Abstract
Background
This study examined the extent to which cigarette smoking and nicotine dependence in adults with alcohol dependence (AD) are associated with adverse childhood experiences. Gender, social support, and an allelic variant in the gene encoding the serotonin transporter (5-HTTLPR) were examined as moderators of this relationship.
Methods
The Semi-Structured Assessment for the Genetics of Alcoholism- Version II (SSAGA-II) was used to assess DSM-IV diagnoses and cigarette smoking characteristics as well as traumatic life events and social support during childhood in 256 AD men (n=149) and women (n=107).
Results
An increase in number of adverse childhood events was associated with heightened risk of cigarette use and nicotine dependence. 5-HTTLPR genotype, gender, and social support did not significantly moderate the relationships among childhood adversity and ever-smoking or nicotine dependence.
Conclusions
Results extend previous findings to suggest that childhood adversity is strongly related to risk for ever-smoking and nicotine dependence in AD individuals. Additional research is needed to examine other potential genetic and environmental moderators and mediators of the relationships among smoking, alcohol use, and childhood trauma.
Keywords: tobacco, substance abuse, trauma, gene-environment interaction
1. Introduction
Almost half of all alcohol dependent adults are also nicotine dependent (Le Strat et al., 2010), and there is a positive correlation between levels of alcohol and nicotine use (Falk et al., 2006). The co-occurrence of nicotine and alcohol dependence is associated with increased psychopathology, morbidity, and mortality, with tobacco-related disease being the leading cause of death in recovering alcohol dependent patients (Hurt et al., 1996; Le Strat et al., 2010).
A variety of biopsychosocial factors contribute to the co-occurrence of alcohol and nicotine dependence. Among the environmental influences that have been linked to alcohol and nicotine dependence, childhood maltreatment is one of the more robust common risk factors for these two disorders. For example, one study found that experiencing four or more adverse childhood events was associated with a two- to four-fold increase in risk for being a current smoker and a five- to eight-fold increase in risk for alcoholism (Felitti et al., 1998). Regarding cigarette smoking, childhood abuse is associated with early-onset smoking, smoking initiation (King et al., 2006; Lloyd and Taylor, 2006; Jun et al., 2008), current smoking (Chartier et al., 2009), heavy smoking (King et al., 2006), and nicotine dependence (Nelson et al., 2002). Among non-alcoholics, research also suggests a positive, graded relationship between the number of adverse childhood events and the severity and prevalence of smoking (Anda et al., 1999; van Loon et al., 2005). With respect to alcohol use, exposure to childhood trauma also predicts an earlier onset of heavy drinking (Waldrop et al., 2007) and is a major risk factor for the development and maintenance of alcohol use disorders (De Bellis, 2002).
Genetic influences contribute to the development and maintenance of substance use disorders in general, including nicotine and alcohol dependence (Nguyen et al., 2011). It has been estimated that 50 to 75% of the total variance in nicotine dependence is explained by genetic factors (Goldman et al., 2005; Vink et al., 2005). The serotonin transporter polymorphism (5-HTTLPR) is one of several genetic variants that have been associated with an increased risk for smoking; however, findings regarding which allelic variant of 5-HTTLPR contributes to smoking behaviors are mixed (Ishikawa et al., 1999; Gerra et al., 2005; Kremer et al., 2005). In addition, growing evidence indicates that genetic vulnerability may only become apparent under particular environmental conditions such as early life stress (Bennett et al., 2002; Barr et al., 2003; Capsi et al., 2003). For example, research examining how childhood trauma may interact with 5-HTTLPR genotype indicates that the homozygous long allele (LL) genotype is associated with a predisposition for increased alcohol use (Barr et al., 2003) and heavier drinking (Laucht et al., 2009). Regarding childhood trauma, the 5-HTTLPR, and cigarette smoking, Nilsson et al. (2009) found that adolescents with the Long-Short (LS) genotype who reported unfavorable family environments were more likely to be a smoker and have higher ratings of nicotine dependence. Lerer et al. (2006), on the other hand, found no association between 5-HTTLPR and smoking in a population of women who had been exposed to a traumatic life event. These two studies are the only reports published to date examining the interaction of 5-HTTLPR and trauma in relation to smoking outcomes, but they are limited because they focused exclusively on women (Lerer et al., 2006) and adolescents (Nilsson et al., 2009), respectively. Additionally, neither study specifically examined traumatic childhood experiences as they interact with 5-HTTLPR.
Interpretation of these findings is even more challenging when one considers that much of the previously published work on gene by environment interactions (e.g., Lerer et al., 2006 and Nilsson et al., 2009) did not incorporate the novel discovery of two distinct variants of the L allele—the higher-expressing LA and the lower-expressing LG variants—which indicates that 5-HTTLPR may be functionally tri-allelic rather than bi-allelic (Wendland et al., 2006). Furthermore, analyzing 5-HTTLPR as tri-allelic rather than bi-allelic may explain some of the mixed findings regarding smoking risk and gene by environment interactions. The study that provided the basis for the tri-allelic grouping in the present study (Wendland et al., 2006) was performed prior to the finding that the —G SNP occurred within the S allele, yet it is still unclear as to whether the SG allele is functionally different that the SA allele. Thus, the present study examines the functionally distinct variants of the L allele and considers 5-HTTLPR by childhood trauma interactions identified in the alcohol literature to predict that the presence of the LA allele would be associated with a greater likelihood of nicotine dependence and ever-smoking in alcohol dependent adults.
Other environmental factors are also likely to moderate the relationship between childhood maltreatment and the later development of alcohol and nicotine dependence. For example, studies have found an inverse relationship between social support and substance use in adolescents (Wills et al., 1992; Wills and Cleary, 1996), and these supportive relationships may act as a protective factor against the risk of smoking initiation. In the context of childhood trauma, research suggests that social support may reduce the risk of smoking by 40% in women who were both physically and sexually abused (Jun et al., 2008).
There is also evidence to suggest that the effects of trauma on risk of smoking may differ by gender. Specifically, women with a history of childhood trauma may exhibit a stronger propensity toward future alcohol use problems than trauma-exposed men (Horwitz et al., 2001; Macmillan et al., 2001; Simpson and Miller, 2002; Hyman et al., 2006). Regarding smoking, women with a trauma history exhibit higher rates of lifetime and current smoking than men who report being traumatized (Hapke et al., 2005).
Despite the importance of these biological and environmental factors in the development and persistence of nicotine and alcohol addiction, relatively few studies have examined factors that may moderate the relationship between childhood trauma and nicotine dependence. Also, no studies have examined the association between childhood trauma and nicotine dependence in an alcohol dependent population despite the frequent co-occurrence of these addictive disorders. Thus, the aim of the present study was to determine the extent to which adverse childhood experiences predict cigarette smoking and nicotine dependence in adults with alcohol dependence in a model that considers relevant genetic and environmental moderators of these relationships. We hypothesized that: 1) childhood adversity would be associated with an increased likelihood of co-occurring cigarette smoking and nicotine dependence among adults with alcohol dependence; and 2) 5-HTTLPR, gender, and social support would moderate the relationship between childhood adversity and ever smoking and nicotine dependence, such that having more of the high-expressing LA alleles, being female, and having less social support would further increase the likelihood of smoking and nicotine dependence among individuals who had been exposed to childhood adversity.
2. Methods
2.1. Participants
Participants were 256 alcohol dependent (AD) men (n=149) and women (n=107) participating in one of two contemporaneously conducted studies performed by the authors examining: (1) the effects of gender and alcohol dependence on physiological stress responsiveness (ClinicalTrials.gov Identifier: NCT00226694); and (2) pharmacogenetic differences in response to citalopram as a treatment for alcohol dependence (ClinicalTrials.gov Identifier: NCT00249405). These studies were reviewed and approved by the University of Cincinnati Medical Center Institutional Review Board and Cincinnati Veterans Affairs Medical Center Research and Development Committee, and written informed consent was obtained in accordance with these policies. A previously published report included a subsample of these same participants (Heffner et al., 2010). A majority of the participants were Caucasian (n=205; 80%) with a mean age of 45.6 (SD=9.22) years. All of the participants met Diagnostic and Statistical Manual for Mental Disorders, 4th edition, Text-Revised (DSM-IV-TR; American Psychiatric Association, 2000) criteria for alcohol dependence. In order to meet inclusion criteria for either study, participants had to be between the ages of 21 and 65, have no current mood, anxiety, or lifetime psychotic disorders, and not be taking any psychotropic medications. Nicotine dependence, substance use disorders in early or sustained full remission, a lifetime or current diagnosis of a substance-induced mood disorder, attention-deficit/hyperactivity disorder, past mood and anxiety disorders, and antisocial personality disorder were not exclusionary. Recruitment of the participants was achieved through radio, television, and newspaper advertisements as well as through presentations to clients in substance abuse treatment programs in the greater Cincinnati area.
2.2. Assessments
The Semi-Structured Assessment for the Genetics of Alcoholism – Version II (SSAGA-II; Bucholz et al., 2004) is a comprehensive, semi-structured interview used to assess major Axis I psychiatric disorders and antisocial personality disorder according to DSM-IV-TR criteria. Validity and reliability of the SSAGA-II are well established (Bucholz et al., 2004), and it is particularly useful in differentiating between substance-induced and independent psychiatric disorders. The Family History Assessment Module (FHAM; Rice et al., 1995) was used to assess major psychiatric diagnoses (i.e., alcohol dependence, drug dependence, depression, mania, schizophrenia, and antisocial personality disorder) in first-, second-, and third-degree relatives of the participant based on DSM-III-R criteria.
Alcohol dependence criteria were assessed via the alcohol section of the SSAGA-II. Similarly, the tobacco section of the SSAGA-II was used to assess nicotine dependence criteria and lifetime smoking patterns. Consistent with epidemiologic definitions, ever-smoking was defined as smoking 100 or more cigarettes during one’s lifetime.
Physical abuse, sexual abuse, poverty, or witnessing domestic violence were assessed from ages 6–13 using the childhood home environment interview section of the SSAGA-II. Parental mood disorder and parental substance abuse/dependence were assessed using the FHAM. These six categories of adverse events are consistent with those used in prior studies examining the relationship between childhood adversity and smoking in general population samples (e.g., Anda et al., 1999). Each category of adverse childhood experiences endorsed was counted as one event, resulting in a range of adverse events from zero to six. Social support was also assessed with the SSAGA-II, and was defined as having a close, confiding relationship with a parent or another adult during ages 6–13.
2.3. Procedures
The data used in the present secondary analysis were obtained as part of the screening procedures for the two studies from which this pooled sample was derived. Individuals underwent a preliminary phone screen to determine eligibility. If eligibility criteria were met, participants completed an in-person screening process involving administration of the SSAGA-II and the FHAM. Both measures were administered by trained research assistants, and diagnostic consensus was achieved through review by a board-certified psychiatrist, psychologist, or postdoctoral clinical psychology trainee working under the supervision of a licensed psychologist. Only individuals who had complete diagnostic data from the SSAGA-II were included in the present sample.
The deletion/insertion polymorphism (L, S), and single nucleotide polymorphism (rs25531) contained in the same promoter region of 5-HTTLPR were genotyped by a modification of the method described by Wendland et al. (2006; see Thompson et al., 2010 for a more detailed description), yielding alleles SA, SG, LA, and LG. As described in Thompson et al. (2010), the 5-HTTLPR genotypes were analyzed under the assumption that the LG allele was functionally equivalent to the low-expressing S alleles (Hu et al., 2006) and resultant genotypes were classified according to the number of high-expressing LA alleles.
2.4. Statistical Analyses
In order to examine gender, genotype, and social support as potential moderators of the relationship between number of adverse childhood events (ACEs) and nicotine dependence, four iterative logistic regression analyses were performed. The ACEs variable was considered as a continuous variable in all analyses. Three preliminary logistic regressions were conducted to examine each potential moderator and its interaction with ACEs. A fourth logistic regression represented the final reduced model, which included the covariates that significantly differed (p<.05) between nicotine dependent and non-nicotine dependent individuals, and any main effects or interactions that emerged as significant predictors (p<.05) in the three prior logistic regression analyses. This procedure was repeated for the analyses to predict ever-smoking.
Prior to conducting the logistic regression analyses, independent samples t-tests and Fisher’s exact tests were used to determine variables related to smoking status and nicotine dependence in this sample. Variables were selected based on prior research demonstrating their association with nicotine dependence and ever-smoking. A two-tailed alpha level of p<.05 was used to establish statistically significant relationships between predictors and the two dependent variables, ever-smoking and nicotine dependence. The predictive value of the full models was estimated using Nagelkerke’s R2. Hosmer and Lemeshow’s procedure (1989) was used to evaluate goodness of fit for each model. Data were analyzed using SPSS v. 17.0.
3. Results
Preliminary analyses revealed differences between nicotine dependent and non-nicotine dependent participants in number of years of education, lifetime substance use disorders, age-at-onset of regular drinking, lifetime anxiety or mood disorder, and conduct disorder with or without antisocial personality disorder (see Table 1). Similarly, ever-smokers and never-smokers differed on all of these characteristics except lifetime anxiety or mood disorders (see Table 2).
Table 1.
Demographic and Clinical Characteristics by Lifetime Nicotine Dependence Diagnosis
| Nicotine Dependent (N=140) | No Diagnosis (N=116) | p | |
|---|---|---|---|
| Age, M (SD) | 39.26 (8.37) | 46.57 (8.31) | .146 |
| Gender (male), no. (%) | 81 (58) | 68 (59) | .999 |
| Race (Caucasian), no. (%) | 115 (82) | 90 (78) | .432 |
| Education (yrs.), M (SD) | 12.21 (1.84) | 13.05 (2.40) | .012 |
| Any substance use disorder (lifetime), no. (%) | 63 (45) | 28 (24) | .001 |
| Age onset of drinking, M (SD) | 18.43 (5.16) | 20.48 (6.20) | .004 |
| Mood and/or anxiety disorders*, no. (%) | 8 (6) | 1 (1) | .043 |
| Conduct Disorder with or without Antisocial Personality Disorder, no. (%) | 24 (17) | 6 (5) | .003 |
Includes lifetime major depressive disorder, posttraumatic stress disorder, and other anxiety disorders.
Table 2.
Demographic and Clinical Characteristics by Lifetime Ever-Smoking
| Ever-Smoker (n=177) | Never Smoker (n=79) | p | |
|---|---|---|---|
| Age, M (SD) | 44.94 (9.64) | 47.24 (8.03) | .065 |
| Gender (male), no. (%) | 99 (56) | 50 (63) | .277 |
| Race (Caucasian), no. (%) | 140 (79) | 65 (82) | .614 |
| Education (yrs.), M (SD) | 13.55 (2.20) | 14.71 (1.81) | <.001 |
| Any substance use disorder (lifetime), no. (%) | 74 (42) | 17 (22) | .002 |
| Age onset of drinking, M (SD) | 18.76 (5.42) | 20.70 (6.21) | .012 |
| Mood and/or anxiety disorders*, no. (%) | 8 (5) | 1 (1) | .282 |
| Conduct Disorder with or without Antisocial Personality Disorder, no. (%) | 28 (16) | 2 (3) | .001 |
Includes lifetime major depressive disorder, posttraumatic stress disorder, and other anxiety disorders.
Thus, these variables were included as covariates in the subsequent logistic regression analyses. In the present sample of alcohol dependent individuals, 69.1% (n=177) met criteria for being an ever-smoker and 54.7% (n=140) met criteria for a lifetime diagnosis of nicotine dependence. In relation to number of LA alleles, six individuals were missing genotype data and were excluded from the analysis examining the interaction between LA alleles and ACEs. Of the remaining participants, 21.2% (n=53) had no LA alleles, 51.2% (n=128) had 1 LA allele, and 27.6% (n=69) were homozygous for LA alleles. With regard to childhood adversity, 11.3% (n=29) of the total sample reported childhood sexual abuse, 12.5% (n=32) reported childhood physical abuse, 12.1% (n=31) reported childhood poverty, 7.4% (n=19) reported witnessing domestic violence during childhood, 7.1% (n=18) reported a parental mood disorder, and 19.7% (n=50) reported parental substance abuse or dependence.
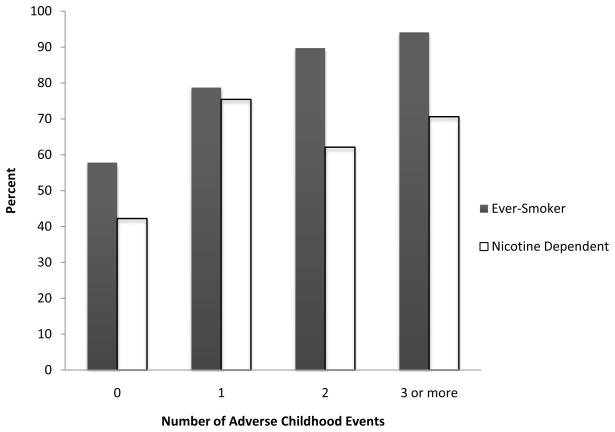
Figure 1 depicts the prevalence of being an ever-smoker or being nicotine dependent by the number of adverse childhood events (ACEs) experienced. As illustrated, an increase in number of ACEs was generally associated with an increase in ever-smoking and nicotine dependence, respectively: 57.8% and 42.2% for participants who reported no ACEs (n=147); 78.7% and 75.4% for those who reported one ACE (n=61); 89.7% and 62.1% for those who reported two ACEs (n=29); and 94.1% and 70.6% for those who reported three or more ACEs (n=17).
Figure 1.
Ever-smoking and nicotine dependence by number of adverse childhood events.
3.1. Nicotine Dependence
After including the previously mentioned covariates (i.e., number of years of education, lifetime substance use disorders, age-at-onset of regular drinking, lifetime anxiety or mood disorder, and conduct disorder with or without antisocial personality disorder), three logistic regression analyses to predict nicotine dependence indicated that the three separate interactions of ACEs with gender (p=.36), number of LA alleles (p=.62), and social support (p=.08) were all nonsignificant. However, number of ACEs emerged as a significant predictor (Wald χ2 =4.55, p=.03, OR=1.40, 95% CI=1.03–1.90). The odds ratio obtained for this variable suggests that, for every additional ACE, the odds of being nicotine dependent increased by 40%. None of the main effects of gender (p=.21), LA alleles (p=.56), or social support (p=.20) were significant. Table 3 portrays the final reduced model (R2=.17).
Table 3.
Results of the Logistic Regression Models to Predict Nicotine Dependence and Ever Smoking Status
| β | SE | Wald χ2 | OR | 95% CI | p | |
|---|---|---|---|---|---|---|
| Nicotine Dependence | ||||||
| Highest Grade Level Achieved | −0.14 | 0.07 | 4.16 | 0.87 | 0.76–1.00 | .042 |
| Age Onset of Regular Drinking | −0.03 | 0.03 | 1.78 | 0.97 | 0.92–1.01 | .185 |
| Other SUD | −0.58 | 0.30 | 3.58 | 0.56 | 0.31–1.02 | .058 |
| Mood and/or Anxiety Disorder | −1.74 | 1.11 | 2.45 | 0.18 | 0.02–1.55 | .175 |
| CD, with or without ASPD | −0.47 | 0.53 | 0.80 | 0.63 | 0.22–1.75 | .372 |
| Number ACEs | 0.34 | 0.16 | 4.55 | 1.40 | 1.03–1.90 | .033 |
| Ever-Smoking | ||||||
| Highest Grade Level Achieved | −0.19 | 0.08 | 5.94 | 0.83 | 0.71–0.96 | .015 |
| Age Onset of Regular Drinking | −0.02 | 0.03 | 0.82 | 0.98 | 0.93–1.03 | .366 |
| Other SUD | − 0.51 | 0.35 | 2.12 | 0.60 | 0.31–1.18 | .140 |
| CD, with or without ASPD | −1.08 | 0.79 | 1.86 | 0.34 | 0.07–1.60 | .172 |
| Number ACEs | 0.71 | 0.22 | 10.31 | 2.04 | 1.32–3.15 | .001 |
Note: SE=standard error; OR=odds ratio; CI=confidence interval; SUD=substance use disorder; ASPD=antisocial personality disorder; CD=conduct disorder; ACE=adverse childhood experience.
3.2. Ever-Smoking
After controlling for the covariates described above, three logistic regression analyses examining the relationship between ever-smoking and the cumulative effects of childhood adversity indicated that the separate gender (p=.37), social support (p=.11), and 5-HTTLPR genotype (p=.78) by ACE interactions were nonsignificant. In the final model (R2=.21), number of adverse childhood events emerged as a significant predictor of ever-smoking (Wald χ2 =10.32, p=.001, OR=2.04, 95% CI=1.32–3.15; see Table 3). Examination of the effect size for number of ACEs indicates that for every additional ACE, the odds of being an ever-smoker are approximately doubled. There were no significant main effects of the other predictor variables (i.e., gender, p=.68; LA alleles, p=.90; or social support, p=.21).
4. Discussion
In this study, we found a positive relationship between the number of adverse childhood events and the prevalence of ever-smoking and nicotine dependence among alcohol dependent men and women. Whereas ever-smoking consistently increased with each category of ACE endorsed, nicotine dependence reached a threshold after just one ACE (see Figure 1). This finding of a positive, graded relationship, particularly between number of adverse childhood events and the likelihood of being an ever-smoker, is consistent with previous research (Anda et al., 1999; De Von Figueroa-Moseley et al., 2004; Nichols and Harlow, 2004; King et al., 2006; Lloyd and Taylor, 2006) and extends these findings to include alcohol dependent adults. Prior studies found that other mental health problems may mediate the relationship between childhood adversity and smoking (De Bellis, 2002; Chartier et al., 2009). However, in this study, the effects of several commonly co-occurring psychiatric disorders were controlled for either methodologically (by exclusion of individuals with current mood, anxiety, or psychotic disorders) or statistically (by covarying for the effects of lifetime psychiatric disorders including other substance use disorders, mood or anxiety disorders and conduct disorder), suggesting that the relationship between childhood adversity and smoking was not influenced by the presence of these other mental health problems.
Contrary to expectations, social support was not a significant moderator in the relationship between childhood adversity and smoking status. This finding is inconsistent with previous research indicating that social support may act as a buffer against some of the adverse effects of childhood trauma and may also help prevent substance use (Wills et al., 1992; Wills and Cleary, 1996; Jun et al., 2008). This discrepancy may be due to limitations associated with the one-item indicator assessing social support in the present study. In addition, the relative homogeneity of the current sample may help explain this discrepancy; this study included only individuals with an alcohol dependence syndrome, possibly missing the protective effects of social support in those who did not go on to develop a diagnosable substance use disorder other than nicotine dependence. The inclusion of a broader spectrum of substance use problems or a control group without alcohol or other non-nicotine substance use disorders may have illuminated this relationship. Furthermore, the buffering effects of social support have been found to be stronger for marijuana and alcohol use than for smoking (Wills and Cleary, 1996), which may help explain why there was no relationship between social support and smoking outcomes in the current study. In addition, the positive effects of social support on prevention of smoking may be dependent upon other factors that were not assessed, such as behavioral coping skills or academic competence (Wills and Cleary, 1996). Due to these limitations, it is possible that the influence of social support on the development of smoking and nicotine dependence was underestimated in our analyses.
Also contrary to expectations, women with a history of childhood adversity were not more vulnerable to ever-smoking and nicotine dependence than men with a similar history. These findings contradict previous research suggesting that women with lifetime exposure to any type of trauma have higher levels of ever-smoking and current smoking than men (Hapke et al., 2005), and suggest that alcohol dependent women and men who have been traumatized are both equally susceptible to smoking and nicotine dependence. It is possible, however, that in the current study the gender-specific effects of childhood sexual abuse were masked by the use of a composite index of childhood adversities that included a variety of potentially traumatic experiences (i.e., physical abuse, parental substance abuse/dependence, parental mood disorder, poverty, or witnessing domestic violence), for which the effects may not be as gender-specific. It is also possible that the reported rates of these events as recorded during the face-to-face interview are artificially low, since other groups have reported rates of ACEs in the range of 30% to 60% in alcohol dependent patients using other ascertainment methods (Moncrieff et al., 1996; Rice et al., 2001; Greenfield et al., 2002).
The current results did not support a 5-HTTLPR genotype by environment interaction in relation to smoking in this sample of alcohol dependent adults with no current mood, anxiety, or psychotic disorders. These findings are in contradiction to findings by Nilsson et al. (2009), who found a significant interaction between the LS genotype and smoking in male and female adolescents. However, the current findings are consistent with previous research which found no relationship between the serotonin transporter gene and life stress in relation to smoking status among women (Lerer et al., 2006), and extend this earlier finding to include both men and women with alcohol dependence. Thus, the results of the current study suggest that the finding of the 5-HTTLPR by early life stress interaction in predicting the development of some mood and substance use disorders (Lesch et al., 1996; Barr et al., 2003; Capsi et al., 2003; Bleich et al., 2007; Kaufman et al., 2007; Laucht et al., 2009) does not generalize to the development of smoking and nicotine dependence in an alcohol dependent population. Notably, Lerer et al. (2006) found associations between stress and other variants of the serotonin receptor genes in terms of smoking, illustrating the importance of further exploring multiple genes that may predict smoking in the presence of adversity.
The current study has several limitations. The aim of this post hoc analysis was not the primary aim of the original investigations from which the participants were drawn; thus, we relied exclusively on retrospective self-reports of childhood adversity and tobacco use, which are subject to recall bias and, in the case of childhood trauma, underreporting of adverse experiences (Johnson et al., 2010). An additional limitation stemming from the secondary nature of the analyses is that data were not collected on other childhood factors that are associated with increased risk of both ACEs and cigarette smoking, such as low socioeconomic status and poor parental monitoring. The relationship between childhood trauma and smoking status may have been more clearly delineated from a prospective study rather than retrospective report, and the lack of a control group without alcohol dependence precluded us from examining the role of alcohol use in the relationship between childhood adversity and smoking. Also, although the SSAGA-II is a valid and reliable measure of substance use disorders and other psychiatric conditions, other instruments may have been able to better tap into a broader range of social support and childhood adversity. Another limitation was that the sample size was relatively small for the analysis of genetic influences; however, it is comparable to the sample sizes utilized in other studies that measured similar gene by environment interactions (e.g., Laucht et al., 2009). In addition, we only examined one single-nucleotide polymorphism (SNP) among multiple ones that may be associated with such phenomena. A final limitation is the reduced generalizability of the current clinical sample to the general population of individuals with alcohol dependence due to the fact that this was a treatment-seeking sample, and most psychiatric comorbidities were exclusionary.
Despite these limitations, we demonstrated a positive, graded relationship between number of adverse childhood events and ever smoking in adults with alcohol dependence using comprehensive, well-validated diagnostic assessments. These results not only provide additional evidence to support the relationship between cumulative adverse childhood events on smoking status and nicotine dependence, but also suggest that further investigation into comorbidities of nicotine dependence may help to advance our understanding of the relationship between trauma and smoking. Additional longitudinal research is needed to explore what are likely to be bidirectional relationships between smoking and alcohol use among individuals who have experienced childhood trauma in order to illuminate risk or protective factors, including peer influence, academic performance, and parenting styles.
Acknowledgments
Role of Funding Sources
This work was supported by National Institute of Alcohol Abuse and Alcoholism grants AA013307 and AA013957 to R.M.A., National Institute on Drug Abuse grants CSP #1022 to R.M.A. and DA026517 to J.L.H., and by the Department of Veterans Affairs. Funding sources had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
The authors would like to thank Candace Johnson, Ph.D.; Patricia Shay; Deonna Suggs, B.S.; Rebecca Kramer, B.A.: Kerri Dawson-Earles, B.S.; Lori Buns, N.P.; Show Lin, M.D., Suzan Winders-Barrett, Ph.D., Giao Tran, Ph.D., and Reene Cantwell for their assistance on this project. In addition, we would like to thank Judith Strong, Ph.D. and Gary Wand, M.D. for their technical assistance with genotyping. We are also indebted to the staff and clients of the Cincinnati VA Substance Dependence Program, Talbert House Pathways for Women Program, First Step Home, Transitions Women’s Recovery Addiction Program, Center for Chemical Addictions Treatment, and the Crossroads Center for their involvement in the study.
Footnotes
Contributors
Drs. Anthenelli and Heffner designed the study. Ms. Mingione and Dr. Heffner managed the literature searches, undertook the data analysis, and wrote the first draft of the manuscript. Mr. Blom contributed to the data analysis. All authors made significant contributions to editing drafts of the manuscript. All authors contributed to and have approved the final manuscript.
Conflicts of Interest
Ms. Mingione and Mr. Blom have no competing interests to disclose. Dr. Heffner provides consultancy services to Pfizer. Dr. Anthenelli provides consultancy and/or advisory board services to Pfizer and GlaxoSmithKline. The Tri-State Tobacco and Alcohol Research Center receives research support from Lilly, Pfizer, Nabi Biopharmaceuticals, and Sanofi-Aventis.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; Washington, DC: 2000. Text-Revision. [Google Scholar]
- Anda RF, Croft JB, Felitti VJ, Nordenberg D, Giles WH, Williamson DF, Giovino GA. Adverse childhood experiences and smoking during adolescence and adulthood. JAMA. 1999;282:1652–1658. doi: 10.1001/jama.282.17.1652. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Becker ML, Champoux M, Lesch KP, Suomi SJ, Goldman D, Higley JD. Serotonin transporter gene variation is associated with alcohol sensitivity in rhesus macaques exposed to early-life stress. Alcohol Clin Exp Res. 2003;27:812–817. doi: 10.1097/01.ALC.0000067976.62827.ED. [DOI] [PubMed] [Google Scholar]
- Bennett AJ, Lesch KP, Heils A, Long JC, Lorenz JG, Shoaf SE, Champoux M, Suomi SJ, Linnoila MV, Higlry JD. Early experience and serotonin transporter gene variation interact to influence primate CNS function. Mol Psychiatry. 2002;7:118–122. doi: 10.1038/sj.mp.4000949. [DOI] [PubMed] [Google Scholar]
- Bleich S, Bonsch D, Rauh J, Bayerlein K, Fiszer R, Frieling H, Hillemacher T. Association of the long allele of the 5-HTTLPR polymorphism with compulsive craving in alcohol dependence. Alcohol Alcohol. 2007;42:509–512. doi: 10.1093/alcalc/agm068. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Capsi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chartier MJ, Walker JR, Naimark B. Health risk behaviors and mental health problems as mediators of the relationship between childhood abuse and adult health. Am J Public Health. 2009;99:847–854. doi: 10.2105/AJPH.2007.122408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD. Developmental traumatology: a contributory mechanism for alcohol and substance use disorders. Psychoneuroendocrinocrinology. 2002;27:155–170. doi: 10.1016/s0306-4530(01)00042-7. [DOI] [PubMed] [Google Scholar]
- De Von Figueroa-Moseley C, Landrine H, Klonoff EA. Sexual abuse and smoking among college student women. Addict Behav. 2004;29:245–251. doi: 10.1016/j.addbeh.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Falk DE, Yi H, Hiller-Sturmhofel S. An epidemiologic analysis of co-occurring alcohol and tobacco use and disorders. Alcohol Res Health. 2006;29:162–171. [PMC free article] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. Am J Prev Med. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Gerra G, Garofano L, Zalmovic A, Mol G, Branchi B, Bussandri M, Brambilla F, Donnini C. Association of the serotonin transporter promoter polymorphism with smoking behavior among adolescents. Am J Med Genet B Neuropsychiatr Genet. 2005;135B:73–78. doi: 10.1002/ajmg.b.30173. [DOI] [PubMed] [Google Scholar]
- Greenfield SF, Kolodziej ME, Sugarman DE, Muenz LR, Vagge LM, He DY, Weiss RD. History of abuse and drinking outcomes following inpatient alcohol treatment: a prospective study. Drug Alcohol Depend. 2002;67:227–234. doi: 10.1016/s0376-8716(02)00072-8. [DOI] [PubMed] [Google Scholar]
- Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nat Rev Genet. 2005;6:521–532. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- Hapke U, Schumann A, Rumpf H, John U, Konerding U, Meyer C. Association of smoking and nicotine dependence with trauma and posttraumatic stress disorder in a general population sample. J Nerv Ment Dis. 2005;193:843–846. doi: 10.1097/01.nmd.0000188964.83476.e0. [DOI] [PubMed] [Google Scholar]
- Heffner JL, Johnson CS, Blom TJ, Anthenelli RM. Relationship between cigarette smoking and childhood trauma symptoms of inattention and hyperactivity/impulsivity in alcohol-dependent adults without attention-deficit hyperactivity disorder. Nicotine Tob Res. 2010;12:243–250. doi: 10.1093/ntr/ntp200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz AV, Widom CS, McLaughlin J, White HE. The impact of childhood abuse and neglect on adult mental health: A prospective study. J Health Soc Behav. 2001;42:184–201. [PubMed] [Google Scholar]
- Hosmer DW, Lemeshow S. Applied Logistic Regression. Wiley; New York: 1989. [Google Scholar]
- Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, Xu K, Arnold PD, Richter MA, Kennedy JL, Murphy DL, Goldman D. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am J Hum Genet. 2006;78:815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt RD, Offord KP, Croghan IT, Gomez-Dahl L, Kottke TE, Morose RM, Melton J. Mortality following inpatient addictions treatment. JAMA. 1996;275:1097–1103. doi: 10.1001/jama.275.14.1097. [DOI] [PubMed] [Google Scholar]
- Hyman SM, Garcia M, Sinha R. Gender specific associations between types of childhood maltreatment and the onset, escalation and severity of substance use in cocaine dependent adults. Am J Drug Alcohol Abuse. 2006;32:655–664. doi: 10.1080/10623320600919193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Ohtsuki T, Ishiguro H, Yamakawa-Kobayasi K, Endo K, Lin Y, Yanagi H, Tsuchiya S, Kawata K, Hamaguchi H, Arinami T. Association between serotonin and transporter gene polymorphism and smoking among Japanese males. Cancer Epidemiol Biomarkers Prev. 1999;8:831–833. [PubMed] [Google Scholar]
- Johnson CS, Heffner JL, Blom TJ, Anthenelli RM. Exposure to traumatic events among treatment-seeking, alcohol-dependent women and men without PTSD. J Trauma Stress. 2010;23:649–652. doi: 10.1002/jts.20563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun HJ, Rich-Edwards JW, Boynton-Jarrett R, Austin SB, Frazier AL, Wright RJ. Child Abuse and smoking among young women: the importance of severity, accumulation, and timing. J Adolesc Health. 2008;43:55–63. doi: 10.1016/j.jadohealth.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Yang B, Douglas-Palumberi H, Crouse-Artus M, Lipschitz D, Krystal JH, Gelernter J. Genetic and environmental predictors of early alcohol use. Biol Psychiatry. 2007;61:1228–1234. doi: 10.1016/j.biopsych.2006.06.039. [DOI] [PubMed] [Google Scholar]
- King G, Guilbert P, Ward DG, Arwidson P, Noubary F. Correlates of sexual abuse and smoking among French adults. Child Abuse Negl. 2006;30:709–723. doi: 10.1016/j.chiabu.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Kremer I, Bachner-Melman R, Reshef A, Broude L, Nemanov L, Gritsenko I, Heresco-Levy U, Elizur Y, Ebstein RP. Association of the serotonin transporter gene with smoking behavior. Am J Psychiatry. 2005;162:924–930. doi: 10.1176/appi.ajp.162.5.924. [DOI] [PubMed] [Google Scholar]
- Laucht M, Treutlein J, Schmid B, Blomeyer D, Becker K, Buchmann AF, Schmidt MH, Esser G, Jennen-Steinmetz C, Rietschel M, Zimmermann US, Banaschewski T. Impact of psychosocial adversity on alcohol intake in young adults: moderation by the ll genotype of the serotonin transporter polymorphism. Biol Psychiatry. 2009;66:102–109. doi: 10.1016/j.biopsych.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Lerer E, Kanyas K, Karni O, Ebstein RP, Lerer B. Why do young women smoke? Role of traumatic life experience, psychological characteristics and serotonergic genes. Mol Psychiatry. 2006;11:771–781. doi: 10.1038/sj.mp.4001855. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1530. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Le Strat Y, Ramoz N, Gorwood P. In alcohol-dependent drinkers, what does the presence of nicotine dependence tell us about psychiatric and addictive disorders comorbidity? Alcohol Alcohol. 2010;45:167–172. doi: 10.1093/alcalc/agp094. [DOI] [PubMed] [Google Scholar]
- Lloyd DA, Taylor J. Lifetime cumulative adversity, mental health and the risk of becoming a smoker. Health (London) 2006;10:95–112. doi: 10.1177/1363459306058990. [DOI] [PubMed] [Google Scholar]
- Macmillan HL, Fleming JE, Streiner DL, Lin E, Boyle MH, Jamieson E, Duku EK, Walsh CA, Wong MY, Beardslee WR. Gender specific associations between types of childhood maltreatment and the onset, escalation and severity of substance use in cocaine dependent adults. Am J Psychiatry. 2001;158:1878–1883. doi: 10.1176/appi.ajp.158.11.1878. [DOI] [PubMed] [Google Scholar]
- Moncrieff J, Drummond C, Candy B, Checinski K, Farmer R. Sexual abuse in people with alcohol problems. Br J Psychiatry. 1996;169:355–360. doi: 10.1192/bjp.169.3.355. [DOI] [PubMed] [Google Scholar]
- Nelson EC, Heath AC, Madden PAF, Cooper ML, Dinwiddie SH, Bucholz KK, Glowinski A, McLaughlin T, Dunne MP, Statham DJ, Martin NG. Association between self-reported childhood sexual abuse and adverse psychosocial outcomes. Arch Gen Psychiatry. 2002;59:139–145. doi: 10.1001/archpsyc.59.2.139. [DOI] [PubMed] [Google Scholar]
- Nguyen TA, Heffner JL, Lin SW, Anthenelli RM. Genetic factors in the risk for substance use disorders. In: Ruiz P, Strain E, editors. Lowinson and Ruiz’s Substance Abuse: A Comprehensive Textbook. 5. Lippincott Williams and Wilkins; Baltimore: 2011. [Google Scholar]
- Nilsson KW, Oreland L, Kronstrand R, Leppert J. Smoking as a product of gene-environment interaction. Ups J Med Sci. 2009;114:100–107. doi: 10.1080/03009730902833406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols HB, Harlow BL. Childhood abuse and risk of smoking onset. J Epidemiol Community Health. 2004;58:402–406. doi: 10.1136/jech.2003.008870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JI, Schuckit MA, Begleiter H. Comparison of direct interview and family history diagnosis of alcohol dependence. Alcohol Clin Exp Res. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Rice C, Mohr CD, Del Boca FK, Mattson ME, Young L, Brady K, Nickless C. Self-reports of physical, sexual, and emotional abuse in an alcoholism treatment sample. J Stud Alcohol. 2001;62:114–123. doi: 10.15288/jsa.2001.62.114. [DOI] [PubMed] [Google Scholar]
- Simpson TL, Miller WR. Concomitance between childhood sexual and physical abuse and substance use problems: a review. Clin Psychol Rev. 2002;22:27–77. doi: 10.1016/s0272-7358(00)00088-x. [DOI] [PubMed] [Google Scholar]
- Thompson RD, Heffner JL, Strong JA, Blom TJ, Anthenelli RM. Relationship between the serotonin transporter and obsessive-compulsive alcohol craving in alcohol dependent adults: a pilot study. Alcohol. 2010;44:401–406. doi: 10.1016/j.alcohol.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Loon AJM, Tijhuis M, Surtees PG, Ormel J. Determinants of smoking status: cross-sectional data on smoking initiation and cessation. Eur J Public Health. 2005;15:256–261. doi: 10.1093/eurpub/cki077. [DOI] [PubMed] [Google Scholar]
- Vink JM, Willemsen G, Boomsma DI. Heritability of smoking initiation and nicotine dependence. Behav Genet. 2005;35:397–406. doi: 10.1007/s10519-004-1327-8. [DOI] [PubMed] [Google Scholar]
- Waldrop AE, Santa Ana EJ, Saladin ME, McRae AL, Brady KT. Differences in early onset alcohol use and heavy drinking among persons with childhood and adult trauma. Am J Addict. 2007;16:439–442. doi: 10.1080/10550490701643484. [DOI] [PubMed] [Google Scholar]
- Wendland JR, Martin BJ, Kruse MR, Lesch KP, Murphy DL. Simultaneous genotyping of four functional loci of human SLC6A4, with a reappraisal of 5-HTTLPR and rs25531. Mol Psychiatry. 2006;11:224–226. doi: 10.1038/sj.mp.4001789. [DOI] [PubMed] [Google Scholar]
- Wills TA, Cleary SD. How are social support effects mediated? A test with parental support and adolescent substance use. J Pers Soc Psychol. 1996;71:937–952. doi: 10.1037//0022-3514.71.5.937. [DOI] [PubMed] [Google Scholar]
- Wills TA, Vaccaro D, McNamara G. The role of life events, family support, and competence in adolescent substance use: a test of vulnerability and protective factors. Am J Community Psychol. 1992;20:349–374. doi: 10.1007/BF00937914. [DOI] [PubMed] [Google Scholar]