Abstract
Background
Allograft function following renal transplantation is commonly monitored using serum creatinine. Multiple cross-sectional studies have shown that serum cystatin C is superior to creatinine for detection of mild to moderate chronic kidney dysfunction. Recent data in adults indicate that cystatin C might also be a more sensitive marker of acute renal dysfunction. This study aims to compare cystatin C and creatinine for detection of acute allograft dysfunction in children using pediatric RIFLE (risk of renal dysfunction, injury to the kidney, failure or loss of kidney function, end stage renal disease) criteria for acute kidney injury.
Methods
Retrospective chart review of post-transplant period in 24 patients in whom creatinine and cystatin C were measured every day. Allograft dysfunction was defined as a sustained rise in marker concentration above the mean of the three preceding measurements.
Results
In total, there were 13 episodes of allograft dysfunction. Maximum RIFLE stages with creatinine were 'R' in 7, 'I' in 4, and 'F' in 2, with cystatin C ‘R’ in 6, ‘I’ in 4 and ‘F’ in 3, respectively. In 9/13 cases, both markers rose simultaneously, in three, the rise in creatinine preceded cystatin C by 1–5 days (median 4). In one case, the rise in cystatin C preceded creatinine by 1 day. The time lag was not statistically different. The maximum relative rise of creatinine was significantly higher than cystatin C. By multiple linear regression analysis, the maximum rise of cystatin C was related to the maximum rise of creatinine, but independent of patient age, gender, steroid dose, and anthropometric data.
Conclusions
In this pediatric population, cystatin C was not superior to creatinine for the detection of acute allograft dysfunction.
Keywords: Allograft dysfunction, Kidney transplantation, Serum creatinine, Serum cystatin C, Children, Acute renal failure, Pharmacokinetic modeling
Introduction
Allograft function following renal transplantation is commonly monitored using serum creatinine. Creatinine is not an ideal marker of kidney function because its production is dependent on age, gender, and muscle mass [1]. Due to tubular secretion and extra-renal elimination via the gut, serum creatinine concentrations may remain within the reference range until about 50% of kidney function has been lost [2]. Because of lower muscle mass, creatinine production in children is reduced compared to adults [3], which is reflected in lower reference values. This may hamper the sensitivity of creatinine for the detection of allograft dysfunction in children [4].
Cystatin C is an emerging endogenous marker of glomerular filtration rate (GFR; for review see [5, 6]), which has been shown to be superior to serum creatinine for the detection of mild to moderate chronic kidney dysfunction in cross-sectional studies both in adults with native kidney disease [7, 8] and renal transplant recipients [9–11]. Recent data in adults admitted to an intensive care unit indicate that cystatin C might also be a more sensitive marker of acute renal dysfunction [12–14].
We therefore hypothesized that serum cystatin C might be more sensitive than creatinine for the detection of acute renal allograft dysfunction in children. As there are no data on the performance of cystatin C in children with rapidly changing GFR, we re-analyzed data from a previously published cohort [15] using the recently developed pediatric RIFLE criteria for acute renal injury [16, 17].
Methods
Patients
We performed a retrospective chart review of the post-transplant period (from day 7 until hospital discharge) of 24 children (14 boys and 10 girls), who had received a functioning graft at Hannover Medical School Children’s Hospital between May 1996 and June 1997. Data on the first 7 days were not included to limit the effect of increased cystatin C synthesis induced by high-dose corticosteroids [18–20]. Patient age was 10.5 ± 5.1 years (mean ± standard deviation), weight 34.2 ± 18.3 kg, height 134 ± 31.7 cm. Half of the organs were from living related donors (four from a father, eight from a mother). The immunosuppressive regimen consisted of the microemulsion formulation of cyclosporine A and prednisolone [15].
Assays
Cystatin C and creatinine were measured in parallel every day. Creatinine was determined enzymatically using the creatininase para-aminophenazone (PAP) assay (Boehringer Mannheim, Mannheim, Germany). Cystatin C concentrations were measured by particle-enhanced immunoturbidimetry using the Cystatin PET-Kit (DAKO, Hamburg, Germany). Both assays were performed on a Hitachi 717 autoanalyzer (Boehringer Mannheim, Mannheim, Germany). Measurements were performed in the same serum samples using remnant material. Interassay precision was better for the creatinine than for the cystatin C assay (coefficient of variation 3.5% vs. 5.5% at concentrations of 13.7 µmol/l and 0.97 mg/l, and 1.6% vs. 4.7% at 79.1 µmol/l and 1.70 mg/l, respectively [20].
Definition of allograft dysfunction
Episodes of allograft dysfunction were identified in analogy to the approach used by Herget-Rosenthal [12] with several modifications: From the daily measurements of cystatin C and creatinine, we calculated the individual baseline concentration for each day as the mean of the three preceding days. Acute allograft dysfunction was defined as a critical rise in marker concentration above baseline on two consecutive days. We used the pediatric RIFLE (risk of renal dysfunction, injury to the kidney, failure or loss of kidney function, end stage renal disease) criteria (pRIFLE) to define critical rises.
The pediatric RIFLE criteria are based on a decrease in estimated glomerular filtration rate by 25% (pRIFLE 'R' indicating risk of renal dysfunction), 50% (pRIFLE 'I', i.e., injury to the kidney) and 75% (pRIFLE ‘F’, i.e., failure of kidney function) [16]. Criteria ‘L’ and ‘E’, which are defined by need for renal replacement therapy, were not applicable in our study, as only patients with primary allograft function were included and no patient required dialysis during our study.
Estimated glomerular filtration rate (eGFRcreat) was calculated from body height and serum creatinine according to Schwartz [21]:
| 1 |
As height was constant during the period of observation, these criteria could be transformed into critical rises in serum creatinine concentration:
| 2 |
Using this transformation, stage 'Rcreat' corresponds to a rise in serum creatinine by more than factor 1.33 above baseline, stage 'Icreat' by more than factor 2 and stage 'Fcreat' by more than factor 4.
For cystatin C, the Grubb equation [22]:
| 3 |
was used to calculate the critical rise corresponding to a decrease in GFR by 25, 50, and 75%. The Grubb equation had been established in children with chronic kidney disease (CKD) and after renal transplantation using the same DAKO immunoturbidimetric assay as in our patients. Critical rises in cystatin C concentration using this definition are 1.19 times baseline ('Rcys'), 1.51 ('Icys'), and 2.28 ('Fcys').
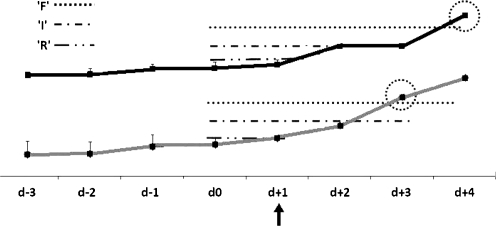
Day 0 of an episode of allograft dysfunction was defined as the day directly preceding a rise in one or both marker concentrations sufficient to meet the pRIFLE criterion ‘R’ (Fig. 1). We determined the time lag (in days) until the other marker met the respective criterion.
Fig. 1.
Method for calculation of time-lag to fulfill RIFLE (risk of renal dysfunction, injury to the kidney, failure or loss of kidney function, end stage renal disease) criteria. The grey marker reaches "RIFLE R" on day + 1 (arrow), which defines the start of the episode. The mean concentration of day –2 to day 0 is used as baseline for calculation of the time-lag. In the example, "RIFLE F" is reached on day +3 by the marker presented in grey and on day +4 by the black marker
Statistics
Statistical analysis was done using SPSS 16.0.1 (SPSS Inc., Chicago, IL, USA). Data are presented as median (interquartile range IQR) unless otherwise indicated. Comparisons were performed using standard non-parametric tests. κ-statistic was calculated for agreement between both markers in the detection of allograft dysfunction. The maximum relative rise in cystatin C concentration during each episode of allograft dysfunction was studied by multiple linear regression analysis including the parameters age, gender, steroid dose, weight, height, and maximum rise in creatinine. p values below 0.05 were considered statistically significant. Due to the small sample size, statistical power is low.
Pharmacokinetic modeling
Kinetics of serum cystatin C and serum creatinine following acute changes in glomerular filtration rate were simulated using a one-compartment pharmacokinetic model with zero-order input for a child with a body weight of 28 kg and a body surface area of 1 m2. For creatinine, we entered total body water (i.e., 0.6 × body weight) as volume of distribution [23] and a production rate of 20 mg/kg/day [1]. For cystatin C, volume of distribution is the extracellular fluid compartment (i.e., 0.2 × body weight) [5] and production rate 0.117 mg/min/1.73 m2 [24]. Clearance was taken as linear with glomerular filtration rate. Besides elimination by glomerular filtration, we also entered extra-renal elimination of cystatin C (i.e., 22.7 ml/min/1.73 m2) into the model [24]. This was not possible for creatinine, as extra-renal elimination of creatinine increases with diminishing GFR and shows large inter-individual variability [1].
Results
The median (IQR) of the mean cystatin C concentrations found in the study subjects during the entire observation period was 2.64 mg/l (1.83–3.65), for creatinine this was 97 µmol/l (56–173). On discharge, the median 24-h creatinine clearance was 66 ml/min/1.73 m2 (36–72), median cystatin C concentration was 2.37 mg/l (1.83–3.29), median creatinine 91 µmol/l (56–135).
In total, there were 13 episodes of kidney dysfunction: nine rejections (six proven by biopsy), one pyelonephritis, and one case of cyclosporine toxicity. In two episodes, no etiology was found. Maximum RIFLE stages were 'Rcreat' in seven, 'Icreat' in four, and 'Fcreat' in two episodes using creatinine compared to ‘R cys', in six, 'Icys' in four, and 'Fcys' in three episodes with cystatin C. Both markers rose simultaneously in nine episodes. In three cases, the rise in creatinine preceded cystatin C by 1 to 5 days (median 4 days). In only one case the rise in cystatin C preceded creatinine by 1 day. The time lag was not statistically different (z = –1.289, p = 0.197; Wilcoxon test).
The median (IQR) of the maximum concentration of cystatin C during the individual episodes of allograft dysfunction was 4.15 mg/l (2.68–4.92). For creatinine, this was 160 µmol/l (98–268). In terms of relative rise above baseline, this was by a factor of 1.59 (1.35–2.28) for cystatin C and 1.9 (1.56–2.95) for creatinine (p = 0.002, Wilcoxon test).
The maximum relative rise of cystatin C above baseline was related to the maximum relative rise of creatinine (Table 1) but independent of patient age, gender, steroid dose, and anthropometric data. The slope of 0.491 reflects the stronger relative rise in creatinine than cystatin C.
Table 1.
Factors influencing the maximum rise in serum cystatin C. Presentation of the slopes (B) representing the correlation with the maximal relative rise of cystatin C, with 95% confidence intervals (95% CI) calculated using multiple linear regression analysis
| B | 95% CI | p value | |
|---|---|---|---|
| Max relative rise Crea | 0.491 | 0.365 to 0.617 | < 0.001 |
| Gender (m = 1) | –0.019 | –0.383 to 0.345 | 0.914 |
| Age (years) | –0.03 | –0.176 to 0.116 | 0.67 |
| Weight (kg) | +0.002 | –0.018 to 0.022 | 0.806 |
| Height (cm) | +0.001 | –0.030 to 0.032 | 0.922 |
| Steroid dose (mg/m2/day) | –0.005 | –0.029 to 0.019 | 0.666 |
Analysis of the agreement between cystatin C and creatinine in the detection of RIFLE stage yielded a κ-value of 0.683.
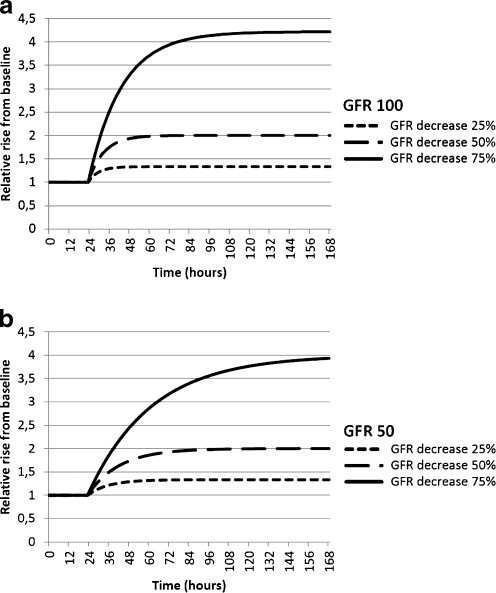
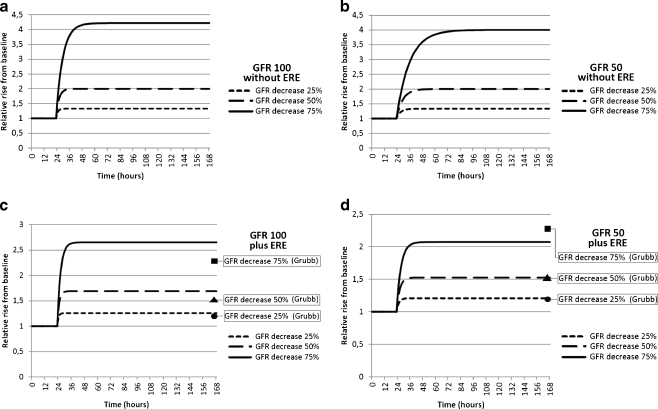
The kinetics of creatinine following an acute decrease in GFR is modeled in Fig. 2a and b. As predicted from Eq. (2), the relative rise in serum creatinine is times 1.33, 2, and 4, and independent of baseline GFR. The time to reach a new steady state is longer with low baseline GFR (Table 2). The pharmacokinetics of cystatin C are shown in Fig. 3a–d. When considering only renal elimination of cystatin C (Fig. 3a and b), the relative rise is identical to creatinine, yet the time to reach a new steady state is considerably shorter due to the smaller volume of distribution (Table 2). When extra-renal elimination of cystatin C is also entered into the model, the relative rise decreases, which is more pronounced at lower baseline GFR (Fig. 3c and d). The model including extra-renal elimination shows better agreement with the steady-state concentrations calculated from the Grubb equation.
Fig. 2.
a, b Pharmacokinetic models illustrating the relative rise of creatinine above baseline at different levels of glomerular filtration rate (GFR) in time. Course of calculated creatinine concentrations following an acute decrease in GFR at time 24 h at two different levels of GFR. Presentation of the relative rise compared to baseline corresponds to a GFR of 100 ml/min/1.73 m2 (Fig. 2a) and a GFR of 50 ml/min/1.73 m2 (Fig. 2b)
Table 2.
Presentation of the time in hours needed by cystatin C and creatinine concentrations to reach a new steady state following acute changes in glomerular filtration rate (GFR) at different levels of baseline GFR: chronic kidney disease (CKD) 1, 100 ml/min/1.73 m2; CKD 2, 75 ml/min/1.73 m2; CKD 3, 50 ml/min/1.73 m2; and CKD 4, 25 ml/min/1.73 m2. For cystatin C, two calculations were done: first, only renal elimination was considered, and second, extra-renal elimination (ERE) was also entered into the model
| Time to new steady state [h] | ||||
|---|---|---|---|---|
| Creatinine | Cystatin C | Cystatin C + ERE | ||
| CKD 1 | R (–25%) | 22 | 7 | 6 |
| I (–50%) | 34 | 11 | 8 | |
| F (–75%) | 71 | 24 | 12 | |
| CKD 2 | R (–25%) | 30 | 10 | 7 |
| I (–50%) | 45 | 15 | 10 | |
| F (–75%) | 89 | 30 | 14 | |
| CKD 3 | R (–25%) | 45 | 15 | 9 |
| I (–50%) | 67 | 22 | 12 | |
| F (–75%) | 134 | 45 | 16 | |
| CKD 4 | R (–25%) | 90 | 30 | 14 |
| I (–50%) | 134 | 45 | 16 | |
| F (–75%) | 268 | 89 | 19 | |
Fig. 3.
a, b, c, and d Pharmacokinetic models illustrating the relative rise of cystatin C above baseline at different levels of glomerular filtration rate (GFR) in time. Course of calculated cystatin C concentrations following an acute decrease in GFR at time 24 h at two different levels of GFR. Presentation of the relative rise compared to baseline corresponds to a GFR of 100 ml/min/1.73 m2 (Fig. 3a and c) and a GFR of 50 ml/min/1.73 m2 (Fig. 3b and d). In Fig. 3c and d, the extrarenal elimination (ERE) of cystatin C (i.e., 22 ml/min/1.73 m2) is taken into account. For comparison, the relative rise of cystatin C calculated according to the Grubb equation is indicated in Fig. 3c and d
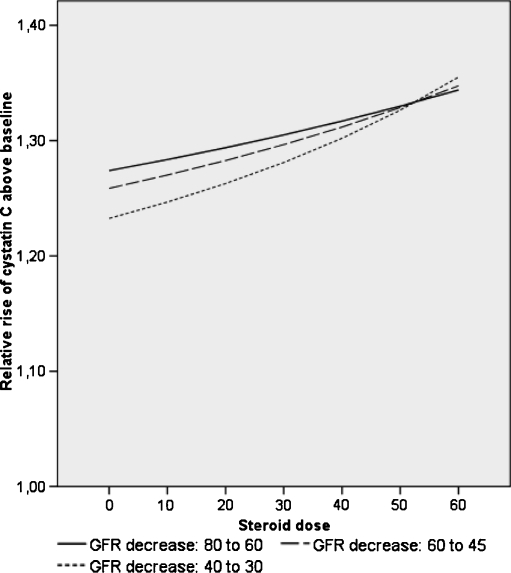
In Fig. 4, the effect of corticosteroids on the relative rise of cystatin C above baseline is illustrated at different levels of GFR. It shows that corticosteroids increase the relative rise in cystatin C, namely in patients with low GFR.
Fig. 4.
Model illustrating the effect of corticosteroids on the relative rise of cystatin C above baseline at different levels of glomerular filtration rate (GFR). Calculation of cystatin C concentrations correspond to three different baseline GFR levels and a decrease by 25% (RIFLE ‘R’) using the Grubb equation. Correction for the dose-dependent increase in serum cystatin C caused by glucocorticoids using the equation 1/cys = 0.0096 * GFR – 0.0023 * prednisone dose (mg/m2/day) [33]
Discussion
Although there is an abundance of cross-sectional studies comparing the diagnostic performance of cystatin C and creatinine, very few have addressed serial changes of these markers in acute renal failure. This can be done using exposure to an acute nephrotoxin such as intravenous radiocontrast fluid or cisplatin [13, 25, 26] or a well-defined insult holding a high risk of acute renal failure, such as major cardiovascular surgery [14]. Using a gold standard GFR measurement, Benöhr et al. found a significant decrease in GFR by 22%, 5 days following cisplatin administration, which was reflected by a significant rise in serum cystatin C by 14% while serum creatinine concentrations remained unchanged. However, following intravenous radiocontrast administration, both Rickli et al. [25] and Poletti et al. [26] found no difference between cystatin C and creatinine during a follow-up of 2 to 4 days.
The present study analyzed serial changes in kidney function during the first weeks after renal transplantation, a period in which allograft function is monitored on a daily basis and where there is a high risk of renal dysfunction. During 13 episodes of acute allograft dysfunction, we observed no difference in sensitivity between both markers, while the relative rise in creatinine concentration was stronger than in cystatin C. Using a similar approach in adults, Herget-Rosenthal [12] found a significantly earlier rise in cystatin C than creatinine allowing prediction of acute renal injury 1 to 2 days before creatinine.
When comparing both studies, several differences have to be considered. (i) The pediatric definition of acute renal injury [16] is based on a decrease in estimated GFR from baseline by 25, 50, and 75%. This corresponds to an increase in serum creatinine by factor 1.33, 2.0, and 4.0 and by factor 1.19, 1.51, and 2.28 for cystatin C, respectively. This should have favored cystatin C in our study, however, as Herget-Rosenthal used the adult RIFLE staging [27] for both creatinine and cystatin C with ‘R’ corresponding to 1.5, ‘I’ to 2.0 and ‘F’ to 3.0 times baseline concentration. (ii) Herget-Rosenthal excluded patients with glucocorticoid therapy or documented thyroid dysfunction, both of which interfere with cystatin C metabolism [28, 29]. Thyroid function was not measured in our patients, but it appears very unlikely that the attenuated rise in cystatin C can be attributed to untreated hypothyroidism in these asymptomatic renal transplant recipients. Being part of the standard immunosuppressive regimen, all of our patients received large doses of glucocorticosteroids known to cause a dose-dependent induction of cystatin C synthesis [30] and higher baseline cystatin C concentrations [15]. Therefore, we excluded the first 7 days post-transplant. From day 8 onwards, mean serum creatinine and cystatin concentrations were constant in relation to each other indicating that a new equilibrium had been reached [15]. Also, corticosteroid dose was not related to the maximum rise in cystatin C during acute allograft dysfunction by multivariate analysis. In fact, corticosteroids increase the relative rise in cystatin C, namely in patients with low GFR, as illustrated in Fig. 4. (iii) The most likely explanation is the difference in kidney function of the patient populations. As shown by our pharmacokinetic model, the effect of the extrarenal elimination of cystatin C increases at lower baseline GFR, leading to an attenuated rise in serum cystatin C. All patients in the cohort of Herget-Rosenthal had normal kidney function at baseline, whereas GFR in our transplant recipients was around 60 to 70 ml/min/1.73 m2. Also, looking at their data, the stronger increase in cystatin C compared to creatinine was most marked at low normal GFR (RIFLE ‘R’) and vanished when kidney failure had progressed to RIFLE ‘F’.
Our study has several limitations. The statistical power is low, calling for larger prospective studies in the critical cohort of patients with pre-existing renal disease and renal transplant recipients. Also, we did not measure GFR by a gold standard technique, as this is not feasible on a daily basis. The diagnosis of acute renal injury was based on the course of kidney function parameters without having a definitive diagnosis of the underlying problem in each case. Still, our definition requiring a sustained increase lasting at least 2 days and the overall good agreement between creatinine and cystatin C indicate that the diagnosis of acute kidney injury was correct. Our strict definition excluded short-term fluctuations of GFR, a potential stronghold of cystatin C [31].
In conclusion, cystatin C was not superior to creatinine for the detection of acute allograft dysfunction in this pediatric series of limited sample size. The potential of cystatin C as a marker of acute renal dysfunction needs further evaluation in adequately powered studies and should be compared with novel markers of renal injury like N-GAL (neutrophil gelatinase-associated lipocalin), which originate from damaged kidney tissue rather than reflecting a decrease in GFR [32].
Acknowledgments
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem. 1992;38:1933–1953. [PubMed] [Google Scholar]
- 2.Vinge E, Lindergard B, Nilsson-Ehle P, Grubb A. Relationships among serum cystatin C, serum creatinine, lean tissue mass and glomerular filtration rate in healthy adults. Scand J Clin Lab Invest. 1999;59:587–592. doi: 10.1080/00365519950185076. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz GJ, Brion LP, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am. 1987;34:571–590. doi: 10.1016/s0031-3955(16)36251-4. [DOI] [PubMed] [Google Scholar]
- 4.Bunchman TE, Fryd DS, Sibley RK, Mauer SM. Manifestations of renal allograft rejection in small children receiving adult kidneys. Pediatr Nephrol. 1990;4:255–258. doi: 10.1007/BF00857670. [DOI] [PubMed] [Google Scholar]
- 5.Grubb AO. Cystatin C—properties and use as diagnostic marker. Adv Clin Chem. 2000;35:63–99. doi: 10.1016/S0065-2423(01)35015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mussap M, Plebani M. Biochemistry and clinical role of human cystatin C. Crit Rev Clin Lab Sci. 2004;41:467–550. doi: 10.1080/10408360490504934. [DOI] [PubMed] [Google Scholar]
- 7.Roos JF, Doust J, Tett SE, Kirkpatrick CM. Diagnostic accuracy of cystatin C compared to serum creatinine for the estimation of renal dysfunction in adults and children—a meta-analysis. Clin Biochem. 2007;40:383–391. doi: 10.1016/j.clinbiochem.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 8.Laterza OF, Price CP, Scott MG. Cystatin C: an improved estimator of glomerular filtration rate? Clin Chem. 2002;48:699–707. [PubMed] [Google Scholar]
- 9.White C, Akbari A, Hussain N, Dinh L, Filler G, Lepage N, Knoll GA. Estimating glomerular filtration rate in kidney transplantation: a comparison between serum creatinine and cystatin C-based methods. J Am Soc Nephrol. 2005;16:3763–3770. doi: 10.1681/ASN.2005050512. [DOI] [PubMed] [Google Scholar]
- 10.Poge U, Gerhardt T, Stoffel-Wagner B, Palmedo H, Klehr HU, Sauerbruch T, Woitas RP. Cystatin C-based calculation of glomerular filtration rate in kidney transplant recipients. Kidney Int. 2006;70:204–210. doi: 10.1038/sj.ki.5001502. [DOI] [PubMed] [Google Scholar]
- 11.Maillard N, Mariat C, Bonneau C, Mehdi M, Thibaudin L, Laporte S, Alamartine E, Chamson A, Berthoux F. Cystatin C-based equations in renal transplantation: moving toward a better glomerular filtration rate prediction? Transplantation. 2008;85:1855–1858. doi: 10.1097/TP.0b013e3181744225. [DOI] [PubMed] [Google Scholar]
- 12.Herget-Rosenthal S, Marggraf G, Husing J, Göring F, Pietruck F, Janssen O, Philipp T, Kribben A. Early detection of acute renal failure by serum cystatin C. Kidney Int. 2004;66:1115–1122. doi: 10.1111/j.1523-1755.2004.00861.x. [DOI] [PubMed] [Google Scholar]
- 13.Benohr P, Grenz A, Hartmann JT, Müller GA, Blaschke S. Cystatin C—a marker for assessment of the glomerular filtration rate in patients with cisplatin chemotherapy. Kidney Blood Press Res. 2006;29:32–35. doi: 10.1159/000092485. [DOI] [PubMed] [Google Scholar]
- 14.Haase-Fielitz A, Bellomo R, Devarajan P, Story D, Matalanis G, Dragun D, Haase M. Novel and conventional serum biomarkers predicting acute kidney injury in adult cardiac surgery—a prospective cohort study. Crit Care Med. 2009;37:553–560. doi: 10.1097/CCM.0b013e318195846e. [DOI] [PubMed] [Google Scholar]
- 15.Bokenkamp A, Ozden N, Dieterich C, Schumann G, Ehrich JH, Brodehl J. Cystatin C and creatinine after successful kidney transplantation in children. Clin Nephrol. 1999;52:371–376. [PubMed] [Google Scholar]
- 16.Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int. 2007;71:1028–1035. doi: 10.1038/sj.ki.5002231. [DOI] [PubMed] [Google Scholar]
- 17.Plötz FB, Bouma AB, van Wijk JA, Kneyber MC, Bökenkamp A. Pediatric acute kidney injury in the ICU: an independent evaluation of pRIFLE criteria. Intensive Care Med. 2008;34:1713–1717. doi: 10.1007/s00134-008-1176-7. [DOI] [PubMed] [Google Scholar]
- 18.Pöge U, Gerhardt T, Bökenkamp A, Stoffel-Wagner B, Klehr HU, Sauerbruch T, Woitas RP. Time course of low molecular weight proteins in the early kidney transplantation period—influence of corticosteroids. Nephrol Dial Transplant. 2004;19:2858–2863. doi: 10.1093/ndt/gfh341. [DOI] [PubMed] [Google Scholar]
- 19.Risch L, Herklotz R, Blumberg A, Huber AR. Effects of glucocorticoid immunosuppression on serum cystatin C concentrations in renal transplant patients. Clin Chem. 2001;47:2055–2059. [PubMed] [Google Scholar]
- 20.Bökenkamp A, Domanetzki M, Zinck R, Schumann G, Byrd D, Brodehl J. Cystatin C—a new marker of glomerular filtration rate in children independent of age and height. Pediatrics. 1998;101:875–881. doi: 10.1542/peds.101.5.875. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grubb A, Nyman U, Björk J, Lindström V, Rippe B, Sterner G, Christensson A. Simple cystatin C-based prediction equations for glomerular filtration rate compared with the modification of diet in renal disease prediction equation for adults and the Schwartz and the Counahan-Barratt prediction equations for children. Clin Chem. 2005;51:1420–1431. doi: 10.1373/clinchem.2005.051557. [DOI] [PubMed] [Google Scholar]
- 23.Schloerb PR. Total body water distribution of creatinine and urea in nephrectomized dogs. Am J Physiol. 1960;199:661–665. doi: 10.1152/ajplegacy.1960.199.4.661. [DOI] [PubMed] [Google Scholar]
- 24.Sjostrom P, Tidman M, Jones I. Determination of the production rate and non-renal clearance of cystatin C and estimation of the glomerular filtration rate from the serum concentration of cystatin C in humans. Scand J Clin Lab Invest. 2005;65:111–124. doi: 10.1080/00365510510013523. [DOI] [PubMed] [Google Scholar]
- 25.Rickli H, Benou K, Ammann P, Fehr T, Brunner-La Rocca HP, Petridis H, Riesen W, Wüthrich RP. Time course of serial cystatin C levels in comparison with serum creatinine after application of radiocontrast media. Clin Nephrol. 2004;61:98–102. doi: 10.5414/cnp61098. [DOI] [PubMed] [Google Scholar]
- 26.Poletti PA, Saudan P, Platon A, Mermillod B, Sautter AM, Vermeulen B, Sarasin FP, Becker CD, Martin PY. I.v. N-acetylcysteine and emergency CT: use of serum creatinine and cystatin C as markers of radiocontrast nephrotoxicity. AJR Am J Roentgenol. 2007;189:687–692. doi: 10.2214/AJR.07.2356. [DOI] [PubMed] [Google Scholar]
- 27.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Acute Dialysis Quality Initiative workgroup Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bjarnadottir M, Grubb A, Olafsson I. Promoter-mediated, dexamethasone-induced increase in cystatin C production by HeLa cells. Scand J Clin Lab Invest. 1995;55:617–623. doi: 10.3109/00365519509110261. [DOI] [PubMed] [Google Scholar]
- 29.Fricker M, Wiesli P, Brändle M, Schwegler B, Schmid C. Impact of thyroid dysfunction on serum cystatin C. Kidney Int. 2003;63:1944–1947. doi: 10.1046/j.1523-1755.2003.00925.x. [DOI] [PubMed] [Google Scholar]
- 30.Bökenkamp A, Laarman CA, Braam KI, van Wijk JA, Kors WA, Kool M, de Valk J, Bouman AA, Spreeuwenberg MD, Stoffel-Wagner B. Effect of corticosteroid therapy on low-molecular weight protein markers of kidney function. Clin Chem. 2007;53:2219–2221. doi: 10.1373/clinchem.2007.094946. [DOI] [PubMed] [Google Scholar]
- 31.Risch L, Blumberg A, Huber A. Rapid and accurate assessment of glomerular filtration rate in patients with renal transplants using serum cystatin C. Nephrol Dial Transplant. 1999;14:1991–1996. doi: 10.1093/ndt/14.8.1991. [DOI] [PubMed] [Google Scholar]
- 32.Mitsnefes MM, Kathman TS, Mishra J, Kartal J, Khoury PR, Nickolas TL, Barasch J, Devarajan P. Serum neutrophil gelatinase-associated lipocalin as a marker of renal function in children with chronic kidney disease. Pediatr Nephrol. 2007;22:101–108. doi: 10.1007/s00467-006-0244-x. [DOI] [PubMed] [Google Scholar]
- 33.Abbink FC, Laarman CA, Braam KI, van Wijk JA, Kors WA, Bouman AA, Spreeuwenberg MD, Stoffel-Wagner B, Bökenkamp A. Beta-trace protein is not superior to cystatin C for the estimation of GFR in patients receiving corticosteroids. Clin Biochem. 2008;41:299–305. doi: 10.1016/j.clinbiochem.2007.11.012. [DOI] [PubMed] [Google Scholar]