Abstract
This study has analysed the generation of 3-(2-deoxy-β-D-erythro-pentafuranosyl) pyrimido [1,2-α] purin-10 (3H)-one deoxyguanosine adduct [M1dG], a biomarker of oxidative stress and lipid peroxidation, in breast fine-needle aspirate samples of 22 patients with breast cancer, at different clinical stages, in respect to 13 controls. The multivariate analysis show that M1dG adduct was higher in cases than in controls (Mean Ratio (MR) = 5.26, 95% CI = 3.16–8.77). Increased M1dG was observed in women with a tumour grade 3 and a pathological diameter 2 (MR = 7.61, 95% CI 3.91–14.80 and MR = 5.75, 95% CI = 3.13–10.59, respectively). A trend with increasing tumour grade and pathological diameter was present (MR = 1.98, 95% CI = 1.57–2.50 and MR = 2.44, 95% CI = 1.71–3.48, respectively). Not significant effects of age and smoking habit were found (MR = 1.58, 95% CI = 0.92–2.72 and MR = 1.68, 95% CI 0.88–3.20, respectively). An increment over the background frequency of M1dG can contribute to breast cancer development. Increasing severity of breast tumour can influence DNA damage level.
Keywords: Breast cancer, free radicals, M1dG, fine-needle aspirate, tumour grade, pathological diameter
Introduction
Breast cancer is a leading cause of cancer death among Western country women and, at the same time, it is a disease difficult to prevent because its aetiology is not well understood [1]. An association between oestrogen exposure, chronic inflammation and increased risk of developing cancer of breast has been suggested [2–4]. Knowledge of how such risk factors induce tumourogenesis in breast tissues is not well defined, but it is possible that an excessive generation of free radicals can be an important mechanism of breast carcinogenesis for oestrogens and inflammation. These reactive intermediates have been shown to cause lipid peroxidation (LPO) and a number of mutagenic DNA adducts, that, if unrepaired, can induce mutations and ultimately cancer [5].
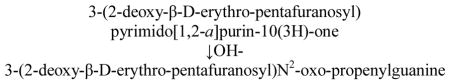
Malondialdehyde (MDA) is a natural product of LPO, which is also formed during prostaglandin E2 biosynthesis via cyclooxygenase [5]. MDA is a reactive by-product capable of interacting with DNA to form different endogenous adducts, including 3-(2-deoxy-β-D-erythro-pentafuranosyl)pyrimido[1,2-a]purin-10(3H)-one deoxyguanosine (M1dG). In addition to these pathways, M1dG adduct can be formed from reactive oxygen species (ROS) through the formation of base propenal intermediaries in DNA [6]. The relevance of M1dG adduct in carcinogenesis is emphasized by their ability to induce base pair mutations and cause frameshift mutations in reiterated sequences [7]. Physiological levels of endogenous DNA damage have been detected in a number of tissues, including larynx, bronchi and peripheral leukocytes [8–13]. Nature and nurture susceptibilities have been reported to modulate M1dG production [8–13]. In addition, some authors, including us, have suggested that enhanced levels of M1dG adduct can be related to cancer development and tumour progression [10–12].
Here, we have undertaken a study to analyse the production of M1dG adduct in breast fine-needle aspirate (FNA) samples of women undergoing breast FNA for diagnostic purpose using 32P-DNA post-labeling and mass spectrometry techniques [13]. We have compared the frequency of M1dG adduct in the breast lesions of 22 patients suffering from breast cancer, at different clinical stages, in respect to 13 controls. Our aim was to evaluate the role of DNA damage induced from free radicals in the target tissue of breast carcinogenesis.
Materials and Methods
Study women and sampling
During 35 consecutive FNA for histological exams, additional samples have been obtained for this study (250 μ1). Breast sample collection was performed in women at the ISPO-Cancer prevention and research Institute, Florence, Italy. This study was conducted according to guidelines of the Declaration of Helsinki. All women signed an informed consent after being informed of the purpose of the study. Ethical clearance for this study was obtained from the institutional ethic committee. Detailed information including demographic data, tobacco smoking and clinical parameters, including histologic tumour grade (G), pathological diameter (pT) and radiological diameter of benign and tumour breast lesions, were collected. FNA samples were obtained from 35 women (Table 1). Twenty-two women who underwent a surgical excision were confirmed as having a cancer of breast after histopathological analysis. FNA samples were shortly frozen after collection, sent to the laboratory and kept frozen until laboratory analysis. Histopathological diagnosis of the tissue were performed in the laboratory of the Unit of Senology, ISPO, Florence. Thirteen women with benign breast lesions were classified such as the control group. Thirty women were classified as non-smokers, five as current smokers (in the last week before examination). The mean age of breast cancer and benign breast disease groups were 66.2 years ± 15 (SD) and 56.1 years ± 15, respectively.
Table 1.
Median of malondialdehyde deoxyguanosine adduct and interquartile range for different variables considered in the study plus the parameter estimates of the multivariate regression mode adjusted by age (continuous), smoking habit, breast cancer case-control status, tumour grade and pathological diameter, as appropriate.
| na | Medianb (25–75) percentiles | Means ratio | 95% CIc | p | |
|---|---|---|---|---|---|
| Age | |||||
| < 50 | 12 | 2.5 (1.8–8.4) | 1 | ||
| 50 –76 | 11 | 7.1 (4.3–11.3) | 1.01 | 0.59–1.72 | 0.971 |
| > 76 | 11 | 12.1 (6.4–23.5) | 1.58 | 0.92–2.72 | 0.095 |
| Smoking habit | |||||
| Never smokersd | 30 | 7.0 (2.3–12.1) | 1 | ||
| Current smokers | 5 | 2.9 (1.9–5) | 1.68 | 0.88–3.20 | 0.111 |
| Status | |||||
| Controlsd | 13 | 1.8 (1.4–2.6) | 1 | ||
| Breast cancer cases | 22 | 9.2 (6.9–15.2) | 5.26 | 3.16–8.77 | < 0.001 |
| Tumour grade | |||||
| Controlsd | 13 | 1.8 (1.4–2.6) | 1 | ||
| G1 | 4 | 8.2 (6.9–10.7) | 4.58 | 2.31–9.09 | < 0.001 |
| G2 | 6 | 8.0 (7.1–11.3) | 5.38 | 2.82–10.25 | < 0.001 |
| G3 | 4 | 15.2 (12.2–15.4) | 7.61 | 3.91–14.80 | < 0.001 |
| Pathologicaldiameter | |||||
| Controlsd | 13 | 1.8 (1.4–2.6) | 1 | ||
| pT1 | 12 | 8.8 (6.9–13.6) | 5.07 | 2.92–8.79 | < 0.001 |
| pT2 | 8 | 13.2 (7.0–20.5) | 5.75 | 3.13–10.59 | < 0.001 |
Some figures do not add up to the total because of missing values.
Level of M1dG per 108 normal nucleotides.
Confidence interval.
Reference level.
M1dG adduct standards
Calf-thymus (CT)-DNA or leukocyte DNA from a blood-donor was treated with 10 mM MDA as previously described [14]. MDA treated CT-DNA was diluted with untreated DNA to obtain decreasing levels of reference standard. Then, we decided to expose human epithelial lung carcinoma A459 cells to 100mM H2o2, a known source of ROS and LPO, to evaluate the formation of M1dG adduct not only in naked DNA but also in experimental in vitro cells, a system that should reflect the physiological conditions of an in vitro organisms.
DNA extraction and purification
Before FNA DNA extraction, 250 μ1 of TE-buffer (10 mM Tris, 1mM EDTA, pH 7.4) containing two anti-oxidats: 6.8 mM 8-hydroxyquinoline and 500 μMN-tert-butyl-a- phenylnitrone were added to FNA samples. DNA was then isolated and purified using a method that requires digestion with ribonuclease A, ribonuclease T1 and proteinase K, extraction with saturated phenol, phenol/chloroform/isoamyl alcohol (25:24:1), chloroform/isoamyl alcohol (24:1) and ethanol precipitation, according to Reddy and Randerath [15]. DNA was gently dissolved in sterile distilled water and its concentration and purity was determined using a Beckman DU 800 spectrophometer (the total amount of FNA DNA recovered ranged from 6–10 μg). Coded DNA was stored at −80°C until laboratory analysis.
P-DNA post-labeling
DNA (2 μg) were digested by micrococcal nuclease and spleen phosphodiesterase. After nuclease P1 treatment, M1dG adduct were labeled with [γ-32P]-ATP. The analysis of M1dG adduct was performed using multidirectional chromatography [13]. Detection and quantification of M1dG adduct and normal nucleotides was performed as previously described[13]. M1dG adduct levels were expressed such as relative adduct labeling (RAL)= screen pixel in adducted nucleotides/screen pixel in total nucleotides. M1dG adduct values were corrected across experiments based on the recovery of the internal standard. The coefficient of variation of the assay was 0.22 and the mean recovery of internal standard was 16.7%.
Mass spectrometry
DNA adducts were also detected in MDA treated CT-DNA by mass spectrometry through the following sequence of steps: (1) react DNA with NaBH4 [16] followed by precipitation with isopropanol; (2) digest with snake venom phosphodiesterase and nuclease P1; (3) extract DNA adducts that are less polar than normal nucleotides (nn) on an OASIS cartridge (Waters Corporation); (4) tag with an isotopologue pair of benzoylhistamines (d0 and d4) in a phosphate-specific labeling reaction in the presence of carbodiimide [17]; (5) remove residual reagents by ion exchange solid-phase extraction; (6) resolve tagged adducts by capillary reversed-phase HPLC with collection of drops onto a MALDI plate; (7) add matrix (a-cyano-4-hydroxycinnamic acid); and (8) analyse by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS).
Alkaline hydrolysis
Since previous studies have shown the hydrolytic ring-opening of M1dG at alkaline pH [18,19], we have evaluated whether the alkaline hydrolysis can induce the autoradiography disappearance of M1dG adduct spots [20]. M1dG adduct spots, that have been directed in the chromatograms of the study women, were excised and incubated with and without increased concentrations of ammonium hydroxide 0.1,0.5,1.0 and 2.0 M, pH 11.7 for 10′ to induce the following ring-opening reaction:
After alkaline hydrolysis, the samples were transferred to the same chromatogram and run side-by-side with 0.24 M urea, pH 6.4.
Statistical analysis
All statistical analyses were performed on log transformed data to stabilize the variance and normalize the distribution of M1dG adduct. A descriptive analysis was initially performed to explore the relationship between individual variables and M1dG adduct. The multivariate analysis was performed using log-normal regression models including terms for age, smoking habit and breast cancer case-control status to estimate the effect of each variable on the outcome adjusting for the concomitant effect of the other variable included in the model. Women were also grouped according to tertiles for age before statistical analyses. The regression parameters estimated from the model are interpreted as ratios between the means of M1dG adduct of each level of the study variables with respect to the reference level, adjusted by age (continuous), smoking habit, breast cancer case-control status, tumour grade and pathological diameter, as appropriate. A p-value of <0.05 (two-tailed) was considered significant for all of the tests. As stated in Greenland and Poole [21], when a trend on the regression coefficients is tested, the inclusion of the category of controls improves the precision of the estimated trend, unless a selection bias is suspected. Since we do not assume that there is a selection bias among our controls, we have included the category of controls in the test for trend. Therefore, to test whether the MR’s of tumour grade and pT were distributed according to a linear trend, we have fitted categorical variables as if they were continuous. In this way, we have obtained the mean MR moving from one level to another of each variable. The data were analysed using SPSS 13.0 (SPSS, Chicago, IL, USA).
Results
Standard M1dG adduct by 32P-DNA post-labelling
A statistically significant increased formation of M1dG adduct was found in MDA treated CT-DNA in respect to untreated CT-DNA (p < 0.001) by 32P-DNA post-labelling assay. The RAL values per 106 were 0.32 ± 0.11 (SE), 1.6 ± 0.38 (SE) and 5.0 ± 0.6 (SE) in 1,4 and 10 mM MDA treated CT-DNA, while a RAL of 0.06 ± 0.01 (SE) was detected in untreated DNA. Figure 1 shows a typical autoradiogram of M1dG adduct in 10 mM MDA treated CT-DNA (Figure 1A). A calibration curve was obtained by the analysis of decreasing level of MDA treated CT-DNA, r2 = 0.99. A statistically significant production of M1dG adduct was also observed in MDA treated leukocyte DNA in respect to control leukocyte DNA (p < 0.001). The RAL values per 106 were 2.2 ± 0.6 (SE) and 0.02 ± 0.01 (SE) in MDA treated and untreated DNA. Then, our results show that the treatment with 100 mM H2O2 induces a statistically significant formation of M1dG adduct in lung carcinoma cells in respect to the unexposed cells (p < 0.001). The RAL values per 106 were 0.25 ± 0.09 (SE) and 0.07 ± 0.02 (SE) in H2O2 treated and untreated lung carcinoma cells.
Figure 1.
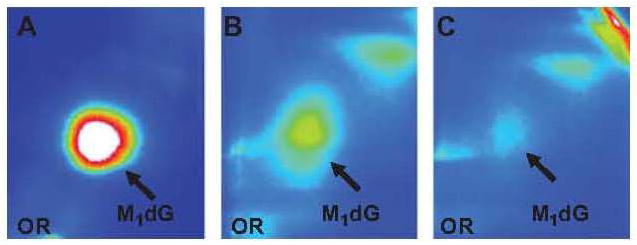
Typical autoradiograms of malondialdehyde deoxy-guanosine adduct in 10 mM malondialdehyde-treated calf thymus DNA (A), in fine needle aspirate of breast cancer case (B) and control (C).
Standard M1dG adduct by mass spectrometry
The presence of M1dG adduct in the 10 mM MDA treated CT-DNA was also confirmed by us subjecting it to analysis by mass spectrometry. M1dG adduct was identified after mass tagging by MALDI-TOF-MS in MDA treated, NaBH4-reduced CT-DNA. Relative to the accurate masses that are seen in the spectrum from one spot, the exact mass of M1dG is as follows using the M nomenclature of Goda and Marnett [16]: M1dG (581.166).
Breast M1dG adduct
The level of M1dG adduct was analysed in 35 women. The intensity of M1dG spots was stronger in the chromatograms of the breast cancer cases, in respect to that observed in benign breast disease patients. Figure 1 shows typical autoradiogams of V adduct in fine needle aspirate of breast cancer case and control (B and C, respectively).
Furthermore, the alkaline hydrolysis experiments showed that the autoradiography disappearance of M1dG adduct was associated to increased OH− concentrations, supporting the identity of breast adduct spots such as M1dG adduct.
The results of the multivariate analysis show that the level of M1dG adduct was significantly higher among breast cancer cases in respect to controls (Mean Ratio (MR) = 5.26, 95% confidence interval (CI) =3.16–8.77, p < 0.001). In addition, when the clinical parameters were analysed, a statistically significant increment of M1dG adduct with increased histological tumour grade was observed. The women with a G3 grade presented the highest MR value (MR = 7.61, 95% CI=3.91–14.80, p < 0.001). A significant trend with increasing tumour grade was found (MR= 1.98, 95% CI= 1.57–2.50, p < 0.001). An enhanced formation of M1dG adduct was also observed with increased pT levels. The breast cancer cases with a pT2 level presented the highest level of DNA damage (MR= 5.75, 95% CI = 3.13– 10.59, p < 0.001). A significant trend with increasing pT was also found (MR=2.44, 95% CI = 1.71–3.48, p < 0.001). When women were grouped according to tertiles for age, a not statistically significant increment was found (MR = 1.58, 95% CI = 0.92–2.72). A not significant effect of smoking habit was also found (MR = 1.68, 95% CI = 0.88–3.20). When the mammography analyses were considered any significant associations were not observed between M1dG adduct and diameters of benign and tumour breast lesions, p = 0.404 and p = 0.486, respectively.
Discussion
The present study shows an increased frequency of M1dG adduct, a biomarker of oxidative stress and LPO, in the breast tissues of women suffering with breast cancer in respect to controls. This is in keeping with previous studies reporting that oxidative stress-related DNA damage is associated with cancer of the breast [22–26]. It is possible that an increment over the physiological level of M1dG adduct generated during physiological processes can contribute to cancer development. Increased levels of M1dG adduct in breast cancer cases can be reflective of impairments in the metabolic detoxification of oxidative by-products of in the DNA repair activity. In fact, the reduced activity of the enzyme 8-oxoguanine DNA N-glycosylase, an enzyme that repairs oxidative DNA lesions, has been shown to be a cancer risk factor [27].
Different mechanisms can be involved in the increased production of M1dG adduct in women with breast cancer. Endocrine factors have been reported to have an important role in hormonal carcinogenesis [28]. Oestrogens can be metabolically activated into catechol oestrogens by cytochrome P450 enzymes [2,3]. Then, metabolic redox cycling between catechol oestrogens and their corresponding quinones can generate oxidative stress and ROS. Inflammation can be also involved in the endogenous induction of breast M1dG adduct by activation of macrophages and neutrophilic response, two processes that can increase the production of hydrogen peroxide and hypochlorite acid. Interestingly, we have recently shown that ROS generated by activated inflammatory neutrophils are involved in M1dG adduct formation [29]
Our next result shows an increasing production of M1dG adduct with increasing the degree of differentiation and severity of cancer. The highest frequency of M1dG adduct was detected in the group of women with the highest pT and G values, parameters that generally identify a type of cancerous breast tumour characterized from poorly differentiated cells. The mechanism by which elevated M1dG can be related to breast cancer progression is unclear. Oxidative stress has been recently associated to an increasing severity of breast tumour [30]. Elevated circulating markers of inflammation have also been associated to reduced breast cancer survival [31]. The relationship between M1dG adduct and the severity of cancer has been previously examined by us in a hospital-based study [12], where elevated levels of DNA damage were associated to worse survival of lung cancer patients, after adjusting for age, gender and packyear (Hazard Ratio = 2.48; 95% CI = 0.65–9.44). The level of oxidative stress could increase in advance stages of cancer as a result of chronic inflammation, and could enhance the generation of M1dG adduct, that, if left unrepaired, can induce additional mutations including in key oncogene and tumour suppressor genes necessary for cancer progression. The small sample size is a limitation of the study, which, however, suggests hypothesis for future investigations. For instance, the evaluation of changes of M1dG adduct level in breast tissues after diminishing breast tumour by surgery and/or chemotherapy would be interesting to pursue this study and could serve to improve our understanding of the mechanisms of tumour progression in the breast.
Tobacco smoke contains a variety of carcinogens and ROS derived from tobacco pyrolysis products that can induce M1dG adduct. Thus, we have analysed the association between smoking and M1dG adduct, but no effect was found, probably due to the small number of smokers. Association of M1dG with smoking has been examined by us in two previous hospital-based studies and higher amount of DNA endogenous lesions have been found in bronchi and larynx of smokers [11,12]. Similar relationships were observed in the leukocytes and oral mucosa of smokers [13,32], but other authors reported no differences for tobacco smoking in breast and colon mucosa[10,22]. A recent study also indicated that cigarette smoking caused the elevations of urinary F2-isoprostanes, an indicator of oxidative stress [4].
Conclusions
This study suggests that an increment over the back ground frequency of M1dG adduct generated during normal physiological processes can contribute to development and progression of cancer of breast. FNA M1dG adduct could be used for evaluating cancer risk in future clinical and epidermiological studies. An additional benefit will be their eventual application to chemoprevention of cancer, i.e. to reduce the levels of mutagenic M1dG adduct to slow the mutation rate of cancer cell.
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of NIEHS and NIH. Contribution number 978 from the Barnett institute.
Abbreviations
- CT
calf-thymus
- FNA
fine-needle aspirate
- LPO
lipid peroxidation
- MDA
malondialdehyde
- M1dG
3-(2-deoxy-β-D-erythro-pentafuranosyl) pyrimido [1,2-α] purin-10(3H)-one deoxyguanosine
- MALDI TOF MS
matrix- assisted laser desorption/ionization time-of flight mass spectrometry
- MR
Mean Ratio
- nn
normal nucleotides
- G
tumour grade
- pT
pathological diameter
- RAL
relative adduct labeling
- ROS
reactive oxygen species
Footnotes
Declaration of interest
This work was supported in part by the ‘Associazione Italiana per la Ricerca sul Cancro’, Milan, Italy and in part by Award Number P42ES017198 from NIEHS. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Wiechmann LKH. The molecular journey from the ductal carcinoma in situ to invasive breast cancer. Cancer. 2008;112:2130–2142. doi: 10.1002/cncr.23430. [DOI] [PubMed] [Google Scholar]
- 2.Bhat HK, Calaf G, Hei TK, Loya T, Vadgama JV. Critical role of oxdative stress in estrogen-induced carcinogenesis. Proc Natl Acad Sci USA. 2003;100:3913–3918. doi: 10.1073/pnas.0437929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolton JL, Thatcher GR. Potential mechanisms of estrogen quinone carcinogenesis. Chem Res Toxicol. 2008;21:93–101. doi: 10.1021/tx700191p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pierce BL, Ballard-Barbash R, Bernstein L, Baumgartner RN, Neuhouser ML, Wener MH, Baumgartner KB, Gilliland FD, Sorensen BE, McTiernan A, Ulrich CM. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J Clin Oncol. 2009;27:3437–3444. doi: 10.1200/JCO.2008.18.9068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marnett LJ. Lipid peroxidation-DNA damage by malondial-dehyde. Mutat Res. 1999;8:83–95. doi: 10.1016/s0027-5107(99)00010-x. [DOI] [PubMed] [Google Scholar]
- 6.Jeong YC, Swenberg JA. Formation of M1G-dR from endogenous and exogenous ROS-inducing chemicals. Free Radic Biol Med. 2005;39:1021–1029. doi: 10.1016/j.freeradbiomed.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 7.VandervVeen LA, Hasmin MF, Shyr Y, Marnett LJ. Induction of frameshift and base pair substitution mutations by the major DNA adduct of the endogenous carcinogen malondialdehyde. Proc Natl Acad Sci. 2003;100:14247–14252. doi: 10.1073/pnas.2332176100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fang JL, Vaca CE, Valsta LM, Mutanen M. Determination of DNA adducts of malonaldehyde in humans: effects of dietary fatty acid composition. Carcinogenesis. 1996;17:1035–1040. doi: 10.1093/carcin/17.5.1035. [DOI] [PubMed] [Google Scholar]
- 9.Bartsch HM, Nair J, Owen RW. Dietary polyunsaturated fatty acids and cancers of the breast and colorectum: emerging evidence for their role as risk modifiers. Carcinogenesis. 1999;20:2209–2218. doi: 10.1093/carcin/20.12.2209. [DOI] [PubMed] [Google Scholar]
- 10.Leuratti C, Watson MA, Deag EJ, Welch A, Singh R, Gottschalg E, Marnett LJ, Atkin W, Day NE, Shuker DE, Bingham SA. Detection of malondialdehyde DNA adducts in human colorectal mucosa: relationship with diet and the presence of adenomas. Cancer Epidemiol Biomarkers Prev. 2002;11:267–273. [PubMed] [Google Scholar]
- 11.Munnia A, Amasio ME, Peluso M. Exocyclic malondialdehyde and aromatic DNA adducts in larynx tissues. Free Radic Biol Med. 2004;37:850–858. doi: 10.1016/j.freeradbiomed.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 12.Munnia A, Bonassi S, Verna A, Quaglia R, Pilucco D, Ceppi M, Neri M, Buratti M, Taioli E, Garte S, Peluso M. Bronchial malondialdehyde DNA adducts, tobacco smoking, and lung cancer. Free Radic Biol Med. 2006;41:1499–1505. doi: 10.1016/j.freeradbiomed.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Peluso M, Srivatanakul P, Munnia A, Jedpiyawongse A, Ceppi M, Sangrajrang S, Piro S, Boffetta P. Malondialdehyde-Deoxyguanosine adducts among workers of a Thai industrial estate and nearby residents. Environ Health Perspect. 2010;118:55–59. doi: 10.1289/ehp.0900907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Helden YG, Keijer J, Heil SG, Picó C, Palou A, Oliver P, Munnia A, Briedé JJ, Peluso M, Franssen-van Hal NL, van Schooten FJ, Godschalk RW. Beta-carotene affects oxidative stress-related DNA damage in lung epithelial cells and in ferret lung. Carcinogenesis. 2009;30:2070–2076. doi: 10.1093/carcin/bgp186. [DOI] [PubMed] [Google Scholar]
- 15.Reddy MV, Randerath K. A comparison of DNA adduct formation in white blood cells and internal organs of mice exposed to benzo[a]pyrene, dibenzo[c,g]carbazole, safrole and cigarette smoke condensate. Mutat Res. 1990;241:37–48. doi: 10.1016/0165-1218(90)90107-d. [DOI] [PubMed] [Google Scholar]
- 16.Goda Y, Marnett LJ. High-performance liquid chromatography with electrochemical detection for determination of the major malondialdehyde-guanine adduct. Chem Res Toxicol. 1991;5:520–524. doi: 10.1021/tx00023a005. [DOI] [PubMed] [Google Scholar]
- 17.Wang P, Giese RW. Phosophate-specific fluorescence labeling under aqueous conditions. Anal Chem. 1993;65:3518–3520. [Google Scholar]
- 18.Schnetz-Boutaud N, Daniels SJ, Hashim MF, Scholl P, Burrus T, Marnett LJ. Pyrimido(1,2-α)purin-10(3H)-one: a reactive electrophile in the genome. Chem Res Toxicol. 2000;13:967–970. doi: 10.1021/tx000135i. [DOI] [PubMed] [Google Scholar]
- 19.Szekely J, Rizzo CJ, Marnett LJ. Chemical properties of oxopropenyl adducts of purine and pyrimidine nucleosides and their reactivity toward amino acid cross-link formation. J Am Chem Soc. 2008;130:2195–2201. doi: 10.1021/ja074506u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bono R, Romanazzi V, Munnia A, Piro S, Allione A, Ricceri F, Guarrera S, Pignata C, Matullo G, Wang P, Giese RW, Peluso M. Malondialdehyde-dG adduct formation in workers of pathology wards: the role of air formaldehyde exposure. Chem Res Toxicol. 2010;23:1342–1348. doi: 10.1021/tx100083x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenland S, Poole C. Interpretation and analysis of differential exposure variability and zero-exposure categories for continuous exposures. Epidemiology. 1995;6:326–328. doi: 10.1097/00001648-199505000-00024. [DOI] [PubMed] [Google Scholar]
- 22.Wang M, Dhingra K, Hittelman WN, Liehr JG, de Andrade M, Li D. Lipid peroxidation-induced putative malondialdehyde-DNA adducts in human breast tissues. Cancer Epidemiol Biomarkers Prev. 1996;5:705–710. [PubMed] [Google Scholar]
- 23.Sinha RJ, Singh R, Mehrotra S, Singh RK. Implications of free radicals and antioxidant levels in carcinoma of the breast: a never-ending battle for survival. Indian J Cancer. 2009;46:146–150. doi: 10.4103/0019-509x.49153. [DOI] [PubMed] [Google Scholar]
- 24.Ray G, Batra S, Shukla NK, Deo S, Raina V, Ashok S, Husain SA. Lipid peroxidation, free radical production and antioxidant status in breast cancer. Breast Cancer Res Treat. 2000;59:163–170. doi: 10.1023/a:1006357330486. [DOI] [PubMed] [Google Scholar]
- 25.Gonenc A, Ozkan Y, Torun M, Simsek B. Plasma malondialdehyde (MDA) levels in breast and lung cancer patients. J Clin Pharm Ther. 2001;26:141–144. doi: 10.1046/j.1365-2710.2001.00334.x. [DOI] [PubMed] [Google Scholar]
- 26.Rajneesh CP, Manimaran A, Sasikala KR, Adaikappan P. Lipid peroxidation and antioxidant status in patients with breast cancer. Singapore Med J. 2008;49:640–643. [PubMed] [Google Scholar]
- 27.Paz-Elizur T, Krupsky M, Blumenstein S, Elinger D, Schechtman E, Livneh Z. DNA repair activity for oxidative damage and risk of lung cancer. J Natl Cancer Inst. 2003;95:1312–1319. doi: 10.1093/jnci/djg033. [DOI] [PubMed] [Google Scholar]
- 28.The Endogenous Hormones and Breast Cancer Collaborative Group. Endogenous sex hormones and breast cancer in post- menopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94:606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 29.Güngör N, Haegens A, Knaapen AM, Godschalk RW, Chiu RK, Wouters EF, Van Schooten FJ. Lung inflammation is associated with reduced pulmonary nucleotide excision repair in vivo. Mutagenesis. 2010;25:77–82. doi: 10.1093/mutage/gep049. [DOI] [PubMed] [Google Scholar]
- 30.Dairkee SH, Nicolau M, Sayeed A, Champion S, Ji Y, Moore DH, Yong B, Meng Z, Jeffrey SS. Oxidative stress pathways highlighted in tumor cell immortalization: association with breast cancer outcome. Oncogene. 2007;26:6269–6279. doi: 10.1038/sj.onc.1210452. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Chen SY, Hsu T, Santella RM. Immunohistochemical detection of malondialdehyde-DNA adducts in human oral mucosa cells. Carcinogenesis. 2002;23:207–211. doi: 10.1093/carcin/23.1.207. [DOI] [PubMed] [Google Scholar]
- 32.Basu S, Helmersson J, Jarosinska D, Sällsten G, Mazzolai B, Barregård L. Regulatory factors of basal F(2)-isoprostane formation: population, age, gender and smoking habits in humans. Free Radic Res. 2009;43:85–91. doi: 10.1080/10715760802610851. [DOI] [PubMed] [Google Scholar]


