 5 min and maintained on ice for 20 min before resuspension in a 25 μg/ml propidium iodide (Sigma) solution. Cells were kept overnight at 4°C and the percentages of cells in G1, S and G2-M phases of the cell cycle were measured with a Coulter Epics XL™ flow cytometer (Becton Dickinson) using 488-nm laser excitation. For apoptosis experiment, MEFs were treated with BZ36 at 25 μM for 24 h, then rinsed in PBS, pelleted at 400g for 5 min and stained with annexinV-FITC (Roche Diagnostics, Meylan, france) for 15 min at 4°C. The percentage of annexin positive cells was immediately analyzed using 488-nm laser excitation. Figures show representative results of at least two independently performed experiments.
Protein extracts and immunoblot analysis
Protein extracts and sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE), electrotransfer and immunoblotting were performed as previously described (18). The primary antibodies rabbit anti-AMPK, rabbit anti-phospho AMPK (Thr172), rabbit anti-AKT and rabbit anti-phospho AKT were purchased from Cell signaling Technology (Ozyme, Saint Quentin Yvelines, France). The primary antibody mouse anti-tubulin α was purchased from Lab Vision (Thermo Fisher Scientific, Microm France, Francheville, France). The primary antibodies rabbit anti-p44/p42 MAPK, rabbit anti-phospho p44/p42 MAPK (Thr202/Tyr204), mouse anti-GSK3α/β, and rabbit anti-phospho GSK3α/β were kindly provided by Dr Gilles Freiss (IRCM-Inserm U896-Université Montpellier 1, France). For phosphoproteome experiment, LNCaP and C4-2 cells treated with BZ36 at 25μM were analyzed for the relative phosphorylation of 46 kinase phosphorylation sites using human phospho-kinase array kit (Proteome Profiler TM) according to the manufacturers’ recommendations (R&D systems Europe, Lille, France). Level of phosphorylation was quantified using ImageJ software.
Fatty acid analysis
Total lipids from human prostate tissues were extracted three times with 5 mL of Folch mixture (chloroform/methanol, 2/1;V/V) and 500 μL of water. Aliquots of the lipid extracts were dried under gaseous nitrogen and were dissolved in 100 μL of the Folch mixture and were separated by thin layer chromatography in different lipid subclasses (CE, TAG and PL) using a hexane/ether/acetic acid (70/30/1; V/V) solvent system. To aid visualization, standards of CE, TAG and PL were co-spotted with samples. Spots were identified under UV light after spraying with 2′, 7′-dichlorofluorescein solution in ethanol and comparing with authentic standards. They were scrapped off the plates and were converted to fatty methyl esters by transesterification with 3 mL of methanol/H2SO4 (19/1;V/V) at 90°C for 30 min. The different solutions were neutralized with a 1 mL of aqueous solution of 10 % of K2CO3 and fatty methyl esters were extracted with 5 mL of hexane. The fatty methyl esters were dried under gaseous nitrogen and were subjected to gas chromatography (GC) and identified by comparison with standards (Sigma). Total lipids from liver tissues and C4-2 tumor xenografts from vehicle- or BZ36- treated animals were extracted three times with folch mixture, converted to fatty methyl esters as previously described, and subjected to gas chromatography (GC) identification. GC was conducted with a Thermo GC fitted with a flame ionization detector. A supelcowax-10 fused silica capillary column (60m × 0.32 mm i.d, 0.25 μM film thickness) was used and oven temperature was programmed from 50°C to 200°C, increased 20°C per minute, held for 50 min, increased 10°C per min to 220°C, and held for 30 min.
Determination of SCD activity
SCD activity was measured as previously described with some modifications (19). Briefly, subconfluent LNCaP, C4-2, PNT2 of Ras SV40 MEFs cells grown in 6-wells plate were incubated with BZ36 at 25 μM for 2 h in serum and fatty acid-free DMEM media supplemented 0.2% BSA. In this environment, the cells are solely dependent on endogenous fatty acid synthesis for production of storage, structural and signaling lipids. Trace amount of [14C] palmitic acid were then added to the culture (0.5 μCi/well), and cells were incubated for 6 more hours. At the end of the incubation, total cell lipids were extracted and saponified, then released fatty acids were esterified with boron trifluoride in methanol for 90 min at 100°C. The derived methyl esters were separated by argentation TLC (Thermo Fisher Scientific) following the procedure of Wilson and Sargen, using a solvent phase consisting of hexane:ethyl ether (90:10; V/V) (20). Pure stearic and oleic methyl ester acids were run in parallel to the samples. Air-dried plates were scanned on a PhosphorImager and fatty acid spots on TLC were analyzed with PhosphorImager software. SCD activity was expressed as the ratio of palmitoleic on palmitic methyl ester acids and normalized to cellular DNA content.
Measurement of de novo fatty acid synthesis
The inhibitor BZ36 was added overnight at a final concentration of 25 μM to subconfluent cultures of cells grown in 6-wells plate in serum and fatty acid- free DMEM media supplemented with 0.2 % BSA. Cultures were then labeled in triplicate with 1.0 μCi of [14C] -palmitate or -stearate for 6 h, and total lipids were folch extracted with chloroform/methanol. Labeled lipids were subjected to TLC in hexane/diethyl ether/acetic acid 90:10:1 (V/V), to separate cholesterol ester, triglycerides and phospholipids. Standards were run for each of the lipid classes. After chromatography, labeled lipid classes were quantified by scintillation counting and radioactivity was normalized to DNA content.
PI(3,4,5)P3 measurement
The production of PI(3,4,5)P3 in LNCaP and C4-2 prostate cancer cells was measured 24 h following exposure to control medium or to medium supplemented with BZ36 at 25 μM. The levels of produced PI(3,4,5)P3 were quantified after cellular lipids extraction using a PI(3,4,5)P3 mass ELISA kit according to the manufacturer’s instruction.
Immunofluorescence (IF) and Immunohistochemistry (IHC) of hSCD1
Immunohistochemical analysis of SCD1 expression was performed using high-density Tissue Microarray (TMA) slide (Accumax array) with 39 prostate adenocarcinoma spots from different patients (Gleason scores from 5 to 9) with corresponding normal tissues. Immunohistochemical analysis of pcna expression was performed on 5 μM paraffin-embedded sections of LNCaP, C4-2 of Ras SV40 MEFs tumor xenografts. Briefly, after antigen retrieval, deparafinized sections were blocked of Fc receptors with PBS containing 5% goat serum and then incubated with corresponding anti-SCD1 mouse antibody, 1:50 (Abcam, Paris, France) or anti-pcna mouse antibody, 1:50 (Santa Cruz) in PBS-Tween 0.1%, overnight at 4°C. SCD1 staining was revealed with a peroxidase-conjugated anti-mouse secondary antibody, 1:100 (Jackson Immunoresearch) and the DAB chromogen (DAKO, Glostrup, Denmark) as substrate. Sections were counterstained with Mayer’s hematoxylin. Pcna staining was revealed by immunofluorescence using a FITC-conjugated anti-mouse secondary antibody, 1:150 (Jackson Immunoresearch). Sections were mounted in mowiol and analyzed rapidly.
Immunocytochemistry
Immunocytochemical analysis of SCD1 expression was performed on PNT2, LNCaP, and C4-2 cells grown on coverslip. Briefly, after fixation in 4% PFA and permeabilization with 0.5% Triton X-100, cells were incubated with blocking buffer (PBS-1% BSA). SCD1 staining was detected with an anti-SCD1 mouse primary antibody, 1:50 (Abcam) for 1 hour at 37°C and revealed with a FITC-conjugated anti-mouse secondary antibody (Jackson Immunoresearch), 1:150 for 30 min at 37°C. Slides were mounted in mowiol and analyzed rapidly.
BrDU staining
LNCaP and C4-2 cells grown on cover slips were incubated for 4 hours with BrdU (100 μM final) at 24 hours and 48 hours following SCD1 knock-down. Cells were then fixed and permeabilized with cold methanol for 10 min at −20°C. After 3 washes with PBS, DNA was denaturized with 4N HCL for 10 min at RT, and cells were incubated with blocking buffer (PBS- 1% BSA). BrDU was then detected with anti-BrDU monoclonal antibody 1:50 (Dako, Carpinteria, CA) for 1 hour at 37°C. After 3 washes with PBS, cells were incubated with an FITC-conjugated anti-mouse secondary antibody 1:150 (Jackson Immunoresearch) for 30 min at 37°C, and slides were mounted in mowiol.
Statistical analysis
Statistical analysis were performed with unpaired Student’s t-test. Differences were considered statistically significant at p < 0.05. (* p < 0.05; ** p < 0.01 and *** p < 0.001). The log-rank test was used to assess the statistical significance of differences in animal survival along the treatment groups.
Results
MUFA content and SCD1 expression are increased during prostate cancer progression
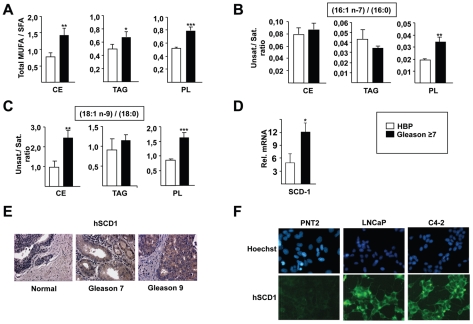
As a first approach to target lipid synthesis pathways in cancer cells we measured FA composition during prostate cancer progression. The ratio of total monounsaturated to saturated FA was significantly increased in CE, TAG and PL lipid fractions in human prostate cancer tissue samples with Gleason score ≥ 7 (Figure 1A), suggesting the participation of desaturase enzymes. Interestingly, among the analyzed MUFAs, those produced by a Δ9-desaturase activity, in majority palmitoleate (16:1n-7) and oleate (18:1n-9), were the most abundant in all lipid subclasses (data not shown). Furthermore, the specific desaturation indexes 16:1n-7/16:0 (Figure 1B) and 18:1n-9/18:0 (Figure 1C) were increased in human prostate samples, supporting the participation of the Δ9-desaturase enzyme. Consistent with this observation, stearoyl-CoA (Δ9-desaturase) 1 (SCD1) was increased in cancerous, compared to normal human prostates both at mRNA (Figure 1D) and protein (Figure 1E) levels, as analyzed by QPCR and immunohistochemistry (IHC) studies respectively. SCD1 expression was also increased, as measured by immunofluorescence in the human prostate cancer cell lines LNCaP and C4-2, compared to the non-tumoral PNT2 benign prostate cell line (Figure 1F).
Figure 1. Monounsaturated fatty acid content and SCD1 expression are increased with prostate cancer progression.
The global desaturation index, which corresponds to the ratio of total monounsaturated to saturated fatty acids (A), and the specific desaturation indexes 16:1n-7/16:0 (B) and 18:1n-9/18:0 (C) in cholesterol esters (CE), triacylglycerides (TAG) and phospholipids (PL) are increased in prostate cancer tissue with Gleason score ≥7. Values are expressed as mean +/− sd. Human SCD1 is overexpressed in prostate cancer tissue with Gleason score ≥7 at mRNA (D) and protein (E) levels. (F): The level of SCD1 expression was compared by immunofluorescence in PNT2 benign prostate cell line, and in LNCaP and C4-2 tumoral prostate cell lines. Statistically significant results are indicated as follows: * p<0.05; ** p<0,01; *** p<0.001 here and in subsequent figures.
Pharmacological and genetic inhibition of SCD1 activity induces growth arrest of CaP cell lines in vitro
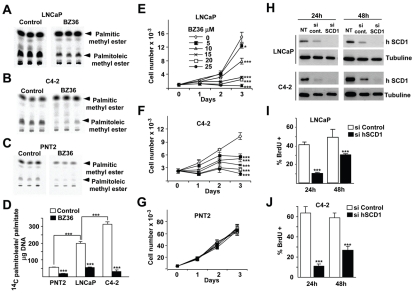
Because MUFA synthesis and SCD1 expression are both increased in prostate cancer, we next evaluated the effects of inhibition of SCD1 activity, measured by the conversion of exogenous [14C] saturated palmitic acid (16:0) substrate to monounsaturated palmitoleic acid (16:1n-7) on lipid synthesis and proliferation in prostate cancer cell lines. In a pharmacological approach, one of the first small molecule SCD1 inhibitor, the 6-[4-(2-Bromo-5-methoxy-benzoyl)-piperazin-1-yl]-N-phenylpropyl-nicotinamide compound (17), was synthesized as BZ36, and used in this study (Supplemental Figure 1). BZ36 compound has been characterized to specifically inhibit the biological activity of stearoyl-coA Δ-9 desaturase, but not Δ-5 desaturase, Δ-6 desaturase or FAS. In all three cell lines tested a marked reduction of the labelled monounsaturated palmitoleic acid was observed in BZ36-treated, compared to control cells (Figure 2A–D). Interestingly, basal SCD1 activity was progressively increased from non-tumoral PNT2 to androgen independent C4-2 cell lines (Figure 2D), further suggesting the implication of SCD1 in prostate cancer progression. Inhibition of SCD1 activity with BZ36 correlated with a dose dependent decrease in cell proliferation of LNCaP, and C4-2 cancer cells, reaching 100% inhibition at the maximal dose used (Figure 2E–F). Flow cytometry analysis further demonstrated the inhibitory effects of BZ36 in CaP cells, showing accumulation of cells in the G0/G1 phase of the cell cycle, concomitant with a decrease in the S phase (Supplemental Figure 2). Strikingly, no effect in proliferation was observed in the non-cancerous PNT2 cell line, even at a maximal dose (Figure 2G). Similar to what observed using SCD1 small molecule inhibitors, genetic SCD1 inhibition using siRNA technology blocked SCD1 protein expression (Figure 2H, and Supplemental Figure 3), and resulted in a marked decrease in proliferation in both LNCaP, and C4-2 cell types (Figure 2I–J). These results suggested that, first, SCD1 activity and lipid synthesis is required in prostate cancer cells in order to proliferate. Second, that non-cancer cells do not require de novo lipid synthesis, and therefore normal cells are not sensitive to inhibition of this pathway. And third, that the inhibitory effects of SCD1 inhibitors are mediated by SCD1 since the same effects are observed when SCD1 is depleted from the cells.
Figure 2. Inhibition of SCD1 activity induces growth arrest of CaP cells in vitro.
(A–D) Autoradiography of TLC showing quantification of SCD1 Δ9-desaturase activity on LNCaP (A), C4-2 (B) and PNT2 (C) cells by measuring the conversion of exogenous [14C]-saturated palmitic acid substrate into monounsaturated palmitoleic acid in control or 25 μM BZ36 treated cells. SCD1 desaturase activity was expressed as the ratio of palmitoleic acid to palmitic acid after normalization to cellular DNA content (D). (E–G): Proliferation of tumoral LNCaP (E) and C4-2 (F) cells, and of benign PNT2 cells (G) were measured with a hematocytometer at 24 h, 48 h and 72 h following culture with increasing doses of BZ36 (H) SCD1 immunoblot in LNCaP and C4-2 cells cell lines 24 h and 48 h after transfection with control or siRNA against hSCD1. (I–J) Proliferation of LNCaP (I) and C4-2 (J) cells was measured by counting the % of BrDU incorporation at 24 h and 48 h after siRNA transfection. Values are expressed as mean +/− sd and are representative of at least two independent experiments performed in triplicates.
To further document the relation between the level of SCD1 expression and the proliferative potential of prostate cancer cells, we analyzed the effect of over-expressing SCD1 in cells. We demonstrate that SCD1 over-expression in LNCaP and C4-2 cells was associated to a 13,7% and 20,5% increase in proliferation, respectively, as assessed by measuring the percentage of BrdU-incorporating proliferative cells at 72h following transient transfection with SCD1 cDNA as compared to mock-transfection (Supplemental Figure S.4). This rather modest increase in proliferation indicates that endogenous SCD1 expression levels are likely sufficient to drive changes in lipid synthesis required for proliferation of cancer cells.
Inhibition of SCD1 activity decreases de novo fatty acid synthesis in CaP cell lines
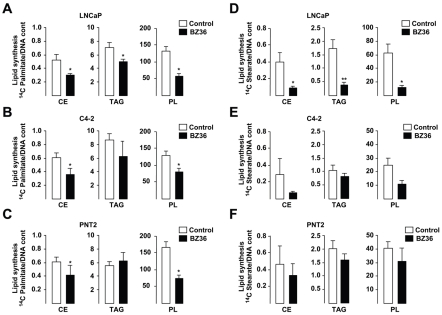
The effects of SCD1 in cell proliferation were likely mediated by the participation of this enzyme in the de novo fatty acid synthesis pathways. This was consistent with the observation that [14C]-palmitate incorporation into CE, TAG and PL, which is a measure of de novo lipid synthesis was respectively inhibited by 39%, 27% and 58% in LNCaP (Figure 3A), and by 33%, 24% and 41%, respectively in C4-2 cells (Figure 3B) treated with BZ36. Similarly, [14C]-stearate incorporation into CE, TAG and PL was also inhibited respectively by 77%, 78%, and 81% in LNCaP (Figure 3D), and by 70%, 19% and 49%, in C4-2 cells (Figure 3E) treated with BZ36. Similar results were observed in PNT2 cells (Figure 3C, and 3F).
Figure 3. Inhibition of SCD1 activity abrogates de novo fatty acid synthesis in CaP cells in vitro.
(A–C) [14C]-palmitate incorporation into CE, TAG and PL was measured in LNCaP (A), C4-2 (B), and PNT2 (C) cells after exposure to control media or to 25 μM of BZ36. (D–F): [14C]-stearate incorporation into CE, TAG and PL was measured in LNCaP (D), C4-2 (E) and PNT2 (F) cells after exposure to control medium or to 25 μM of BZ36 inhibitor. Values are expressed as mean +/− sd and are representative of at least two independent experiments performed in triplicates.
SCD1 inhibition interferes with major signaling pathways in prostate cancer cells
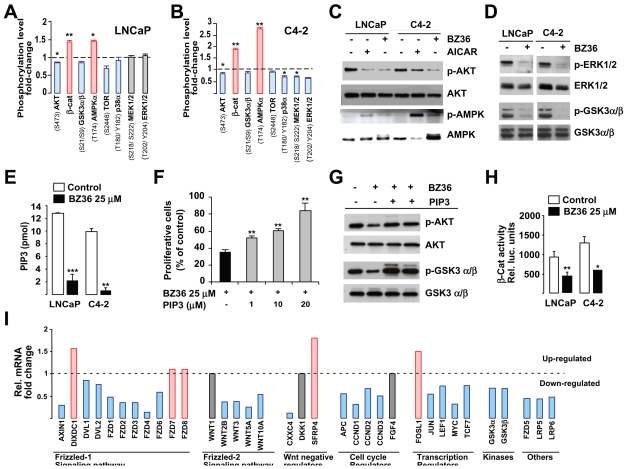
Lipids are important signaling molecules that actively participate in triggering specific phosphorylation pathways. Since inhibition of SCD1 activity was associated with a significant reduction in de novo lipids synthesis in prostate cancer cells (Figure 3), we expected also a decrease in these pathways. The relative phosphorylation status of several kinases in both LNCaP and C4-2 cells in response to treatment with BZ36 showed similar important changes as compared to control (Figure 4A–B and Supplemental Figure 5). Relevant for our study was the decrease in AKT phosphorylation (S473/T308) levels in BZ36-treated LNCaP (Figure 4A) and C4-2 (Figure 4B), compared to non-treated cells. Significant decreases were also observed in the phosphorylation levels of ERK1/2 (T202/Y204, T185/Y187) and MEK1/2 (S218/S222, S222/S226) in BZ36-treated C4-2 cells as compared to control cells. Interestingly, phosphorylation of AMPKα (T174) was increased following BZ36 treatment in both LNCaP and C4-2 cancer cells (Figure 4A–B). Western blot analysis further proved phosphorylation changes in these proteins (Figure 4C–D). Inhibition of AKT phosphorylation was not the result of decreased expression of AKT because total AKT protein levels were similar in treated and not treated cells (Figure 4C). In contrast to AKT, BZ36 treatment induced a significant increase in phosphorylated AMPKα in both LNCaP and C4-2 cells (Figure 4C). Interestingly, BZ36 activated AMPK more efficiently than the classical AMPK activator AICAR (Figure 4C). We next investigated the active phosphorylation status of downstream signaling proteins, such as ERK1/2. Immublot analysis revealed decreased phosphorylation of ERK1/2 in both LNCaP and C4-2 cells following exposure to BZ36, whereas total ERK expression was not changed (figure 4D). Moreover, we found that phosphorylation of GSK3α/β (S21/S9), which is inhibited by phosphorylation by AKT, was also abrogated by BZ36 treatment in LNCaP and C4-2 cells (figure 4D). These results were consistent with the abrogation of, at least AKT signaling in BZ36 treated cells.
Figure 4. Inhibition of SCD1 activity decreases AKT/PIP3 and GSK3α/β/β-catenin signaling pathways in CaP cells in vitro.
(A–B) Proteome analysis of the change in the phosphorylation status of indicated kinases in LNCaP (A) and C4-2 (B) cells 24 h after culture with 25 μM of BZ36 inhibitor. Level of phosphorylation was quantified using ImageJ software. Values are represented as relative fold-change of phosphorylation level in cells treated with 25 μM of BZ36 inhibitor compared to cells cultured in control medium, and are expressed as mean +/− sd. (C) Immunoblot analysis of p-AKT, total AKT, p-AMPK, and total AMPK in LNCaP and C4-2 cells at 24 h following exposure to control media, to 25 μM of BZ36 inhibitor, or to 1 mM AICAR. (D): Immunoblot analysis of p-ERK1/2, total ERK1/2, p-GSK3α/β, and total GSK3α/β in LNCaP and C4-2 cells at 24 h following exposure to control media or to 25 μM of BZ36 inhibitor. (E) The levels of PI(3,4,5)P3 produced were measured by ELISA on lipid extracts from LNCaP and C4-2 cells 24 hours following treatment with BZ36 at 25μM or with control medium. Data are expressed in pmol as mean +/− sd. (F): Rescue of BZ36- treated C4-2 cells proliferation by increasing PI(3,4,5)P3 amounts was measured by MTT assay. Forty-eight hours after treatment, the enzymatic reduction of MTT to formazan was quantified by optical density reading at 540 nm. Data are expressed as mean +/− sd. (G): Rescue of BZ36- treated C4-2 cells signaling by increasing PI(3,4,5)P3 amounts was evaluated by immunoblot analysis of p-AKT, total AKT, p-GSK3α/β, and total GSK3α/β 24 h following exposure to 25 μM of BZ36 inhibitor. (H): Measurement of β-catenin luciferase reporter activity in LNCaP and C4-2 cells 24 h after exposure to control media or to 25 μM of BZ36 inhibitor. Data are expressed as mean +/− sd. (I): Analysis of mRNA expression levels variation of genes related to the Wnt/β-catenin signaling pathway in C4-2 cells 24 h following exposure to 25 μM of BZ36 inhibitor. Genes are grouped according to their belonging to cell-surface receptors (members of the Frizzled-1 signaling pathway), glycosylated extracellular signaling molecules (members of the Frizzled-2 signaling pathway), Wnt binding antagonists and regulators of cell cycle, proliferation and transcription relative to the Wnt signaling pathway. Genes that are upregulated are indicated in red and genes that are downregulated are indicated in blue. Data are representative of at least two independent experiments.
AKT is activated by PI(3,4,5)P3, which is the result of PIP2 phosphorylation by PI3K. Since SCD1 inhibition induced a dramatic decrease in de novo synthesis of phospholipids, which are PI(3,4,5)P3 precursors, we anticipated that BZ36 would have an impact in PIP3 concentration in these prostate cancer cells. ELISA test demonstrated that PI(3,4,5)P3 concentration was strongly decreased by 84 % and 92 % respectively in LNCaP and C4-2 BZ36-treated, compared to non-treated cells (Figure 4E). These results suggested that inhibition of AKT activity in these cells was mediated, at least partially by decreased synthesis of PI(3,4,5)P3 precursors after BZ36 treatment. To further document the implication of PI(3,4,5)P3 in mediating SCD1 effect, we asked whether addition of external PI(3,4,5)P3 could rescue cells from the effect of SCD1 inhibition on cell proliferation and signaling. As shown in figure 4F, the percentage of proliferative C4-2 cells 48h following treatment with 25 μM of SCD1 inhibitor BZ36 was only 35% that of untreated control cells. In contrast, addition of increasing doses of PI(3,4,5)P3 at 1, 5 or 10 μM in presence of 20μM BZ36 significantly rescued cell proliferation in a dose-dependent manner, at 52%, 61% and 84 % of control, respectively (Figure 4F). The increase in cell proliferation following PI(3,4,5)P3 treatment in the presence of BZ36 was correlated with an increase of the AKT activity, as measured by an increase of the phosphorylated AKT (S473/T308) and GSKα/β proteins (Figure 4G). Increase of phosphorylation was not the result of increased expression of AKT and GSKα/β because total AKT and GSKα/β protein levels were similar in treated and not treated cells.
It was also particularly interesting the effects on GSK3α/β, which is further downstream AKT pathway. Activation of GSK3α/β by SCD1 inhibitors, resulted in the disruption of β-catenin signaling, demonstrated by the decreased activity of a β-cat reporter in response to BZ36 in both LNCaP, and C4-2 cells (Figure 4H). This was fully consistent with changes in the expression of genes in the β-cat pathway, including, but not limited to decreased expression of several members of the frizzled family, or decreased cyclin D1, D2, D3, myc, or c-jun expression, or decreased expression of several Wnt family members in C4-2 cells treated with BZ36 (Figure 4I). Taken together these results proved that SCD1 inhibition, directly or indirectly results in a strong disruption of major signaling pathways implicated in cell proliferation, migration, and survival.
Inhibition of SCD1 activity inhibit LNCaP and C4-2 tumor growth in vivo
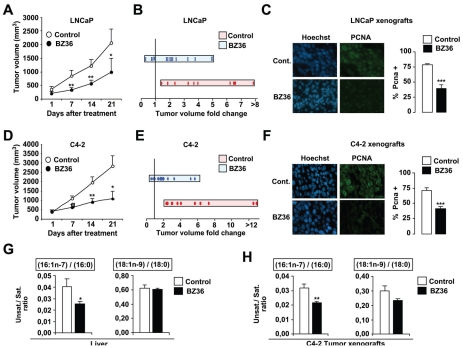
We next evaluated whether reduction of SCD1 activity by BZ36 could inhibit the growth of pre-established PC tumors in mice, a clinically relevant situation. LNCaP and C4-2 cells were injected subcutaneously in male athymic nude mice. Treatment of each individual mouse started when tumor nodules were measurable. In two independent experiments, we observed that treatment of mice with 80 mg/kg of BZ36 5 days of week during 21 days significantly inhibited both androgen-dependent LNCaP (Figures 5A–B) and androgen-insensitive C4-2 (Figures 5D–E) tumor volume and tumor growth rate, as compared with control mice that received vehicle only. Moreover, we observed that BZ36 treatment at 80 mg/kg induced LNCaP and C4-2 tumor regression in 27% of LNCaP (Figure 5B) and 19% of C4-2 (Figure 5E) xenografted mice, whereas no tumor regression was observed in control mice. On the opposite, we observed that LNCaP or C4-2 tumor volume was increased more than 4-fold in 42% of vehicle-treated mice, whereas this was the case in only 18% (LNCaP) and 12% (C4-2) of BZ36-treated mice (Figure 5B, and E). Immunostaining of tumors from LNCAP (Figure 5C) and C4-2 (Figure 5F) xenografts for the proliferation-associated marker PCNA confirmed the impact of BZ-36 on tumor cell proliferation with an average 50% decrease of PCNA-positive cells in tumors from animals treated with BZ36 at 80mg/kg compared to tumors from control animals. We next investigated whether the reduction of tumor growth following SCD1 inhibition was associated to changes in fatty acids composition in vivo. Analyze of total lipids extracted from both liver and tumor tissues revealed that the specific ratios of 16:1n-7/16:0 and 18:1n-9/18:0 FA were significantly reduced in both tissues in mice receiving BZ36 treatment, as compared to control mice (Figure 5G). This indicates that the observed effects of BZ36 treatment on tumor growth are specific to inhibition of SCD1 Δ9-desaturase activity.
Figure 5. Inhibition of SCD1 activity decreases tumor growth of CaP xenografts in vivo.
Tumor volume progression of subcutaneously implanted LNCaP (A–B) or C4-2 (D–E) cells in nude athymic mice was measured weekly following daily i.p. injection with BZ36 at 80mg/kg or with vehicle. (A;D): Values are expressed as the mean tumor volume (mm3) +/− sem from day 1 to day 21. (B;E): Values are expressed as the fold change to initial tumor volume (day 14/day 1). Quantification of PCNA immunostaining of proliferative LNCaP (C) and C4-2 (F) cells subcutaneously implanted 14 days following daily i.p. injection with BZ36 at 80mg/kg or with vehicle. Six fields per section were analyzed for PCNA immunostaining indicative of cell proliferation. Sections of tumors of all mice were analyzed. At least 300 cells were counted per tumor. Data are representative of at least three independent experiments. (G–H): The specific desaturation indexes 16:1n-7/16:0 and 18:1n-9/18:0 were analyzed in total lipids extracted from liver (G) and C4-2 tumor (H) tissues. Values are expressed as mean +/− sem.
We next examined whether BZ36 treatment improved the survival of mice with pre-established androgen-independent PC tumors derived from C4-2 cells. Nude mice were treated with vehicle or two doses of BZ36, 80 mg/kg or 160 mg/kg, and followed until death or until tumor volume did reach 2000 mm3. Among mice bearing subcutaneous C4-2 tumors, BZ36 treatment of animals result in significant and dose-dependant prolongation of animal survival in comparison to vehicle control (p=0.038, Supplemental Figure 6). Indeed, after the treatment was start, the median survival in the control group was of 14 days although it was of 21 days in animals treated with 80 mg/kg BZ36. At 14 days of treatment, 37.5% of animals did survived in the control group against 75% and 100% in the groups treated with 80mg/kg BZ36 and 160mg/kg BZ36 respectively. At 28 days of treatment, 75% of animals receiving 160mg/kg BZ36 did survived against only 12.5% in the control group.
Importantly, no significant weight loss or other toxicity was observed in mice following daily i.p. injection of BZ36 compound (Supplemental Figure 7A–C). Histological examination confirmed the absence of toxicity in liver and skin, and no difference of tissue integrity between control and BZ36- treated animals (Supplemental Figure 7D). Moreover, biochemical analysis of mice sera confirmed the absence of toxicity in liver and kidney. Liver function, assessed by measurement of albumin and bilirubin levels, as well as the enzymes phosphatase alkaline (ALP), aminotransferase alanine (ALT), and aminotransferase aspartate (AST) activities, was similar between control and BZ36-treated animals. Creatinin and urea blood levels were also similar between control and BZ36-treated animals, revealing an intact kidney metabolism (Supplemental Figure 7E).
Inhibition of SCD-like activities results in p53-independent loss of viability and reduce tumorigenicity of transformed MEFs
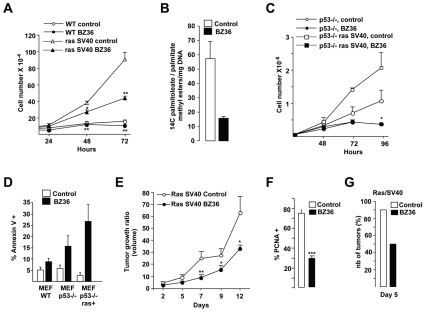
To further document the strong antiproliferative effects of SCD inhibitors on Ras-transformed cells we next tested their impact on Mouse embryonic fibroblasts (MEFs) transformed by genetically defined elements or genetic background that cooperate with Ras in transformation. Proliferation index of MEFs fully transformed by activated Ras (HaRasV12) and SV40 Large T was strongly decreased by BZ36 treatment (Figure 6A) whereas that of the slowly growing normal MEFs (WT) was only moderately reduced. Similar to prostate cancer cells, inhibition of SCDs activity in these transformed MEFs resulted in decreased lipogenesis as measured by the decrease of [14C]-palmitoleic to palmitic methyl ester acids ratio (Figure 6B).
Figure 6. Inhibition of SCD1 activity restrains proliferation, increases apoptosis in Ras SV40 transformed MEFs in vitro, and decreases tumor growth of Ras SV40 MEFs xenografts in vivo.
(A) Proliferation of WT-, SV40- immortalized, or Ras-SV40 transformed MEFs was measured with a hematocytometer at 24h, 48h and 72 h following culture with control media or with 25 μM of BZ36 inhibitor. (B): SCD1 Δ9-desaturase activity was analyzed by measuring the conversion of exogenous [14C]-saturated palmitic acid substrate into monounsaturated palmitoleic acid following exposure to control media or to 25 μM of BZ36 inhibitor. (C): Proliferation of p53 KO- or Ras-transformed p53 KO- MEFs was measured with a hematocytometer at 24h, 48h and 72 h following culture with control media or with 25 μM of BZ36 inhibitor. (D): Effect of the inhibition of SCD1 activity on apoptosis induction was analyzed by FACS by measuring the percentage of annexin positive Ras SV40 MEFs at 24h after exposure to control media or to 25 μM of BZ36 inhibitor. (E): Tumor volume progression of subcutaneously implanted Ras SV40 MEFs in nude athymic mice was measured weekly following daily i.p. injection with BZ36 at 80mg/kg or with vehicle. (F) PCNA immunostaining of proliferative Ras SV40 MEFs subcutaneously implanted 14 days following daily i.p. injection with BZ36 at 80mg/kg or with vehicle. Six fields per section were analyzed for PCNA immunostaining indicative of cell proliferation. Sections of tumors of all mice were analyzed. At least 300 cells were counted per tumor. (G) The delay in tumor apparition following subcutaneously injection of 5x 105 Ras SV40-tranformed MEFs was compared in mice that received daily injection of vehicle or of BZ36 at 80mg/kg during one week prior to tumor cell injection. Values are expressed as mean +/− sd and are representative of at least two independent experiments performed in triplicates.
Importantly, the potent antiproliferative effect of BZ36 on cells containing Large T confirmed that SCD inhibition has impact on cells with altered pRB- and p53-pathways, a hallmark of most cancer cells. Consistently, BZ36 also efficiently blocked the proliferation of p53−/− MEFs fully transformed by HaRasV12 (Figure 6C). Interestingly, these antiproliferative effects of BZ36 were associated with a strong induction of cell death (annexin V-positive cells) in all transformed cells, including p53−/− MEFS transformed by activated Ras, suggesting that the end result of SCD inhibition in cancer cells is a p53-independent cell death (Figure 6D).
To further investigate the effects of BZ36 on transformed Mefs, we next tested its impact on the capacity of these cells to form tumors when injected in nude mice. Two days after their injection with HaRasV12-SV40 Large T–transformed Mefs, mice were treated with BZ36 or with vehicle only. This treatment significantly and reproducibly reduced the size of tumors induced in these animals all along the 12 days of this experiment (Figure 6E). Accordingly, PCNA immunostaining on these tumors demonstrated a significant 61% decrease in the number of proliferative cells (PCNA-positive) in BZ36-treated tumors compared to vehicle-treated tumors (Figure 6F). Strikingly, pre-treatment of mice with BZ36, one week prior injection with transformed cells, also reduced tumor formation in these animals. Indeed, five days after cells were grafted, almost all of vehicle treated mice developed tumors, whereas tumors were observed in only 50% of BZ36 treated mice (Figure 6G). Collectively, these data confirmed our observation with LNCaP and C4-2 human cancer cell lines, and demonstrate that inhibition of SCD activity has a potent inhibitory effect on the cell viability and tumorigenicity of p53-deficient and Ras-transformed cells.
Discussion
Many efforts have been directed during the last years towards more efficient ways to ablate pathways implicated in signaling from growth receptors in cancer cells. These new kinase-directed therapies have been of some success in several cancers, whereas still long lists of cancers, including prostate cancer are unfortunately refractory to these treatments. Although dramatic metabolic differences have been described from long time ago between normal and cancer cells, little efforts have been invested in targeting metabolic pathways for the treatment of cancer. We have previously demonstrated a close relationship between metabolic responses and proliferative stimuli (21–23). Changes in this coordinated response might lead to abnormal metabolic changes during tumor development and cancer progression. In the present study, we demonstrate that targeting SCD1, a key lipogenic enzyme required for the biosynthesis of MUFA, might be of great benefit for the treatment of prostate cancer.
One important finding in our study is the increase in MUFA/SFA ratio in prostate cancer tissue from patients with gleason ≥7, compared to HBP, and in particular the increase of the monounsaturated palmitoleate and oleate, which are the major products of SCD1. Consistent with this observation, we demonstrate for the first time the overexpression of SCD1 in human prostate cancer tissues as well as androgen-sensitive and androgen-resistant prostate cancer cell lines. We thus have analyzed the effects of SCD1 inhibition in prostate cancer cells and prostate tumor xenografts in mice, and we have dissected the molecular mechanisms underlying these effects. Inhibition of MUFA synthesis through inactivation of SCD1 enzymatic activity results in major changes in complex lipid composition of the cell, decreases cell proliferation, abrogates growth of prostate tumor xenografts in mice and significantly increases tumor-bearing mice survival. Importantly, we demonstrate that SCD1 inhibition represses proliferation of both androgen-sensitive LNCaP and androgen-resistant C4-2 prostate cancer cells in vitro and in vivo, suggesting that it may represent a potent target during the progression of prostate cancer toward an androgen-independent status. Interestingly, it has been previously noticed that while FAS expression in prostate cancer is initially sensitive to androgens, this sensitivity is lost in parallel with the emergence of the androgen independent-phenotype (24, 25) Furthermore, the changes in lipid synthesis following SCD1 repression are translated into the inhibition of the AKT pathway, which is certainly one of the major signaling pathways implicated in advanced PC (26–28) Indeed, oncogenic signaling converges in the AKT pathway (29) and this pathway functions to promote tumor growth and the emergence of hormone refractory disease (30, 31). SCD1 actively participates in the generation of phosphatidyl inositols, which are precursors of PI(3,4,5)P3, a known AKT activator. Consistent with this role of SCD1 we found decreased concentration of PI(3,4,5)P3 in prostate cancer cells treated with BZ36, concomitant with a decrease in AKT phosphorylation. Furthermore, targeting AKT pathway results in the inhibition of prostate tumors in mice (27). On the other hand, AKT activation has been shown to be important for triggering the metabolic switch in cancer cells. First, regulating hexokinase (HK) and ACL activity, which results in increased lipid synthesis substrates. Hexokinase accelerates glycolysis, resulting in increased formation of citrate, which is catalyzed to acetyl CoA in the cytoplasm for lipid synthesis (32, 33). And second, AKT stimulates the transcriptional activity of SREBP, finally resulting in increased mRNA expression of key enzymes for lipid synthesis such as FAS, or SCD1 (34, 35). Our results strongly suggest that SCD1 inhibition creates a negative loop in which AKT inactivation results, not only in decreased proliferation and apoptosis of cancer cells, but also in decreased lipid synthesis. Inhibition of de novo fatty acid synthesis may also overcome inactivation of PTEN, which is frequently mutated in prostate cancer (36). Since PTEN inactivates AKT by degrading PI(3,4,5)P3, the PTEN inactivation found in cancers demonstrate that elevated PI(3,4,5)P3 levels contribute to oncogenesis (37). Since PTEN is not expressed in prostate cancer LNCaP and C4-2 cells, our study could be also representative of prostate tumors that carry mutations in PTEN (36). Interestingly, PNT2 cells express functional PTEN protein, and SCD1 inhibitor does almost not affect proliferation of these cells, which suggest that the effects of this treatment are mediated by decreased production of PI(3,4,5)P3. This is further supported by our observation that prostate cancer cell proliferation potential and AKT signaling were rescued from the repressive effect of SCD1 inhibitor following addition of external PI(3,4,5)P3.
AKT also inhibits GSK3β (38). We found that GSK3β is activated following SCD1 inhibition, likely through AKT pathway abrogation (Figure 4C). Consistent with this finding are the decrease in β-catenin activity (Figure 4F), and the general decrease in the β-catenin growth promoting trancriptional targets (Figure 4G). Complementary to this finding is the observed activation of the AMP-activated kinase (AMPK) pathway. It has been previously shown that oncogenic signaling impairs AMPK activation through LKB1 inhibition (39). Conversely, AMPK activation inhibits prostate cancer cell proliferation (40), although the precise mechanism whereby AMPK activation induces growth arrest in cancer cells is complex. Several pathways are likely implicated, including p53, p21, or p27 (41, 42). Our results further support that AMPK activation may interfere with cancer cell metabolism, at least in large part, through the AKT pathway. Concerning the energy status of the cell, the AMP/ATP ratio is expected to be low in cancer cells, a condition in which AMPK is inactive. As a result of lipid synthesis inhibition and AKT pathway abrogation, AMPK may become active. Activated AMPK inhibits on its turn the AKT target and activator mTOR, through both TSC1-2 activation (43) and direct AKT inhibition (40). In addition, AMPK also inhibits lipid synthesis pathways, which are essential for AKT activation (44). Summarizing, AMPK activation results in a concomitant inhibition of AKT pathway.
Finally we show that cell transformation by oncogenic signaling, such as Ras-mediated tumorigenesis requires intact lipid synthesis pathways, since inhibition of lipogenesis, such as observed when SCD1 inhibitor is used prevents Ras-mediated transformation, and significantly delays Ras-mediated tumorigenesis of primary fibroblastic cells (Figure 6). This finding strongly suggests that oncogenic signaling triggers a metabolic response that directs the cell to particular lipid synthesis pathways that will facilitate cancer cell growth and survival. Interestingly, in addition of modification of growth factor receptor systems in the cellular membrane, and generating signaling lipids, this modification in lipid synthesis modulates, on its turn the activation of Ras, and other oncogenic pathways through protein lipidation, such as N-myristoylation and S-palmitoylation (45). Strikingly, it was recently reported that some Wnt proteins are modified by palmitoleate, which is the direct product of SCD1 activity, and that this modification is required for secretion and/or signaling activities of secreted signaling proteins, such as the Wingless proteins Wnt-1 and Wnt-3a (46). This creates a positive feed-back loop that contributes to sustained cancer cell growth and proliferation. Interesting for our study, although Ras mutations are rare in prostate cancer, activation of the Ras pathway is a common feature of this cancer (47, 48). Moreover, it has been shown that in PC a cooperative Ras and Wnt oncogenic signaling exists (49). Indeed, our finding that SCD1 activity is required for Ras-mediated tumorigenesis in Mefs may be of great importance for understanding oncogenic signaling in PC and may support further investigations.
Treatment of prostate cancer is a major goal to be achieved, and we believe that SCD1 targeting should be considered as an alternative or complementary treatment of prostate cancer. Perhaps the most important finding in our study is the inhibition of prostate tumor growth, even tumor remission in a mice model of prostate cancer following pharmacological inhibition of SCD1 with the small molecule BZ36. Consistent with our work, increased expression of SCD1 was found in lung and liver cancers (19, 50).
Acknowledgments
Dr L. Le Cam, and members of the Fajas’ lab are acknowledged for support and discussions. We thank Denis Greuet for animal care. This work was supported by grants from Association pour la Recherche contre le Cancer, Fondation pour la Recherche Médicale (FRM), and Institut National du Cancer (INCA).
References
- 1.Nelson PS, Clegg N, Arnold H, et al. The program of androgen-responsive genes in neoplastic prostate epithelium. Proc Natl Acad Sci U S A. 2002;18:11890–5. doi: 10.1073/pnas.182376299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huggins C. Endocrine-induced regression of cancers. Science. 1967;3778:1050–4. doi: 10.1126/science.156.3778.1050. [DOI] [PubMed] [Google Scholar]
- 3.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 4.Warburg O. Metabolism of Tumors. Arnold Constable; London: 1930. [Google Scholar]
- 5.Warburg O. On respiratory impairment in cancer cells. Science. 1956;3215:269–70. [PubMed] [Google Scholar]
- 6.Warburg O. On the origin of cancer cells. Science. 1956;3191:309–14. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 7.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;5930:1029–33. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medes G, Thomas A, Weinhouse S. Metabolism of neoplastic tissue. IV. A study of lipid synthesis in neoplastic tissue slices in vitro. Cancer Res. 1953;1:27–9. [PubMed] [Google Scholar]
- 9.Mashima T, Seimiya H, Tsuruo T. De novo fatty-acid synthesis and related pathways as molecular targets for cancer therapy. Br J Cancer. 2009;9:1369–72. doi: 10.1038/sj.bjc.6605007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuhajda FP. Fatty-acid synthase and human cancer: new perspectives on its role in tumor biology. Nutrition. 2000;3:202–8. doi: 10.1016/s0899-9007(99)00266-x. [DOI] [PubMed] [Google Scholar]
- 11.Kuhajda FP, Jenner K, Wood FD, et al. Fatty acid synthesis: a potential selective target for antineoplastic therapy. Proc Natl Acad Sci U S A. 1994;14:6379–83. doi: 10.1073/pnas.91.14.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagy P, Vereb G, Sebestyen Z, et al. Lipid rafts and the local density of ErbB proteins influence the biological role of homo- and heteroassociations of ErbB2. J Cell Sci. 2002;(Pt 22):4251–62. doi: 10.1242/jcs.00118. [DOI] [PubMed] [Google Scholar]
- 13.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;10:763–77. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 14.Swinnen JV, Brusselmans K, Verhoeven G. Increased lipogenesis in cancer cells: new players, novel targets. Curr Opin Clin Nutr Metab Care. 2006;4:358–65. doi: 10.1097/01.mco.0000232894.28674.30. [DOI] [PubMed] [Google Scholar]
- 15.Miyazaki M, Flowers MT, Sampath H, et al. Hepatic stearoyl-CoA desaturase-1 deficiency protects mice from carbohydrate-induced adiposity and hepatic steatosis. Cell Metab. 2007;6:484–96. doi: 10.1016/j.cmet.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 16.Patra SK. Dissecting lipid raft facilitated cell signaling pathways in cancer. Biochim Biophys Acta. 2008;2:182–206. doi: 10.1016/j.bbcan.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Fu JM, Kodomuru V, Sun S, Winther MD, Fine RM, Harvey DF, Klebansky B, Gray-Keller MP, Gschwend HW, Li W. US 2005/0119251 A1 Nicotinamide derivatives and their use as therapeutic agents.
- 18.Sarruf DA, Iankova I, Abella A, Assou S, Miard S, Fajas L. Cyclin D3 promotes adipogenesis through activation of peroxisome proliferator-activated receptor gamma. Mol Cell Biol. 2005;22:9985–95. doi: 10.1128/MCB.25.22.9985-9995.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scaglia N, Igal RA. Inhibition of Stearoyl-CoA Desaturase 1 expression in human lung adenocarcinoma cells impairs tumorigenesis. Int J Oncol. 2008;4:839–50. [PubMed] [Google Scholar]
- 20.Wilson R, Sargent JR. Chain separation of monounsaturated fatty acid methyl esters by argentation thin-layer chromatography. J Chromatogr A. 2001;1-2:251–7. doi: 10.1016/s0021-9673(00)01006-2. [DOI] [PubMed] [Google Scholar]
- 21.Abella A, Dubus P, Malumbres M, et al. Cdk4 promotes adipogenesis through PPARgamma activation. Cell Metab. 2005;4:239–49. doi: 10.1016/j.cmet.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Fajas L, Egler V, Reiter R, et al. The retinoblastoma-histone deacetylase 3 complex inhibits the peroxisome proliferator-activated receptor gamma and adipocyte differentiation. Developmental Cell. 2002:903–910. doi: 10.1016/s1534-5807(02)00360-x. [DOI] [PubMed] [Google Scholar]
- 23.Fajas L, Egler V, Reiter R, Miard S, Lefebvre AM, Auwerx J. PPARgamma controls cell proliferation and apoptosis in an RB-dependent manner. Oncogene. 2003;27:4186–93. doi: 10.1038/sj.onc.1206530. [DOI] [PubMed] [Google Scholar]
- 24.Pizer ES, Pflug BR, Bova GS, Han WF, Udan MS, Nelson JB. Increased fatty acid synthase as a therapeutic target in androgen-independent prostate cancer progression. Prostate. 2001;2:102–10. doi: 10.1002/pros.1052. [DOI] [PubMed] [Google Scholar]
- 25.Rossi S, Graner E, Febbo P, et al. Fatty acid synthase expression defines distinct molecular signatures in prostate cancer. Mol Cancer Res. 2003;10:707–15. [PubMed] [Google Scholar]
- 26.Davies MA, Koul D, Dhesi H, et al. Regulation of Akt/PKB activity, cellular growth, and apoptosis in prostate carcinoma cells by MMAC/PTEN. Cancer Res. 1999;11:2551–6. [PubMed] [Google Scholar]
- 27.Kinkade CW, Castillo-Martin M, Puzio-Kuter A, et al. Targeting AKT/mTOR and ERK MAPK signaling inhibits hormone-refractory prostate cancer in a preclinical mouse model. J Clin Invest. 2008;9:3051–64. doi: 10.1172/JCI34764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Souza P, Russel P, Kearsley J. Role of the Akt pathway in prostate cancer. Cuurent Cancer Drug Targets. 2009;2:163–175. doi: 10.2174/156800909787581006. [DOI] [PubMed] [Google Scholar]
- 29.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;7:1261–74. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kreisberg JI, Malik SN, Prihoda TJ, et al. Phosphorylation of Akt (Ser473) is an excellent predictor of poor clinical outcome in prostate cancer. Cancer Res. 2004;15:5232–6. doi: 10.1158/0008-5472.CAN-04-0272. [DOI] [PubMed] [Google Scholar]
- 31.Shen MM, Abate-Shen C. Pten inactivation and the emergence of androgen-independent prostate cancer. Cancer Res. 2007;14:6535–8. doi: 10.1158/0008-5472.CAN-07-1271. [DOI] [PubMed] [Google Scholar]
- 32.Elstrom RL, Bauer DE, Buzzai M, et al. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 2004;11:3892–9. doi: 10.1158/0008-5472.CAN-03-2904. [DOI] [PubMed] [Google Scholar]
- 33.Bauer DE, Hatzivassiliou G, Zhao F, Andreadis C, Thompson CB. ATP citrate lyase is an important component of cell growth and transformation. Oncogene. 2005;41:6314–22. doi: 10.1038/sj.onc.1208773. [DOI] [PubMed] [Google Scholar]
- 34.Van de Sande T, De Schrijver E, Heyns W, Verhoeven G, Swinnen JV. Role of the phosphatidylinositol 3′-kinase/PTEN/Akt kinase pathway in the overexpression of fatty acid synthase in LNCaP prostate cancer cells. Cancer Res. 2002;3:642–6. [PubMed] [Google Scholar]
- 35.Bandyopadhyay S, Pai SK, Watabe M, et al. FAS expression inversely correlates with PTEN level in prostate cancer and a PI 3-kinase inhibitor synergizes with FAS siRNA to induce apoptosis. Oncogene. 2005;34:5389–95. doi: 10.1038/sj.onc.1208555. [DOI] [PubMed] [Google Scholar]
- 36.Li J, Yen C, Liaw D, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;5308:1943–7. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 37.Wymann MP, Schneiter R. Lipid signalling in disease. Nat Rev Mol Cell Biol. 2008;2:162–76. doi: 10.1038/nrm2335. [DOI] [PubMed] [Google Scholar]
- 38.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;6559:785–9. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 39.Zheng B, Jeong JH, Asara JM, et al. Oncogenic B-RAF negatively regulates the tumor suppressor LKB1 to promote melanoma cell proliferation. Mol Cell. 2009;2:237–47. doi: 10.1016/j.molcel.2008.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rattan R, Giri S, Singh AK, Singh I. 5-Aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside inhibits cancer cell proliferation in vitro and in vivo via AMP-activated protein kinase. J Biol Chem. 2005;47:39582–93. doi: 10.1074/jbc.M507443200. [DOI] [PubMed] [Google Scholar]
- 41.Jones RG, Plas DR, Kubek S, et al. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell. 2005;3:283–93. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 42.Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;10:774–85. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 43.Inoki K, Ouyang H, Zhu T, et al. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;5:955–68. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 44.Long YC, Zierath JR. AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest. 2006;7:1776–83. doi: 10.1172/JCI29044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nadolski MJ, Linder ME. Protein lipidation. Febs J. 2007;20:5202–10. doi: 10.1111/j.1742-4658.2007.06056.x. [DOI] [PubMed] [Google Scholar]
- 46.Takada R, Satomi Y, Kurata T, et al. Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Dev Cell. 2006;6:791–801. doi: 10.1016/j.devcel.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 47.Voeller HJ, Wilding G, Gelmann EP. v-rasH expression confers hormone-independent in vitro growth to LNCaP prostate carcinoma cells. Mol Endocrinol. 1991;2:209–16. doi: 10.1210/mend-5-2-209. [DOI] [PubMed] [Google Scholar]
- 48.Gioeli D. Signal transduction in prostate cancer progression. Clin Sci (Lond) 2005;4:293–308. doi: 10.1042/CS20040329. [DOI] [PubMed] [Google Scholar]
- 49.Pearson HB, Phesse TJ, Clarke AR. K-ras and Wnt signaling synergize to accelerate prostate tumorigenesis in the mouse. Cancer Res. 2009;1:94–101. doi: 10.1158/0008-5472.CAN-08-2895. [DOI] [PubMed] [Google Scholar]
- 50.Yamashita T, Honda M, Takatori H, et al. Activation of lipogenic pathway correlates with cell proliferation and poor prognosis in hepatocellular carcinoma. J Hepatol. 2009;1:100–10. doi: 10.1016/j.jhep.2008.07.036. [DOI] [PubMed] [Google Scholar]