Abstract
Surveillance biopsies are increasingly used in the post-transplant monitoring of pediatric renal allograft recipients. The main justification for this procedure is to diagnose early and presumably modifiable acute and chronic renal allograft injury. Pediatric recipients are theoretically at increased risk for subclinical renal allograft injury due to their relatively large adult-sized kidneys and their higher degree of immunological responsiveness. The safety profile of this procedure has been well investigated. Patient morbidity is low, with macroscopic hematuria being the most common adverse event. No patient deaths have been attributed to this procedure. Longitudinal surveillance biopsy studies have revealed a substantial burden of subclinical immunological and non-immunological injury, including acute cellular rejection, interstitial fibrosis and tubular atrophy, microvascular lesions and transplant glomerulopathy. The main impediment to the implementation of surveillance biopsies as the standard of care is the lack of demonstrable benefit of early histological detection on long-term outcome. The considerable debate surrounding this issue highlights the need for multicenter, prospective, and randomized studies.
Keywords: Pediatric, Kidney transplantation, Protocol renal allograft biopsies
Introduction
The surveillance biopsy, also known as the ‘protocol biopsy,’ is defined as the sampling of renal tissue in patients with stable allograft function at predetermined time points [1, 2], typically between 1-12-months post-transplantation. Surveillance biopsies are increasingly used to diagnose subtle (i.e., subclinical) acute and chronic pathology in renal allografts. In some centers, they are also performed to evaluate baseline histology at implantation (i.e., ‘donor’ or ‘implantation’ biopsies) or to determine the efficacy of acute rejection (AR) therapy (i.e., ‘follow-up’ biopsies) [3]. The main justification for this procedure is to detect early and presumably modifiable renal allograft injury. However, in the pediatric renal transplant community, considerable debate about the clinical utility of this invasive procedure remains, particularly in the low immunological risk recipient [2, 4–10]. Similarly, in the absence of obvious graft dysfunction at predetermined time points, private insurers may be reluctant to provide coverage for this procedure.
Rationale for surveillance biopsies in pediatric renal transplant recipients
Several unique factors merit a higher index of suspicion for subclinical renal allograft injury in pediatric recipients. The first is the large mass of the adult-sized kidney (ASK) relative to the small pediatric recipient [11]. In one study in the pre-surveillance biopsy era, less than 50% of young pediatric recipients with acute rejection on biopsy actually manifested an appreciable increase in their baseline sCr values [12]. In the original Winnipeg pediatric cohort, AR, diagnosed on surveillance biopsy but without functional deterioration (i.e., subclinical acute rejection, SCR), was observed in 19% of low immunological risk patients managed on antibody, steroids, tacrolimus, and mycophenolate mofetil [13]. In this cohort, neither the estimated GFR (eGFR), nor the presence of proteinuria was predictive of interstitial fibrosis and tubular atrophy (IF/TA) [13], formerly known as ‘CAN’ [14].
Pediatric renal transplant recipients also exhibit a high degree of immunological responsiveness. Young peritoneal dialysis patients manifest higher total lymphocyte counts, CD4/CD8 ratios and increased blastogenesis when compared to their older counterparts [15]. Similarly, following sensitizing events such as blood transfusions, pediatric patients are five times more likely to develop anti-HLA antibodies than older patients [16]. Thus, it has been postulated that the large renal mass of an ASK may conceal incipient acute and chronic renal allograft injury in the pediatric recipient [3, 10, 13]. Since children have more robust immunological responses, they are potentially at higher risk for SCR [10, 13].
Biopsy procedure
The surveillance biopsy is typically performed under conscious sedation in an outpatient unit [17]. Depending on center expertise, the procedure is performed by a pediatric nephrologist or an interventional radiologist. Conscious sedation (e.g., intravenous midazolam or propofol) is administered by an anesthesiologist or an intensivist. Specimen adequacy is determined by a histopathology technician who is also present during the procedure.
A renal pole situated away from the main transplant vessels is localized with ultrasound in real-time. Ideally, two tissue cores are obtained using an 18-gauge or a 16-gauge disposable needle [17]. While the utilization of a larger diameter needle improves specimen adequacy (at least seven glomeruli and two arteries) [18], its use is associated with a higher incidence of post-biopsy hemorrhage [17, 19]. Patients are recovered for a minimum of 4 h, as the majority of biopsy-related complications manifest within the first 4 h of biopsy [17].
Renal tissue specimens are fixed in formalin and embedded in paraffin. For Banff scoring, paraffin sections are processed with hematoxylin and eosin (H&E), periodic acid Schiff (PAS), periodic acid methenamine Schiff (PAMS) and Masson’s trichrome (MT) stains [18]. To facilitate the diagnosis of antibody-mediated rejection (AMR), most centers also perform C4d staining on frozen tissue. When the protocol biopsy is used for research purposes, upon procurement, a portion of the core (e.g., 1/3 or 1/2) is snap-frozen in liquid nitrogen and stored at −80°C for future analyses [19].
Adverse events related to surveillance biopsies
The safety profile of surveillance biopsies has been documented in more than 1,900 adult and 250 pediatric recipients who underwent approximately 5,000 and 700 biopsy procedures, respectively (Table 1). Importantly, the incidence of major adverse events such as allograft loss is extremely low and no deaths have been reported in these series [17, 20–25]. Macroscopic hematuria was the most-commonly reported adverse event. Its frequency increased with the use of a larger diameter needle (16-gauge vs. 18-gauge) [17, 20] and the penetration of renal medulla or highly inflamed arteries [21]. It should be noted that since many centers do not routinely perform post-biopsy ultrasound, the incidence of perinephric hematomas and arteriovenous fistulae is likely to be under-reported. In all of these series, major (i.e., highly invasive) post-biopsy interventions were rare [17, 20–25] (Table 2).
Table 1.
Incidence of adverse events following surveillance biopsies in adult and pediatric kidney transplant recipients [17, 20–25]
| Incidence (%) | ||
|---|---|---|
| Adverse event | Adult kidney transplant recipients | Pediatric kidney transplant recipients |
| Macroscopic hematuria | 2.8-3.1 | 2.7-8.8 |
| Perinephric hematoma* | 3.3 | 13.4 |
| Arteriovenous fistula* | 9.0 | 1.3 |
| Bowel perforation | 0.04 | 0 |
| Vasovagal reaction | 0.8 | 0 |
| Allograft loss | 0.04-0.3 | 0 |
| Death | 0 | 0 |
*May be under-reported
Table 2.
| Incidence (%) | ||
|---|---|---|
| Intervention | Adult kidney transplant recipients | Pediatric kidney transplant recipients |
| Blood transfusion | 0.1-0.7 | 0 |
| Bladder catheterization | 0.3-0.6 | 2.3 |
| Radiological procedures | 0.04 | 2.3 |
| Surgical procedures | 0.09-0.4 | 0 |
| Prolonged hospitalization | 2.0% | 3.5% |
Surveillance biopsies for the detection of subclinical acute cellular rejection
Definition of subclinical acute cellular rejection
Rush et al. [26] originally described the condition, “subclinical rejection” (SCR), in which one-third of adult renal transplant recipients managed on steroids, cyclosporine, and azathioprine had acute cellular rejection on surveillance biopsy. Notably absent was a concomitant increase in their baseline serum creatinine (sCr) values. These findings led to the implementation of biopsies 1, 2, 3, 6, and 12 months post-transplantation as standard of care for renal allograft monitoring [27].
In the last decade, the definition of SCR has been subclassified to include: (1) Acute subclinical rejection (A-SCR), in which the degree of cellular interstitial and tubular infiltration reach Banff criteria for AR (≥ i2t2, respectively) (Figs. 1a and 2). Borderline subclinical rejection (B-SCR), characterized by milder degrees of inflammation (i0-1 and/or t1-t3) [2, 28] (Fig. 1b). However, as the differences in cellular infiltration (e.g., activated macrophages) and pro-inflammatory gene expression (e.g., tumor necrosis factor alpha, interleukin (IL)-1 beta, transforming growth factor beta, interferon gamma, IL-2, IL-4, IL-10, and IL-15, granzyme B, perforin, Fas ligand, and CD152 costimulation molecule) are quantitative rather than qualitative, it is likely that A-SCR and B-SCR merely represent different potencies of the same acute inflammatory process [19, 29, 30].
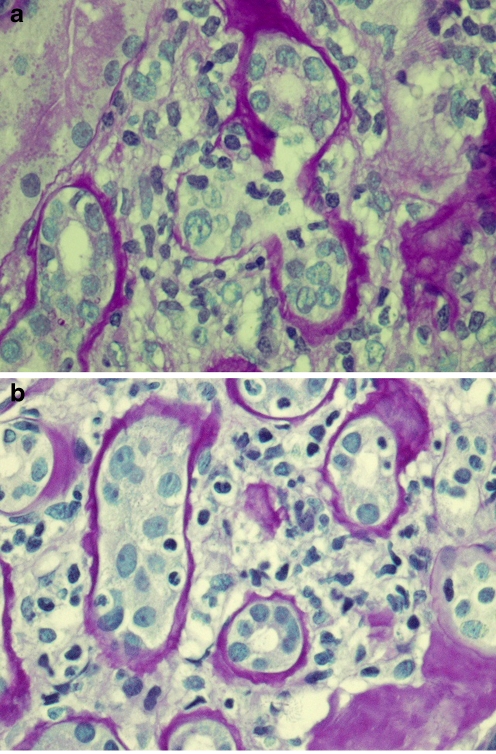
Fig. 1.
a Surveillance renal allograft biopsy showing acute cellular subclinical rejection (A-SCR) with tubulitis (t2), PAS stain. b Surveillance renal allograft biopsy showing borderline cellular subclinical rejection (B-SCR) with minimal interstitial infiltrates (i1) and mild tubulitis (t1), PAS stain
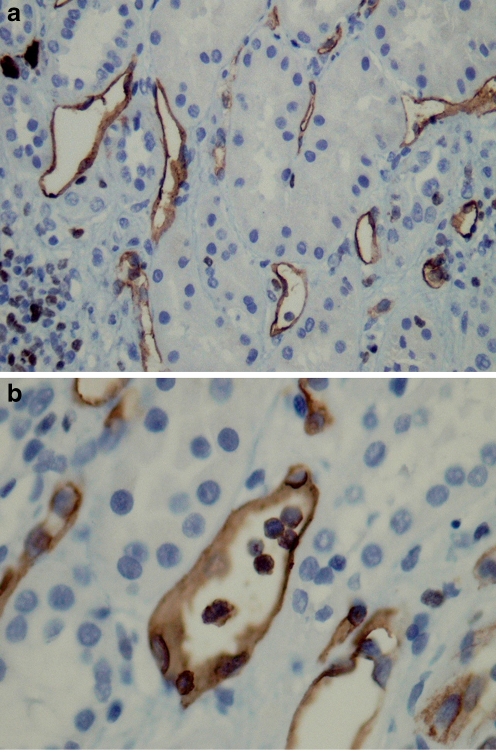
Fig. 2.
a Surveillance renal allograft biopsy showing subclinical AMR with diffuse C4d + staining of peritubular capillaries and b Peritubular capillaritis (ptc2)
Epidemiology of subclinical acute cellular rejection
The incidence of all forms of SCR is influenced by both the amount and potency of immunosuppression [2, 28]. In the cyclosporine/azathioprine era, SCR was detected in approximately 30% adult recipients during the first 3 months post-transplant [26, 27]. Similarly, in the landmark Australian report of adult kidney-pancreas recipients undergoing 1,000 surveillance biopsies over 10 years, the incidence of SCR was 46% at 3 months post-transplant. By 1 year post-transplant, the incidence had decreased to 18% [2], possibly due to the phenomenon of accommodation. The use of more potent immunosuppressive medications such as antibody induction, tacrolimus, and mycophenolate mofetil has led to a marked reduction in the overall prevalence of SCR (3-5% for A-SCR and 11% for B-SCR) [31, 32]. The paucity of acute inflammation reported in these recent studies have led some investigators to question the value of the surveillance biopsy in the low immunological risk adult recipient [31, 32].
In contrast, in children, the incidence and prevalence of SCR remains high. In a recent report of pediatric recipients managed on basiliximab induction, steroids, tacrolimus or sirolimus and mycophenolate mofetil, 29% patients had either A-SCR or B-SCR at 3 months post-transplant [33]. However, in this study, the dosage of mycophenolate mofetil (600–900 mg/m2/day) was lower than a recent task force dosage recommendation of 1,200 mg/m2/day [34]. Thus, the higher prevalence of SCR observed in this particular study may reflect under-immunosuppression.
In the Winnipeg cohort (in which patients receive induction, steroids, tacrolimus, or sirolimus and mycophenolate mofetil 1,200 mg/m2/day), by 7–12 months post-transplant, the incidence of A-SCR had declined to 8%, with an additional 13% ‘spike’ occurring in nonadherent adolescents after 3 years post-transplantation. Borderline SCR was observed in up to 20% of surveillance biopsies, with a peak incidence occurring at 7–12 months post-transplant [35]. In another pediatric report, A-SCR was seen in the late post-transplant period (19% at 3 years and 16% at 4 years post-transplant) [36].
Pathogenicity of subclinical acute cellular rejection
The potential for SCR to cause significant renal allograft injury continues to be debated. In both adult and pediatric recipients, A-SCR and B-SCR have been associated with the development and progression of IF/TA [13, 27, 35–41], impaired glomerular adaptation [42], late allograft dysfunction [36–38] and decreased allograft survival [39, 43–46]. Given these findings, it is not unreasonable to assume that the immunosuppressive treatment of SCR would improve renal allograft outcomes. However, studies in adult recipients are collectively equivocal. In some reports, steroid treatment of SCR resulted in lower IF/TA scores at 6 months and 2 years post-transplant [40, 41, 47]; and fewer subsequent AR episodes and lower sCr values at 2 years post-transplant [47]. Other studies, however, showed no significant differences in chronic histology or in renal allograft function and survival [32, 48, 49]. These conflicting results reflect, in part, variable definitions of SCR and a lack of recognition of antibody-mediated rejection (AMR). To date, no pediatric studies have evaluated the immunosuppressive treatment of SCR using a randomized and prospective study design.
In one pediatric retrospective study, increasing the dosage of mycophenolate mofetil by 50% resulted in a significantly reduced prevalence of SCR from 44–29% [33]. However, this reduction was accompanied by a marked increase in polyoma (BK) viremia from 3–30%. Thus, in the management of SCR, the risk of pathogenicity must be weighed against the risk of over-immunosuppression, which includes opportunistic infections, and possibly, post-transplant lymphoproliferative disease.
Surveillance biopsies for the detection of chronic renal allograft injury
Patterns of chronic renal allograft injury
Longitudinal observational surveillance studies demonstrate that chronic renal allograft injury develops early after transplantation [35, 37], with 89% patients manifesting grade 1 or higher IF/TA by 7–12 months [35]. Both adult and pediatric surveillance biopsy studies reveal a biphasic pattern of pathological changes [35, 37]. Chronic tubulointerstitial injury develops within the first 12 months post-transplant, followed by chronic microvascular injury (vascular fibrous intimal thickening, arteriolar hyalinosis and glomerulosclerosis) at 2 years post-transplant and beyond [35, 37, 50–52].
Risk factors for chronic renal allograft injury
In children, multivariate analyses provide insights into the etiology of chronic renal allograft lesions. Interstitial fibrosis/tubular atrophy and arteriolar hyalinosis are associated with low recipient BSA, which is a surrogate for the renal hypoperfusion resulting from the transplantation of ASK’s into small recipients [35, 50]. The development of glomerulosclerosis lesions parallels the onset of vascular lesions, implicating CNI-mediated ischemia as a contributing factor [35, 51, 52]. Other potentially modifiable risk factors include AR and all types of SCR (IF/TA), donor hypertension (vascular fibrous intimal thickening) and post-transplant obesity (IF/TA) [35].
Surveillance biopsies for the detection of antibody-mediated rejection
Antibody-mediated rejection is characterized by the variable presence of: 1. Acute tissue injury, such as glomerulitis and peritubular capillaritis (ptc); 2. Complement degradation product (C4d) staining; and 3. Circulating donor-specific antibody (DSA) [53]. In a primate alloantibody model, the progression of AMR to renal allograft failure begins with the formation of DSA (most commonly anti-HLA Class II antibodies), followed by complement activation and C4d deposition in glomeruli and ptc. The sequelae of persistent glomerular inflammation is basement membrane duplication, mesangial matrix expansion and mesangial cell interposition, a condition known as transplant glomerulopathy (TG) [54, 55].
In adult recipients, AMR is more deleterious than acute cellular rejection (35% renal allograft survival, compared to 100% renal allograft survival, respectively, at 4 years post-transplant) [56]. Similarly, in pediatric recipients, C4d-positive ptc is associated with a higher prevalence of TG and late renal allograft loss [57]. Subclinical acute AMR is increasingly recognized on surveillance biopsy (Figs. 2a and b), but its precise incidence is currently unknown. However, subclinical TG is well described in adult recipients, with a cumulative incidence of 3% (1 year), 6% (2 years), 9% (3 years) and 12% (5 years) [55]. By 1 year post-transplant, TG was already associated with reduced eGFR and increased proteinuria [55]. In the Winnipeg pediatric cohort, the overall prevalence of TG was 22% in patients with a mean follow-up of 44 ± 5 months. Among these, 44% were C4d-positive on surveillance biopsy and 22% had DSA [35].
Limitations of surveillance biopsy findings
A typical biopsy core represents only 0.04% of the renal allograft [4]. Sampling error has been estimated to affect up to 25% of surveillance biopsies [58], leading to erroneous diagnoses. Noninvasive technologies (e.g., microarrays, proteonomics, and NMR spectroscopy) utilizing blood or urine can potentially obviate sampling error, but must be validated in large and heterogeneous populations. Thus, the surveillance biopsy, while flawed, remains the gold standard for the diagnosis of renal allograft pathology.
Surveillance biopsy data are also susceptible to the ‘era effect.’ The largest and most frequently cited surveillance biopsy study describing the natural history study of ‘CAN’ in kidney-pancreas recipients was performed before the routine use of C4d staining and solid-phase assays for DSA detection. In light of the subsequent observation of the negative impact of antibody-mediated injury on renal allograft survival, it is likely that some proportion of the IF/TA reported in this study actually resulted from AMR [59]. In adult recipients managed in the tacrolimus/sirolimus era, there is a growing body of evidence showing that mild IF/TA is minimally progressive [60] and in the absence of acute inflammation, it is not associated with renal allograft dysfunction or diminished graft survival [61].
Conclusions
Pediatric recipients are theoretically at increased risk for subclinical renal allograft injury due to their relatively large adult-sized kidneys and their higher degree of immunological responsiveness. In these patients, longitudinal surveillance biopsy studies have revealed a substantial burden of subclinical immunological and non-immunological injury, including acute cellular rejection, interstitial fibrosis and tubular atrophy, microvascular lesions and transplant glomerulopathy. The main impediment to the implementation of surveillance biopsies as standard of care is the lack of demonstrable benefit of early histological detection on long-term outcome. The considerable debate surrounding this issue speaks to the need for multicenter, prospective and randomized studies, which are currently lacking. In the absence of a direct benefit of longitudinal screening, the most pragmatic use of the surveillance biopsy is in guiding the post-transplant management of the higher immunological risk pediatric recipient.
Questions (answers are provided after references)
- The following statements about subclinical acute cellular rejection (SCR) are true except:
- The incidence of SCR is dependent on the amount and potency of immunosuppression
- In adult renal transplant recipients, the incidence of SCR is decreasing
- In pediatric renal transplant recipients, the incidence of SCR is decreasing
- SCR is defined as Banff histology showing acute rejection in patients with stable allograft function
True or False: Acute subclinical rejection (A-SCR) is defined as acute rejection by Banff criteria (≥i2t2) with a concomitant increase in the serum creatinine.
True or False: Borderline subclinical rejection (B-SCR) is defined as acute rejection which does not meet Banff criteria (i0-i1 and/or t1-t3) without a concomitant increase in the serum creatinine.
- The following are complications of surveillance biopsies except:
- Macroscopic hematuria
- Arteriovenous fistula
- Death
- Bowel perforation
- All of the following increase the risk of post-biopsy hemorrhage except:
- Acute rejection with vascular involvement
- Adult-sized kidney (ASK)
- Penetration of the renal medulla
- Use of a 16-gauge needle
- Which of the following lesions have been observed in surveillance biopsies?
- Acute cellular rejection
- Interstitial fibrosis and tubular atrophy (IF/TA)
- Transplant glomerulopathy (TG)
- All of the above
Footnotes
Answers
1. c
2. False
3. True
4. c
5. b
6. d
References
- 1.Wigmore SJ, Forsythe JLR. To biopsy or not to biopsy. Transplantation. 2003;76:909–910. doi: 10.1097/01.TP.0000082543.17341.67. [DOI] [PubMed] [Google Scholar]
- 2.Nankivell BJ, Chapman JR. The significance of subclinical rejection and the value of protocol biopsies. Am J Transplant. 2006;6:2006–2012. doi: 10.1111/j.1600-6143.2006.01436.x. [DOI] [PubMed] [Google Scholar]
- 3.Birk PE, Rush DN. Protocol biopsies should be standard of care for pediatric renal alloallograft recipients! Pediatr Transplantation. 2006;10:760–765. doi: 10.1111/j.1399-3046.2006.00573.x. [DOI] [PubMed] [Google Scholar]
- 4.Rush D. Pro: Protocol biopsies should be part of the routine management of kidney transplant recipients. Am J Kid Dis. 2002;40:671–673. doi: 10.1053/ajkd.2002.36427. [DOI] [PubMed] [Google Scholar]
- 5.Salomon D. Con: Protocol biopsies should be part of the routine management of kidney transplant recipients. Am J Kid Dis. 2002;40:674–677. doi: 10.1053/ajkd.2002.36426. [DOI] [PubMed] [Google Scholar]
- 6.Rush D. Protocol transplant biopsies: An underutilized tool in kidney transplantation. J Am Soc Nephrol. 2006;1:138–143. doi: 10.2215/CJN.00390705. [DOI] [PubMed] [Google Scholar]
- 7.Wilkinson A. Protocol transplant biopsies: Are they really needed? J Am Soc Nephrol. 2006;1:130–137. doi: 10.2215/CJN.00350705. [DOI] [PubMed] [Google Scholar]
- 8.Racusen L. Protocol transplant biopsies in kidney alloallografts: Why and when are they indicated? J Am Soc Nephrol. 2006;1:144–147. doi: 10.2215/CJN.01010905. [DOI] [PubMed] [Google Scholar]
- 9.Shapiro R. Protocol biopsies should not (yet) be standard of care for pediatric renal alloallograft recipients. Pediatr Transplantation. 2006;10:766–767. doi: 10.1111/j.1399-3046.2006.00572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birk PE. What do we need to do to make protocol biopsies standard of care or should we discontinue doing? Pediatr Transplantation. 2009;13:797–801. doi: 10.1111/j.1399-3046.2009.01229.x. [DOI] [PubMed] [Google Scholar]
- 11.Salvatierra O, Singh T, Shifrin R, Conley S, Alexander S, Tanney D, Lemley S, Sarwal M, Mackie F, Alfrey E, Orlandi P, Zarins C, Herfkens R. Successful transplantation of adult-sized kidneys into infants requires maintenance of high aortic blood flow. Transplantation. 1998;66:819–823. doi: 10.1097/00007890-199810150-00001. [DOI] [PubMed] [Google Scholar]
- 12.Bunchman TE, Fryd DS, Sibley RK, Mauer M. Manifestations of renal allograft rejection in small children receiving adult kidneys. Pediatr Nephrol. 1990;4:255–258. doi: 10.1007/BF00857670. [DOI] [PubMed] [Google Scholar]
- 13.Birk PE, Stannard KM, Konrad HB, Blydt-Hansen TD, Ogborn MR, Cheang MS, Gartner JG, Gibson IW. Surveillance biopsies are superior to functional studies for the diagnosis of acute and chronic renal alloallograft pathology in children. Pediatr Transplant. 2004;8:29–38. doi: 10.1046/j.1397-3142.2003.00122.x. [DOI] [PubMed] [Google Scholar]
- 14.Solez K, Colvin RB, Racusen LC, Sis B, Halloran PF, Birk PE, Campbell PM, Cascalho M, Collins AB, Demetris AJ, Drachenberg CB, Gibson IW, Grimm PC, Haas M, Lerut E, Liapis H, Mannon RB, Marcus PB, Mengel M, Mihatsch MJ, Nankivell BJ, Nickeleit V, Papadimitriou JC, Platt JL, Randhawa P, Roberts I, Salinas-Madriga L, Salomon DR, Seron D, Sheaff M, Weening JJ. Banff '05 Meeting Report: differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy ('CAN') Am J Transplant. 2007;7:518–526. doi: 10.1111/j.1600-6143.2006.01688.x. [DOI] [PubMed] [Google Scholar]
- 15.Ettenger RB, Blifeld C, Prince H, Gradus DB-E, Cho S, Sekiya N, Salusky I, Fine RN. The pediatric nephrologist’s dilemma: growth after renal transplantation and its interaction with age as a possible immunologic variable. J Pediatr. 1987;111:1022–1025. doi: 10.1016/S0022-3476(87)80049-5. [DOI] [PubMed] [Google Scholar]
- 16.Scornik J, Pfaff WW, Howard RJ, Fennell RS, III, Ramos E, Peterson JC, Neiberger R. Increased antibody responsiveness to blood transfusions in pediatric patients. Transplantation. 1994;58:1361–1365. [PubMed] [Google Scholar]
- 17.Birk PE, Blydt-Hansen TD, Dart AB, Kaita LM, Proulx C, Taylor G. Low incidence of adverse events in outpatient pediatric protocol renal alloallograft biopsies. Pediatr Transplant. 2007;11:196–200. doi: 10.1111/j.1399-3046.2006.00659.x. [DOI] [PubMed] [Google Scholar]
- 18.Racusen L, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, Croker BP, Demetris AJ, Drachenberg CB, Fogo A, Furness P, Gaber LW, Gibson IW, Glotz D, Goldberg J, Grande J, Halloran PF, Hansen HE, Hartley B, Haryry P, Hill CM, Hoffman EO, Hunsicker LG, Lindblad AS, Marcusssen N, Mihatsch MJ, Nadasdy T, Nickerson P, Olsen TS, Papadimitriou JC, Randhawa PS, Rayner DC, Roberts I, Rose S, Rush D, Salinas-Madrigal L, Salomon DR, Sund S, Taskinen E, Trpkov K, Yamaguchi Y. The Banff 97 working classification of kidney transplant pathology. Kidney Int. 1999;55:713–723. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
- 19.Lipman ML, Shen Y, Jeffery JR, Gough J, McKenna RM, Grimm PC, Rush DN. Immune-activation gene expression in clinically stable renal allograft biopsies: molecular evidence for subclinical rejection. Transplantation. 1998;66:1673–1681. doi: 10.1097/00007890-199812270-00018. [DOI] [PubMed] [Google Scholar]
- 20.Furness PN, Philpott CM, Chorbadjian MT, Nicholson ML, Bosmans J-L, Corthouts BL, Bogers JJPM, Schwarz A, Gwinner W, Haller H, Mengel M, Seron D, Moreso F, Canas C. Protocol biopsy of the stable renal transplant: a multicenter study of methods and complication rates. Transplantation. 2003;76:969–973. doi: 10.1097/01.TP.0000082542.99416.11. [DOI] [PubMed] [Google Scholar]
- 21.Mengel M, Chapman JR, Cosio FG, Cavaille-Coll MW, Haller H, Halloran PF, Kirk AD, Mihatsch MJ, Nankivell BJ, Racusen LC, Roberts IS, Rush DN, Schwarz A, Seron D, Stegall M, Colvin RB. Protocol biopsies in renal transplantation: insights into patient management and pathogenesis. Am J Transplant. 2007;7:512–517. doi: 10.1111/j.1600-6143.2006.01677.x. [DOI] [PubMed] [Google Scholar]
- 22.Wilczek HE. Percutaneous needle biopsy of the renal alloallograft. A clinical safety evaluation of 1129 biopsies. Transplantation. 1990;50:790–797. doi: 10.1097/00007890-199011000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Schwarz A, Gwinner W, Hiss M, Radermacher J, Mengel M, Haller H. Safety and adequacy of renal transplant protocol biopsies. Am J Transplant. 2005;5:1992–1996. doi: 10.1111/j.1600-6143.2005.00988.x. [DOI] [PubMed] [Google Scholar]
- 24.Benfield MR, Herrin J, Feld L, Rose S, Stablein D, Tejani A. Safety of kidney biopsy in pediatric transplantation: a report of the controlled clinical trials in pediatric transplantation trial of induction therapy study group. Transplantation. 1999;67:544–547. doi: 10.1097/00007890-199902270-00010. [DOI] [PubMed] [Google Scholar]
- 25.Vidhun J, Masciandro J, Varich L, Salvatierra O, Sarwal M. Safety and risk stratification of percutaneous biopsies of adult-sized renal alloallografts in infant and older pediatric recipients. Transplantation. 2003;76:552–557. doi: 10.1097/01.TP.0000076097.90123.21. [DOI] [PubMed] [Google Scholar]
- 26.Rush DN, Henry S, Jeffery JR, Schroeder TJ, Gough J. Histological findings in early routine biopsies of stable renal allloallograft recipients. Transplantation. 1994;57:208–211. doi: 10.1097/00007890-199401001-00009. [DOI] [PubMed] [Google Scholar]
- 27.Rush DN, Jeffery JR, Gough J. Sequential protocol biopsies in renal transplant patients. Transplantation. 1995;59:511–514. [PubMed] [Google Scholar]
- 28.Kuypers DRJ. Immunosuppressive drug therapy and subclinical acute renal alloallograft rejection: impact and effect. Transplantation. 2008;85:S25–S30. doi: 10.1097/TP.0b013e318169c48d. [DOI] [PubMed] [Google Scholar]
- 29.Grimm PC, McKenna R, Nickerson P, Russell ME, Gough J, Gospodarek E, Lui B, Jeffery J, Rush DN. Clinical rejection is distinguished from subclinical rejection by increased infiltration by a population of activated macrophages. J Am Soc Nephrol. 1999;10:1582–1589. doi: 10.1681/ASN.V1071582. [DOI] [PubMed] [Google Scholar]
- 30.Hoffman SC, Hale DA, Kleiner DE, Mannon RB, Kampen RL, Jacobson LM, Cendales LC, Swanson SJ, Becker BN, Kirk AD. Functionally significant renal allograft rejection is defined by transcriptional criteria. Am J Transplant. 2005;5:573–581. doi: 10.1111/j.1600-6143.2005.00719.x. [DOI] [PubMed] [Google Scholar]
- 31.Gloor JM, Cohen A, Lager DJ, Grande JP, Fidler ME, Velosa JA, Larson TS, Schwab TR, Griffin MD, Prieto M, Nyberg S, Sterioff S, Kremers WK, Stegall MD. Subclinical rejection in tacrolimus-treated renal transplant recipients. Transplantation. 2002;73:1965–1968. doi: 10.1097/00007890-200206270-00023. [DOI] [PubMed] [Google Scholar]
- 32.Rush D, Arlen D, Boucher A, Busque S, Cockfield SM, Girardin C, Knoll G, Lachance JG, Landsberg D, Shapiro J, Shoker A, Yilmaz S. Lack of benefit of early protocol biopsies in renal transplant patients receiving TAC and MMF: a randomized study. Am J Transplant. 2007;7:2538–2545. doi: 10.1111/j.1600-6143.2007.01979.x. [DOI] [PubMed] [Google Scholar]
- 33.Hymes LC, Warshaw BL, Hennigar RA, Amaral SG, Greenbaum LA. Prevalence of clinical rejection after surveillance biopsies in pediatric renal transplants: does early subclinical rejection predispose to subsequent rejection episodes? Pediatr Transplant. 2009;13:823–826. doi: 10.1111/j.1399-3046.2009.01200.x. [DOI] [PubMed] [Google Scholar]
- 34.Kuypers RJ, Meur L, Cantarovich M, Tredger MJ, Tett SE, Cattaneo D, Tonshoff B, Holt DW, Chapman J, Gelder T. Consensus report on therapeutic drug monitoring of mycophenolic acid in solid organ transplantation. Clin J Am Soc Nephrol. 2010;5:341–358. doi: 10.2215/CJN.07111009. [DOI] [PubMed] [Google Scholar]
- 35.Dart AD, Schall A, Gibson IW, Blydt-Hansen TD, Birk PE. Patterns of chronic injury in pediatric renal allografts. Transplantation. 2010;89:334–340. doi: 10.1097/TP.0b013e3181bc5e49. [DOI] [PubMed] [Google Scholar]
- 36.Shishido S, Asanuma H, Nakai H, Mori Y, Satoh H, Kamimaki I, Hataya H, Ikeda M, Honda M, Hasegawa A. The impact of repeated subclinical acute rejection on the progression of chronic allograft nephropathy. J Am Soc Nephrol. 2003;14:1046–1052. doi: 10.1097/01.ASN.0000056189.02819.32. [DOI] [PubMed] [Google Scholar]
- 37.Nankivell BJ, Borrows RJ, Fung CL-S, O’Connell PJ, Allen RDM, Chapman JR. The natural history of chronic allograft nephropathy. N Engl J Med. 2003;349:2326–2333. doi: 10.1056/NEJMoa020009. [DOI] [PubMed] [Google Scholar]
- 38.Nankivell BJ, Borrows RJ, Fung CL, O’Connell PJ, Allen RD, Chapman JR. Natural history, risk factors and impact of subclinical rejection in kidney transplantation. Transplantation. 2004;78:242–249. doi: 10.1097/01.TP.0000128167.60172.CC. [DOI] [PubMed] [Google Scholar]
- 39.Nankivell BJ, Fenton-Lee CA, Kuypers DR, Allen RD, O’Connell PJ, Chapman JR. Effect of histological damage on long-term kidney transplant outcome. Transplantation. 2001;71:515–523. doi: 10.1097/00007890-200102270-00006. [DOI] [PubMed] [Google Scholar]
- 40.Masin-Spasovska J, Spasovska G, Dzikova S, Petrusevska G, Lekovski L, Ivanovski N, Popov Z. Do we have to treat subclinical rejections in early protocol renal allograft biopsies? Transplant Proc. 2007;39:2550–2553. doi: 10.1016/j.transproceed.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 41.Masin-Spasovska J, Spasovska G, Polenakovic M, Dzikova S, Petrusevska G, Dimova B, Lekovski L, Popov Z, Ivanovski N. Chronic allograft nephropathy (CAN) in early renal protocol biopsies: does treatment of borderline and subclinical acute rejections prevent development and progression of CAN? Prilozi. 2005;26:91–103. [PubMed] [Google Scholar]
- 42.Ibernon M, Goma M, Moreso F, Fulladosa X, Hueso M, Cruzado JM, Torras J, Bestard O, Grinyo JM, Seron D. Subclinical rejection impairs glomerular adaptation after renal transplantation. Kidney Int. 2006;70:557–561. doi: 10.1038/sj.ki.5001582. [DOI] [PubMed] [Google Scholar]
- 43.Ishikawa A, Flechner SM, Goldfarb DA, Myles JL, Modlin CS, Boparai N, Papajcik D, Mastroianni B, Novick AC. Quantitative assessment of the first acute rejection as a predictor of renal transplant outcome. Transplantation. 1999;68:1318–1324. doi: 10.1097/00007890-199911150-00017. [DOI] [PubMed] [Google Scholar]
- 44.Choi BS, Shin MJ, Shin SJ, Kim YS, Choi YJ, Kim YS, Moon IS, Kim SY, Koh YB, Bang BK, Yang CW. Clinical significance of an early protocol biopsy in living-donor renal transplantation: ten-year experience at a single center. Am J Transplant. 2005;5:1354–1360. doi: 10.1111/j.1600-6143.2005.00830.x. [DOI] [PubMed] [Google Scholar]
- 45.Cosio FG, Grande JP, Wadei H, Larson TS, Griffin MD, Stegall MD. Predicting subsequent decline in kidney allograft function from early surveillance biopsies. Am J Transplant. 2005;5:2464–2472. doi: 10.1111/j.1600-6143.2005.01050.x. [DOI] [PubMed] [Google Scholar]
- 46.Moreso F, Ibernon M, Goma M, Carrera M, Fulladosa X, Hueso M, Gil-Vernet S, Cruzado JM, Torras J, Grinyo JM, Seron D. Subclinical rejection associated with chronic allograft nephropathy in protocol biopsies as a risk factor for late graft loss. Am J Transplant. 2006;6:747–752. doi: 10.1111/j.1600-6143.2005.01230.x. [DOI] [PubMed] [Google Scholar]
- 47.Nickerson P, Jeffery J, Gough J, McKenna R, Grimm P, Cheang M, Rush D. Identification of clinical and histopathologic risk factors for diminished renal function 2 yrs posttransplant. J Am Soc Nephrol. 1998;9:482–487. doi: 10.1681/ASN.V93482. [DOI] [PubMed] [Google Scholar]
- 48.Scholten EM, Rowshani AT, Cremers S, Bemelman FJ, Eikmans M, Kan E, Mallat MJ, Florquin S, Surachno J, Berge IJ, Bajema IM, Fijter JW. Untreated rejection in 6-month protocol biopsies is not associated with fibrosis in serial biopsies or with loss of allograft function. J Am Soc Nephrol. 2006;17:2622–2632. doi: 10.1681/ASN.2006030227. [DOI] [PubMed] [Google Scholar]
- 49.Roberts IS, Reddy S, Russell C, Davies DR, Friend PJ, Handa AI, Morris PJ. Subclinical rejection and borderline changes in early protocol biopsy specimens after renal transplantation. Transplantation. 2004;77:1194–1198. doi: 10.1097/01.TP.0000118905.98469.91. [DOI] [PubMed] [Google Scholar]
- 50.Naessens M, Kambham N, Concepcion W, Salvatierra O, Sarwal M. The evolution of nonimmune histological injury and its clinical relevance in adult-sized kidney grafts in pediatric recipients. Am J Transplant. 2007;7:2504–2514. doi: 10.1111/j.1600-6143.2007.01949.x. [DOI] [PubMed] [Google Scholar]
- 51.Nankivell BJ, Borrows RJ, Fung CL, O’Connell PJ, Chapman JR, Allen RDM. Calcineurin inhibitor nephrotoxicity: longitudinal assessment by protocol histology. Transplantation. 2004;78:557–565. doi: 10.1097/01.TP.0000128636.70499.6E. [DOI] [PubMed] [Google Scholar]
- 52.Nankivell BJ, Borrows RJ, Fung CL, O’Connell PJ, Allen RD, Chapman J. Evolution and pathophysiology of renal-transplant glomerulosclerosis. Transplantation. 2004;78:461–468. doi: 10.1097/01.TP.0000128612.75163.26. [DOI] [PubMed] [Google Scholar]
- 53.Sis B, Mengel M, Haas M, Colvin RB, Halloran PF, Racusen LC, Solez K, Baldwin WM, 3rd, Bracamonte ER, Broecker V, Cosio F, Demetris AJ, Drachenberg C, Einecke G, Gloor J, Glotz D, Kraus E, Legendre C, Liapis H, Mannon RB, Nankivell BJ, Nickeleit V, Papadimitriou JC, Randhawa P, Regele H, Renaudin K, Rodriguez ER, Seron D, Seshan S, Suthanthiran M, Wasowska BA, Zachary A, Zeevi A. Banff ’09 meeting report: antibody mediated graft deterioration and implementation of Banff working groups. Am J Transplant. 2010;10:464–471. doi: 10.1111/j.1600-6143.2009.02987.x. [DOI] [PubMed] [Google Scholar]
- 54.Smith RN, Kawai BS, Nadaazdin O, Sachs DH, Cosimi AB, Colvin RB. Four stages and lack of stable accommodation in chronic alloantibody-mediated renal allograft rejection Cynomolgus monkeys. Am J Transplant. 2008;8:1662–1667. doi: 10.1111/j.1600-6143.2008.02303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gloor JM, Sethi S, Stegall MD, Park WD, Moore SB, DeGoey S, Griffin MD, Larson TS, Cosio FG. Transplant glomerulopathy: subclinical incidence and association with alloantibody. Am J Transplant. 2007;7:2124–2132. doi: 10.1111/j.1600-6143.2007.01895.x. [DOI] [PubMed] [Google Scholar]
- 56.Everly MJ, Everly JJ, Arend LJ, Brailey P, Susskind B, Govil A, Rike A, Roy-Chaudhury P, Mogilishetty G, Alloway RR, Tevar A, Woodle ES. Reducing de novo donor-specific antibody levels during acute rejection diminishes renal allograft loss. Am J Transplant. 2009;9:1063–1071. doi: 10.1111/j.1600-6143.2009.02577.x. [DOI] [PubMed] [Google Scholar]
- 57.Herman J, Lerut E, Damme-Lombaerts R, Emonds MP, Damme B. Capillary deposition of complement C4d and C3d in pediatric renal allograft biopsies. Transplantation. 2005;79:1435–1440. doi: 10.1097/01.TP.0000158420.26623.0F. [DOI] [PubMed] [Google Scholar]
- 58.Seron D, Moreso F, Fulladosa X, Hueso M, Carrera M, Grinyo JM. Reliability of chronic allograft nephropathy diagnosis in sequential protocol biopsies. Kidney Int. 2002;61:727–733. doi: 10.1046/j.1523-1755.2002.00174.x. [DOI] [PubMed] [Google Scholar]
- 59.Brouard S, Renaudin K, Soulillou JP. Revisting the natural history of IF/TA in renal transplantation. Am J Transplant. 2011;11:647–649. doi: 10.1111/j.1600-6143.2011.03456.x. [DOI] [PubMed] [Google Scholar]
- 60.Stegall MD, Park WD, Larson TS, Gloor JM, Cornell LD, Sethi S, Dean PG, Prieto P, Amer H, Textor S, Schwab T, Cosio FG. The histology of solitary renal allografts at 1 and 5 years after transplantation. Am J Transplant. 2011;11:698–707. doi: 10.1111/j.1600-6143.2010.03312.x. [DOI] [PubMed] [Google Scholar]
- 61.Mannon RB, Matas AJ, Grande J, Leduc R, Connett J, Kasiske B, Cecka JM, Gaston RS, Cosio F, Gourishankar S, Halloran PF, Hunsicker L, Rush D. Inflammation in areas of tubular atrophy in kidney allograft biopsies: a potent predictor of allograft failure. Am J Transplant. 2010;10:2066–2073. doi: 10.1111/j.1600-6143.2010.03240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]