Abstract
The pubertal increase in GnRH secretion resulting in sexual maturation and reproductive competence is a complex process involving kisspeptin stimulation of GnRH neurons and requiring Ca2+ and possibly other intracellular messengers. To determine whether the expression of Ca2+ channels, or small-conductance Ca2+ -activated K+ (SK) channels, whose activity reflects cytoplasmic free Ca2+ concentration, changes at puberty in GnRH neurons, Ca2+ and SK currents in GnRH neurons were recorded in brain slices of juvenile [postnatal day (P) 10–21], pubertal (P28–P42), and adult (≥ P56) male GnRH-green fluorescent protein transgenic mice using perforated-patch and whole-cell techniques. Ca2+ currents were inhibited by the Ca2+ channel blocker Cd2+ and showed marked heterogeneity but were on average similar in juvenile, pubertal, and adult GnRH neurons. SK currents, which were inhibited by the SK channel blocker apamin and enhanced by the SK and intermediate-conductance Ca2+ -activated K+ channel activator 1-ethyl-2-benzimidazolinone, were also on average similar in juvenile, pubertal, and adult GnRH neurons. These findings suggest that whereas Ca2+ and SK channels may participate in the pubertal increase in GnRH secretion and there may be changes in Ca2+ or SK channel subtypes, overall Ca2+ and SK channel expression in GnRH neurons remains relatively constant across pubertal development. Hence, the expected increase in GnRH neuron cytoplasmic free Ca2+ concentration required for increased GnRH secretion at puberty appears to be due to mechanisms other than altered Ca2+ or SK channel expression, e.g. increased membrane depolarization and subsequent activation of pre-existing Ca2+ channels after increased excitatory synaptic input.
Puberty, the period between childhood and adulthood during which sexual maturity and reproductive competence are attained, begins with an increase in the pulsatile release of the decapeptide GnRH from a network of approximately 800 mainly hypothalamic GnRH-synthesizing neurons into the portal vasculature connecting the hypothalamus and anterior pituitary (1–3). GnRH binds to GnRH receptors on pituitary gonadotrophs and stimulates the release into the general circulation of LH and FSH, which are required for gonadal steroid secretion and the production of mature gametes in males and females. The GnRH neurosecretory system is active during the neonatal period in primates and rodents but in primates enters a dormant state in the juvenile period. At puberty there is a gonadal-independent increase in the amplitude and frequency of GnRH and LH pulses to adult levels, which represents a reactivation or reawakening of the GnRH neurosecretory system in primates and a further activation in rodents (3–8).
One approach to elucidate the mechanism of the pubertal increase in GnRH secretion is to investigate the pubertal increase in cytoplasmic free Ca2+ concentration ([Ca2+]i) in GnRH neurons. Increased [Ca2+]i, perhaps accompanied by changes in other intracellular messengers including cAMP, cGMP, and lipid-derived signaling molecules, is required for increased GnRH secretion in GnRH neurons (9–12), probably for secretory vesicle or granule docking and fusion, as in other neurons and endocrine cells (13, 14). The pubertal increase in GnRH secretion depends on the stimulatory actions of the neurotransmitter kisspeptin via the G protein-coupled receptor 54 (15, 16) and presumably occurs by Ca2+ entry through voltage-gated Ca2+ channels or by Ca2+ release from intracellular stores (10–14, 17) after kisspeptin excitation of GnRH neurons. Kisspeptin (15, 16), along with other neurotransmitters and hormones (18–23), may convey information from presynaptic neurons about age, growth, availability of metabolic fuels such as glucose and fats (perhaps through insulin and leptin), circadian rhythm, and other factors.
Two groups demonstrated that postnatal GnRH neurons express voltage-gated Ca2+ channels, but whether total Ca2+ channel expression in GnRH neurons changes at puberty remained unclear. One of the groups (24) used whole-cell recording of acutely dissociated green fluorescent protein (GFP)-labeled GnRH neurons from gonadal-intact juvenile, aged postnatal day (P) 4–10, and ovariectomized adult female GnRH-GFP mice. They found that maximum, peak Ca2+ current density (i.e. maximum, peak Ca2+ current divided by cell capacitance, which is proportional to membrane surface area) increased significantly (P < 0.025) at puberty from 21.0 ± 2.1 (n = 10 juvenile GnRH neurons) to 28.4 ± 2.2 pA/pF (n = 17 adult GnRH neurons), which suggested increased Ca2+ channel expression. However, the other group (25), using perforated-patch recordings of overnight cultures of GFP-labeled GnRH neurons from gonadal-intact neonatal/juvenile (P1–P7) and pubertal (P35–P40) male and female GnRH-GFP transgenic rats, found no change (P > 0.05) in maximum, peak Ca2+ current density.
GnRH neurons also appear to express voltage-independent Ca2+-activated K+ [K(Ca)] channels, which have no intrinsic voltage dependence but do obtain voltage dependence from the voltage dependence of Ca2+ entry through Ca2+ channels (13, 26, 27). In other cells, voltage-independent K(Ca) channels aid in the prolonged afterhyperpolarization after action potential firing and associated Ca2+ influx, and they participate in rhythmic electrical activity (13, 26, 27). They may regulate the frequency of action potential firing in GnRH neurons, determining the subsequent amount of Ca2+ influx and GnRH secretion. K(Ca) channel activity in GnRH neurons may change developmentally due to changes in Ca2+ channel expression/activity or K(Ca) channel expression as in some other neurons (28–30). Two types of voltage-independent K(Ca) currents may be responsible for the afterhyperpolarization, an apamin (a toxin from honeybee venom)-sensitive, medium afterhyperpolarization current with a decay time constant in the range of 100–200 msec, which is mediated by small-conductance (SK) K(Ca) channels, and an apamin-insensitive slow afterhyperpolarization current with a decay time constant ranging from hundreds of milliseconds to several seconds (26, 27). Immortalized mouse (31) and adult female guinea pig (32) GnRH neurons have been reported to express SK channels. However, because they are derived from tumors of embryonic GnRH neurons (33), immortalized GnRH neurons are immature neurons and do not permit the study of developmental changes. In the guinea pig study (32), although SK channel subunit mRNA was found to be expressed, it is unclear whether the afterhyperpolarizations recorded were mediated by SK channels or apamin-insensitive K(Ca) channels because apamin was not tested on GnRH neurons. Recently Kato et al. (34) reported that apamin inhibits a slow rather than a medium afterhyperpolarization current in overnight cultures of GnRH neurons from gonadal-intact adult female rats, providing the first evidence for the expression of functional SK channels in postnatal GnRH neurons. Nevertheless, the question of whether SK channel expression or activity in GnRH neurons changes at puberty was not addressed.
To determine whether Ca2+ or SK currents change at puberty and whether the discrepant results reported previously (24, 25) were due to species differences (rat vs. mouse) or recording technique (perforated patch vs. whole cell), and preserving more of the dendritic and axonal processes that may contribute to channel activity and avoiding artifacts that may arise from cell dissociation and culture, perforated-patch and whole-cell recordings of Ca2+ or SK currents in GnRH neurons in brain slices of juvenile, pubertal, and adult male GnRH-GFP mice were obtained. Males, rather than females, were selected to avoid possible confounding effects of estrous cycle stage on Ca2+ or SK currents and because neither Ca2+ nor SK currents in GnRH neurons of male mice had been previously characterized. No significant differences were found in Ca2+ or SK currents between GnRH neurons of juvenile, pubertal, and adult males, suggesting that Ca2+ and SK channel expression in GnRH neurons remains relatively constant across pubertal development.
Materials and Methods
Use and care of mice
Juvenile (P10–P21), pubertal (P28–P42), and adult (≥ P56) male homozygous GnRH-GFP transgenic mice in which GFP is genetically targeted to GnRH neurons (35) were used for experiments. The time periods associated with the juvenile, pubertal, and adult groups are based on previously observed stages of mouse reproductive development (36) and were chosen to avoid borderline periods in which it may be difficult to distinguish between groups. To confirm pubertal development during these time periods, testes from some of the GnRH-GFP mice were removed and weighed. Testes weights (wet weights of combined testes) were 39.4 ± 4.7, 170.3 ± 10.7, and 205.8 ± 6.0 mg for P21, P42, and P88 mice (n = 3 mice at each age), respectively, the greater than 4-fold higher testicular weight at P42 compared with P21 indicating that pronounced testicular growth occurs between P21 and P42 in GnRH-GFP mice. Mice were housed in a temperature (22 C)- and light (on 0600–1800 h)-controlled room with ad libitum access to food and water. All procedures were approved by the Animal Care and Use Committee of the University of Chicago and conducted within the guidelines of the National Research Council publication Guide for Care and Use of Laboratory Animals.
Brain slice preparation for electrophysiology experiments
Mice were anesthetized with isoflurane (Baxter Healthcare, Deerfield, IL) and then decapitated. Brains were removed, dissected in ice-cold Ringer’s solution gassed with 95% O2-5% CO2, containing (in mM) 125 NaCl, 25 NaHCO3, 1.25 NaH2PO4, 2.5 KCl, 2 CaCl2, 1 MgCl2, and 25 glucose, 316 mOsm (pH 7.4), and cut sagittally into 300-μm-thick slices with a vibratome (Campden Instruments Integraslice 7550MM; Lafayette Instruments, Lafayette, IN) as described (35). Slices were transferred using the back end of a Pasteur pipette to an incubation chamber with Ringer’s solution (gassed with 95% O2-5% CO2) for at least 30 min at 35 C and then stored at room temperature (22 C) in the same chamber until electrophysiological recording.
Fluorescence and infrared microscopy for visualizing and recording from GFP-labeled GnRH neurons
For visualizing and recording from GFP-labeled GnRH neurons, slices were transferred to a 3-ml, temperature-controlled recording chamber (Luigs and Neumann, Ratingen, Germany), fixed in place with a grid, and superfused at a rate of 1 ml/min with Ringer’s solution. Slices were viewed with an upright, motorized fluorescence microscope (Axioskop 2 FS; Zeiss Microimaging, Thornwood, NY) equipped with an infrared filter set and a Senarmont system for differential interference contrast, first in bright field with a ×5 objective (Plan-Neofluar; Zeiss). To visualize GFP, the white light from the bright-field halogen lamp was blocked and a fluorescent lamp (HBO 100 W; Osram, Berlin, Germany) was switched on. The intensity of the fluorescent lamp was regulated by a FluoArc power supply (Zeiss). GFP filter set 41017, consisting of excitation filter HQ470/40, dichroic mirror Q495LP, and emission filter HQ525/50 (Chroma Technology, Brattleboro, VT), was used. Upon locating a fluorescent neuron with the ×5 objective, a ×40 (0.8 NA) water-immersion objective (Achroplan IR; Zeiss) was used for further imaging and to view cells for perforated-patch and whole-cell recording. Infrared differential interference contrast imaging was performed subsequent to fluorescence observation. After viewing a fluorescent neuron, magnification was increased by ×2.5 with an intermediate phototube (Optovar; Zeiss), the light from the fluorescent lamp was blocked, and infrared light transmitted via the Senarmont system was directed to a near-infrared charge-coupled device camera (C7500–50; Hamamatsu Photonic Systems, Bridgewater, NJ). A neuron viewed with infrared optics was considered to be the same as that viewed with fluorescence optics when the infrared image and the fluorescent image of the neuron had the same position and orientation through the eyepiece of the microscope (fluorescent image) and with the infrared imaging system (infrared image).
Perforated-patch and whole-cell recording of Ca2+ and SK currents
Nystatin perforated-patch and conventional whole-cell recordings were used to assess Ca2+ and SK channel activity. Ca2+ and SK currents were recorded from GFP-labeled neurons in the diagonal band of Broca and preoptic area as described (35). Experiments were performed at 35 C.
The bath solution for recording Ca2+ currents consisted of (in mM) 117.5 NaCl, 25 NaHCO3, 1.25 NaH2PO4, and 10 tetraethylammonium (TEA)-Cl (to block K+ channels), 2 CaCl2, 1 MgCl2, and 25 glucose, gassed with 95% O2-5% CO2 (pH 7.4, 307 mOsm) and supplemented with 0.5 μM tetrodotoxin (TTX; to block TTX-sensitive Na+ channels), 50μM kynurenic acid (to block ionotropic glutamate receptors), and 50 μM picrotoxin (to block A-type γ-aminobutyric acid receptors). Substituting 2.5 mM Cs+, which blocks K+ channels as well as hyperpolarization-activated cation channels (13), for 2.5 of the 117.5 mM NaCl did not affect Ca2+ currents (data not shown).
The pipette solution for perforated-patch recording of Ca2+ currents contained (in mM) 130 Cs-MeSO3, 5 CsCl, 10 TEA-Cl, 5 NaCl, 1 MgCl2, and 10 HEPES (pH 7.28, 284 mOsm). Pipettes were briefly immersed in this solution and then backfilled with the same solution supplemented with 500 μg/ml nystatin. The latter was prepared fresh for each experiment by dissolving 2 mg nystatin in 2 μl dimethyl sulfoxide in a microfuge tube, adding 998 μl pipette solution to obtain a 2 mg/ml nystatin stock solution, sonicating for 1 min, placing 250 μl of the stock solution in a second tube containing 750 μl pipette solution, filtering with a syringe filter, and then storing on ice and in the dark until use. Nystatin was selected as the membrane perforating substance for perforated-patch recordings rather than amphotericin B, which had been used previously to record Ca2+ and SK currents in GnRH neurons (25, 34) because nystatin had been reported to perforate faster at similar concentrations, thereby reducing the time required for complete opening of the pores and for initiation of experiments (37). Complete opening in the present study, as assessed by monitoring series resistance, was achieved within 5–20 min after gigaseal formation.
The pipette solution for whole-cell recording of Ca2+ currents consisted of (in mM) 110 CsCl, 30 TEA-Cl, 5 NaCl, 1 CaCl2, 2 Mg-ATP, 10 EGTA, and 10 HEPES (pH 7.25, 295 mOsm).
The bath solution for perforated-patch recording of SK currents consisted of (in mM) 125 NaCl, 25 NaHCO3, 1.25 NaH2PO4, 2.5 KCl, 2 CaCl2, 1 MgCl2, and 25 glucose, equilibrated with 95% O2-5% CO2 (pH 7.4, 316 mOsm) and supplemented with 50 μM kynurenic acid and 50 μM picrotoxin.
The pipette solution for perforated-patch recording of SK currents contained (in mM) 130 K-acetate, 15 KCl, 5 NaCl, 1 MgCl2, and 10 HEPES (pH 7.27, 293 mOsm). As with the pipette solution for perforated-patch recording of Ca2+ currents, pipettes were briefly immersed in this solution and then backfilled with the same solution supplemented with 500 μg/ml nystatin.
Recording pipettes were made from thick-walled borosilicate glass capillary tubes (length of 75 mm, outer diameter of 2 mm, inner diameter of 1 mm, and wall thickness of 0.5 mm; Hilgenberg, Malsfeld, Germany) using a P-97 Flaming/Brown micropipette puller (Sutter Instrument, Novato, CA) and had resistances ranging from 3 to 5 MΩ when filled with either of the two pipette solutions. Pipettes were connected via an Ag-AgCl wire to the headstage of an EPC-10 patch clamp amplifier (HEKA Electronics, Mahone Bay, Nova Scotia, Canada). The reference electrode was an Ag-AgCl pellet (IVM, Healdsburg, CA) immersed in bath solution. The EPC-10 amplifier and PatchMaster software (HEKA) were used to acquire (10 kHz), filter (Bessel, 2.9 kHz), and analyze patch clamp data, which were stored on a Power Macintosh G4 computer. The patch clamp amplifier was also used to compensate pipette and cell capacitance. Series resistance was uncompensated but was always less than 50 MΩ in recordings selected for analysis. Series resistance (mean ± SD) in perforated-patch recordings of Ca2+ and SK current was 26.2 ± 8.5 MΩ for juvenile (n = 23), 16.7 ± 8.6 MΩ for pubertal (n = 21), and 26.3 ± 8.9 MΩ for adult (n = 20) GnRH neurons. Series resistance (mean ± SD) in whole-cell recordings of Ca2+ current was 21.5 ± 11.2 MΩ for juvenile (n = 15), 12.7 ± 2.5 pF for pubertal (n = 14), and 22.9 ± 12.0 MΩ for adult (n = 14) GnRH neurons. The similar values of series resistance in recordings from juvenile and adult GnRH neurons suggest that uncompensated series resistance did not mask possible changes in Ca2+ or SK current during pubertal development. Cell capacitance (mean ± SD) was 11.1 ± 2.3 pF for juvenile (n = 41), 12.3 ± 2.7 pF for pubertal (n = 35), and 12.6 ± 3.4 pF for adult (n = 34) GnRH neurons (values from perforated-patch and whole-cell recordings pooled). Capacitative and leak currents were subtracted using a P/4 protocol. Traces were processed for presentation using Igor Pro 4.0 (Wavemetrics, Lake Oswego, OR) and Canvas 8.0.5 software (Deneba Systems, Miami, FL).
Drug application
To inhibit Ca2+ or SK channel activity or enhance SK channel activity, CdCl2 (200 μM) apamin (10 or 100 nM), or 1-ethyl-2-benzimidazolinone (1-EBIO; 200 μM) was added to the bath solution as indicated. After the establishment of a perforated-patch or whole-cell recording, brain slices were superfused (at a rate of 1 ml/min) for at least 10 min with bath solution lacking these drugs to record control Ca2+ or SK currents and then for 10 min with bath solution containing these drugs to record the Ca2+ or SK currents in their presence.
Statistics
Data are expressed as mean ± SEM unless indicated otherwise. Statistical comparisons were performed using Kruskal-Wallis one-way ANOVA for multiple independent groups (38) with the help of GB-STAT 5.06 software (Dynamic Microsystems, Silver Spring, MD). A difference between groups was significant if P obtained from the χ2 distribution associated with the Kruskal-Wallis one-way ANOVA was less than 0.05.
Reagents
Except for 1-EBIO and TTX, which were obtained from Tocris Bioscience (Ellisville, MO), all reagents used in this study were purchased from Sigma-Aldrich (St. Louis, MO).
Results
Ca2+ currents in juvenile, pubertal, and adult GnRH neurons
Ca2+ currents, evoked by voltage steps from −60 mV, near the resting membrane potential (16, 35, 39, 40), to membrane potentials of −50 to +20 mV in nystatin perforated-patch recordings from GnRH neurons in brain slices of male GnRH-GFP mice were inward, rapidly activating, slowly inactivating, and rapidly deactivating (Fig. 1A). Ca2+ channel activity was inhibited by the broad-spectrum Ca2+ channel blocker Cd2+ (Fig. 1B) and had a V-shaped peak current-voltage relationship (Fig. 1C) as in dissociated GnRH neurons (24, 25). Activity began around a membrane potential of −50 mV, was highest at membrane potentials between −20 and 0 mV, and declined at more depolarized potentials. Ca2+ channel activity evoked from a holding potential of −100 mV was similar to that evoked from −60 mV and slightly larger than that evoked from −40 mV (Fig. 1, D–F). At 0 mV in adult GnRH neurons, for example, averaged peak Ca2+ current densities were −33 ± 3, −35 ± 3, and −29 ± 2 pA/pF from holding potentials of −100, −60, and −40 mV, respectively (n = 11 GnRH neurons from four mice). The weak dependence of the channel activity on holding potential suggests that Ca2+ currents in GnRH neurons are carried mainly by high-voltage activated Ca2+ channels (13), in accord with previous findings (24, 25).
Fig. 1.
GnRH neurons express Ca2+ channels. A, Ca2+ currents in an adult GnRH neuron evoked by 50-msec voltage pulses at 1 Hz from a holding potential (Vh) of −60 mV to membrane potentials (Vm) of −80, −70, −60, −50, −40, −30, −20, −10, 0, +10, and +20 mV. B, Inhibition of Ca2+ currents in the GnRH neuron in A by the broad-spectrum Ca2+ channel inhibitor Cd2+. Outward currents in B are most likely Cs+ currents. C, Current-voltage (I–V) relationship for the Ca2+ channel activity in A and B. D, Ca2+ currents in a different adult GnRH neuron from that in A, evoked from a Vh of −100 mV. E, Ca2+ currents from the same GnRH neuron as in D, evoked from a Vh of −60 mV. F, Ca2+ currents from the same neuron as in D, evoked from a Vh of −40 mV. Note that changing Vh from −100 to −60 or −40 mV had little effect on Ca2+ currents.
Ca2+ currents varied markedly in peak amplitude among juvenile (Fig. 2, A and B), pubertal (Fig. 2, C and D), and adult (Figs. 1, A and E, and 2, E and F) GnRH neurons (range of peak current densities at 0 mV: −17 to −48, −22 to −58, and −16 to −42 pA/pF, respectively), even among those from the same mouse or slice.
Fig. 2.
Ca2+ currents vary markedly in amplitude among juvenile, pubertal, and adult GnRH neurons. A, Ca2+ currents in a juvenile GnRH neuron. B, Ca2+ currents in a different juvenile GnRH neuron than in A. C, Ca2+ currents in a pubertal GnRH neuron. D, Ca2+ currents in a different pubertal GnRH neuron than in C. E, Ca2+ currents in an adult GnRH neuron. F, Ca2+ currents in a different adult GnRH neuron than in E. These traces illustrate the wide range of Ca2+ current amplitudes in GnRH neurons. The voltage protocol, shown in A and used to obtain the traces in A–F, is identical with that in Fig. 1, A, B, and E.
Yet there were no significant differences in Ca2+ currents among juvenile (Fig. 3A), pubertal (Fig. 3B), and adult (Fig. 3C) GnRH neurons in perforated-patch recordings. Averaged peak (Fig. 3D), sustained (Fig. 3E), and tail (Fig. 3F) Ca2+ current densities were similar (P > 0.05) between juvenile (n = 12 GnRH neurons from four mice), pubertal (n = 12 GnRH neurons from eight mice), and adult (n = 12 GnRH neurons from five mice) GnRH neurons from −50 to +20 mV. Peak Ca2+ current densities at 0 mV, for example, were −31.1 ± 2.2, −35.5 ± 2.9, and −34.8 ± 2.8 pA/pF in juvenile, pubertal, and adult GnRH neurons, respectively, in perforated-patch recordings. In addition, no significant differences (P > 0.05) were found in the voltage corresponding to the maximum, peak current (−4.6 ± 1.9, −5.0 ± 1.9, and −4.6 ± 1.7 mV in juvenile, pubertal, and adult GnRH neurons, respectively). These data suggest that Ca2+ channel expression in GnRH neurons remains relatively constant across pubertal development.
Fig. 3.
Averaged Ca2+ currents are similar in juvenile, pubertal, and adult GnRH neurons in perforated-patch recordings. A, Ca2+ currents in a juvenile GnRH neuron. B, Ca2+ currents in a pubertal GnRH neuron. C, Ca2+ currents in an adult GnRH neuron. The currents in A–C are representative of the averaged Ca2+ currents in juvenile, pubertal, and adult GnRH neurons in perforated-patch recordings, obtained using the same voltage protocol as in Figs. 1, A, B, and E, and 2. D, Averaged peak Ca2+ current density as a function of membrane potential (Vm) in juvenile, pubertal, and adult GnRH neurons. E, Averaged sustained Ca2+ current density as a function of Vm in juvenile, pubertal, and adult GnRH neurons. F, Averaged tail Ca2+ current density as a function of Vm in juvenile, pubertal, and adult GnRH neurons. P, Peak; S, sustained; T, tail.
Ca2+ currents varied even more among juvenile, pubertal, and adult GnRH neurons (range of peak current densities at 0 mV: −12 to −57, −11 to −43, and −6 to −55 pA/pF, respectively) in whole-cell than perforated-patch recordings, possibly due to rundown associated with whole-cell recording (13).
However, for the most part, and as with the Ca2+ currents recorded using the perforated-patch technique, there were no significant differences in averaged Ca2+ currents between juvenile (Fig. 4A), pubertal (Fig. 4B), and adult (Fig. 4C) GnRH neurons in whole-cell recordings. Averaged peak (Fig. 4D), sustained (Fig. 4E), and tail (Fig. 4F) Ca2+ current densities were similar (P > 0.05) between juvenile (n = 15 GnRH neurons from four mice), pubertal (n = 14 GnRH neurons from four mice), and adult (n = 14 GnRH neurons from four mice) GnRH neurons from −50 to +20 mV in whole-cell recordings. Peak Ca2+ current densities at 0 mV, for example, were −26.3 ± 3.3, −29.4 ± 2.8, and −25.8 ± 3.5 pA/pF in juvenile, pubertal and adult GnRH neurons, respectively, in whole-cell recordings. Unlike in perforated-patch recordings, there were significant differences (P < 0.05) in peak Ca2+ current density between juvenile and pubertal GnRH neurons at −30 and −20 mV, at or near the steepest part of the V-shaped current-voltage relationship, although not between juvenile and adult or between pubertal and adult GnRH neurons. These differences may stem from the greater variability in peak current amplitude in whole-cell recordings. Also unlike in perforated-patch recordings, there was a small but significant pubertal leftward shift (P < 0.05) in the voltage required to elicit maximum, peak current (from −6.0 ± 1.2 mV in juvenile to −12.1 ± 1.9 mV in pubertal and −12.1 ± 1.5 mV in adult GnRH neurons). Nonetheless, taken together with the perforated-patch data, these whole-cell data suggest that Ca2+ channel expression in GnRH neurons remains relatively constant across pubertal development.
Fig. 4.
Averaged Ca2+ currents are similar in juvenile, pubertal, and adult GnRH neurons in whole-cell recordings. A, Ca2+ currents in a juvenile GnRH neuron. B, Ca2+ currents in a pubertal GnRH neuron. C, Ca2+ currents in an adult GnRH neuron. The currents in A–C are representative of the averaged Ca2+ currents in juvenile, pubertal, and adult GnRH neurons in whole-cell recordings, obtained using the same voltage protocol as in Figs. 1, A, B, and E, 2, and 3. D, Averaged peak Ca2+ current density as a function of membrane potential (Vm) in juvenile, pubertal, and adult GnRH neurons. E, Averaged sustained Ca2+ current density as a function of Vm in juvenile, pubertal, and adult GnRH neurons. F, Averaged tail Ca2+ current density as a function of Vm in juvenile, pubertal, and adult GnRH neurons. P, Peak; S, sustained; T, tail.
SK currents in juvenile, pubertal, and adult GnRH neurons
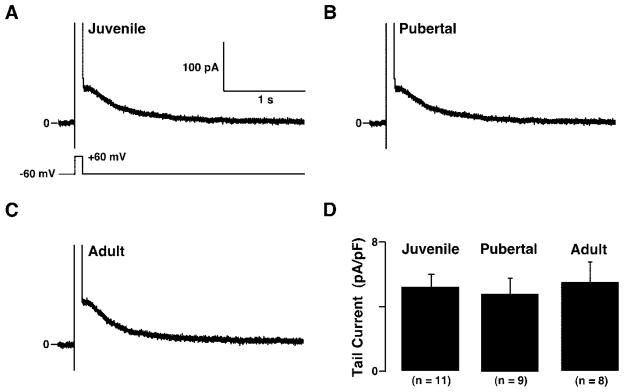
In addition to Ca2+ channel activity, GnRH neurons exhibited SK channel activity, recorded as the tail current upon returning to −60 mV after a voltage step from −60 mV to +60 mV, in perforated-patch recordings (Fig. 5A). The tail current was inhibited (Fig. 5, A and B) by the SK channel blocker apamin (41) in a concentration-dependent manner and enhanced (Fig. 5C) by the SK and intermediate-conductance Ca2+ -activated (IK) channel activator 1-EBIO (42), as summarized in Fig. 5D. Because on average only approximately 7% of the tail current was resistant to apamin (Fig. 5D), the tail current appeared to be carried almost exclusively by SK channels. Although slower than in other cell types (26, 27), the apamin-sensitive SK channels in mouse GnRH neurons displayed similar kinetics as those in adult rat GnRH neurons in brain slices (34).
Fig. 5.
GnRH neurons express SK channels. A, SK current, recorded as the tail current upon repolarization to −60 mV after a 100-msec depolarizing pulse from −60 to +60 mV, in the absence and presence of 10 nM apamin, an SK channel inhibitor. B, SK current recorded from a different GnRH neuron than in A, in the absence and presence of 100 nM apamin. C, SK current, recorded from a different GnRH neuron than those in A and B in the absence and presence of the SK and IK channel activator 1-EBIO. D, Averaged SK current inhibition by apamin and activation by 1-EBIO.
Like the Ca2+ currents, SK currents varied markedly in amplitude among juvenile (1.5–9.5 pA/pF; n = 11 GnRH neurons from eight mice), pubertal (1.4–10.0 pA/pF; n = 9 GnRH neurons from six mice), and adult (2.4–12.3 pA/pF; n = 8 GnRH neurons from five mice) GnRH neurons, possibly due to heterogeneous Ca2+ channel activity.
However, again like the Ca2+ currents, there were no significant differences in averaged SK current between juvenile (Fig. 6A), pubertal (Fig. 6B), and adult (Fig. 6C) GnRH neurons in perforated-patch recordings. Averaged SK current density was similar (P > 0.05) among juvenile, pubertal, and adult GnRH neurons (Fig. 6D). Because SK channel activity in GnRH and other neurons reflects changes in [Ca2+]i resulting from Ca2+ channel activity (27, 34), the lack of significant differences in SK current in GnRH neurons among juvenile, pubertal, and adult GnRH neurons was consistent with the lack of significant differences in total Ca2+ current and provided a further indication that Ca2+ channel expression in GnRH neurons remains relatively constant across pubertal development.
Fig. 6.
Averaged SK current is similar in juvenile, pubertal, and adult GnRH neurons. A, SK current in a juvenile GnRH neuron. B, SK current in a pubertal GnRH neuron. C, SK current in an adult GnRH neuron. D, Averaged peak amplitude of SK current in juvenile, pubertal, and adult GnRH neurons. The voltage protocol, shown in A and used to obtain the traces in A–C is identical with that in Fig. 5, A–C.
Discussion
This is the first report on Ca2+ and SK currents in GnRH neurons in mouse brain slices. Previous studies of GnRH neuron Ca2+ and/or SK currents were performed using GnRH neurons in embryonic olfactory placode explant cultures (43), immortalized GnRH neurons (31, 44–46), which do not permit investigation of developmental changes, or dissociated GnRH neurons without, or having only very short, dendritic, and axonal processes (24, 25, 34). In contrast, GnRH neurons in brain slice preparations and in vivo possess extensive, spiny, dendritic processes (47), as well as axon terminals, that possibly contain Ca2+ and SK channels in addition to those expressed in the soma, and the dendritic processes appear to undergo remodeling at puberty (48). The present study involving GnRH neurons in brain slices permitted the analysis of GnRH neuron combined somatic, dendritic, and axonal Ca2+ and SK currents before, during, and after puberty, although dendritic and axonal currents measured in the soma would likely have been small due to the reduced ability to control the voltage in those processes, compared with the soma when recording from the soma, as well as attenuation associated with passive spread of the currents in those processes.
The identities of the Ca2+ and SK currents recorded from GnRH neurons in brain slices in the present study were confirmed with pharmacological agents. Ca2+ currents were inhibited by the broad spectrum Ca2+ channel blocker Cd2+. SK currents were inhibited by the SK channel blocker apamin and enhanced by the SK and IK channel activator 1-EBIO.
The Ca2+ and SK currents in the present study varied markedly in their peak amplitude independent of the age of the GnRH neuron from which they were recorded. Variation in peak amplitude may be due to heterogeneity in channel expression, episodic changes in channel activity linked to GnRH pulsatility, or differences among GnRH neurons in the contributions of dendritic and axonal channels to channel activity, depending on the degree to which dendritic and axonal processes were retained during slicing. Previous whole-cell current-clamp recordings of evoked membrane potential changes in GnRH neurons suggested heterogeneity in Ca2+ channel expression in a development-specific manner (40). However, some of the neurons recorded from in that study may have been non-GnRH neurons, which would have accounted for some of the observed heterogeneity because they were identified based on location and morphology (rather than GnRH promoter-driven GFP fluorescence), and most but not all contained GnRH mRNA as determined by postrecording characterization with single-cell RT-PCR.
Despite the apparent heterogeneity, averaged Ca2+ and SK currents in juvenile, pubertal, and adult mouse GnRH neurons recorded with the perforated-patch or whole-cell technique in the present study were similar. The only statistically significant differences, which were observed in whole-cell recordings, were an increase in Ca2+ current density in pubertal, compared with juvenile, GnRH neurons at membrane potentials of −30 and −20 mV (rather than in maximum, peak Ca2+ current density, which occurred at membrane potentials between −20 and 0 mV), and a small leftward shift in the voltage, from −6 to −12 mV, required to evoke maximum, peak current. However, the first of these two differences was not maintained in adult GnRH neurons, and neither difference was observed in perforated-patch recordings. Nevertheless, these results contrast with the small but statistically significant increase in maximum, peak Ca2+ current density in adult vs. juvenile mouse GnRH neurons reported by Nunemaker et al. (24) using the whole-cell technique. On the other hand, they are in accord with the lack of a pubertal change in maximum, peak Ca2+ current density in rat GnRH neurons reported by Kato et al. (25) using the perforated-patch technique, which unlike the whole-cell technique allows for retention of cytoplasmic factors that could regulate Ca2+ or SK currents and is therefore more physiological. Hence, it appears that the discrepancy between the two previous reports on total Ca2+ currents in GnRH neurons (24, 25) is probably due more to a methodological than a species difference.
The similarities in Ca2+ and SK currents among juvenile, pubertal, and adult GnRH neurons suggest that Ca2+ and SK channel expression in GnRH neurons remains relatively constant across pubertal development. Total Ca2+ or SK current (I) at any given membrane potential equals the number of Ca2+ or SK channels (N) multiplied by the single-channel open probability (p) and single-channel current (i), i.e. I = Npi (13). A physiological change in i has yet to be described. Thus, for I not to change at puberty in GnRH neurons, which seems to be the case based on the present results, there would need to be equal and opposite changes in N and p. More likely is that neither N nor p changes, although there may be changes in N or p of individual Ca2+ or SK channel subtypes in GnRH neurons as suggested by previous studies (24, 25, 32). This implies that rather than being due to an alteration in the overall expression of Ca2+ or SK channels, which would affect N, or to a change in channel gating as may occur by phosphorylation, which would affect p (13), the pubertal increase in GnRH secretion is probably due to other mechanisms such as greater membrane depolarization and subsequent increased activation of preexisting Ca2+ channels after increased excitatory synaptic input.
Acknowledgments
This work was supported by the Louis B. Block Fund and Children’s Research Foundation of the University of Chicago.
Abbreviations
- [Ca2+]i
Cytoplasmic free Ca2+ concentration
- 1-EBIO
1-ethyl-2-benzimidazolinone
- GFP
green fluorescent protein
- I
current
- i
single-channel current
- IK
intermediate-conductance Ca2+-activated
- K(Ca)
Ca2+-activated K+
- N
number of Ca2+ or SK channels
- p
open probability
- P
postnatal day
- SK
small-conductance Ca2+-activated K+
- TEA
tetraethylammonium
- TTX
tetrodotoxin
Footnotes
Disclosure Statement: The author has nothing to disclose.
References
- 1.Grumbach MM. The neuroendocrinology of human puberty revisited. Horm Res. 2002;57(Suppl 2):2–14. doi: 10.1159/000058094. [DOI] [PubMed] [Google Scholar]
- 2.Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci. 2004;7:1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- 3.Ebling FJP. The neuroendocrine timing of puberty. Reproduction. 2005;129:675–683. doi: 10.1530/rep.1.00367. [DOI] [PubMed] [Google Scholar]
- 4.Plant TM. A study of the role of the postnatal testes in determining the ontogeny of gonadotropin secretion in the male rhesus monkey (Macaca mulatta) Endocrinology. 1985;116:1341–1350. doi: 10.1210/endo-116-4-1341. [DOI] [PubMed] [Google Scholar]
- 5.Matsumoto AM, Karpas AE, Southworth MB, Dorsa DM, Bremner WJ. Evidence for activation of the central nervous system-pituitary mechanism for gonadotropin secretion at the time of puberty in the male rat. Endocrinology. 1986;119:362–369. doi: 10.1210/endo-119-1-362. [DOI] [PubMed] [Google Scholar]
- 6.Urbanski HF, Ojeda SR. Gonadal-independent activation of enhanced afternoon luteinizing hormone release during pubertal development in the female rat. Endocrinology. 1987;121:907–913. doi: 10.1210/endo-121-3-907. [DOI] [PubMed] [Google Scholar]
- 7.Sisk CL, Richardson HN, Chappell PE, Levine JE. In vivo gonadotropin releasing hormone secretion in female rats during peripubertal development and on proestrus. Endocrinology. 2001;142:2929–2936. doi: 10.1210/endo.142.7.8239. [DOI] [PubMed] [Google Scholar]
- 8.Harris GC, Levine JE. Pubertal acceleration of pulsatile gonadotropin-releasing hormone release in male rats as revealed by microdialysis. Endocrinology. 2003;144:163–171. doi: 10.1210/en.2002-220767. [DOI] [PubMed] [Google Scholar]
- 9.Krsmanovic LZ, Stojilkovic SS, Merelli F, Dufour SM, Virmani MA, Catt KJ. Calcium signaling and episodic secretion of gonadotropin-releasing hormone in hypothalamic neurons. Proc Natl Acad Sci USA. 1992;89:8462–8466. doi: 10.1073/pnas.89.18.8462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stojilkovic SS, Krsmanovic LZ, Spergel DJ, Catt KJ. GnRH neurons: intrinsic pulsatility and receptor-mediated regulation. Trends Endocrinol Metab. 1994;5:201–209. doi: 10.1016/1043-2760(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 11.Terasawa E, Keen KL, Mogi K, Claude P. Pulsatile release of luteinizing hormone-releasing hormone (LHRH) in cultured LHRH neurons derived from the embryonic olfactory placode of the rhesus monkey. Endocrinology. 1999;140:1432–1441. doi: 10.1210/endo.140.3.6559. [DOI] [PubMed] [Google Scholar]
- 12.Martínez de la Escalera G, Clapp C. Regulation of gonadotropin-releasing hormone secretion: insights from GT1 immortal GnRH neurons. Arch Med Res. 2001;32:486–498. doi: 10.1016/s0188-4409(01)00320-4. [DOI] [PubMed] [Google Scholar]
- 13.Hille B. Ion channels of excitable membranes. 3. Sunderland, MA: Sinauer; 2001. [Google Scholar]
- 14.Ghijsen WEJM, Leenders AGM. Differential signaling in presynaptic neurotransmitter release. Cell Mol Life Sci. 2005;62:937–954. doi: 10.1007/s00018-004-4525-0. [DOI] [PubMed] [Google Scholar]
- 15.Tena-Sempere M. KiSS-1 and reproduction: focus on its role in the metabolic regulation of fertility. Neuroendocrinology. 2006;83:275–281. doi: 10.1159/000095549. [DOI] [PubMed] [Google Scholar]
- 16.Han S-K, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watanabe M, Sakuma Y, Kato M. High expression of the R-type voltage-gated Ca2+ channel and its involvement in Ca2+ -dependent gonadotropin-releasing hormone release in GT1–7 cells. Endocrinology. 2004;145:2375–2383. doi: 10.1210/en.2003-1257. [DOI] [PubMed] [Google Scholar]
- 18.Spergel DJ, Krsmanovic LZ, Stojilkovic SS, Catt KJ. Glutamate modulates [Ca2+]i and gonadotropin-releasing hormone secretion in immortalized hypothalamic GT1–7 neurons. Neuroendocrinology. 1994;59:309–317. doi: 10.1159/000126672. [DOI] [PubMed] [Google Scholar]
- 19.Spergel DJ, Krsmanovic LZ, Stojilkovic SS, Catt KJ. L-type Ca2+ channels mediate joint modulation by γ-aminobutyric acid and glutamate of [Ca2+]i and neuropeptide secretion in immortalized gonadotropin-releasing hormone neurons. Neuroendocrinology. 1995;61:499–508. doi: 10.1159/000126873. [DOI] [PubMed] [Google Scholar]
- 20.Uemura T, Nishimura J, Yamaguchi H, Hiruma H, Kimura F, Minaguchi H. Effects of noradrenaline on GnRH-secreting immortalized hypothalamic (GT1–7) neurons. Endocr J. 1997;44:73–78. doi: 10.1507/endocrj.44.73. [DOI] [PubMed] [Google Scholar]
- 21.Terasawa E, Keen KL, Grendell RL, Golos TG. Possible role of ATP in synchronization of Ca2+ oscillations in primate luteinizing hormone releasing hormone (LHRH) neurons. Mol Endocrinol. 2005;19:2736–2747. doi: 10.1210/me.2005-0034. [DOI] [PubMed] [Google Scholar]
- 22.Wada K, Hu L, Mores N, Navarro CE, Fuda H, Krsmanovic LZ, Catt KJ. 5-HT receptor subtypes mediate specific modes of serotonin-induced signaling and regulation of neurosecretion in GnRH neurons. Mol Endocrinol. 2006;20:125–135. doi: 10.1210/me.2005-0109. [DOI] [PubMed] [Google Scholar]
- 23.Martinez-Fuentes AJ, Hu L, Krsmanovic LZ, Catt KJ. Gonadotropin-releasing hormone (GnRH) receptor expression and membrane signaling in early embryonic GnRH neurons: role in pulsatile neurosecretion. Mol Endocrinol. 2004;18:1808–1817. doi: 10.1210/me.2003-0321. [DOI] [PubMed] [Google Scholar]
- 24.Nunemaker CS, DeFazio RA, Moenter SM. Calcium current subtypes in gonadotropin-releasing hormone neurons. Biol Reprod. 2003;69:1914–1922. doi: 10.1095/biolreprod.103.019265. [DOI] [PubMed] [Google Scholar]
- 25.Kato M, Ui-Tei K, Watanabe M, Sakuma Y. Characterization of voltage-gated calcium currents in gonadotropin-releasing hormone neurons tagged with green fluorescent protein in rats. Endocrinology. 2003;144:5118–5125. doi: 10.1210/en.2003-0213. [DOI] [PubMed] [Google Scholar]
- 26.Vogalis F, Storm JF, Lancaster B. SK channels and the varieties of slow after-hyperpolarizations in neurons. Eur J Neurosci. 2003;18:3155–3166. doi: 10.1111/j.1460-9568.2003.03040.x. [DOI] [PubMed] [Google Scholar]
- 27.Stocker M. Ca2+ -activated K+ channels: molecular determinants and function of the SK family. Nat Rev Neurosci. 2004;5:758–770. doi: 10.1038/nrn1516. [DOI] [PubMed] [Google Scholar]
- 28.Rossi P, D’Angelo E, Magistretti J, Toselli M, Taglietti V. Age-dependent expression of high-voltage activated calcium currents during cerebellar granule cell development in situ. Pflügers Arch. 1994;429:107–116. doi: 10.1007/BF02584036. [DOI] [PubMed] [Google Scholar]
- 29.Elsen FP, Ramirez J-M. Postnatal development differentially affects voltage-activated calcium currents in respiratory rhythmic versus nonrhythmic neurons of the pre-Bötzinger complex. J Neurophysiol. 2005;94:1423–1431. doi: 10.1152/jn.00237.2005. [DOI] [PubMed] [Google Scholar]
- 30.Cingolani LA, Gymnopoulos M, Boccaccio A, Stocker M, Pedarzani P. Developmental regulation of small-conductance Ca2+ -activated K+ channel expression and function in rat Purkinje neurons. J Neurosci. 2002;22:4456–4467. doi: 10.1523/JNEUROSCI.22-11-04456.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Goor F, Krsmanovic LZ, Catt KJ, Stojilkovic SS. Coordinate regulation of gonadotropin-releasing hormone neuronal firing patterns by cytosolic calcium and store depletion. Proc Natl Acad Sci USA. 1999;96:4101–4106. doi: 10.1073/pnas.96.7.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bosch MA, Kelly MJ, Rønnekleiv OK. Distribution, neuronal colocalization, and 17β-E2 modulation of small conductance calcium-activated K+ channel (SK3) mRNA in the guinea pig brain. Endocrinology. 2002;143:1097–1107. doi: 10.1210/endo.143.3.8708. [DOI] [PubMed] [Google Scholar]
- 33.Mellon PL, Windle JJ, Goldsmith PC, Padula CA, Roberts JL, Weiner RI. Immortalization of hypothalamic GnRH neurons by genetically targeted tumorigenesis. Neuron. 1990;5:1–10. doi: 10.1016/0896-6273(90)90028-e. [DOI] [PubMed] [Google Scholar]
- 34.Kato M, Tanaka N, Usui S, Sakuma Y. The SK channel blocker apamin inhibits slow afterhyperpolarization currents in rat gonadotropin-releasing hormone neurons. J Physiol. 2006;574:431–442. doi: 10.1113/jphysiol.2006.110155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spergel DJ, Krüth U, Hanley DF, Sprengel R, Seeburg PH. GABA-and glutamate-activated channels in green fluorescent protein-tagged gonadotropin-releasing hormone neurons in transgenic mice. J Neurosci. 1999;19:2037–2050. doi: 10.1523/JNEUROSCI.19-06-02037.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rugh R. The mouse: its reproduction and development. New York: Oxford University Press; 1991. [Google Scholar]
- 37.Akaike N, Harata N. Nystatin perforated patch recording and its applications to analyses of intracellular mechanisms. Jap J Physiol. 1994;44:433–473. doi: 10.2170/jjphysiol.44.433. [DOI] [PubMed] [Google Scholar]
- 38.Winer BJ. Statistical principles in experimental design. 2. New York: McGraw-Hill; 1971. [Google Scholar]
- 39.Suter KJ, Song WJ, Sampson TL, Wuarin J-P, Saunders JT, Dudek FE, Moenter SM. Genetic targeting of green fluorescent protein to gonadotropin-releasing hormone neurons: characterization of whole-cell electrophysiological properties and morphology. Endocrinology. 2000;141:412–419. doi: 10.1210/endo.141.1.7279. [DOI] [PubMed] [Google Scholar]
- 40.Sim JA, Skynner MJ, Herbison AE. Heterogeneity in the basic membrane properties of postnatal gonadotropin-releasing hormone neurons in the mouse. J Neurosci. 2001;21:1067–1075. doi: 10.1523/JNEUROSCI.21-03-01067.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Romey G, Hughes M, Schmid-Antomarchi H, Lazdunski M. Apamin: a specific toxin to study a class of Ca2+ -dependent K+ channels. J Physiol (Paris) 1984;79:259–264. [PubMed] [Google Scholar]
- 42.Syme CA, Gerlach AC, Singh AK, Devor DC. Pharmacological activation of cloned intermediate- and small-conductance Ca2+ -activated K+ channels. Am J Physiol Cell Physiol. 2000;278:C570–C581. doi: 10.1152/ajpcell.2000.278.3.C570. [DOI] [PubMed] [Google Scholar]
- 43.Kusano K, Fueshko S, Gainer H, Wray S. Electrical and synaptic properties of embryonic luteinizing hormone-releasing hormone neurons in explant cultures. Proc Natl Acad Sci USA. 1995;92:3918–3922. doi: 10.1073/pnas.92.9.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bosma MM. Ion channel properties and episodic activity in isolated immortalized gonadotropin-releasing hormone (GnRH) neurons. J Membr Biol. 1993;136:85–96. doi: 10.1007/BF00241492. [DOI] [PubMed] [Google Scholar]
- 45.Hales TG, Sanderson MJ, Charles AC. GABA has excitatory actions on GnRH-secreting immortalized hypothalamic (GT1–7) neurons. Neuroendocrinology. 1994;59:297–308. doi: 10.1159/000126671. [DOI] [PubMed] [Google Scholar]
- 46.Van Goor F, Krsmanovic LZ, Catt KJ, Stojilkovic SS. Control of action potential-driven calcium influx in GT1 neurons by the activation status of sodium and calcium channels. Mol Endocrinol. 1999;13:587–603. doi: 10.1210/mend.13.4.0261. [DOI] [PubMed] [Google Scholar]
- 47.Campbell RE, Han SK, Herbison AE. Biocytin filling of adult gonadotropin-releasing hormone neurons in situ reveals extensive, spiny, dendritic processes. Endocrinology. 2005;146:1163–1169. doi: 10.1210/en.2004-1369. [DOI] [PubMed] [Google Scholar]
- 48.Cottrell EC, Campbell RE, Han S-K, Herbison AE. Postnatal remodeling of dendritic structure and spine density in gonadotropin-releasing hormone (GnRH) neurons. Endocrinology. 2006;147:3652–3661. doi: 10.1210/en.2006-0296. [DOI] [PubMed] [Google Scholar]