Abstract
Mycoplasmas comprise a conglomerate of pathogens and commensals occurring in humans and animals. The genus Mycoplasma alone contains more than 120 species at present, and new members are continuously being discovered. Therefore, it seems promising to use a single highly parallel detection assay rather than develop separate tests for each individual species. In this study, we have designed a DNA microarray carrying 70 oligonucleotide probes derived from the 23S rRNA gene and 86 probes from the tuf gene target regions. Following a PCR amplification and biotinylation step, hybridization on the array was shown to specifically identify 31 Mycoplasma spp., as well as 3 Acholeplasma spp. and 3 Ureaplasma spp. Members of the Mycoplasma mycoides cluster can be recognized at subgroup level. This procedure enables parallel detection of Mollicutes spp. occurring in humans, animals or cell culture, from mono- and multiple infections, in a single run. The main advantages of the microarray assay include ease of operation, rapidity, high information content, and affordability. The new test's analytical sensitivity is equivalent to that of real-time PCR and allows examination of field samples without the need for culture. When 60 field samples from ruminants and birds previously analyzed by denaturing-gradient gel electrophoresis (DGGE) were tested by the microarray assay both tests identified the same agent in 98.3% of the cases. Notably, microarray testing revealed an unexpectedly high proportion (35%) of multiple mycoplasma infections, i.e., substantially more than DGGE (15%). Two of the samples were found to contain four different Mycoplasma spp. This phenomenon deserves more attention, particularly its implications for epidemiology and treatment.
Introduction
The genus Mycoplasma, one of the major taxa in the class Mollicutes, currently comprises more than 120 species [1]. These bacteria, which are regarded as the smallest self-replicating organisms, have unique characteristics including reduced genome size, lack of a rigid cell wall and limited number of functional metabolic pathways. Therefore, mycoplasmas have been considered models of minimal cells [2]. However, despite their apparent simplicity, several Mycoplasma species are significant pathogens. In humans, for instance, atypical pneumonia is associated with Mycoplasma (M.) pneumoniae, and genital disorders with M. genitalium and Ureaplasma (U.) urealyticum. Four mycoplasmoses are included in the list of notifiable diseases of the World Organisation for Animal Health (OIE), i.e. contagious bovine pleuropneumonia (CBPP) with the causative agent M. mycoides subsp. mycoides (formerly Small Colony type), contagious caprine pleuropneumonia (M. capricolum subsp. capripneumoniae), contagious agalactia (M. agalactiae), and avian mycoplasmosis (M. gallisepticum, M. synoviae). Other economically important diseases include respiratory and mammary infections of cattle caused by M. bovis, ocular and respiratory infection in small ruminants caused by M. conjunctivae or M. ovipneumoniae, respectively, as well as enzootic pneumonia (M. hyopneumoniae), arthritis and polyserositis (M. hyorhinis, M. hyosynoviae) in swine.
Mycoplasma contamination of cell culture is a major concern to researchers and pharmaceutical companies [3], because the unwanted presence of M. arginini, M. hyorhinis, Acholeplasma [A.] laidlawii, M. orale, or M. fermentans can distort the results of in vitro tests [4].
Although studies addressing dissemination and transmission pathways of the above-mentioned pathogens have been conducted [5], [6], [7], [8], [9], [10], [11], [12], [13], data on the current epidemiological situation is absent or, at best, available for selected regions only. This is partly due to the general difficulties in mycoplasma diagnosis resulting from their slow growth and requirement of fastidious culture media, as well as limitations of available tests in terms of sensitivity and specificity.
Epidemiological and clinical studies in a given animal species usually considered only the main and best characterized mycoplasmal agent, while disregarding minor or aberrant mollicutes. For instance, most projects on mycoplasmosis in cattle focused on M. bovis or M. mycoides subsp. mycoides, thus ignoring the possible presence of related species, such as M. bovigenitalium, M. bovirhinis, M. bovoculi, M. californicum, M. canadense, M. dispar, M. leachii and others. In poultry, M. gallisepticum is the most prominent pathogen, but M. synoviae, M. iowae and M. imitans should be considered as well. Likewise, small ruminants can harbor a variety of mycoplasmas besides M. agalactiae, e.g. members of the Mycoplasma mycoides cluster, M. ovipneumoniae, and M. conjunctivae. Diagnostic evidence from recent years clearly suggests that host specificity of animal mycoplasmas is generally not stringent [14], but more comprehensive investigations are required.
Very little is known about the frequency of co-infection by two or more mycoplasmal agents [15], [16], both at single-animal and herd levels, thus preventing proper assessment of the synergetic and/or competitive effects involved. The diagnostic challenges resulting from the multitude of mycoplasma organisms potentially involved can be efficiently addressed only if adequate detection methods are available. A single PCR or ELISA test would not necessarily identify atypical or co-infecting agents present in a sample. While PCR combined with denaturing-gradient gel electrophoresis (DGGE) can detect mixed infections, it is laborious and complex to perform and interpret [17].
In contrast, DNA microarray testing opens up new possibilities for laboratory diagnosis. The possibility of using a large number of detection probes covering discriminatory gene segments and/or multiple genomic regions of many different microbial agents confers a high degree of parallelity to this technology. Therefore, DNA microarray assays can attain far higher diagnostic resolution than PCR. The broad use of array technology in rapid diagnosis of bacterial and viral pathogens, however, is only emerging.
In the present study, we developed a rapid DNA microarray assay capable of identifying at least 37 Mollicutes spp., among them important human and animal pathogens and cell culture contaminants.
Materials and Methods
Mycoplasma strains
The type or reference strains used are listed in Table 1. Field strains were from the collection of the National Reference Laboratory for CBPP (Head: MH). Culture was conducted according to standard methodology [18].
Table 1. Summary of hybridization test results of 44 Mollicutes organisms on two genomic target sites.
| Species/Taxon | Type strain | Specific detn. 23S rDNA | Specific detn. tuf gene | Field strains tested | Comment |
| A. axanthum | S743 | + | + | 2 | |
| A. laidlawii | PG8 | + | + | 5 | |
| A. modicum | PG49 | + | + | 2 | |
| M. adleri | G145 | + | n.d. | 0 | |
| M. agalactiae | PG2 | + | +* | 3 | * M. bovis |
| M. alkalescens | PG51 | + | + | 5 | |
| M. alvi | ILSLEY | + | + | 3 | |
| M. arginini | G230 | +* | +* | 3 | * M.gateae |
| M. bovigenitalium | PG11 | + | + | 3 | |
| M. bovirhinis | PG43 | + | + | 6 | |
| M. bovis | PG45 | + | +* | 24 | * M. agalactiae |
| M. bovoculi | M165/69 | + | + | 2 | |
| M. californicum | ST-6 | n.d. | + | 2 | |
| M. canadense | 275C | n.d. | + | 3 | |
| M. canis | PG14 | + | + | 4 | |
| M. capricolum subsp. capricolum | California Kid | Mmyc. cluster | Mmyc. cluster | 2 | |
| M. capricolum subsp. capripneumoniae | F38 | Mmyc. cluster | Mmyc. cluster | 2 | |
| M. conjunctivae | HRC581 | + | + | 4 | |
| M. dispar | 462/2 | + | + | 4# | #mixed culture |
| M. fermentans | PG18 | + | + | 2 | |
| M. gallinarum | PG16 | + | + | 3 | |
| M. gallisepticum | PG31 | +* | + | 6 | * M.imitans |
| M. gateae | CS | n.d. | +* | 0 | * M.arginini |
| M. genitalium | G37 | +* | + | 0 | * M.pneumoniae |
| M. hominis | PG21 | + | + | 6 | |
| M. hyopneumoniae | J | + | + | 1 | |
| M. hyorhinis | BTS-7 | n.d. | + | 4 | |
| M. imitans | 4229 | + | + | 0 | |
| M. iowae | 695 | + | + | 6 | |
| M. leachii | PG50 | Mmyc. cluster | Mmyc. cluster | 2 | |
| M. meleagridis | N17529 | + | + | 6 | |
| M. mycoides subsp. capri | PG3 | Mmyc. cluster | Mmyc. cluster | 2 | |
| M. mycoides subsp. mycoides (SC) | PG1 | Mmyc. cluster | Mmyc. cluster | 2 | |
| M. orale | CH19299 | + | + | 3 | |
| M. ovipneumoniae | Y98 | + | + | 7 | |
| M. pneumoniae | FH | +* | + | 1 | * M.genitalium |
| M. pulmonis | Ash/PG34 | + | + | 1 | |
| M. putrefaciens | KS-1 | + | + | 2 | |
| M. salivarium | PG20 | + | + | 0 | |
| M. synoviae | WVU1853 | + | + | 3 | |
| M. verecundum | 107 | + | + | 0 | |
| U. diversum | A417/C (NCTC10182) | n.d. | + | 2 | |
| U. parvum | 27 | + | +* | 2 | * U.urealyticum |
| U. urealyticum | 960 | + | +* | 2 | * U.parvum |
cross-reaction with related species, n.d. not done (no specific probes identified in that locus).
Field samples
The majority of the field samples tested originated from investigations by the AHVLA Regional Laboratories in England and Wales between 2009 and 2011, where disease investigations and, in some cases, post-mortems had been performed. Clinical samples as detailed in Table 2 and File S1 were submitted to the Mycoplasma Group (AHVLA, Weybridge, UK) in Eaton's media [19]. DNA was extracted directly from the sample using a Maxwell 16 automated system and Maxwell tissue DNA purification kit (Promega, Southampton, UK) and stored at −20°C until testing. Other samples included in Table 2, were sample references: 14F11, 20F11, 22F11, 23F11, 33F11, and 39F11, which were submitted as freeze-dried clinical samples from Iran for identification by the Mycoplasma Group as they are the OIE Contagious Agalactia Reference Laboratory (Head: RN). Sample SR00 came from a culture collection, and samples 82A10 and 83A10 were submitted as freeze-dried culture samples as part of a proficiency test.
Table 2. Summary of test results of DNA microarray and DGGE assays on 51 clinical tissue samples and 9 cultures from field samples.
| Sample ID | Sample type | DGGE | DNA microarray | Comment |
| 100SR10 | ovine, lung | M. arginini, M. ovipneumoniae | M. arginini 3, M. ovipneumoniae | concordant, dual infection |
| 34 B 10 | bovine, lung | M. bovis, M. alkalescens | M. bovis, M. alkalescens | concordant, dual infection |
| 108 B 10 | bovine, lung | M. bovis, M. alkalescens 1 | M. bovis, M. alkalescens 1 | concordant, dual infection |
| 13SR11 | ovine, nasal swab | M. ovipneumoniae, M. arginini | M. ovipneumoniae, M. arginini 3 | concordant, dual infection |
| 33F11 | culture, ovine, milk | M. agalactiae | M. agalactiae, M. putrefaciens | more species by AS |
| 19B10 | bovine, lung | M. alkalescens, M. bovis | M. alkalescens, M. bovis, M. dispar 2 | more species by AS |
| 490 B 09 | bovine, vaginal swab | M. bovigenitalium | M. bovigenitalium 3, M. alkalescens 1 | more species by AS |
| 485 B 09 | bovine, vaginal swab | M. bovigenitalium | M. bovigenitalium, M. alkalescens 1 | more species by AS |
| 53B10 | bovine, swab | M. bovirhinis | M. bovirhinis, M. bovis 1, M. arginini 2 , 3 | more species by AS |
| 365B10 | bovine, nasal swab | M. bovirhinis, M. dispar | M. bovirhinis, M. dispar, M. bovis 1, M.arginini 2 , 3 | more species by AS |
| 36B10 | bovine, lung | M. bovis | M. bovis, M. arginini 1 ,4 | more species by AS |
| 49B10 | bovine, lung | M. bovis | M. bovis, M. dispar 1 | more species by AS |
| 279B11 | bovine, lung | M. bovis | M. bovis, M. dispar 1 | more species by AS |
| 669 B 09 | bovine, eye swab | M. bovoculi | M bovoculi, M. canadense | more species by AS |
| 128SR09 | ovine, eye swab | M. conjunctivae | M. conjunctivae, M. ovipneumoniae 1, M. arginini 2 | more species by AS |
| 32 B 10 | bovine, lung | M. dispar | M. dispar, M. bovis 1, M. alkalescens 1, M. bovirhinis 2 | more species by AS |
| 39 B 10 | bovine, lung | M. dispar | M. dispar 1, M. bovis 1, M. alkalescens 1 | more species by AS |
| 265B10 | bovine, nasal swab | M. dispar | M. dispar, M. arginini 2 , 3 | more species by AS |
| 83A10 | culture from strain collection | M. meleagridis | M. meleagridis, M. dispar 2 | more species by AS |
| 120A10 | chicken, eyelid | M. synoviae | M. synoviae, M. iners | more species by AS |
| 95SR10 | ovine, swab | unidentified bands | M. conjunctivae | AS more specific |
| 79O10 | caprine, lung | M. arginini, M. ovipneumoniae 2 | M arginini 3 | more species by DGGE |
| 142 B 09 | bovine, lung | M. bovirhinis, M. alkalescens 1 | M. bovirhinis | more species by DGGE |
| 89 B 10 | bovine, lung | M. bovirhinis, M. alkalescens 1 | M. bovirhinis, M. dispar 1 | discordance in second agent |
| 36 samples | tissue (29) and culture (7) | M. agalactiae, M. alkalescens, M. arginini, M. bovirhinis, M. bovis, M. canadense, M. capricolum subsp. capricolum, M. conjunctivae, M. gallisepticum, M. mycoides subsp. capri, M. ovipneumoniae, M. putrefaciens, M. synoviae | concordant, monoinfections |
confirmed by species-specific PCR,
species-specific PCR negative,
not distinguishable from M. gateae.
DNA extraction
Cultured mycoplasma strains were DNA extracted using the High Pure PCR Template Preparation Kit (Roche Diagnostics, Mannheim, Germany) according to the instructions of the manufacturer.
Sequencing the 23S rRNA and tuf genes
Partial 23S rDNA sequences of mycoplasma strains were determined. Primers F1388 (5′-GTT TCC TGG GCA AGG TTC G-3′) and R1982 (5′-CCG TTA TAG TTA CGG CCG CC-3′) were used to amplify a 600-bp segment in the central domain of the gene.
Primer pair tuf-064F (5′-ATGCCNCAAACWMGWGAACAC-3′)/tuf-681R (5′-TRTGACKWCCACCTTCWTCTT-3′) was selected from sites of highest homology in the alignment. The 614-bp central region flanked by these primers was the final target region in the tuf gene that was further analyzed for probe design. All sequencing was conducted by Eurofins MWG Operon (Ebersberg, Germany). New sequences determined in the present study have been deposited in the GenBank database under the following accession numbers: JQ390341–JQ390384 (23S rDNA), JQ390385–JQ390408 (tuf gene).
In silico sequence analysis and selection of hybridization probes
An alignment of experimentally determined 23S rDNA sequences from 44 taxa listed in Table 1 was processed using the Vector NTI Advance 11 software (Invitrogen, Carlsbad, CA, USA), which is based on the ClustalW algorithm. The 471-nt alignment is provided in File S2.
A total of 19 tuf gene sequences of Mollicutes included in Table 1 were available from GenBank (18 as part of complete genomes and the CDS of M. canis). These sequences were combined with 24 de novo sequences in an alignment of the central 614-nt region (File S3). The sequence of M. adleri was not available at the time of array production.
Two different probe selection strategies were used. a) In the case of the 23S rDNA target region, probe binding sites were selected manually on the basis of uniqueness, i.e. each probe was designed to be specific for its eponymous mycoplasma species and checked by BLAST analysis. b) For the tuf target, hybridization probe design included processing of the alignment from File S3 using the program E-INS-I of the MAFFT package [20], version 6.853b (2011/04/27), which is available from http://mafft.cbrc.jp/alignment/software/. Subsequently, the in-house software package Clondiag ArrayDesign (Alere Technologies, Jena, Germany) was used to fine-tune and select the best-discriminating probes.
The following basic selection criteria were used for the hybridization probes: i) specificity of the target sequence (i.e. uniqueness, at least one nucleotide difference to second best match in the case of 23S probes), ii) melting temperature in the range from 54 to 62°C, and iii) absence of significant self-complementarity. The Oligonucleotide Properties Calculator (http://www.unc.edu/~cail/biotool/oligo/) was used to check these parameters. The selected oligonucleotides (70 for 23S rDNA; 86 for tuf) had an average size of 28.4 nt (min. 23/max. 34), a melting temperature of 59.0°C (54/62), and a G+C content of 40.3 mol-% (27/57).
Nucleotide sequences and basic physical parameters of all probes are provided in File S4. Each substance was spotted three-fold onto the microarray. Biotinylated oligonucleotide probes (staining controls), and spotting buffer (background control) were also included. Production of the microarrays was described previously [21].
Pre-hybridization amplification (Biotinylation PCR)
The 5′-biotinylated primers F1388 and R1982 were used to amplify an approximately 600-bp segment containing the signature region of the 23S rRNA gene. The 620-bp tuf target region was amplified using 5′-biotinylated primers tuf-064F and tuf-681R. Pre-hybridization amplification reactions were run on either real-time (two simplex reactions) or conventional (duplex) protocols.
In real-time PCR, the reaction mix for each target contained 1 µl (10–100 ng) of mycoplasma chromosomal DNA, 500 nM of both forward and reverse primer, 10 µl of DyNAmo™ Flash SYBR® Green qPCR Mastermix (Finnzymes, Vantaa, Finland), and was made up to 20 µl with deionized water. After initial denaturation at 95°C for 10 min, 40 cycles (95°C for 30 s, 52°C for 30 s and 72°C for 60 s) with subsequent dissociation curve analysis were run on a Mx3000P® thermocycler and processed using the MxPro™ 4.10 software (both from Agilent, Waldbronn, Germany).
In conventional PCR experiments, both primer pairs were used in a duplex amplification protocol. Each reaction mix contained 1 µl (10–100 ng) of mycoplasma chromosomal DNA, 400 nM of each primer, 2.5 mM MgCl2, 1 mM dNTP mix, 2.5 µl of 10× PCR Buffer, 0.5 U of Taq DNA polymerase (reagents from 5 Prime, VWR, Darmstadt, Germany), and was made up to 25 µl with water. The cycling profile included initial denaturation at 95°C for 60 s, 40 cycles (95°C for 30 s, 52°C for 30 s and 72°C for 60 s) and final elongation at 72°C for 60 s on a Thermocycler T3 (Biometra, Göttingen, Germany). For inspection, products were separated on 1.5% agarose gels, stained with ethidium bromide and visualized by UV illumination.
DNA microarray hybridization
Optimal hybridization conditions were determined empirically by varying hybridization temperatures from 50°C to 60°C and washing step temperatures immediately after hybridization from 35°C to 47°C. The Identibac Hybridisation Kit (Alere) was used according to the instructions of the manufacturer. Briefly, the AS vessels were conditioned by washing with 200 µl of deionized water and 100 µl of hybridization buffer C1 at 50°C for 5 min. All incubations were conducted upon shaking at 550 rpm on a BioShake iQ (Quantifoil Instruments Jena, Germany). One µl of the PCR product (0.5 µl of each simplex product from real-time PCR) was diluted in 99 µl Hybridization Buffer in a separate tube, heated at 95°C for 5 min and put on ice for 30 s. Once transferred into the AS, DNA reassociation was allowed at 50°C for 60 min. Supernatants were discarded and the array was washed twice with 200 µl of washing buffer C2 at 45°C for 10 min. Subsequently, 100 µl of horse radish peroxidase conjugate solution (1 µl C3 and 99 µl C4) were added to the tubes and incubated at 30°C for 10 min. The vessels were then washed with 200 µl of washing buffer C5 at 30°C for 4 min before reactive spots were finally visualized using 100 µl of Seramun Grün (D1) as peroxidase substrate. Hybridization signals were measured using the ArrayMate transmission reader (Alere).
Processing of AS hybridization data using the PatternMatch algorithm
Hybridization signals were processed using the Iconoclust software, version 3.3 (Alere). Normalized intensities of the spots were calculated automatically by the software using the following equation: NI = 1−(M/BG) (where NI is normalized intensity, M is average intensity of the automatically recognized spot, and BG is intensity of local background). NI values would theoretically range from 0 (no signal) to 1 (maximum signal).
A global specificity table listing the number of mismatches of each probe to all mycoplasma species per target (“Probe matching matrix”, File S4) was used to construct theoretical hybridization patterns (i.e. signal intensity of 0.9 for perfect match, 0.6 for 1 mismatch, 0.3 for 2 mismatches, 0.1 for 3 mismatches, no signal for more mismatches, at medium stringency).
The assignment of hybridization patterns obtained from the 23S rDNA and tuf gene sectors of the array was based on probe-by-probe comparison of the measured signals of a given sample with theoretically expected signals of all reference strains. For this operation, the PatternMatch algorithm was used, which is an integral part of the Partisan ArrayLIMS database software system (Alere). The final numerical output is given as the matching score (MS), which represents the sum of differences between corresponding signal intensities of sample and reference. Thus, the MS value is a measure of dissimilarity between two hybridization patterns. An ideal match of two patterns based on the same set of oligonucleotide probes will yield MS = 0, whereas values above 40 require critical scrutiny because they may indicate a poor match or multiple infection. In the latter case, additional manual assignment is necessary. The Delta MS value, defined as the arithmetic difference between best and second best match [22], served as measure for the accuracy of mycoplasma species identification. A value higher than 0.5 was considered as sufficient for unambiguous distinction between two patterns.
Denaturing gradient gel electrophoresis (DGGE)
DNA preparations from field tissue samples were amplified by PCR and analyzed by DGGE as described previously [17].
Confirmatory PCR testing
Field samples giving different results in DGGE and DNA microarray assay were additionally examined using species-specific PCR protocols from the literature for M. bovis [23], M. bovirhinis, M. alkalescens [24], M. dispar [25], M. ovipneumoniae [26], and M. arginini [27].
Results
Analysis of the 23S ribosomal RNA gene region and probe selection
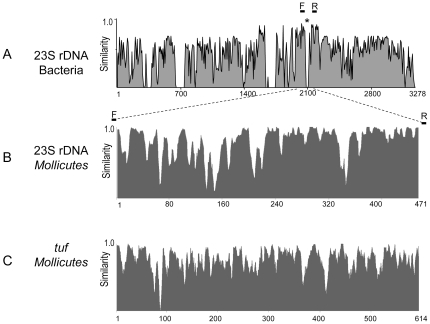
An alignment of 23S rDNA sequences from eubacteria and mycoplasmas revealed an alternate distribution of highly conserved and variable segments over the entire gene locus, which is illustrated in the similarity plot in Fig. 1A. Unlike the cell-walled bacterial species, all mycoplasmas examined showed a 23–26 nt deletion in the segment around position 2100. This observation prompted us to sequence type strains of 38 Mycoplasma, 3 Acholeplasma and 3 Ureaplasma spp. in this variable region. The similarity plot of the aligned segments from 44 Mollicutes spp. in Fig. 1B shows the considerable sequence diversity (alignment given in File S2). Subsequently, we systematically explored this domain for discriminatory sites. Manual selection of species-specific hybridization probe binding sites led to the definition of 107 oligonucleotide probes, of which 70 were confirmed after two rounds of specificity testing (data not shown), i.e. 64 probes for 41 species, 4 for the Mycoplasma mycoides cluster, as well as genus-specific probes for Mycoplasma (6), Acholeplasma (1) and Ureaplasma (1). It was not possible to find functional probes in this target region for the following species: M. californicum, M. canadense, M. gateae, M. hominis, M. hyorhinis, and U. diversum.
Figure 1. Sequence similarity plots of the target regions used in the present microarray.
Numbers on the abscissa denote positions in the sequence alignment. The diagrams were produced using Vector NTI 11 and are based on alignments of A) complete 23S rRNA genes of 10 selected eubacterial species and 9 mycoplasmas (asterisk showing the location of the 23–26-nt deletion found in all Mollicutes spp.), B) the 471-nt signature region of all 44 mycoplasmas included in this study (alignment in File S2), and C) the central 614-nt region of the tuf gene of 43 mycoplasma species (alignment in File S3). Bars denoted F and R indicate the positions of forward and reverse primers, respectively, that were used for amplification. MVW 1–3 indicate the positions of most variable windows.
To facilitate automatic assignment of measured signals to individual species, theoretical hybridization patterns were constructed based on the expected dependence of signal intensity on the number of nucleotide mismatches between target and probe. Previous studies had shown that, depending on the level of stringency of the hybridization reaction, signal reductions caused by one, two or three mismatches were directly measurable [28], [29]. All 44 theoretical hybridization patterns from the 23S rDNA sector were placed in the database.
Analysis of the tuf gene region and probe selection
To extend the discriminatory capacity of the microarray, the tuf gene was considered as an additional target. Nineteen gene sequences were retrieved from GenBank and combined in an alignment with de novo sequences of further 24 taxa from Table 1 (alignment given in File S3). As the extent and nature of sequence diversity did not allow a consistent selection of strictly species-specific probes as in the case of 23S rDNA, the objective was to define combinatorial sets of probes leading to characteristic hybridization patterns that can be assigned to individual species. The alignment of 614-nt segments of 43 tuf genes was processed using E-INS-I, which led to the definition of three most variable windows whose positions are indicated in Fig. 1C. Within these three windows, the software identified 86 oligonucleotide probes satisfying the basic selection criteria. All theoretically expected tuf patterns were entered in the database.
Validation of the 23S rRNA and tuf probes
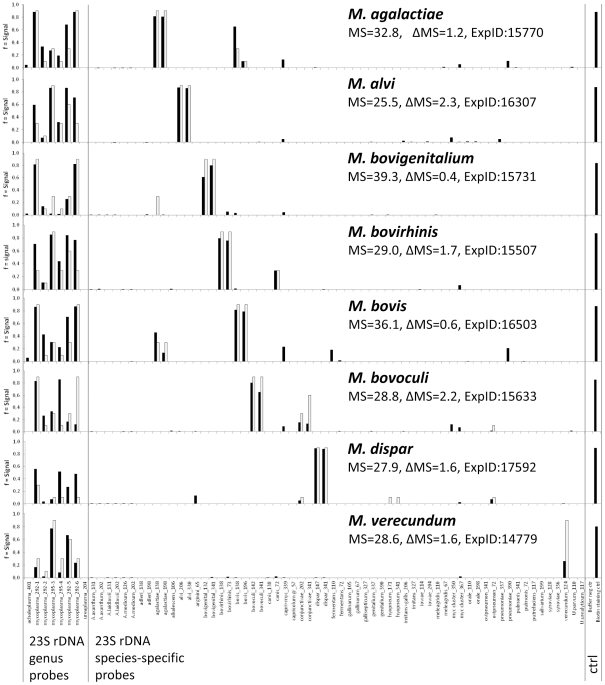
A total of 44 type strains of the Mollicutes organisms were examined using the present DNA microarray assay protocol. In addition, 129 field strains from 37 species were examined, whose identity had been previously established using DGGE, DNA sequencing or immunofluorescence. The results are summarized in Table 1. In the case of 27 species, the 23S rDNA probe panel gave rise to theoretically expected species-specific hybridization patterns consisting of genus and species probe signals only. The discriminatory potential of this target region is illustrated in Fig. 2, where the distinction of eight different bovine mycoplasmas based on specific probes for genus and species is shown.
Figure 2. Differentiation based on 23S rDNA probes among eight Mycoplasma species potentially occurring in cattle.
Black bars denote experimental signals, gray bars denote theoretically predicted signals. Each hybridization experiment is characterized by matching score (MS) and accuracy (Delta MS, see Materials and Methods). Control bars at the right-hand margin show spotting buffer (background control) and biotinylated oligonucleotide (staining control).
However, 23S rDNA probes alone did not allow the identification of all organisms on the list. In addition to the six species lacking specific probes (see above), four species could not be unambiguously recognized by their probe signals, i.e. M. arginini, M. gallisepticum, M. genitalium, and M. pneumoniae, because of cross-reactions with closely related species. Furthermore, M. mycoides with its subspecies mycoides and capri, M. capricolum with its subspecies capricolum and capripneumoniae, as well as M. leachii could be identified as members of the Mycoplasma mycoides cluster, and differentiation between mycoides and capricolum subgroups was possible.
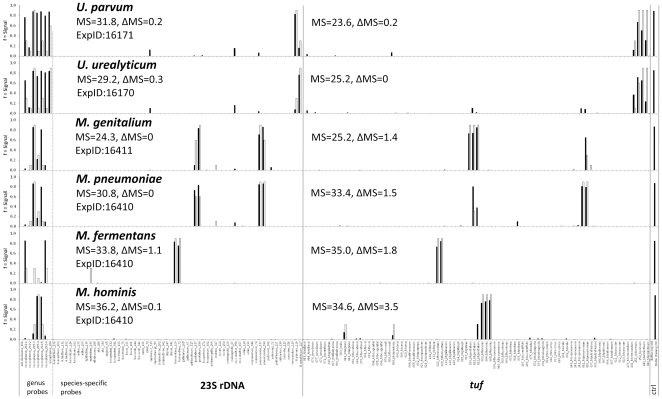
The tuf gene segment offered additional discriminatory capacity. It can be seen from the test results in Table 1 that 34 species have been unambiguously identified by probes from this target. The sister species M. pneumoniae and M. genitalium, both relevant human pathogens, show identical hybridization patterns each for the 23S rDNA target, but are well distinguishable by their specific hybridization pattern on tuf probes (Fig. 3). Cell culture contaminants, such as A. laidlawii, M. arginini, M. hyorhinis, and M. orale, can also be readily identified. Conversely, the closely related pairs of M. bovis/M. agalactiae (Fig. 2) and U. urealyticum/U. parvum showed very similar patterns on tuf (data not shown), whereas the signals from 23S rDNA probes were straightforward and discriminatory.
Figure 3. Differentiation among human Mycoplasma and Ureaplasma spp. based on the combination of probes from 23S rDNA and the tuf gene.
Matching scores (MS) and Delta MS values are given for both gene loci in each hybridization experiment. Black bars denote experimental signals, gray bars denote theoretically predicted signals. Control bars at the right-hand margin show spotting buffer (background control) and biotinylated oligonucleotide (staining control).
When combining the results from both target genes, a total of 37 type strains were correctly assigned at species level. Similar to the findings from the 23S rDNA site, the tuf probes failed to completely differentiate among members of the Mycoplasma mycoides cluster, but the two subgroups of the cluster could be differentiated as above. The high sequence homology did not allow the selection of discriminatory probes for each of the five members. The remaining critical pairs include the closely related M arginini and M. gateae from the M. hominis cluster, which were not distinguishable using the present set of probes.
Analytical sensitivity
The sensitivity of the combined PCR-microarray assay was evaluated by examining decimal dilutions of spectrophotometrically quantified genomic DNA (100 pg to 1 fg) from the type strains of M. bovis and M. dispar. When these mycoplasmas were analyzed separately, 100 fg of DNA corresponding to approximately 100 genome copies were found to be sufficient to obtain species-specific hybridization patterns. To assess the technique's capability to detect co-infections, the two test DNAs were mixed at different ratios, co-amplified by SYBR Green real-time PCR and hybridized on ArrayStrips. The presence of a 103-fold excess of M. bovis DNA did not result in a deteriorated detection limit for M. dispar (data not shown). This illustrates the usability of the test for parallel and simultaneous detection of multiple mycoplasma species with differing loads in a clinical sample.
Testing of field samples
A panel of 60 samples (31 from cattle, 25 from small ruminants and 4 from birds) previously examined by DGGE was tested blind on the present microarray. The results in Table 2 show that concordant results were obtained in the majority of cases. Mycoplasma organisms identified by DGGE were confirmed by the microarray test in 59 (98.3%) instances, among them 36 samples with a mycoplasma monoinfection. One sample with an unclear DGGE result was unambiguously identified as M. conjunctivae.
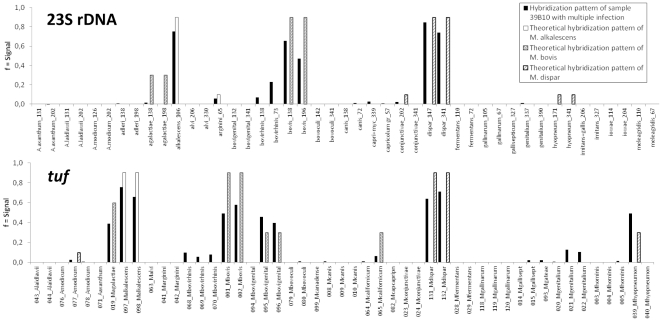
Notably, the microarray revealed a large proportion of samples containing multiple mycoplasma infection (21/60 = 35.0%) compared with DGGE (9/60 = 15.0%). There were identical results in both tests for 4 samples harboring two different species. The microarray test detected additional mycoplasmas in 16 cases (10 confirmed by independent test), and DGGE in 2 cases (1 confirmed). In 6 samples, the microarray identified more than two mycoplasma species (3 confirmed). As an example, detection of M. alkalescens, M. bovis and M. dispar in a bovine lung tissue sample is shown in Fig. 4.
Figure 4. Detection of multiple mycoplasma infection in a DNA extract from bovine lung tissue (sample 39 B 10, Table 2 ).
The diagram shows the combined pattern match of sample and three matching Mycoplasma spp. Black bars denote the hybridization signals of the sample, while theoretically predicted signals for M. alkalescens, M. bovis and M. dispar are represented by empty, dashed and dotted bars, respectively. This close-up presentation shows only the relevant sections of the diagram.
Discussion
DNA microarray assays using the present platform have already been used for a number of microbial pathogens [21], [30], [31]. For the detection of mycoplasmas, Volokhov et al. [32] described an alternative array technology. The paper featured a slide-based microarray system with probes derived from the 16S–23S intergenic transcribed spacer region and involved fluorescence labeling of targets. The system was shown to identify cultured strains of 24 Mollicutes spp. The novelty of the present approach consists in the combination of two basically different probe sets for differentiation among related species, i) the 23S rRNA gene probes picked manually in similarity minima of the signature region, and ii) the combinatorial set of probes derived from the central region of the tuf gene. While the former probe set allows direct identification according to the hybridization signals of the species-specific probes (yes/no decision), the latter was designed to produce hybridization patterns that can be assigned to individual species. The use of different probe design strategies resulted from the observed differences in inter-species sequence diversity within the 23S ribosomal and tuf gene loci. Similarity plots in Fig. 1 show the higher abundance of low-similarity sites in the ribosomal target, all of which represent potential binding sites for highly discriminatory probes.
While the present analysis of the 23S rRNA gene region in Mollicutes for diagnostic purposes is new, the remarkable potential of the gene encoding elongation factor Tu was recognized more than a decade ago. Kamla and co-workers [33] already showed that it represented a better phylogenetic marker than the 16S rRNA locus. Later on the tuf gene was used as target in a broad-range real-time PCR assay for detection of a group of mycoplasma species [34]. In the present study, we took advantage of the locus' discriminatory potential for Mollicutes through the combinatorial approach. This potential can be further exploited in future studies as it will allow the addition of probes for more mycoplasma species of interest on an extended version of the microarray. Altogether, the two-target approach led to an increase of the microarray assay's discriminatory capacity, as well as an improvement of the accuracy of species identification.
The findings of the present study demonstrate the excellent diagnostic potential of the microarray-based methodology. For instance, the possibility of running a single test to monitor all mycoplasmas in human samples (Fig. 3) can be a promising time-saving and economical alternative. The same applies to testing for cell culture contaminants. In veterinary diagnosis, simultaneous detection of different mycoplasma agents occurring in cattle (Fig. 2), in small ruminants including M. agalactiae, M. conjunctiviae, M. ovipneumoniae, mycoides cluster members, or in poultry including M. gallisepticum, M. meleagridis, M. synoviae, M. iowae, M. imitans, renders the setup of individual tests for each agent unnecessary.
Furthermore, the present assay is an efficient tool to investigate dual and multiple mycoplasma infections in individual animals. The present panel of samples was found to contain an unexpectedly high proportion of these infections, i.e. 35%. Although we cannot rule out that the panel has a bias towards multiple infections, the findings indicate that the simultaneous presence of different Mycoplasma spp. is no rare event. In addition, the present data is raising intriguing questions on interactions and synergies between individual microorganisms, as well as their consequences for epidemiology and therapy, which have to be addressed in future studies.
Differentiation among members of the Mycoplasma mycoides cluster remains a particularly difficult problem. Even after the recent revision of its taxonomy [35], the remaining five member organisms are still closely related. In addition, intra-taxon heterogeneity is poorly investigated, but probably not negligible. Unambiguous identification of all cluster members based on combined 23S rDNA and tuf gene targets was not possible.
Direct identification of pathogens from clinical samples is an important asset of the ArrayStrip assay. In accordance with our previous finding that the sensitivity of the present DNA microarray platform was equivalent to that of real-time PCR [36], examination of 60 field samples (Table 2) confirmed that a valid hybridization pattern was obtained as soon as a sample contained sufficient DNA template to yield a PCR amplicon. In this context, the microarray test can supersede time-consuming culture experiments and, in the absence of a clear idea about the identity of the pathogen in the sample, avoid the necessity of running several different tests.
The high specificity of the present assay results from the large number of oligonucleotide probes on the array, which simultaneously interrogate the sample DNA during hybridization. For each mycoplasma organism, there are multiple probes, i.e. one to three from the 23S rDNA and a combinatorial set from the tuf gene.
Alternative approaches to parallel detection tests include melting curve analysis [37] and bead-based Luminex assays [38], [39]. However, while rapid, highly specific and reasonably sensitive, the parallelity of these technologies is practically limited to about ten different species in a single test.
All in all, the ArrayStrip microarray test is suitable for routine diagnosis as shown for other pathogens [30], [40], [41], mainly for its ease of operation, rapidity, potential to high throughput, high information content, and affordability. The major steps include DNA extraction using a commercial kit, amplification by duplex biotinylation PCR, as well as hybridization, washing and staining, and results are available within a working day. Apart from the ArrayStrips and the transmission reader, the assay requires only standard laboratory equipment. The fact that the final output is based on automatic comparison of measured hybridization signals with reference patterns in the database adds a reasonable degree of objectivity.
We conclude that we have developed a promising diagnostic tool for rapid detection of mono- and multiple infections of 42 Mollicutes spp., including subgroup identification of Mycoplasma mycoides cluster members. The combination of species-specific and combinatorial probes, which has facilitated differentiation between closely related species, can be further extended in this open system to include additional organisms of interest. The present DNA microarray assay can be used in diagnosis of human and animal infections, as well as cell culture contamination.
Supporting Information
Complete list of field samples and individual test results of DGGE and DNA microarray testing
(XLS)
Alignment of 23S rDNA sequences
(MSF)
Alignment of tuf sequences
(MSF)
List of oligonucleotide probes on the array and Probe Matching Matrix
(XLS)
Acknowledgments
We thank Peter Slickers (Alere) for advising and supporting us in the use of software for systematic selection of hybidization probes and the generation of theoretical hybridization patterns in silico. We thank Susann Bahrmann, Ines Engelmann, Christine Grajetzki, Simone Bettermann, Sophie Bisgaard-Frantzen, Lizelle Cronje and Graeme Barden for excellent technical assistance. We are also grateful to Jörg Stülke, Göttingen, Birgit Henrich, Düsseldorf, and Roger Dumke, Dresden, for providing DNA of mycoplasma strains.
Footnotes
Competing Interests: Ralf Ehricht is an employee of Alere Technologies GmbH. There are no patents, products in development or marketed products to declare. This does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials, as detailed online in the guide for authors. All other authors have declared that no competing interests exist.
Funding: This study was supported by grant no. 0315859 “Improvements in the diagnosis and control of bovine mycoplasmosis” of the European Initiative EMIDA ERA-Net. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Brown DR, May M, Bradbury JM, Balish MF, Calcutt MJ, et al. Genus I. Mycoplasma Nowak 1929. Bergey's Manual of Systematic Bacteriology, 2nd edition, vol 4, Eds NR Krieg, JT Staley, DR Brown, BP Hedlund, BJ Paster, NL Ward, W Ludwig, WB Whitman), Springer, Heidelberg. 2011;4:575–613. [Google Scholar]
- 2.Razin S. The minimal cellular genome of mycoplasma. J Biochem Biophys. 1997;34:124–130. [PubMed] [Google Scholar]
- 3.Volokhov DV, Graham LJ, Brorson KA, Chizhikov VE. Mycoplasma testing of cell substrates and biologics: Review of alternative non-microbiological techniques. Mol Cell Probes. 2011;25:69–77. doi: 10.1016/j.mcp.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Garner CM, Hubbold LM, Chakraborti PR. Mycoplasma detection in cell cultures: a comparison of four methods. Br J Biomed Sci. 2000;57:295–301. [PubMed] [Google Scholar]
- 5.Nicholas RA, Bashiruddin JB. Mycoplasma mycoides subspecies mycoides (small colony variant): the agent of contagious bovine pleuropneumonia and member of the “Mycoplasma mycoides cluster”. J Comp Pathol. 1995;113:1–27. doi: 10.1016/s0021-9975(05)80065-9. [DOI] [PubMed] [Google Scholar]
- 6.Bergonier D, Berthelot X, Poumarat F. Contagious agalactia of small ruminants: current knowledge concerning epidemiology, diagnosis and control. Rev Sci Tech. 1997;16:848–873. doi: 10.20506/rst.16.3.1062. [DOI] [PubMed] [Google Scholar]
- 7.Thiaucourt F, Bolske G, Leneguersh B, Smith D, Wesonga H. Diagnosis and control of contagious caprine pleuropneumonia. Rev Sci Tech. 1996;15:1415–1429. doi: 10.20506/rst.15.4.989. [DOI] [PubMed] [Google Scholar]
- 8.Kleven SH. Mycoplasmas in the etiology of multifactorial respiratory disease. Poult Sci. 1998;77:1146–1149. doi: 10.1093/ps/77.8.1146. [DOI] [PubMed] [Google Scholar]
- 9.Pfützner H, Sachse K. Mycoplasma bovis as an agent of mastitis, pneumonia, arthritis and genital disorders in cattle. Rev Sci Tech. 1996;15:1477–1494. doi: 10.20506/rst.15.4.987. [DOI] [PubMed] [Google Scholar]
- 10.Giacometti M, Nicolet J, Johansson KE, Naglic T, Degiorgis MP, et al. Detection and identification of Mycoplasma conjunctivae in infectious keratoconjunctivitis by PCR based on the 16S rRNA gene. Zentralbl Veterinarmed B. 1999;46:173–180. doi: 10.1046/j.1439-0450.1999.00218.x. [DOI] [PubMed] [Google Scholar]
- 11.Jones GE, Foggie A, Sutherland A, Harker DB. Mycoplasmas and ovine keratoconjunctivitis. Vet Rec. 1976;99:137–141. doi: 10.1136/vr.99.8.137. [DOI] [PubMed] [Google Scholar]
- 12.Marois C, Le Carrou J, Kobisch M, Gautier-Bouchardon AV. Isolation of Mycoplasma hyopneumoniae from different sampling sites in experimentally infected and contact SPF piglets. Vet Microbiol. 2007;120:96–104. doi: 10.1016/j.vetmic.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 13.Ross DF. Mycoplasmal diseases. In: Leman AD, Straw BE, Mengeling WL, D'Allaire S, Taylor DJ, editors. Diseases of Swine, 7th edition. Ames, IA: Iowa State University Press; 1992. pp. 537–551. [Google Scholar]
- 14.Pitcher DG, Nicholas RA. Mycoplasma host specificity: fact or fiction? Vet J. 2005;170:300–306. doi: 10.1016/j.tvjl.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Levisohn S, Garazi S, Gerchman I, Brenner J. Diagnosis of a mixed mycoplasma infection associated with a severe outbreak of bovine pinkeye in young calves. J Vet Diagn Invest. 2004;16:579–581. doi: 10.1177/104063870401600615. [DOI] [PubMed] [Google Scholar]
- 16.Rhoades KR. Turkey airsacculitis: effect of mixed mycoplasmal infections. Avian Dis. 1981;25:131–135. [PubMed] [Google Scholar]
- 17.McAuliffe L, Ellis RJ, Lawes JR, Ayling RD, Nicholas RA. 16S rDNA PCR and denaturing gradient gel electrophoresis; a single generic test for detecting and differentiating Mycoplasma species. J Med Microbiol. 2005;54:731–739. doi: 10.1099/jmm.0.46058-0. [DOI] [PubMed] [Google Scholar]
- 18.Freundt EA. Culture media for classic mycoplasmas. In: Razin S, Tully JG, editors. Methods in Mycoplasmology Vol I: Mycoplasma characterization. London: Academic Press; 1983. pp. 127–135. [Google Scholar]
- 19.Nicholas R, Baker S. Recovery of mycoplasmas from animals. Methods Mol Biol. 1998;104:37–43. doi: 10.1385/0-89603-525-5:37. [DOI] [PubMed] [Google Scholar]
- 20.Katoh K, Kuma K, Miyata T, Toh H. Improvement in the accuracy of multiple sequence alignment program MAFFT. Genome Inform. 2005;16:22–33. [PubMed] [Google Scholar]
- 21.Sachse K, Hotzel H, Slickers P, Ellinger T, Ehricht R. DNA microarray-based detection and identification of Chlamydia and Chlamydophila spp. Mol Cell Probes. 2005;19:41–50. doi: 10.1016/j.mcp.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Sachse K, Laroucau K, Vorimore F, Magnino S, Feige J, et al. DNA microarray-based genotyping of Chlamydophila psittaci strains from culture and clinical samples. Vet Microbiol. 2009;135:22–30. doi: 10.1016/j.vetmic.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 23.Sachse K, Salam HS, Diller R, Schubert E, Hoffmann B, et al. Use of a novel real-time PCR technique to monitor and quantitate Mycoplasma bovis infection in cattle herds with mastitis and respiratory disease. Vet J. 2010;186:299–303. doi: 10.1016/j.tvjl.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi H, Hirose K, Worarach A, Paugtes P, Ito N, et al. In vitro amplification of the 16S rRNA genes from Mycoplasma bovirhinis, Mycoplasma alkalescens and Mycoplasma bovigenitalium by PCR. J Vet Med Sci. 1998;60:1299–1303. doi: 10.1292/jvms.60.1299. [DOI] [PubMed] [Google Scholar]
- 25.Marques LM, Buzinhani M, Yamaguti M, Oliveira RC, Ferreira JB, et al. Use of a polymerase chain reaction for detection of Mycoplasma dispar in the nasal mucus of calves. J Vet Diagn Invest. 2007;19:103–106. doi: 10.1177/104063870701900118. [DOI] [PubMed] [Google Scholar]
- 26.McAuliffe L, Hatchell FM, Ayling RD, King AI, Nicholas RA. Detection of Mycoplasma ovipneumoniae in Pasteurella-vaccinated sheep flocks with respiratory disease in England. Vet Rec. 2003;153:687–688. doi: 10.1136/vr.153.22.687. [DOI] [PubMed] [Google Scholar]
- 27.Timenetsky J, Santos LM, Buzinhani M, Mettifogo E. Detection of multiple mycoplasma infection in cell cultures by PCR. Braz J Med Biol Res. 2006;39:907–914. doi: 10.1590/s0100-879x2006000700009. [DOI] [PubMed] [Google Scholar]
- 28.Sachse K, Laroucau K, Hotzel H, Schubert E, Ehricht R, et al. Genotyping of Chlamydophila psittaci using a new DNA microarray assay based on sequence analysis of ompA genes. BMC Microbiol. 2008;8:63. doi: 10.1186/1471-2180-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wiehlmann L, Wagner G, Cramer N, Siebert B, Gudowius P, et al. Population structure of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 2007;104:8101–8106. doi: 10.1073/pnas.0609213104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monecke S, Coombs G, Shore AC, Coleman DC, Akpaka P, et al. A field guide to pandemic, epidemic and sporadic clones of methicillin-resistant Staphylococcus aureus. PLoS One. 2011;6:e17936. doi: 10.1371/journal.pone.0017936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geue L, Schares S, Mintel B, Conraths FJ, Muller E, et al. Rapid microarray-based genotyping of enterohemorrhagic Escherichia coli serotype O156:H25/H-/Hnt isolates from cattle and clonal relationship analysis. Appl Environ Microbiol. 2010;76:5510–5519. doi: 10.1128/AEM.00743-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Volokhov DV, George J, Liu SX, Ikonomi P, Anderson C, et al. Sequencing of the intergenic 16S–23S rRNA spacer (ITS) region of Mollicutes species and their identification using microarray-based assay and DNA sequencing. Appl Microbiol Biotechnol. 2006;71:680–698. doi: 10.1007/s00253-005-0280-7. [DOI] [PubMed] [Google Scholar]
- 33.Kamla V, Henrich B, Hadding U. Phylogeny based on elongation factor Tu reflects the phenotypic features of mycoplasmas better than that based on 16S rRNA. Gene. 1996;171:83–87. doi: 10.1016/0378-1119(95)00884-5. [DOI] [PubMed] [Google Scholar]
- 34.Stormer M, Vollmer T, Henrich B, Kleesiek K, Dreier J. Broad-range real-time PCR assay for the rapid identification of cell-line contaminants and clinically important mollicute species. Int J Med Microbiol. 2009;299:291–300. doi: 10.1016/j.ijmm.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 35.Manso-Silvan L, Vilei EM, Sachse K, Djordjevic SP, Thiaucourt F, et al. Mycoplasma leachii sp. nov. as a new species designation for Mycoplasma sp. bovine group 7 of Leach, and reclassification of Mycoplasma mycoides subsp. mycoides LC as a serovar of Mycoplasma mycoides subsp. capri. Int J Syst Evol Microbiol. 2009;59:1353–1358. doi: 10.1099/ijs.0.005546-0. [DOI] [PubMed] [Google Scholar]
- 36.Ehricht R, Slickers P, Goellner S, Hotzel H, Sachse K. Optimized DNA microarray assay allows detection and genotyping of single PCR-amplifiable target copies. Mol Cell Probes. 2006;20:60–63. doi: 10.1016/j.mcp.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 37.Rebelo AR, Parker L, Cai HY. Use of high-resolution melting curve analysis to identify Mycoplasma species commonly isolated from ruminant, avian, and canine samples. J Vet Diagn Invest. 2011;23:932–936. doi: 10.1177/1040638711416846. [DOI] [PubMed] [Google Scholar]
- 38.Battaglia A, Schweighardt AJ, Wallace MM. Pathogen detection using a liquid array technology. J Forensic Sci. 2011;56:760–765. doi: 10.1111/j.1556-4029.2011.01708.x. [DOI] [PubMed] [Google Scholar]
- 39.Righter DJ, Rurangirwa FR, Call DR, McElwain TF. Development of a bead-based multiplex PCR assay for the simultaneous detection of multiple Mycoplasma species. Vet Microbiol. 2011;153:246–256. doi: 10.1016/j.vetmic.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 40.Borel N, Kempf E, Hotzel H, Schubert E, Torgerson P, et al. Direct identification of chlamydiae from clinical samples using a DNA microarray assay: a validation study. Mol Cell Probes. 2008;22:55–64. doi: 10.1016/j.mcp.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 41.Ruettger A, Feige J, Slickers P, Schubert E, Morre SA, et al. Genotyping of Chlamydia trachomatis strains from culture and clinical samples using an ompA-based DNA microarray assay. Mol Cell Probes. 2011;25:19–27. doi: 10.1016/j.mcp.2010.09.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Complete list of field samples and individual test results of DGGE and DNA microarray testing
(XLS)
Alignment of 23S rDNA sequences
(MSF)
Alignment of tuf sequences
(MSF)
List of oligonucleotide probes on the array and Probe Matching Matrix
(XLS)