Abstract
Cumulating evidence has demonstrated that mu opioid receptor (MOR) agonists promote spinal glial activation, lead to synthesis and release of proinflammatory cytokines and chemokines, and contribute to opioid-induced hyperalgesia and development of opioid tolerance and dependence. However, whether these MOR agonists directly or indirectly act on spinal cord astrocytes and microglial cells in vivo is unclear. In the present study, by combining the techniques of in situ hybridization of MOR mRNA with immunohistochemistry of GFAP (an astrocyte marker) and Iba1 (a microglial marker), we examined expression and distribution of GFAP, Iba1, and MOR mRNA in the spinal cord of rats under chronic morphine tolerance conditions. Twice daily intrathecal injections of morphine for 7 days reduced morphine analgesic effect and increased both GFAP and Iba1 immunostaining densities in spinal cord. Unexpectedly, neither GFAP nor Iba1 colocalized with MOR mRNA in spinal cord cells. Our findings indicate that MOR expression is absent from spinal cord astrocytes and microglia, suggesting that these cell types are indirectly activated by MOR agonists under chronic opioid tolerance conditions.
Keywords: mu opioid receptor, astrocyte, microglial cells, expression, spinal cord, morphine tolerance
Introduction
Opioids remain the gold standard for pharmacologic treatment of moderate to severe pain in the clinical setting. Among the multiple subtypes of opioid receptor (μ, δ, and κ,), μ opioid receptor (MOR) agonists (e.g., morphine) are the most commonly used agents. However, the use of MOR agonists in pain management is often hindered by adverse effects such as development of tolerance, hyperalgesia, respiratory depression, nausea, sedation, and immune modulation [1]. In particular, the development of tolerance to MOR agonists constitutes a major limitation to opioid use in patients with chronic pain who require long-term pharmacologic treatment [1].
Traditionally, studies of tolerance to MOR agonists have focused principally on neuronal mechanisms. However, growing evidence indicates that spinal cord glial cell (e.g., astrocytes and microglia) activation plays a pivotal role in the development and maintenance of chronic opioid tolerance [2]. Some previous studies have suggested that MOR agonists might bind directly to MORs expressed in spinal cord glial cells and thereby contribute to glial activation [3,4]. MORs are expressed in cultured astrocytes derived from brain [5] in a region-specific manner. For example, the level of MOR mRNA in cultured cortical astrocytes was higher than that in cultured striatal and cerebellar astrocytes [6] and was undetectable in cultured hippocampal glial cells [6]. In vivo, very small proportions (2–4%) of astrocytes in limited areas of the central nervous system express MORs at a very low level under normal conditions [7]. A recent study revealed that MOR immunofluorescent staining overlapped some CD11b (a microglia marker) immunofluorescent staining in spinal cord [8]. However, immunofluorescent staining detects MOR protein predominantly in fibers and terminals in the superficial dorsal horn [8,9]. Most of these fibers and terminals are generally believed to originate from the dorsal root ganglion, although the possibility that they come from the processes of spinal cord neurons and glial cells and the descending afferents from the supraspinal regions cannot be ruled out [10]. Therefore, it is still unclear whether spinal cord glial cells express MORs. In the present study, by combining the techniques of in situ hybridization of MOR mRNA with immunofluorescent staining of astrocyte and microglial markers, we examined whether MOR mRNA is expressed in spinal cord astrocytes and microglia under chronic spinal morphine tolerance conditions.
Materials and Methods
Animal preparations
Adult male Sprague-Dawley rats weighing 250–300 g were used in accordance with protocols that were approved by the Animal Care and Use Committee at the Johns Hopkins University. All efforts were made to minimize animal suffering and to reduce the number of animals used. Rats received intrathecal (i.th.) implantation with PE-10 catheter as described previously [11]. Rats with neurologic deficits were excluded from the study during 7-day recovering period. The position of the PE-10 catheter was confirmed after behavioral testing. All drugs were administrated intrathecally in a 10-μL volume followed by 12 μL of saline to flush the catheter.
Induction of chronic spinal morphine tolerance
Chronic spinal morphine tolerance was induced as described previously [11]. In brief, rats received i.th. injections of 10 μg morphine sulfate (n = 5) or saline (n = 5) twice daily for 7 days. The tail-flick behavioral test as described below was performed 1 day before morphine or saline injection and at 0.5 h after injection on mornings 1 and 8.
Tail-flick assay
A tail-flick apparatus (Model 33B Tail Flick Analgesia Meter, IITC Life Science, Woodland Hills, CA, USA) with a radiant heat source connected to an automatic timer was used to assess the analgesic response. A cutoff time latency of 10 s was used to avoid tissue damage to the tail. Tail-flick latencies were measured as the time required to induce a tail flick after applying radiant heat to the skin of the tail. The antinociceptive effects were expressed as the percentage of maximal possible analgesic effect (%MPAE): %MPAE = [(response latency − baseline latency)/(cutoff latency − baseline latency)] × 100%.
Tissue preparation
After behavioral testing, rats were deeply anesthetized and transcardially perfused with 0.9% sodium chloride followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). L4-5 spinal cord was dissected out, post-fixed in the same fixative for 4 h, and cryoprotected in 30% sucrose in 0.01 M phosphate buffered saline (PBS) overnight at 4°C. The cryostat sections were cut at a thickness of 20 μm, and four groups of sections (at least 12–14 sections/group) were collected from each spinal cord by grouping every fourth section. The sections were mounted onto slides and stored at −80°C until use.
In situ hybridization and immunofluorescent staining
Full-length rat MOR plasmid provided by Dr. H. Akil (University of Michigan) was linearized with Acc65I or SacI to obtain the antisense and sense probes. The MOR riboprobe was a 229 bp fragment spanning the coding sequence of MOR (699–927 bp), which corresponds to the sequence in exon 3 of the rat MOR gene. The rat MOR gene has 9 splice variants. Except for MOR-1S [12], all remaining variants contain exon 3 in their gene structures [12]. Thus, the antisense probe designed should detect most MOR splice variants. Briefly, the antisense and sense cRNA riboprobes were synthesized with a digoxigenin RNA labeling kit according to the manufacturer’s instructions (Roche Diagnostics, Indianapolis, IN). Both probes were purified with MicroSpin G-50 Columns (GE Healthcare Life Sciences, Piscataway, NJ).
In situ hybridization histochemistry was conducted by using the IsHyb in Situ Hybridization kit (BioChain Institute, Inc., Hayward, CA) following the manufacturer’s instructions. Briefly, the sections were digested with 20 μg/mL proteinase K (QIAGEN Inc., Valencia, CA) at 37°C for 8–10 min, fixed again with 4% paraformaldehyde in PBS for 15 min, and prehybridized with ready-to-use prehybridization solution for 3–4 h at 60°C. The first and third groups of sections were hybridized with 2 ng/μL digoxigenin-labeled antisense riboprobe, and the second and fourth groups of sections were hybridized with 2 ng/μL digoxigenin-labeled sense riboprobe, each in ready-to-use hybridization solution for 16 h at 60°C. After hybridization, the sections were washed at 60°C with 2× saline sodium citrate (SSC) for 10 min, 1.5× SSC for 10 min, and 0.2× SSC twice for 20 min. Finally, the sections were incubated with 1× blocking solution for 1 h at room temperature and alkaline phosphatase-conjugated anti-digoxigenin antibody (1:1,000) overnight at 4°C. After being washed, the fluorescent signals were developed with Fast Red (Roche, Indianapolis, IN).
Immediately after the development of fluorescent signals, immunofluorescent staining was carried out as described [13]. Briefly, after being blocked in PBS containing 0.3% Triton X-100 and 10% goat serum for 1 h at 37°C, the first and third groups of sections were incubated with mouse monoclonal anti-glial fibrillary acidic protein (GFAP) antibody (1:200, Sigma, St. Louis, MO) and rabbit polyclonal anti-ionized calcium binding adaptor molecule (Iba1) antibody (1:1,000, DAKO, Carpinteria, CA), respectively, overnight at 4°C. The sections were lastly incubated with goat anti-rabbit or anti-mouse IgG conjugated with Cy2 (1:300; Jackson ImmunoResearch, West Grove, PA) for 1 h at 37°C. Control experiments included substitution of normal rabbit or mouse serum for the primary antiserum and omission of the primary antiserum.
Statistical analysis
Data from behavioral tests were analyzed with a one-way analysis of variance (ANOVA) and are shown as means ± SEM. When ANOVA showed significant differences, pair-wise comparisons between means were tested by the post hoc Tukey method. Significance was set at P < 0.05. All statistical analyses were carried out with the statistical software package of SigmaStat.
Results
Induction of morphine analgesic tolerance in rats by repeated i.th. morphine injections
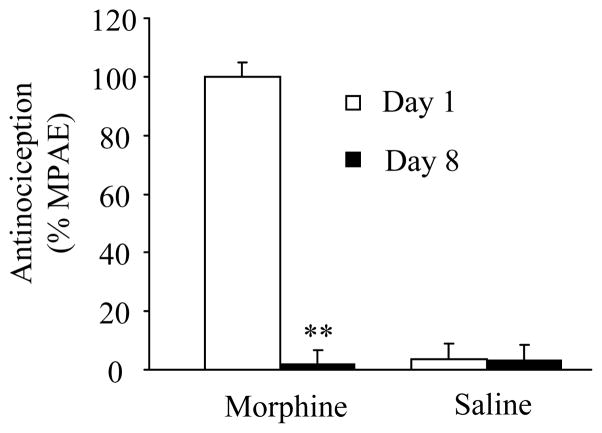
To ensure that all rats developed morphine analgesic tolerance, we measured tail-flick latency in response to heat stimulation. Consistent with previously published data [11], the 7-day regimen of injections induced morphine analgesic tolerance, as indicated by a significant decrease in %MPAE of morphine on day 8 compared to that on day 1 (Fig. 1, n = 5, P < 0.01). Saline injections did not cause changes in basal tail-flick latency (Fig. 1, n = 5, P > 0.05).
Fig. 1.
Development of morphine-induced analgesic tolerance. Rats received intrathecal injections of 10 μg morphine sulfate (n = 5) or saline (n = 5) twice daily for 7 days. The tail-flick behavioral test was performed at 0.5 h after saline or morphine injection on mornings 1 and 8. ** P < 0.01 vs the corresponding day 1. %MPAE, percentage of maximal possible analgesic effect.
Expression and distribution of MOR mRNA-positive cells in rat lumbar spinal cord
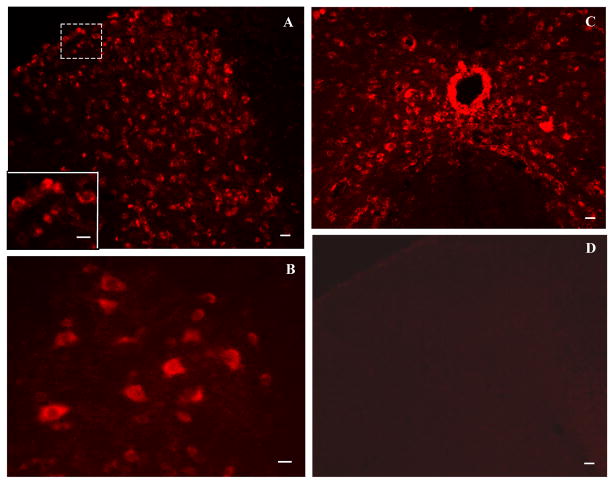
The MOR antisense probe showed very clear and intense fluorescent signals in spinal cord cells (Fig. 2A–C), and the sense probe revealed almost no signal (Fig. 2D) in L4-5 spinal cord of the saline-treated rats. Furthermore, with the antisense probe, fluorescent signals for MOR mRNA were abundant in the gray matter of spinal cord, whereas fluorescent signals were distributed only sparely in the spinal cord white matter (Fig. 2A–C). Intense signals were observed in dorsal horn laminae I–VI (Fig. 2A), ventral horn laminae VII and VIII (Fig. 2B), and the region around the central canal (lamina X, Fig. 2C), Immunofluorescent reactivity partially or fully occupied intracellular plasma. No significant differences were apparent in optical density of fluorescent signals or number and distribution of labeled cells for MOR mRNA in dorsal horn, ventral horn, and the region around the central canal of L4-5 spinal cord between saline-treated and morphine-treated groups (data not shown).
Fig. 2.
Distribution of mu opioid receptor mRNA-labeled cells in spinal cord of saline-treated rats revealed by fluorescent in situ hybridization histochemistry. A–C: The antisense probe shows very clear and intense fluorescent signals, which were (developed with Fast Red) in dorsal horn (A), ventral horn lamina VIII (B), and the region around the central canal (C). The inset in A shows a higher magnification of the region outlined by dashes. Cells labeled for mu opioid receptor mRNA are clearly visible. D: The sense probe exhibited no signals in spinal cord. Scale bars: 50 μm in A, C, and D; 30 μm in A inset and B.
Double labeling of MOR mRNA and GFAP protein in lumbar spinal cord
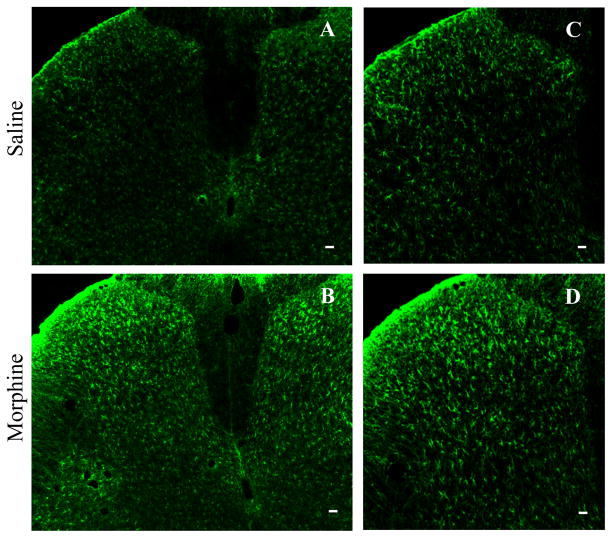
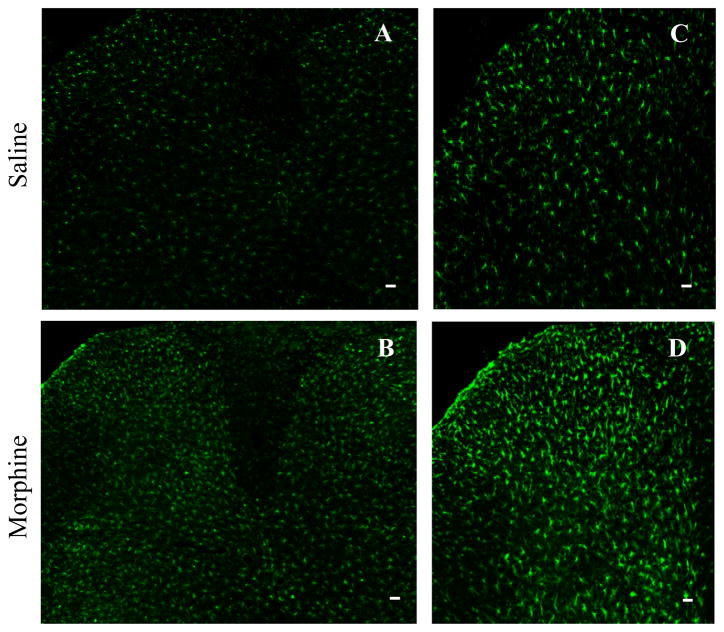
Consistent with previous studies [14,15], repeated i.th. injections of morphine produced a robust, bilateral increase in GFAP immunoreactivity in L4-5 (Fig. 3). Each individual GFAP-labeled cell was hypertrophied in the morphine-treated group compared to cells in the saline-treated group (Fig. 3).
Fig. 3.
Repeated intrathecal injections of morphine produces a robust, bilateral increase in immunoreactivity for the astrocyte marker GFAP inL4-5 spinal cord dorsal horn. Each individual GFAP-labeled cell was hypertrophied in sections from morphine-treated rats compared to those from the saline-treated group (A and B). C shows a higher magnification of part of A. D shows a higher magnification of part of B. Scale bars: 75 μm in A and B; 50 μm in C and D.
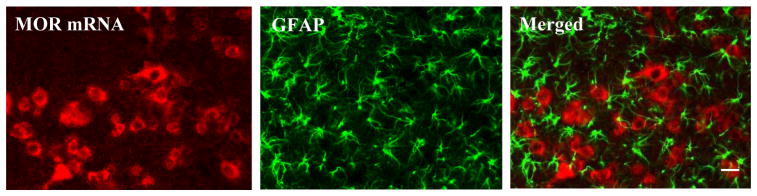
The double-labeling experiments showed that MOR mRNA-labeled cell bodies did not overlap with GFAP-positive cell bodies in either the saline-treated or morphine-treated rats (Fig. 4). However, we observed that some GFAP-positive cell bodies were closely adjacent to MOR mRNA-labeled cell bodies (Fig. 4). In addition, a few processes of GFAP-positive cells extended to MOR mRNA-labeled cell bodies (Fig. 4).
Fig. 4.
Mu opioid receptor (MOR) mRNA does not colocalize with GFAP in deep dorsal horn neurons of morphine-treated rats. In double-labeling experiments, mu opioid receptor mRNA-labeled cell bodies did not overlap with GFAP-positive cell bodies. However, some GFAP-positive cell bodies were closely adjacent to mu opioid receptor mRNA-labeled cell bodies. In addition, a few processes of GFAP-positive cells extended to mu opioid receptor mRNA-labeled cell bodies. Scale bar: 60 μm.
Double labeling of MOR mRNA and Iba1 protein in lumbar spinal cord
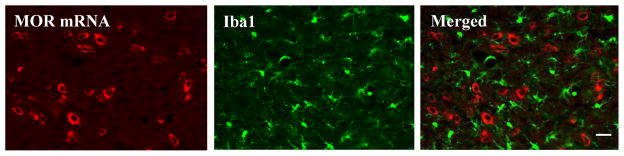
Repeated i.th. injections of morphine also led to a robust bilateral increase in lba1 immunoreactivity in L4-5 spinal cord (Fig. 5) [14,15]. Cells in the morphine-treated group had a hypertrophic morphology and increased density of Iba1 immunostaining compared to those in the saline-treated group (Fig. 5). In addition, MOR mRNA-labeled cell bodies did not overlap with Iba1-positive cell bodies in either the saline-treated or morphine-treated rats (Fig. 6).
Fig. 5.
Repeated intrathecal injections of morphine produces a robust, bilateral increase in immunoreactivity of the microglial marker Iba-1 inL4-5 spinal cord dorsal horns. Each individual Iba1-labeled cell was hypertrophied in sections from the morphine-treated group compared to those from the saline-treated group (A and B). C shows a higher magnification of part of A. D shows a higher magnification of part of B. Scale bars: 75 μm in A and B; 50 μm in C and D.
Fig. 6.
Mu opioid receptor (MOR) mRNA does not colocalize with Iba1 in deep dorsal horn neurons of morphine-treated rats. In double-labeling experiments mu opioid receptor mRNA-labeled cell bodies did not overlap with Iba1-positive cell bodies. Scale bar: 60 μm.
Discussion
In situ hybridization histochemistry is a powerful tool used to illustrate the expression and distribution of the target mRNA with a high degree of cellular resolution in tissue sections. The probes used can be labeled by either a radioactive isotope or fluorophore, but using fluorescence reduces experimental time, enhances visualization of the probes, and makes automated cell counting possible [16]. In situ hybridization histochemistry can be coupled to other anatomical techniques, such as histochemistry and immunohistochemistry, to gather multiple kinds of data, including gene coexpression.
In the present study, we combined the use of fluorescent in situ hybridization histochemistry of MOR mRNA with immunofluorescence of GFAP and Iba1 to examine the expression and distribution of MOR mRNA, GFAP, and Iba1 in spinal cord cells of rats that had undergone repeated injections of saline or morphine. The expression and distribution of MOR mRNA-positive cells in spinal cord of the saline-treated rats were consistent with those reported in previous studies that used radioactive probes in naïve animals [17]. Morphine tolerance did not change the density, number, or distribution of MOR mRNA-positive cells in spinal cord but led to significant hypertrophy of astroglial cells and microglial cells in spinal cord, similar to what has been reported previously [14,15]. Unexpectedly, MOR mRNA did not colocalize with either GFAP or Iba1 in spinal cord cell bodies in either the saline-treated or morphine-treated groups. To exclude the possibility that in situ hybridization steps (such as proteinase K treatment) denatured and/or digested the antigenic sites of GFAP and Iba1, we compared the expression of GFAP and Iba1 in spinal cord sections labeled by immunofluorescence alone with that in sections labeled by the combination of in situ hybridization and immunofluorescence. We observed no marked difference in number or density of GFAP-positive and Iba1-positive cells between these sections (data not shown). These findings suggest that MOR mRNA is not expressed in spinal cord astrocytes or microglia. Based on the morphology of MOR mRNA-positive cells, it is very likely that MOR mRNA is expressed in spinal cord neurons. Unfortunately, we were unable to detect the neuronal marker NeuN in MOR mRNA-positive cells of spinal cord in our pilot study because the antigenic sites of NeuN are very sensitive to proteinase K pretreatment (even <4 min). Similar observations have been reported recently in brain sections [18].
Growing evidence indicates that chronic morphine exposure produces hypertrophy of spinal cord astrocytes and microglia and that spinal glial inhibition attenuates the development of morphine tolerance [2]. These findings suggest that astrocytes and microglia are activated in spinal cord. Our double-labeling study revealed that astrocytes and microglia may be activated indirectly under chronic morphine tolerance conditions. Indeed, it has been demonstrated that the development of morphine tolerance is associated with changes in number or functional activity of MOR receptors [19] and their downstream signals (isoform-specific synthesis and phosphorylation of adenylyl cyclase and phosphorylation of the Gβ subunit of Gβγ) [20]. Moreover, chronic morphine exposure also induces plasticity changes in other receptors (e.g., glutamate receptors) and cellular signals (e.g., PKC, nNOS) in the central nervous system [20,21]. Hypertrophy of astrocytes and microglia and release of glial-produced cytokines and chemokines in spinal cord may be some of the changes undergone in the central nervous system after repeated morphine injections.
In summary, by combining in situ hybridization of MOR mRNA with immunofluorescent staining of GFAP and Iba1, we have shown that neither GFAP nor Iba1 colocalize with MOR mRNA in spinal cord cells after repeated i.th. saline or morphine injections. Our findings suggest that astrocytes and microglia in spinal cord do not express MOR mRNA and cannot be directly activated by MOR agonists under chronic morphine tolerance conditions.
Acknowledgments
Sources of Funding: This work was supported by NIH Grants (NS 058886 and NS072206); the Rita Allen Foundation; Mr. David Koch and the Patrick C. Walsh Prostate Cancer Research Fund; and the Blaustein Pain Research Fund.
Footnotes
Conflicts of Interest: The authors declare no conflict of interests.
References
- 1.Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, et al. Opioid complications and side effects. Pain Physician. 2008;11:S105–S120. [PubMed] [Google Scholar]
- 2.Watkins LR, Hutchinson MR, Milligan ED, Maier SF. “Listening” and “talking” to neurons: implications of immune activation for pain control and increasing the efficacy of opioids. Brain Res Rev. 2007;56:148–169. doi: 10.1016/j.brainresrev.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horvath RJ, DeLeo JA. Morphine enhances microglial migration through modulation of P2X4 receptor signaling. J Neurosci. 2009;29:998–1005. doi: 10.1523/JNEUROSCI.4595-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horvath RJ, Landry RP, Romero-Sandoval EA, DeLeo JA. Morphine tolerance attenuates the resolution of postoperative pain and enhances spinal microglial p38 and extracellular receptor kinase phosphorylation. Neuroscience. 2010;169:843–854. doi: 10.1016/j.neuroscience.2010.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turchan-Cholewo J, Dimayuga FO, Gupta S, Keller JN, Knapp PE, Hauser KF, et al. Morphine and HIV-Tat increase microglial-free radical production and oxidative stress: possible role in cytokine regulation. J Neurochem. 2009;108:202–215. doi: 10.1111/j.1471-4159.2008.05756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruzicka BB, Fox CA, Thompson RC, Meng F, Watson SJ, Akil H. Primary astroglial cultures derived from several rat brain regions differentially express mu, delta and kappa opioid receptor mRNA. Brain Res Mol Brain Res. 1995;34:209–220. doi: 10.1016/0169-328x(95)00165-o. [DOI] [PubMed] [Google Scholar]
- 7.Stiene-Martin A, Knapp PE, Martin K, Gurwell JA, Ryan S, Thornton SR, et al. Opioid system diversity in developing neurons, astroglia, and oligodendroglia in the subventricular zone and striatum: impact on gliogenesis in vivo. Glia. 2001;36:78–88. [PMC free article] [PubMed] [Google Scholar]
- 8.Horvath RJ, Romero-Sandoval EA, De Leo JA. Inhibition of microglial P2X4 receptors attenuates morphine tolerance, Iba1, GFAP and mu opioid receptor protein expression while enhancing perivascular microglial ED2. Pain. 2010;150:401–413. doi: 10.1016/j.pain.2010.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohno T, Ji RR, Ito N, Allchorne AJ, Befort K, Karchewski LA, et al. Peripheral axonal injury results in reduced mu opioid receptor pre- and post-synaptic action in the spinal cord. Pain. 2005;117:77–87. doi: 10.1016/j.pain.2005.05.035. [DOI] [PubMed] [Google Scholar]
- 10.Abbadie C, Lombard MC, Besson JM, Trafton JA, Basbaum AI. Mu and delta opioid receptor-like immunoreactivity in the cervical spinal cord of the rat after dorsal rhizotomy or neonatal capsaicin: an analysis of pre- and postsynaptic receptor distributions. Brain Res. 2002;930:150–162. doi: 10.1016/s0006-8993(02)02242-4. [DOI] [PubMed] [Google Scholar]
- 11.Liaw WJ, Zhang B, Tao F, Yaster M, Johns RA, Tao YX. Knockdown of spinal cord postsynaptic density protein-95 prevents the development of morphine tolerance in rats. Neuroscience. 2004;123:11–15. doi: 10.1016/j.neuroscience.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Pan YX. Diversity and complexity of the mu opioid receptor gene: alternative pre-mRNA splicing and promoters. DNA Cell Biol. 2005;24:736–750. doi: 10.1089/dna.2005.24.736. [DOI] [PubMed] [Google Scholar]
- 13.Xu JT, Zhao X, Yaster M, Tao YX. Expression and distribution of mTOR, p70S6K, 4E-BP1, and their phosphorylated counterparts in rat dorsal root ganglion and spinal cord dorsal horn. Brain Res. 2010;1336:46–57. doi: 10.1016/j.brainres.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui Y, Chen Y, Zhi JL, Guo RX, Feng JQ, Chen PX. Activation of p38 mitogen-activated protein kinase in spinal microglia mediates morphine antinociceptive tolerance. Brain Res. 2006;1069:235–243. doi: 10.1016/j.brainres.2005.11.066. [DOI] [PubMed] [Google Scholar]
- 15.Narita M, Suzuki M, Narita M, Niikura K, Nakamura A, Miyatake M, et al. mu-Opioid receptor internalization-dependent and -independent mechanisms of the development of tolerance to mu-opioid receptor agonists: Comparison between etorphine and morphine. Neuroscience. 2006;138:609–619. doi: 10.1016/j.neuroscience.2005.11.046. [DOI] [PubMed] [Google Scholar]
- 16.Sonomura T, Nakamura K, Furuta T, Hioki H, Nishi A, Yamanaka A, et al. Expression of D1 but not D2 dopamine receptors in striatal neurons producing neurokinin B in rats. Eur J Neurosci. 2007;26:3093–3103. doi: 10.1111/j.1460-9568.2007.05923.x. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, Wessendorf MW. Equal proportions of small and large DRG neurons express opioid receptor mRNAs. J Comp Neurol. 2001;429:590–600. doi: 10.1002/1096-9861(20010122)429:4<590::aid-cne6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 18.Nehme B, Henry M, Mouginot D. Combined fluorescent in situ hybridization and immunofluorescence: limiting factors and a substitution strategy for slide-mounted tissue sections. J Neurosci Methods. 2011;196:281–288. doi: 10.1016/j.jneumeth.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 19.Raehal KM, Bohn LM. Mu opioid receptor regulation and opiate responsiveness. AAPS J. 2005;7:E587–E591. doi: 10.1208/aapsj070360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gintzler AR, Chakrabarti S. Post-opioid receptor adaptations to chronic morphine; altered functionality and associations of signaling molecules. Life Sci. 2006;79:717–722. doi: 10.1016/j.lfs.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 21.Yu L, Xue FS, Li CW, Xu YC, Zhang GH, Liu KP, et al. N(omega)-nitro-L-arginine methyl ester inhibits the up-regulated expression of neuronal nitric oxide synthase/NMDA receptor in the morphine analgesia tolerance rats. Sheng Li Xue Bao. 2006;58:593–598. [PubMed] [Google Scholar]