Abstract
Objectives/Hypothesis
This study examined the speech perception skills of a younger and older group of cochlear implant recipients to determine the benefit that auditory and visual information provides for speech understanding.
Study Design
Retrospective review.
Methods
Pre- and postimplantation speech perception scores from the Consonant-Nucleus-Consonant (CNC), the Hearing In Noise sentence Test (HINT), and the City University of New York (CUNY) tests were analyzed for 34 postlingually deafened adult cochlear implant recipients. Half were elderly (i.e., >65 y old) and other half were middle aged (i.e., 39–53 y old). The CNC and HINT tests were administered using auditory-only presentation; the CUNY test was administered using auditory-only, vision-only, and audiovisual presentation conditions
Results
No differences were observed between the two age groups on the CNC and HINT tests. For a subset of individuals tested with the CUNY sentences, we found that the preimplantation speechreading scores of the younger group correlated negatively with auditory-only postimplant performance. Additionally, older individuals demonstrated a greater reliance on the integration of auditory and visual information to understand sentences than did the younger group
Conclusions
On average, the auditory-only speech perception performance of older cochlear implant recipients was similar to the performance of younger adults. However, variability in speech perception abilities was observed within and between both age groups. Differences in speechreading skills between the younger and older individuals suggest that visual speech information is processed in a different manner for elderly individuals than it is for younger adult cochlear implant recipients.
Keywords: Cochlear implant, speech perception, aging
INTRODUCTION
Evidence has suggested that older profoundly deaf individuals can significantly improve their speech perception performance after receiving a cochlear implant.1,2 Additionally, mean speech perception scores for this population are very similar to the scores obtained from younger adult implant recipients.1,3,4 However, the specific communication requirements of the older cochlear implant population has not been adequately examined and defined. Considering that the number of individuals over the age of 65 is dramatically increasing (U.S. Administration on Aging) and that individuals within this population are at significant risk for a hearing loss (HL),5 further exploration into the area of auditory processing in the aging cochlear implant population seems warranted.
In the non-implanted population multimodal factors, and the contributions they provide for speech understanding, have been studied to determine the specific listening requirements of individuals over the age of 60 years old. Sommers et al.6 reported that normal hearing older individuals experienced more difficulty compared with younger individuals when identifying words and sentences using speechreading-alone cues. In addition, Spehar et al.7 found that older adults experienced more difficulty with the visual-only identification of words. A number of studies have demonstrated that information from both the visual and auditory modalities is superadditive.6,8 That is, the gain in performance provided by the use of both visual and auditory cues is much larger than the separate contributions of auditory-only and visual-only cues for speech understanding. Examining the impact that multimodal factors have on speech understanding within the elderly cochlear implant population, therefore, might provide some insight into the particularly challenging aspects of communication for these individuals.
Presently, there are very limited data on the particular difficulties that the aging cochlear implant population might experience while communicating with others in normal listening situations. It is known that on average, older recipients perform similarly to their younger adult counterparts on tests of word recognition in controlled listening environments.1,3,4 However, it is not known how auditory and visual speech information is integrated within the central nervous system for elderly compared with younger adult cochlear implant recipients, and in addition, how these multimodal factors contribute to communication skills. The main objectives of this study, therefore, were to complete a retrospective examination of the speech perception skills of a younger and older group of cochlear implant recipients and to determine the contributions that both auditory and visual information provide for overall speech perception performance in these two populations. Specifically, speechreading skills of elderly and younger adult cochlear implant population were examined to assess the impact they have on speech perception abilities both pre- and postimplantation.
MATERIALS AND METHODS
Participants
Study participants received either an Advanced Bionics Corporation Clarion cochlear implant, a Cochlear Corporation Nucleus 24 cochlear implant, or a MedEl Combi 40+ cochlear implant at the Indiana University Medical Center between the years of 1996 and 2003. Individuals were selected if they had completed at least two speech perception tests at two separate clinical visits. The 17 participants in the younger group had a mean age of 46 years, with a range of 39 years to 53 years; the 17 participants in the older group had a mean age of 74 years, with a range of 65 years to 83 years. Demographic data for both groups of individuals are presented in Table I.
Table I.
Study Participant Demographics.
| Study Participant | Age at Implantation | Etiology/Length of Hearing Loss | Device | Study Participant | Age at Implantation | Etiology/Length of Hearing Loss | Device |
|---|---|---|---|---|---|---|---|
| Y1 | 46 | Unknown 2 years | CI24R | E1 | 83 | Unknown 20 years | Clarion |
| Y2 | 49 | LVA 1 year | MedEl C40+ | E2 | 80 | Hereditary 9 years | CI24R |
| Y3 | 53 | Unknown 10 years | MedEl C40+ | E3 | 75 | Meniere’s 23 years | CI24R |
| Y4 | 51 | Hereditary 3 years | CI24R | E4 | 67 | Hereditary 20 years | MedEL C40+ |
| Y5 | 49 | Unknown 41 years | CI24R | E5 | 80 | Unknown 25 years | CI24R |
| Y6 | 44 | Unknown 10 years | MedEl C40+ | E6 | 66 | Unknown 26 years | MedEl C40+ |
| Y7 | 51 | Unknown 5 years | MedEl C40+ | E7 | 76 | Hereditary 30 years | CI24M |
| Y8 | 45 | Ménière’s 10 years | MedEl C40+ | E8 | 81 | Unknown 15 years | MedEl C40+ |
| Y9 | 53 | Unknown 7 years | CI24R | E9 | 82 | Unknown 30 years | CI24R |
| Y10 | 43 | Unknown 30 years | MedEl C40+ | E10 | 75 | Unknown 1 year | MedEl C40+ |
| Y11 | 43 | German Measles 38 years | CI24R | E11 | 75 | Hereditary 23 years | CI24R |
| Y12 | 42 | Ototoxicity 13 years | MedEl C40+ | E12 | 63 | Otosclerosis 32 years | MedEl C40+ |
| Y13 | 42 | Hereditary 32 years | CI24R | E13 | 77 | Unknown 10 years | MedEl C40+ |
| Y14 | 39 | Otosclerosis, meningitis 21 years | MedEl C40+ | E14 | 68 | Noise exposure 23 years | MedEl C40+ |
| Y15 | 40 | Hereditary 32 years | MedEl C40+ | E15 | 65 | Scarlet fever 56 years | MedEl C40+ |
| Y16 | 40 | Meningitis 34 years | MedEl C40+ | E16 | 68 | Unknown 12 years | HiFocus CII |
| Y17 | 40 | Unknown 26 years | MedEl C40+ | E17 | 69 | Unknown 32 years | MedEl C40+ |
| Mean | 46 | 19 years | Mean | 74 | 23 years |
Length of hearing loss is defined as the time when a hearing loss was first diagnosed to the time of implantation. LVA = large vestibular aqueduct.
Procedures and Testing Materials
Three open-set word recognition tests were analyzed before implantation and at 6 months, 12 months, and 24 months postimplantation. These tests were administered as part of the testing battery for individual clinical visits, and, consequently, not every participant received all tests at each testing interval. Measures obtained from the Consonant-Nucleus-Consonant (CNC) monosyllabic word test, the Hearing In Noise sentence Test (HINT), and the City University of New York (CUNY) sentence test were used for this study. All testing was conducted in a double-walled sound attenuated IAC booth at 70 dB sound pressure level. The HINT test was administered in quiet and at a +10 signal-to-noise ratio. The CUNY sentence test was administered in quiet using three different presentation conditions, auditory-only (A), vision-only (V) and auditory-visual (AV).
Data Analysis
A repeated measures analysis of variance (ANOVA) was used to determine significant differences in speech perception performance between the two participant groups and between pre- and postimplant testing intervals. Data from the 12 month postimplant session was used for this calculation because of the large number of data points obtained at this interval. In the few cases where data were missing for that interval, data from the 6-month postimplant testing interval were used. In addition, to assess the impact that speechreading skills before implantation might have had on postimplantation performance, Pearson correlation coefficients were calculated using the preimplant CUNY V scores and the 12-month postimplant auditory-only HINT and CNC scores for both groups of study participants.
For individuals who had CUNY data from a pre- and postimplantation testing interval, the A, V and AV speech recognition scores were used to assess how both auditory and visual cues enhance overall speech understanding. Data from a subset of six study participants were used to calculate the gain scores from audition-alone (i.e., AV-V/100-V), and the vision-alone (i.e., AV-A/100-A) presentations.6 These scores were analyzed to assess the contributions that auditory and visual information provide for overall word recognition. Specifically, the “A gain” score represents the improvement in speech perception caused by the addition of visual information to the auditory signal, and, conversely, the “V gain” score represents the improvement in speech perception caused by the addition of auditory cues to the visual signal.
To determine the overall integration of the information provided by the auditory and visual modalities, the combined AV score was divided by the sum of the A and V scores alone (i.e., AV gain = AV/A+V). If the combined AV performance was the same as the addition of the A-alone and V-alone scores, then AV gain would be 1, and little AV enhancement would be indicated. Alternatively, if the combined AV scores were greater than the sum of the A and V scores, this finding would suggest that the integration of auditory and visual information is beneficial for speech understanding. Essentially, this measurement addresses the superadditive nature of the combination of auditory and visual cues.
RESULTS
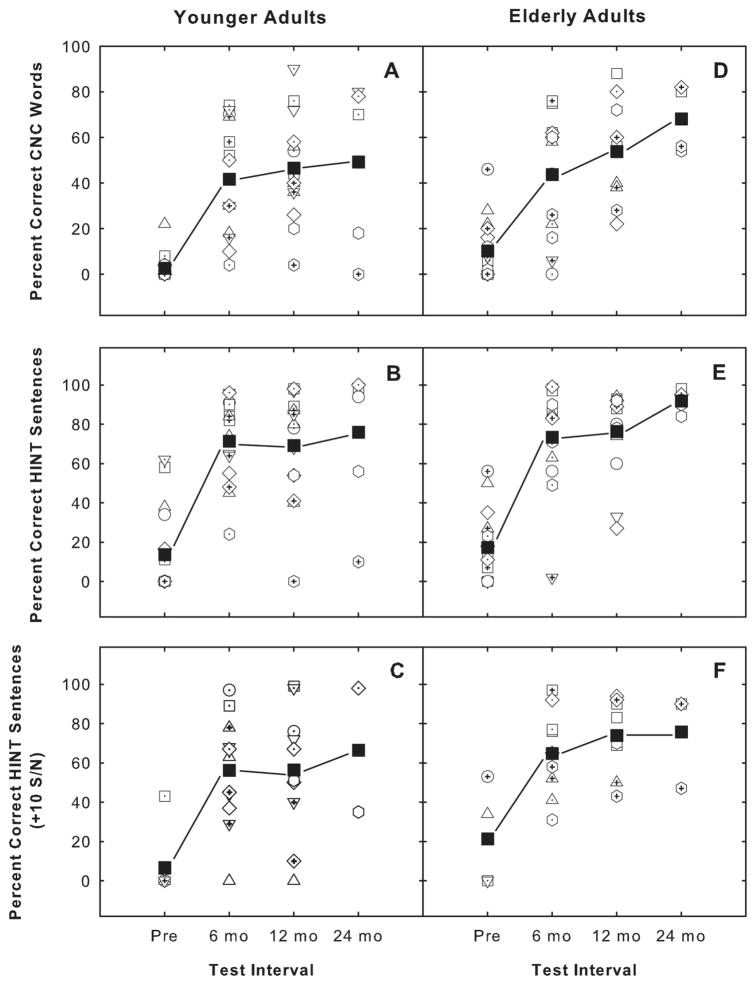
Figure 1 shows the HINT and CNC scores for the two groups of participants as a function of testing interval. Results from the 39- to 53-year-old group are presented in panels A, B, and C, and results from the over 65-year-old group are shown in panels D, E, and F. Open symbols represent individual scores, and solid symbols show the mean data. For the majority of individuals in both age groups, the postimplant performance for each test is better than the preimplant performance. A two-way repeated measures ANOVA revealed a significant difference from the pre- to 12 month postimplant interval for the HINT sentences in quiet (F = 98.175, df = 62, P < .001) and for the CNC words (F = 62.676, df = 59, P <.001). There was no significant difference between the two groups or a significant interaction between groups or test interval. For the HINT sentences in noise, an ANOVA could not be reliably performed because of the number of individual omitted data points from the pre- to posttesting interval.
Fig. 1.
Auditory-only speech recognition scores, (i.e., Hearing In Noise sentence Test and Consonant-Nucleus-Consonant data) for the two groups of participants as a function of testing interval. Results from the 39- to 53-year-old group (A, B, and C), and results from the over 65-year-old group (D, E, and F are shown). Each open symbol represents data from one study participant. The solid square at each testing interval represents the mean score.
The data presented in Figure 1 also reveal the variability in performance that is observed across participants. For example, the CNC data presented in panel A show that for some individuals, a substantial degree of improvement in speech recognition is observed postimplantation. For others, however, less improvement is shown across testing sessions. ANOVA analyses revealed that the type of implant used did not significantly affect the scores obtained on these tests across individuals. A number of alternate factors, such as the age of onset and duration of HL before implantation, have been shown to impact postimplantation speech perception.9,10 As is displayed in Table I, three individuals in the younger age group acquired their HL over a short duration (i.e., less than 3 years), but only one individual in the older age group experienced a short duration HL. Also, when examining the results in the younger age group, the lowest word recognition scores were obtained by those individuals who were postlingually deafened between the ages of 5 and 10 and, therefore, had experienced a long-term progressive HL (i.e., 20 years or more). However, in the older age group, all of the individuals had acquired their HL in middle age or older. That is, none of the elderly individuals had acquired their HL as young children. Because of the limited number of individuals who had experienced short duration HL in both groups and because of the limited number of individuals who had acquired their HL from an early age, we were unable to assess the statistical significance of these factors on speech perception performance.
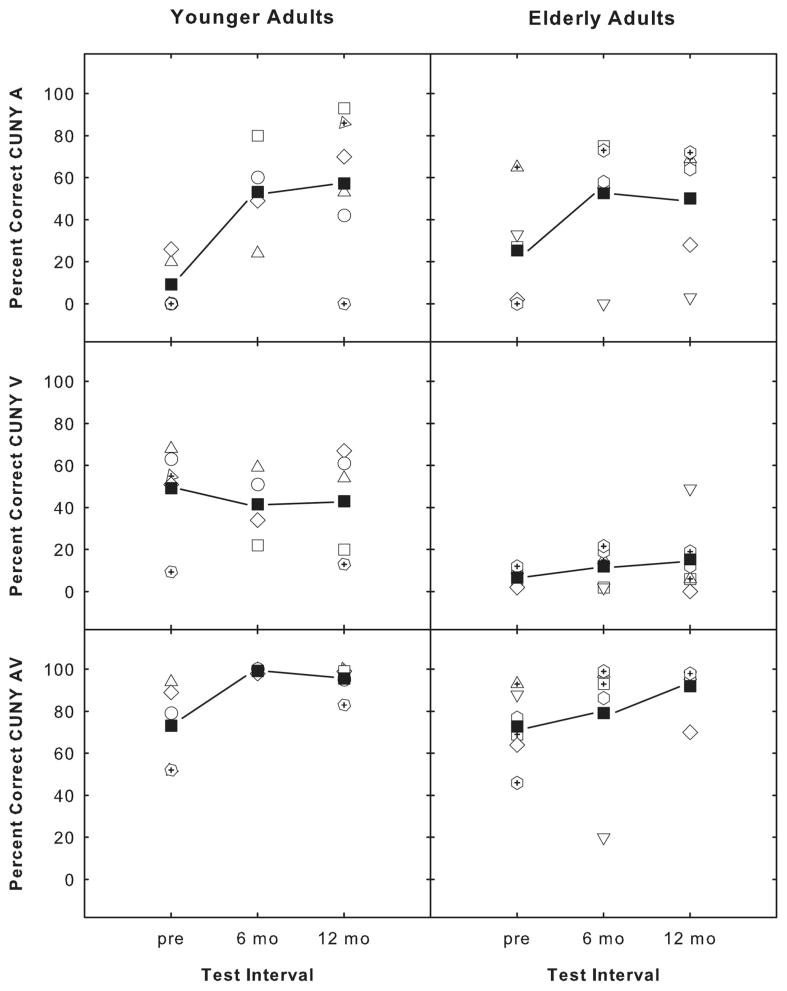
Results from the CUNY sentence test for the subset of six study participants are presented in Figure 2. The CUNY A, V, and AV scores for three testing intervals are presented in panels A, B, and C, respectively, for the younger adults and in panels D, E, and F, respectively, for the elderly adults. Open symbols represent individual data and solid squares represent the mean score. As observed from the figure, performance variability is observed for both experimental groups for each of the presentation conditions. A two-way repeated measures ANOVA revealed significant differences from pre- to postimplantation for both age groups for the CUNY A (F = 8.286, df = 21, P =.021) and the CUNY AV (F = 14.236, df = 22, P =.004) presentations. No significant differences, however, were indicated between the two groups for the A and AV presentations. For the visual-only condition, significant differences in performance were not revealed from pre- to postimplantation but were revealed between the two age groups (F = 11.373, df = 21, P =.007).
Fig. 2.
City University of New York (CUNY) A, V, and AV scores presented for three testing intervals. (A, B, and C) Younger adult data; (D, E, and F) elderly data. Open symbols represent individual data and solid squares represent the mean score.
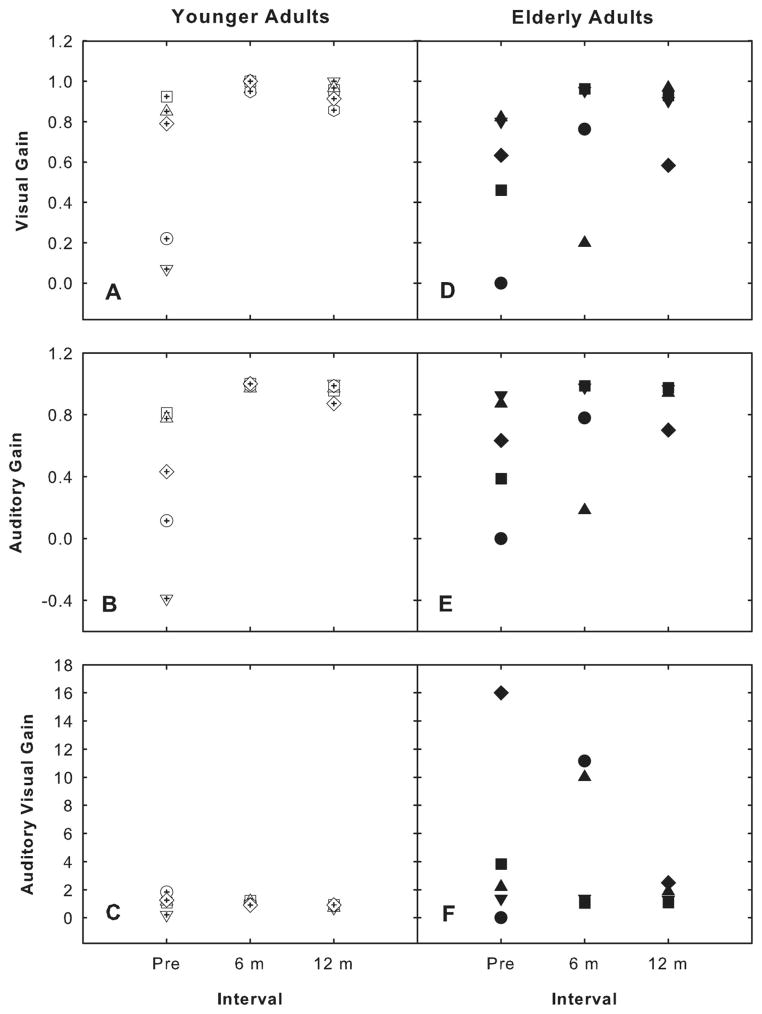
Figure 3 shows the individual enhancement scores that were derived through the auditory (i.e., A gain = AV-V/100-V) and visual modalities (i.e., V gain = AV-A/100-A) for the CUNY sentence test pre- and postimplantation for the subset of six study participants. The overall pre- and postimplantation AV gain scores (i.e., AV gain = AV/A+V) also are presented in this figure. For the younger age group, ceiling effects are present for the postimplantation A gain scores, and near ceiling effects are observed for V gain scores (see panels A and B). As a result, the observed AV gain is limited for this group compared with the AV gain observed for the older age group (panels C and F). For the over 65-year-old group, variability in the A and V gain scores was present, and ceiling effects were not observed for all participants (panels D and E). For the individuals who did not reach ceiling in this group, a substantial audiovisual benefit was observed (panel F). A two-way repeated measures ANOVA revealed a significant difference in A gain from pre- to postimplantation for both age groups (F = 4.587, df +27, P =.031). No significant differences from pre- to postimplantation were observed for either the V gain or AV gain scores for both age groups. However, a significant difference between the two age groups was found for the AV gain scores (F = 6.429, df = 27, P =.032). These data suggest that the older age group did not use the information provided through the visual modality to the same degree as the younger adults to assist them with sentence understanding.
Fig. 3.
Benefit derived through the auditory (i.e., A gain = AV-V/100-V) and visual modalities (i.e., V gain = AV-A/100-A) for the CUNY sentence test pre- and postimplantation. In addition, the overall pre- and postimplantation audiovisual gain (i.e., AV gain = AV/A+V) scores are presented. Data from the 6 younger individuals (open symbols) (A, B, and C), and data from the 6 elderly individuals (closed symbols) (D, E, and F are displayed).
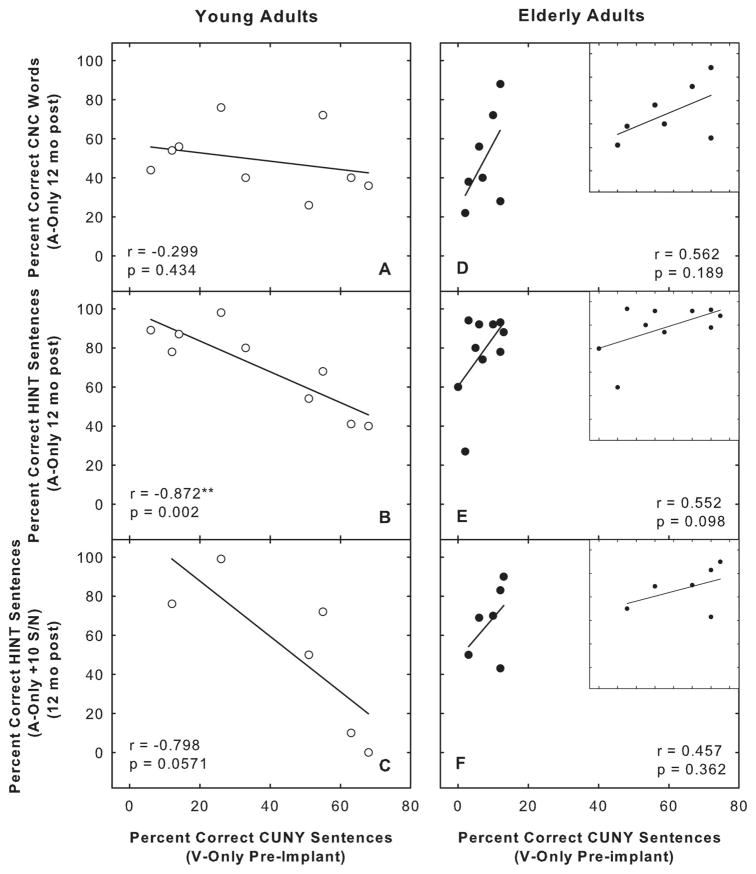
The CNC and HINT 12 month postimplantation scores are presented as a function of the preimplant CUNY sentences vision-only scores in Figure 4. Note that the range of performance for the CUNY V scores is much larger for the younger age group (i.e., 6% correct to 68% correct) compared with the older age group (i.e., 0% correct to 13% correct). To more effectively show the individual elderly data, an inset figure is shown in panels D, E, and F using a much narrower scale on the abscissa. Pear-son correlation coefficients and linear regression lines are included in each panel. None of the correlations was statistically significant except for the scores shown in panel B (r = −0.872, P =.002). Data in this panel suggest that better speechreading skills preimplantation for the younger individuals are associated with poorer sentence recognition postimplantation for the HINT sentence test in quiet. When HINT sentences are presented with a +10 signal-to-noise ratio (panel C), this trend is also present but does not reach significance because of the small sample size. To determine the effects that the duration of HL before implantation and the age of onset of the HL had on the obtained results, these factors were correlated with both the CUNY V scores obtained preimplantation and the HINT scores obtained in quiet postimplantation. The duration of HL before implantation, which ranged from 2 to 41 years, was positively correlated with the CUNY V score (r = 0.858, P =.003) and negatively correlated with the HINT in quiet scores (r = −0.903, P =.0008). The age of onset of the HL was positively correlated with the HINT scores in quiet (r = 0.921, P =.0004) and negatively correlated with the CUNY V scores (r = −0.873, P =.002). Age of implantation was not correlated with either the CUNY V or HINT scores. These findings suggest that for the younger age group, the earlier the onset, and the longer the duration of HL before cochlear implantation, the better the speechreading skills and the poorer the performance on auditory-only sentence perception tasks.
Fig. 4.
Auditory-only 12 month post-implantation Consonant-Nucleus-Consonant (CNC) and Hearing In Noise sentence Test (HINT) scores presented as a function of preimplant City University of New York (CUNY) sentence speechreading scores. Data from the 39- to 53-year-old group (A, B, and C), and data from the over 65-year-old group (D, E, and F are displayed). Data for the older adult population is also provided in the inset figures in D, E, and F using a narrower abscissa scale. Pearson correlation coefficients and linear regression lines are included in each panel.
The pattern observed in the younger age group was not found for the older group of participants. The data shown in panel E reveal that the over 65-year-old group had much poorer speech reading skills overall than the younger age group. In addition, the duration of HL before implantation, ranging from 9 to 26 years, the age of onset of HL, and the age of implantation did not correlate with either the CUNY V preimplantation scores or with the 12 month postimplantation HINT scores.
DISCUSSION
The results from this study suggest that older and younger adult cochlear implant recipients are able to obtain similar degrees of speech understanding. However, as shown in Figure 1, variability among individuals for both auditory-only and vision-only speech recognition performance was observed. Also, differences in speechreading scores between the two age groups were revealed from the data shown in Figures 2 and 4. These data suggest that older adults are poorer speechreaders, on average, than younger adults. However, the more proficient speechread-ers in the younger group generally had poorer auditory-only sentence recognition skills than did the poorer speechreaders in this group. The relationship between speechreading and auditory-only sentence recognition skills was not observed in the older group of implant recipients. Finally, from the limited data presented in Figure 3, the degree of AV gain achieved by the older individuals was generally larger than the AV gain achieved by the younger cochlear implant recipients.
Previous studies examining the benefit that elderly profoundly deaf individuals receive from their cochlear implant also have found that auditory-only speech recognition for both words and sentences significantly improves after 12 months of cochlear implant use.1,2 Chatelin et al.2 reported that in a group of 65 cochlear implant recipients over the age of 70, CNC word scores were 36%, and Central Institute for the Deaf (CID) sentence scores were 61%. In addition, Djalilian et al.1 demonstrated that in a population of 33 cochlear implant recipients over the age of 60, mean Northwestern University (NU-6) word test and CID sentence test responses were 17% and 40%, respectively. In both of these studies, there were no significant differences in performance noted between a younger group of cochlear implant recipients and the test population. In the present study, the older group of implant recipients achieved a mean 12 month postoperative CNC score of 53.8% correct and a HINT sentence score of 76.3% correct. These scores were not significantly different from those obtained by the younger group of study participants, who obtained mean scores of 46.6% and 69.2% correct for the CNC and HINT sentence test, respectively.
The higher scores obtained in this study compared with the data reported in earlier studies can be partially attributed to the less stringent candidacy criteria used during the years when individuals in this study were implanted. Typically, more relaxed candidacy criteria implies that cochlear implant recipients have greater amounts of residual hearing than earlier implanted individuals. Evidence has suggested that individuals with greater degrees of residual hearing preoperatively tend to have higher speech understanding scores postimplanta-tion.11,12 In the Chatelin et al.2 study, individuals were implanted between 1991 and 2002, and in the Djalilian et al.1 study, individuals received their implant between 1989 and 2002. Earlier candidacy criteria required individuals to present with a severe-to-profound HL and no speech perception benefit from hearing aids.13 In the mid 1990s, individuals who had a severe-to-profound HL and displayed the ability to identify 30% of words in sentences were considered for implantation.13,14 More recently, individuals with 50% or less word in sentence identification in the implanted ear are considered for implantation.15
The findings from this study suggest that younger individuals who had shorter durations of deafness before implantation, and concomitantly poorer speechreading skills, also had better speech understanding skills. This finding, however, was not observed in the older group of cochlear implant recipients. Battmer et al.9 also reported that adults with cochlear implants and early onset HL tended to have better speechreading skills compared with individuals with later onset HL. Their results suggested a negative relationship between the degree of speechreading skills and auditory-only speech recognition performance. Specifically, individuals with better speechreading skills were generally observed to have poorer speech perception skills compared with individuals with poor speechreading skills.
We suspect that the confounding factors of age of onset and duration of HL before implantation could be responsible for these observed findings. That is, the individuals in the younger group who had long durations of deafness were young children at the onset of their HL and were in the process of developing spoken language and communication skills. In contrast, individuals in the older group with relatively longer durations of deafness were in their 30s or 40s and were no longer developing communication skills. Thus, the younger individuals might have had to rely more on visual cues from speechreading than the older individuals merely to become proficient users of spoken language.
The lower speechreading skills in the older cochlear implant population also suggest declines in the processing of visual speech information with aging. Reduced speechreading abilities in older individuals have been widely demonstrated in the published audio-visual speech perception literature.6,16,17 For example, Sommers et al.,6 working with two groups of normally hearing-impaired individuals, one older and one younger, demonstrated that older individuals have poorer speechreading skills compared with those of younger individuals. The auditory-only abilities, however, were comparable for both groups, a finding that also was observed in the present study. Sommers et al.6 suggested, therefore, that aging is most likely associated with declines in sensory and cognitive capacities other than hearing impairment, which are necessary for the encoding of visual speech information.
The results from the present study suggest that A gain and V gain scores are comparable for the two age groups. However, we found that the older group demonstrated greater AV gain than the younger group. Possibly, therefore, the integration of visual and auditory information is carried out differently for the younger and older adults. More data, however, are required to determine the mechanism behind the integration of auditory and visual information in older cochlear implant recipients.
CONCLUSIONS
The results of this study revealed similar word and sentence perception scores for older and younger adult cochlear implant recipients. A large degree of variability in performance, however, was observed within and between these two groups. In addition, it was observed that older cochlear implant recipients have poorer speechreading skills than younger cochlear implant recipients. The better speechreading abilities in the younger group of individuals were associated with poorer speech perception scores. These findings suggest that the processing of visual speech information may be carried out in a fundamentally different manner for elderly individuals than for younger individuals after cochlear implantation.
Acknowledgments
Supported by Training Grant T32 DC00012 from the NIH/NIDCD.
Footnotes
Presented at the Twenty-Eighth MidWinter Research Meeting of the Association for Research in Otolaryngology, New Orleans, Louisiana, U.S.A., February 19–24, 2005.
BIBLIOGRAPHY
- 1.Djalilian HR, King TA, Smith SL, Levine SC. Cochlearim-plantation in the elderly: Results and quality-of-life assessment. Ann Otol Rhinol Laryngol. 2002;111:890–895. doi: 10.1177/000348940211101005. [DOI] [PubMed] [Google Scholar]
- 2.Chatelin V, Kim EJ, Driscoll C, et al. Cochlear implant outcomes in the elderly. Otol Neurotol. 2004;25:298–301. doi: 10.1097/00129492-200405000-00017. [DOI] [PubMed] [Google Scholar]
- 3.Shin YJ, Fraysse B, Deguine O, et al. Benefits of cochlear implantation in elderly patients. Otolaryngol Head Neck Surg. 2000;122:602–606. doi: 10.1067/mhn.2000.98317. [DOI] [PubMed] [Google Scholar]
- 4.Pasanisi E, Bacciu A, Vincenti V, et al. Speech recognition in elderly cochlear implant recipients. Clin Otolaryngol. 2003;28:154–157. doi: 10.1046/j.1365-2273.2003.00681.x. [DOI] [PubMed] [Google Scholar]
- 5.Cruickshanks KJ, Tweed TS, Wiley TL, et al. The 5-year incidence and progression of hearing loss. Arch Otolaryn-gol Head Neck Surg. 2003;129:1041–1046. doi: 10.1001/archotol.129.10.1041. [DOI] [PubMed] [Google Scholar]
- 6.Sommers M, Tye-Murray N, Spehar B. Auditory-visual speech perception and auditory-visual enhancement in normal-hearing younger and older adults. Ear Hear. 2005;26:263–274. doi: 10.1097/00003446-200506000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Spehar B, Tye-Murray N, Sommers M. Time-compressed visual speech and age: a first report. Ear Hear. 2004;25:565–572. doi: 10.1097/00003446-200412000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Sumby WH, Pollack I. Visual contributions to speech intelligibility in noise. Acoust Soc Am. 1954;26:212–215. [Google Scholar]
- 9.Battmer RD, Gupta SP, Allum-Mcklenburg DJ, Lenarz T. Factors influencing cochlear implant perceptual performance in 132 adults. Ann Otol Rhinol Laryngol. 1995;166 (Suppl):185–187. [PubMed] [Google Scholar]
- 10.Gantz BJ, Tyler RS, Knutson JF, et al. Evaluation of five different cochlear implant designs: audiologic assessment and predictors of performance. Laryngoscope. 1988;98:1100–1106. doi: 10.1288/00005537-198810000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Gantz BJ, Woodworth GG, Knutson JF, et al. Multivariate predictors of audiological success with multichannel co-chlear implants. Ann Otol Rhinol Laryngol. 1993;102:909–916. doi: 10.1177/000348949310201201. [DOI] [PubMed] [Google Scholar]
- 12.van Dijk JE, van Olphen AF, Langereis MC, et al. Predictors of cochlear implant performance. Audiology. 1999;38:109–116. doi: 10.3109/00206099909073010. [DOI] [PubMed] [Google Scholar]
- 13.NIH Consensus Development Panel. Cochlear implants in adults and children. JAMA. 1995;274:1955–1961. [PubMed] [Google Scholar]
- 14.UK Cochlear Implant Study Group. Criteria of candidacy for unilateral cochlear implantation in postlingually deafened adults. III. Prospective evaluation of an actuarial approach to defining a criterion. Ear Hear. 2004;25:361–374. doi: 10.1097/01.aud.0000134551.13162.88. [DOI] [PubMed] [Google Scholar]
- 15.Zeng FG. Trends in cochlear implants. Trends Amplification. 2004;8:1–34. doi: 10.1177/108471380400800102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Middelweerd MJ, Plomp R. The effect of speechreading on the speech-reception threshold of sentences in noise. J Acoust Soc Am. 1987;82:2145–2147. doi: 10.1121/1.395659. [DOI] [PubMed] [Google Scholar]
- 17.Lyxell B, Ronnberg J. Word discrimination and chronological age related to sentence-based speech-reading skill. Br J Audiol. 1991;25:3–10. doi: 10.3109/03005369109077858. [DOI] [PubMed] [Google Scholar]