Abstract
Objectives
To use consensus methods and the considerable expertise contained within the Children’s Arthritis and Rheumatology Research Alliance (CARRA) organization, to extend the 3 previously developed treatment plans for moderate juvenile dermatomyositis (JDM) to span the full course of treatment.
Methods
A consensus meeting was held in Chicago on April 23–24, 2010 involving 30 pediatric rheumatologists and 4 lay participants. Nominal group technique was used to achieve consensus on treatment plans which represented typical management of moderate JDM. A pre-conference survey of CARRA, completed by 151/272 (56%) members, was used to provide additional guidance to discussion.
Results
Consensus was reached on timing and rate of steroid tapering, duration of steroid therapy, and actions to be taken if patients were unchanged, worsening, experiencing medication side effects or disease complications. Of particular importance, a single, consensus steroid taper was developed.
Conclusions
We were able to develop consensus treatment plans which describe therapy for moderate JDM throughout the treatment course. These treatment plans can now be used clinically, and data collected prospectively regarding treatment effectiveness and toxicity. This will allow comparison of these treatment plans and facilitate the development of evidence-based treatment recommendations for moderate JDM.
Juvenile dermatomyositis (JDM) is a rare, autoimmune illness characterized by muscle and skin involvement, with less frequent involvement of other systems, including the gastrointestinal tract and lungs. Although previously associated with significant mortality (1), morbidity is a much greater problem since the introduction of corticosteroids as a mainstay of therapy. Many children experience complications of their underlying disease such as contractures, weakness, disfiguring skin lesions and painful calcinosis. They may also develop complications secondary to prolonged courses of treatment, in particular those side effects related to chronic corticosteroid use(2).
There is very little data on which to base treatment decisions in JDM. Even the most commonly used therapies, corticosteroids and/or methotrexate, have not been studied in clinical trials. In fact, at the time of this writing, no randomized clinical trials of therapy in JDM have been published. This lack of data has resulted in wide variation in treatment of children with JDM. This has been documented by Stringer et al. who reported the results of the Childhood Arthritis and Rheumatology Research Alliance (CARRA) JDM treatment survey (3). Using a series of representative clinical scenarios of children with JDM, members of CARRA were surveyed with regard to investigations and therapies. The survey was answered by 141 CARRA members (84% response rate), and revealed a remarkable degree of variation in dose, duration and route of corticosteroids, and use of methotrexate and other immunosuppressive agents (3).
In 2007, 12 pediatric rheumatologists experienced in the care of children with JDM participated in a CARRA-initiated consensus conference in Toronto, Ontario, Canada (4). This meeting had the explicit goal of defining a small number of consensus treatment plans for the initial treatment (up to 2 months) of children with moderate JDM. Using data from the CARRA JDM treatment survey (3) and expert opinion, the meeting participants were able to develop 3 consensus treatment plans. It is important to note that these treatment plans were not intended to be innovative. Rather, the goal was to develop treatment plans which were similar to approaches that were being used commonly in the pediatric rheumatology community. It was hoped that by developing consensus treatment plans, variation in treatment approaches could be decreased and data could be prospectively collected. This would allow for comparative research and would be the first step in defining evidence-based treatments for moderate JDM.
After the success of the Toronto consensus meeting, it was recognized that for these treatment plans to be studied, they needed to be extended beyond 2 months of treatment. The goal of the present effort was to use consensus methods, and the considerable expertise contained within the CARRA organization, to extend the 3 previously developed treatment plans to span the full course of treatment of children with moderate JDM.
Methods
CARRA
CARRA is a North American organization of pediatric rheumatologists whose mission is to prevent, treat and cure rheumatic diseases in children and adolescents through fostering, facilitating and conducting high quality research. CARRA currently has more than 304 members from 92 centres, and includes the majority of pediatric rheumatologists in North America.
Pre-meeting Survey
Prior to the consensus meeting, which was held during the 2010 annual CARRA meeting, an electronic survey was sent to all CARRA members. This survey collected information about treatment strategies beyond the initial 2 months and what information respondents used to make treatment decisions. Several options were provided for each question, with an open text field available for each question. Treatment-related questions from this survey are listed in Appendix 2. One hundred and fifty-one of 272 (56 % response rate) CARRA members responded to the questionnaire. The results were used to inform discussion during the consensus meeting.
Appendix 2.
Pre-consensus meeting JDM treatment survey questions. Additional questions concerning response to complications of disease and medication are not presented here.
| Question | Key responses | % (N) | |
|---|---|---|---|
| 1 | What criteria should be used to decide that a patient has improved and a taper can proceed? | Physician judgment based on physician global, patient global, and laboratory measures available. | 70.7% (104) |
| The definition of improvement (DOI) proposed by IMACS [ref] | 17.7% (26) | ||
| 2 | When should the first taper of prednisone be attempted (assuming patient has met improvement criteria)? | At 4 weeks | 41.5% (61) |
| When the patient first meets improvement criteria (possibly less than 4 weeks) | 20.4% (30) | ||
| After 4 weeks from when the patient first meets improvement criteria | 13.6% (20) | ||
| At 6 weeks | 12.2% (18) | ||
| 3 | At what interval should prednisone be tapered? | More frequently when on high dose, less frequently | 33.3% (49) |
| No fixed time, rather based only on clinical response | 20.4% (30) | ||
| Every 4 weeks | 16.3% (24) | ||
| Every 2–4 weeks | 15.0% (22) | ||
| Every 2 weeks | 11.6% (17) | ||
| 4 | By what dose should prednisone be tapered at each taper step? (If possible, choose the regimen which most closely matches your usual regimen.) | 10 mg per drop until the dose is 20 mg; 5 mg per drop thereafter | 23.8% (35) |
| If at 60 mg, drop by 10 mg until 50 mg; thereafter 5 mg per drop until 25 mg; thereafter 2.5 mg per drop until 10 mg; thereafter 1 mg per drop. | 23.1% (34) | ||
| 5 mg per drop until the dose is 25 mg; 2.5 mg per drop thereafter | 20.4% (30) | ||
| 10 mg per drop until the dose is 1 mg/kg; 5 mg per drop thereafter. | 15.6% (23) | ||
| Other (please specify) | 17.0% (25) | ||
| 5 | What criteria should be used to decide that a patient has worsened and a taper cannot proceed? | Physician judgment based on physician global, patient global, and laboratory measures available. | 71.9% (105) |
| The definition of worsening proposed by IMACS [ref Oddis 2005 A&R 52(9):2607–15] | 16.4% (24) | ||
| 6 | What should be done with the prednisone taper when a patient meets criteria for worsening/flare (mild or moderate)? | Physician judgment based on severity of worsening. | 53.4 % (78) |
| 7 | What other steps would you take when a patient meets criteria for worsening/flare (mild or moderate)? | Give “pulse” intravenous methylprednisolone (30 mg/kg, maximum 1000 mg) | 44.5% (65) |
| Add an additional anti-inflammatory/immunosuppressive agent. | 32.9% (48) | ||
| 8 | What should be done when a patient meets criteria for severe worsening/flare? | Physician judgment based on severity of worsening | 43.8% (64) |
| 9 | What other steps would you take when a patient meets criteria for severe worsening/flare? | Give “pulse” intravenous methylprednisolone (30 mg/kg, maximum 1000 mg) | 51.4% (75) |
| Add an additional anti-inflammatory/immunosuppressive agent | 22.6% (33) | ||
| 10 | What criteria should be used to decide that a patient is unchanged? | The patient is unchanged on either the patient or physician global measure (depending on availability) AND the patient is not in remission. | 55.9% (81) |
| The patient is unchanged on physician global measures AND the patient is not in remission. | 35.9% (52) | ||
| 11 | What should be done when a patient meets criteria for being unchanged (assuming patient is not in remission)? | Escalate dose of prednisone or add another treatment. | 37.2% (54) |
| Hold dose steady for 1 month. | 26.9% (39) | ||
| Hold dose steady until next planned taper. | 13.1% (19) |
The respondents to this survey had graduated from medical school on average in 1991 (range 1961–2006, standard deviation 11 years), were 61% female (N=92), had a mean age of 45.2 (range 28–73 years, standard deviation 10.5) and were actively managing a median of 8 (range 0–210, 25th percentile 5, 75th percentile 15) patients with JDM at the time of the survey.
Consensus meeting
The 2-day consensus meeting (April 23–24, 2010) was attended by 30 pediatric rheumatologists, one of whom acted as facilitator (Adam Huber). The facilitator participated in all discussions, but did not vote. There were also 4 lay participants with particular interest and/or experience with JDM who participated in discussions, but did not vote.
Despite the challenge imposed by the large number of meeting participants, nominal group technique was used to achieve consensus for all questions considered during the meeting. The same format was used for each question. The facilitator framed the question to be discussed, and then presented data from the survey relevant to that question. Each participant then had an opportunity to express their opinion for 1–2 minutes without interruption. Potential responses to the question were recorded on flip charts at the front of the room, and detailed notes were kept. Participants were then given the opportunity to vote for their preferred responses to the questions. Each participant had 5 stickers, which they attached to the flip charts; stickers could be distributed in any way (e.g. all 5 on one answer or 1 sticker each for 5 answers). If there was a clear preferred response, this was considered to be the consensus answer to the question. If not, participants were once again given the opportunity to speak uninterrupted for 1–2 minutes. After discarding answers which clearly were not preferred, there was another round of voting by each participant with another 5 stickers. This process continued until there was clear consensus or a stalemate was reached. At some points, if the choices had been reduced to 2, a show-of-hands vote was conducted if all participants agreed to an open vote. Consensus was defined as at least 80%.
The process was dynamic, with discussion and decisions from earlier in the meeting influencing the questions discussed and the overall goals as the meeting progressed. In fact, the overall approach to the goal of extending the treatment plans beyond 2 months changed during the meeting. It became clear on day one of the meeting that the most important component of the extended treatment plan was the approach to the corticosteroid taper. Initially, it was intended that the corticosteroid taper would be based on the answers to the following questions:
When should the first taper of prednisone be attempted, assuming patient has met improvement criteria?
At what interval should corticosteroids be tapered?
By what dose should prednisone be tapered at each step?
During the first day, it became clear that there were some logistic issues with this approach. For example, some proposed tapering protocols resulted in differences in corticosteroid duration based on body weight. As discussion progressed on day one, most consensus participants also realized that they were more interested in total time on corticosteroids than in the tapering details. It was then agreed, unanimously, that the corticosteroid taper would be based on achieving targets (e.g. reaching certain percentages of the starting corticosteroid dose at specified times, such as 75% of starting dose by time 1).
The questions which were considered during this consensus meeting are summarized in Table 1. Table 2 shows the patient characteristics considered to be consistent with moderate JDM and Table 3 shows the initial 2 month treatments, as decided during the Toronto consensus meeting (4).
Table 1.
Questions and results considered during the consensus meeting.
| Question | Method | Responses* | Votes | |
|---|---|---|---|---|
| 1-initial vote | What criteria should be used to decide that a patient has improved and a taper can proceed? | Sticker vote | Physician judgment based on core set (improvement/unacceptable toxicity) | 50 |
| Strength improved or normal | 23 | |||
| 1-final vote | What criteria should be used to decide that a patient has improved and a taper can proceed? | Hand vote | Patient improved or unacceptable toxicity (defined as strength improved or normal AND enzymes improved or normal AND rash stable/improved/absent AND additional criteria consistent improvement) | 25 |
| Physician judgment based on core set improvement or unacceptable toxicity | 1 | |||
| 2-initial vote | When should first taper of prednisone be attempted (assuming patient has met improvement criteria)? | Sticker vote | 4 weeks | 60 |
| When 1st meets improvement criteria | 48 | |||
| 2-final vote | When should first taper of prednisone be attempted (assuming patient has met improvement criteria)? | Hand vote | 4 weeks | 20 |
| When 1st meets improvement criteria | 4 | |||
| 3-initial vote | At what interval should prednisone be tapered? | Sticker vote | Every 2 weeks from 2-0.5mg/kg, then every 4 weeks | 43 |
| Ever 2 – 4 weeks | 25 | |||
| Every 4 weeks, then when less than 0.2 mg/kg every 8 weeks | 24 | |||
| Every 2 weeks | 20 | |||
| 3-second vote | At what interval should prednisone be tapered? | Sticker vote | Ever 2 weeks at 2-0.5mg/kg, then q4 wks | 66 |
| Every 2 weeks | 29 | |||
| Every 4 weeks at 2.0-0.2 mg/kg, then every 8 weeks | 28 | |||
| 3-final vote | At what interval should prednisone be tapered? | Hand vote | Every 2 weeks at 2-0.5mg/kg, then q4 wks | 24 |
| Disagree | 3 | |||
| 4-initial vote | By what dose should prednisone be tapered at each taper step? | Sticker vote | 20% of current dose | 56 |
| 10% of current dose | 38 | |||
| 0.25mg/kg x4, 0.125mg/kg x4, then 0.05mg/kg x4 to 5mg, then off | 15 | |||
| 5 | When should children come off corticosteroids? | No voting | 4–8 months | n/a |
| >12 months | n/a | |||
| 6 | What criteria should be used to decide that a patient is unchanged? | Hand vote | Not improved and not worse (physician judgment | 19 |
| Physician judgment | 1 | |||
| Physician and parent judgment (combined) | 1 | |||
| 7-initial vote | What should be done when a patient meets criteria for being unchanged (assuming patient is not in remission)? | Sticker vote | Hold dose for 4 weeks, then escalate therapy if still unchanged | 45 |
| Physician judgment | 38 | |||
| 7-final vote | What should be done when a patient meets criteria for being unchanged (assuming patient is not in remission)? | Hand vote | Hold dose for 4 weeks, then escalate therapy if still unchanged | 14 |
| Physician judgment | 3 |
only responses with substantial support
Table 2.
Patient characteristics for moderate JDM.
|
Patients should have:
| |
| Rash (Gottron’s rash, heliotrope rash, or extensor surface rash) | |
| Muscle weakness | |
| Evidence of myositis (by biopsy, magnetic resonance imaging, or electromyography) | |
| Age <16 years at onset | |
| Physician global assessment of moderate (on a 3-category scale of mild, moderate, or severe) | |
|
Patients should NOT have:
| |
| Severe disability as defined by can’t get out of bed, CMAS score <15, or MMT8 score <30 | |
| Parenchymal lung disease | |
| Gastrointestinal vasculitis (as determined by imaging or presence of bloody stools) | |
| Other autoimmune or mimicking disease (as determined by the treating physician) | |
| Requirement for intensive care unit management | |
| Presence of aspiration or dysphagia to the point of inability to swallow | |
| Central nervous system disease (defined as decreased level of consciousness or seizures) | |
| Skin ulceration | |
| Medication contraindication | |
| Myocarditis | |
| Pregnancy | |
| Significant calcinosis (as determined by the treating physician) | |
| Age <1 year | |
CMAS=Childhood Myositis Assessment Score; MMT8=Manual Muscle Testing, 8-muscle group score
Table 3.
Initial treatment plans for first 4 weeks (4).
|
Treatment A:
| |
| Intravenous methylprednisolone: 30 mg/kg/day (max 1 g) once a day for 3 days. May continue 1x per week (optional) | |
| Methotrexate (subcutaneous unless only oral possible): Lesser of 15 mg/m2 or 1 mg/kg (max 40 mg) once weekly | |
| Prednisone 2 mg/kg/day (max 60 mg) once daily × 4 weeks, then follow schedule in Table 4 | |
|
Treatment B:
| |
| Intravenous methylprednisolone: 30 mg/kg/day (max 1 g) once a day for 3 days. May continue 1x per week (optional) | |
| Methotrexate (subcutaneous unless only oral possible): Lesser of 15 mg/m2 or 1 mg/kg (max 40 mg) once weekly | |
| Prednisone 2 mg/kg/day (max 60 mg) once daily × 4 weeks, then follow schedule in Table 4 | |
| Intravenous immunoglobulin 2 g/kg (max 70 g), q 2 weeks × 3, then monthly (Optional intravenous methylprednisolone × 1 with each dose) | |
|
Treatment C:
| |
| Methotrexate (subcutaneous unless only oral possible): Lesser of 15 mg/m2 or 1 mg/kg (max 40 mg) once weekly | |
| Prednisone 2 mg/kg/day (max 60 mg) once daily × 4 weeks, then follow schedule in Table 4 | |
Results
The first question discussed concerned what criteria could be used to determine that a patient was improved and the corticosteroid taper could begin. There was considerable discussion about the role of physician judgment and how to quantify this. It was agreed (25/26 votes, 96%) that although physician judgment was needed to determine this point, it should be based on the following criteria: strength improved or normal AND enzymes improved or normal AND rash stable/improved/absent AND additional criteria consistent with improvement (not specifically defined, and could vary depending on patient and physician factors).
The second question concerned when corticosteroid tapering could begin, assuming improvement criteria were met. The discussion largely centered on whether tapering could begin at the first point where improvement was documented, or if one should wait until a certain amount of time had elapsed. Consensus was reached (20/24 votes, 83%) for waiting until 4 weeks of treatment (assuming improvement criteria were met).
The third question concerned the frequency of corticosteroid weaning. There was considerable variation in opinion during the first round of voting. However, in the ensuing discussion, several participants indicated that their choice for longer intervals was based on concerns that patients might not be ready for a dose reduction due to a variety of clinical or biochemical factors. When it was emphasized that weaning of corticosteroid would only occur when the physician thought it was appropriate, they indicated they would be comfortable with a shorter weaning period. After 2 further rounds of voting, consensus was reached (24/27 votes, 89%) on a weaning interval of every 2 weeks on corticosteroid doses from 2-0.5 mg/kg, and then every 4 weeks thereafter.
The fourth question addressed how much corticosteroid should be tapered at each step. This question resulted in considerable discussion. There were two main issues. First, it became clear that there were two camps among the participants—a group which favored an aggressive, faster tapering of corticosteroid (off corticosteroid by 4–8 months) and another group which favored a slower taper (greater than 12 months to discontinuation). Second, while the participants were willing to discuss the approach set out for the meeting (deciding on how often to wean and by how much), many were more comfortable with setting specific targets (i.e. how long to come off corticosteroids, how long to get to 1 mg/kg etc.). Much of this discussion occurred near the end of the first day. On day two, it was agreed that the approach would be changed, and that participants would attempt to reach consensus on weaning targets (unanimous). Question 4 was not discussed further.
Before proceeding to the next question, there was discussion about which percentages should be used as targets. It was agreed that the targets would be the following: 75%, 50%, 25%, 10% and 0% of initial starting corticosteroid dose.
The fifth question concerned when children would come off corticosteroids, and formed the basis for establishing the steroid tapering schedule. It initially appeared that it would not be possible to reach consensus, as there were substantial numbers of participants who favored either a short or a long period of corticosteroid. At this point, these 2 groups were separated and asked to independently develop tapering regimens. Despite not being instructed to do so, both groups developed very similar plans. There was unanimity that the facilitator would generate a corticosteroid weaning plan which summarized the 2 proposed plans. This consensus steroid weaning plan is found in Table 4.
Table 4.
Consensus steroid tapering schedule. Treating physicians may either use the “any weight column” to calculate steroid targets or the weight column which is closest to the patient in question.
| Any Weight | 10 kg | 15 kg | 20 kg | 25 kg | ≥30 kg | |
|---|---|---|---|---|---|---|
| 0 weeks (100%) | 2 mg/kg | 20 | 30 | 40 | 50 | 60 |
| 4 weeks | 17.5 | 27.5 | 35 | 45 | 55 | |
| 6 weeks | 25 | 32.5 | 40 | 50 | ||
| 8 weeks (75%) | 1.5 mg/kg | 15 | 22.5 | 30 | 37.5 | 45 |
| 10 weeks | 12.5 | 20 | 25 | 32.5 | 40 | |
| 12 weeks | 17.5 | 22.5 | 27.5 | 35 | ||
| 14 weeks (50%) | 1.0 mg/kg | 10 | 15 | 20 | 25 | 30 |
| 16 weeks | 12.5 | 17.5 | 20 | 25 | ||
| 18 weeks | 7.5 | 10 | 15 | 17.5 | 20 | |
| 20 weeks | 12.5 | 15 | 17.5 | |||
| 22 weeks (25%) | 0.5 mg/kg | 5 | 7.5 | 10 | 12.5 | 15 |
| 24 weeks | 8 | 10 | 12.5 | |||
| 26 weeks | 4 | 5 | 7.5 | 10 | ||
| 28 weeks | 6 | 7.5 | ||||
| 30 weeks | 3 | 5 | ||||
| 32 weeks | 2.5 | 4 | 5 | |||
| 34 weeks | 2 | |||||
| 36 weeks (10%) | 0.2 mg/kg | 1.5 | 2 | 2.5 | 3 | |
| 38 weeks | 1 | |||||
| 40 weeks | 1 | 2 | ||||
| 42 weeks | 1 | |||||
| 44 weeks | 1 | |||||
| 46 weeks | 1 | |||||
| 50 weeks (off) | off | 0 | 0 | 0 | 0 | 0 |
At this point, there was little time left in the meeting. The final discussions concerned how to define whether a patient’s disease status was unchanged, and what actions to take if patients were unchanged on visits where corticosteroid tapering was planned. The results of these discussions are summarized in Table 1 (Question 6 and 7). Finally, participants agreed that other issues that were not discussed (e.g. what to do if patients are worsening, responses to drug toxicity or disease complications) would be left to the judgment of the treating physician. This is consistent with the results of the pre-meeting survey.
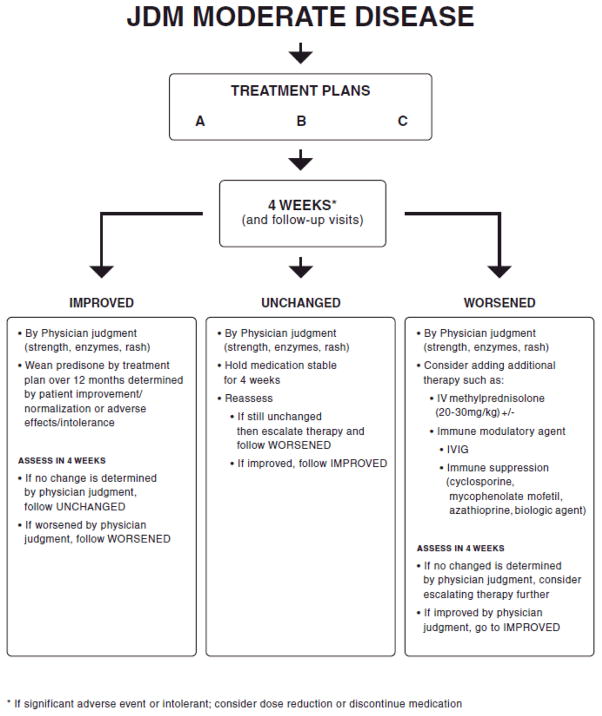
A summary of patient flow when using these treatment plans is found in Figure 1. The complete treatment plans including assessment recommendations, can be downloaded from the CARRA website at www.carragroup.org.
Figure 1.
Patient flow diagram for consensus treatment plans.
* If significant adverse event or intolerant; consider dose reduction or discontinue medication
Discussion
Through a consensus process, members of CARRA who participated in this meeting were able to develop consensus treatment plans which extended therapy beyond the initial two months. In combination with the results of the first consensus meeting (4), the resulting 3 complete treatment plans describe typical therapy for children with moderate JDM from treatment initiation to discontinuation of corticosteroids. Considering the amount of variation in treatment approaches that has been previously documented (3), this is a remarkable achievement.
It is important to point out that these treatment plans are not to be considered standard of care and are not innovative or cutting-edge therapy for moderate JDM. These treatment plans will not be appropriate for all patients with moderate JDM, depending on a variety of difficult to quantify factors. It is also possible that some patients will need to deviate from a treatment plan, depending on their disease course and the opinion of the treating physician. The intent was to develop treatment plans that were similar to the care that would commonly be provided by the majority of pediatric rheumatologists. This will limit treatment variation and facilitate prospective, meaningful comparisons between treatments and assist in future development of evidence-based recommendations. It is emphasized that these are not treatment recommendations. Approaches different than these treatment plans may be equally or more safe and effective. Given that these treatment plans are based on expert opinion of usual practice, they can be considered as representing a low level of evidence (Level3 according to the Strength of Recommendation Taxonomy (5)).
If these treatment plans are not intended as recommendations, the ultimate goal of developing them requires discussion and explanation. There have been no randomized clinical trials published for treatment of new onset JDM, related partly to the rarity of the illness and partly to difficulties in assessing this complex illness. We note that there is an ongoing clinical trial involving corticosteroids, methotrexate and cyclosporin in new-onset JDM (http://www.printo.it/project_ongoing_detail.asp?ProjectID=14). However, it is unlikely that there will be substantial numbers of clinical trials in the future. This will continue to limit the development of evidence-based treatment recommendations in JDM. For this reason, CARRA has chosen the following alternative approach to determine the best treatments in JDM. Comparative Effectiveness Research (CER) includes powerful methods of evaluating therapies through analysis of prospectively collected data obtained during the provision of routine clinical care (6, 7). This approach is being used widely and promoted by the National Institutes of Health, insurers and policy makers as an alternative to the traditional clinical trial (8, 9). CER has significant advantages over randomized clinical trials in the areas of cost and efficiency, and given that data are collected within the context of clinical care, may be more generalizable.
Our intent is that treating pediatric rheumatologists will be able to use a treatment plan which is similar to their usual practice. By doing so, variation will be reduced and large numbers of children will be treated with one of these treatment plans. Prospective data collected on these patients can then be used for CER to determine which treatment approach is the most successful, with regard to both effectiveness and toxicity. Subsequent iterations of CER using updated and revised treatment plans will ultimately lead to determination of the optimal care for children with moderate JDM. In the future, this methodology will be expanded to include other forms of JDM (mild, severe, ulcerative, etc.) and to include other rheumatic illnesses.
The assessment of children with JDM is challenging. Progress has been made in the description of core sets assessments (10, 11) and preliminary definitions of improvement (12–14). These recommendations include multiple assessments, and are best suited to clinical trials with appropriate infrastructure support. Unfortunately, the combination of these assessments is probably not practical in the typical clinician’s office. For this reason, we have previously recommended a reduced, minimum data collection to be used as part of the CER data collection, with the more detailed assessments left as an option (4).
There are some potential limitations to this work. Although the consensus meeting involved a large number of pediatric rheumatologists, there are likely some who would disagree with the decisions made. There was considerable discussion about some issues within our small group. It is recognized that not all physicians will be able to identify a treatment plan which is similar to their usual care of moderate JDM and that it is impossible for a treatment plan to accommodate all possible courses that a patient may take. These treatment plans do not replace the clinical judgment of treating physicians. Also, while statistical methods exist which are intended to control for patient factors which influence treatment decisions, there is no guarantee that these methods will be able to completely eliminate bias in the comparison of these treatment plans.
In conclusion, we present here the completed CARRA Consensus Treatments for children with new onset moderate JDM. This has been a collaborative effort of the investigators of CARRA, and as such should have wide appeal and acceptability to pediatric rheumatologists across North America and beyond. The next steps are to prospectively collect data on patients treated with these plans as part of routine clinical care, and through an iterative, analytic process identify the treatments with the best outcomes and least side effects for children with JDM.
Appendix 1.
Members of the Juvenile Dermatomyositis Sub-Committee of the Children’s Arthritis and Rheumatology Research Alliance who participated in the Chicago Consensus Meeting, April 23–24, 2010.
| Leslie Abramson |
| Barbara Adams |
| Sharon Bout-Tabaku |
| Ruy Carrasco |
| Megan Curran |
| Peter Dent |
| Barbara Edelheit |
| Brian Feldman |
| Adam Huber |
| Josephine Isgro |
| Harry Gewanter |
| Thomas Griffin |
| Kathleen Haines |
| Mark Hoeltzel |
| Philip Kahn |
| Dan Kingsbury |
| Ann Kunkel |
| Bianca Lang |
| Angela Robinson |
| Heinrike Schmeling |
| Kara Schmidt |
| Rosie Scuccimarri |
| Bracha Shaham |
| Michael Shishov |
| Elizabeth Stringer |
| Heather Van Mater |
| Carol Wallace |
| Lay participants |
| John Hayhurst |
| Patti Lawler |
| Debbie Wright |
| Julie Wohrley |
Significance and Innovation.
we have successfully identified consensus treatment plans for moderate juvenile dermatomyositis which reflect commonly used treatment approaches
these treatment plans include consensus on corticosteroid weaning
this is an important step in the development of evidence-based treatment recommendations for moderate juvenile dermatomyositis
Acknowledgments
This work was completed with support from the National Institute of Arthritis and Musculoskeletal and Skin Disease at the National Institutes of Health (NIH RC1AR058605-01 and R13-AR053058-04), the Children’s Arthritis and Rheumatology Research Alliance (CARRA), the Arthritis Foundation, the Wasie Foundation and Friends of CARRA.
Our sincere thanks to Drs. Lisa Rider, Lauren Pachman and Dan Solomon for their valuable input into this project and to Audrey Hendrickson and Vaishali Tenkale for their outstanding administrative assistance.
Contributor Information
Adam M. Huber, IWK Health Centre and Dalhousie University, Halifax, NS.
Angela B. Robinson, Rainbow Babies and Children’s Hospital, Cleveland, OH.
Ann M. Reed, Mayo Clinic School of Medicine and Mayo Foundation, Rochester, MN.
Leslie Abramson, University of Vermont College of Medicine and Fletcher Allen Health Care, Burlington, VT.
Sharon Bout-Tabaku, Nationwide Children’s Hospital, Columbus, OH.
Ruy Carrasco, Dell Children’s Hospital, Austin, TX.
Megan Curran, Children’s Memorial Hospital, Chicago, IL.
Brian M. Feldman, Hospital for Sick Children and University of Toronto, Toronto, ON.
Harry Gewanter, Pediatric and Adolescent Health Partners, Richmond, VA.
Thomas Griffin, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH.
Kathleen Haines, Children’s Hospital, Hackensack University Medical Center, Hackensack, NJ.
Joseph M. Sanzari, Children’s Hospital, Hackensack University Medical Center, Hackensack, NJ
Mark F. Hoeltzel, Children’s Mercy Hospital, Kansas City, MO.
Josephine Isgro, Morgan Stanley Children’s Hospital of New York – Presbyterian, New York, NY.
Philip Kahn, New York University School of Medicine, New York, NY.
Bianca Lang, IWK Health Centre and Dalhousie University, Halifax, NS.
Patti Lawler, The Cure JM Foundation.
Bracha Shaham, Children’s Hospital Los Angeles and USC Keck School of Medicine, Los Angeles, CA.
Heinrike Schmeling, Alberta Children’s Hospital and University of Calgary, Calgary, AB.
Rosie Scuccimarri, Montreal Children’s Hospital/McGill University Health Centre, Montreal, QC.
Michael Shishov, Phoenix Children’s Hospital, Phoenix, AZ.
Elizabeth Stringer, IWK Health Centre and Dalhousie University, Halifax, NS.
Julie Wohrley, The Cure JM Foundation.
Norman T. Ilowite, Children’s Hospital at Montefiore and Albert Einstein College of Medicine, Bronx, NY.
Carol Wallace, Seattle Children’s Hospital and University of Washington, Seattle, WA.
References
- 1.Bitnum S, Daeschner C, Jr, Travis LB, Dodge WF, Hopps HC. Dermatomyositis. J Pediatr. 1964;64(1):101–31. doi: 10.1016/s0022-3476(64)80325-5. [DOI] [PubMed] [Google Scholar]
- 2.Huber AM, Lang B, LeBlanc CM, Birdi N, Bolaria R, Malleson P, et al. Medium- and Long-Term Functional Outcomes in a Multicenter Cohort of Children with Juvenile Dermatomyositis. Arthritis Rheum. 2001;43(3):541–9. doi: 10.1002/1529-0131(200003)43:3<541::AID-ANR9>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 3.Stringer E, Ota S, Bohnsack J, Bowyer SL, Griffin TA, Huber AM, et al. Treatment approaches to juvenile dermatomyositis across North America: The Childhood Arthritis and Rheumatology Research Alliance (CARRA) JDM treatment survey. J Rheumatol. 2009;37(9):1953–61. doi: 10.3899/jrheum.090953. [DOI] [PubMed] [Google Scholar]
- 4.Huber AM, Giannini EH, Bowyer SL, Kim S, Lang B, Lindsley CB, et al. Protocols for the initial treatment of moderately severe juvenile dermatomyositis: results of a Children’s Arthritis and Rheumatology Research Alliance Consensus Conference. Arthritis Care Res (Hoboken) 2010 Feb;62(2):219–25. doi: 10.1002/acr.20071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ebell MH, Siwek J, Weiss BD, Woolf SH, Susman J, Ewigman B, et al. Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. Am Fam Physician. 2004 Feb 1;69(3):548–56. [PubMed] [Google Scholar]
- 6.Sox HC. Defining comparative effectiveness research: the importance of getting it right. Med Care. 2010 Jun;48(6 Suppl):S7–8. doi: 10.1097/MLR.0b013e3181da3709. [DOI] [PubMed] [Google Scholar]
- 7.Tunis SR, Benner J, McClellan M. Comparative effectiveness research: Policy context, methods development and research infrastructure. Stat Med. 2010 Aug 30;29(19):1963–76. doi: 10.1002/sim.3818. [DOI] [PubMed] [Google Scholar]
- 8.Sox HC. Comparative effectiveness research: a progress report. Ann Intern Med. 2010 Oct 5;153(7):469–72. doi: 10.7326/0003-4819-153-7-201010050-00269. [DOI] [PubMed] [Google Scholar]
- 9.Luce BR, Kramer JM, Goodman SN, Connor JT, Tunis S, Whicher D, et al. Rethinking randomized clinical trials for comparative effectiveness research: the need for transformational change. Ann Intern Med. 2009 Aug 4;151(3):206–9. doi: 10.7326/0003-4819-151-3-200908040-00126. [DOI] [PubMed] [Google Scholar]
- 10.Ruperto N, Ravelli A, Murray K, Lovell DJ, Andersson-Gare B, Feldman BM, et al. Preliminary core sets of measures for disease activity and damage assessment in juvenile systemic lupus erythematosus and juvenile dermatomyositis. Rheumatology (Oxford) 2003;42(12):1452–9. doi: 10.1093/rheumatology/keg403. [DOI] [PubMed] [Google Scholar]
- 11.Miller FM, Rider LG, Chung Y, Cooper R, Danko K, Farewell V, et al. Proposed preliminary core set measures for disease outcome assessment in adult and juvenile idiopathic inflammatory myopathy. Rheumatology (Oxford) 2001;40:1262–73. doi: 10.1093/rheumatology/40.11.1262. [DOI] [PubMed] [Google Scholar]
- 12.Ruperto N, Pistorio A, Ravelli A, Rider LG, Pilkington C, Oliveira S, et al. The Paediatric Rheumatology International Trials Organisation provisional criteria for the evaluation of response to therapy in juvenile dermatomyositis. Arthritis Care Res (Hoboken) 2010 Nov;62(11):1533–41. doi: 10.1002/acr.20280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rider LG, Giannini EH, Brunner HI, Ruperto N, James-Newton L, Reed AM, et al. International consensus on preliminary definitions of improvement in adult and juvenile myositis. Arthritis Rheum. 2004;50(7):2281–90. doi: 10.1002/art.20349. [DOI] [PubMed] [Google Scholar]
- 14.Rider LG, Giannini EH, Harris-Love M, Joe G, Isenberg D, Pilkington C, et al. Defining clinical improvement in adult and juvenile myositis. J Rheumatol. 2003;30:603–17. [PubMed] [Google Scholar]