SUMMARY
Central tolerance can be mediated by peripheral dendritic cells (DCs) that transport innocuous antigens (Ags) to the thymus for presentation to developing T cells, but the responsible DC subsets remain poorly defined. Immature plasmacytoid DCs (pDCs) express CCR9, a chemokine receptor involved in migration of T cell precursors to the thymus. We show here that CCR9 mediated efficient thymic entry of endogenous or i.v. transfused pDCs. pDCs activated by Toll-like receptor (TLR) ligands downregulated CCR9 and lost their ability to home to the thymus. Moreover, endogenous pDCs took up subcutaneously injected fluorescent Ag and, in the absence of TLR signals, transported Ag to the thymus in a CCR9-dependent fashion. Injected, Ag-loaded pDCs effectively deleted Ag-specific thymocytes, and this thymic clonal deletion required CCR9-mediated homing and was prevented by infectious signals. Thus peripheral pDCs can contribute to immune tolerance through CCR9-dependent transport of peripheral Ags and subsequent deletion of Ag-reactive thymocytes.
Keywords: Plasmacytoid dendritic cells, CCR9, central tolerance, thymic homing, clonal deletion, toll-like receptor ligands
INTRODUCTION
Central (thymic) tolerance requires that self-antigens, including tissue-specific Ags (TSAs), be available in the thymus to be processed and presented on MHC molecules to developing thymocytes. Some soluble antigens (Ags) can access the thymus from the blood, and be presented to thymocytes by thymic dendritic cell (DC) populations (Wu and Shortman, 2005). Other peripheral TSAs can be expressed by medullary thymic epithelial cells (mTECs) under the control of the transcription factor AIRE and deficiencies in this gene have been shown to lead to organ-specific autoimmunity (Anderson et al., 2002). AIRE-independent mechanisms for thymic epithelial expression of peripheral Ags may also exist (Derbinski et al., 2005), but these transcriptional mechanisms are not likely to allow expression of all self-Ags present within the body at sufficient levels for presentation to developing T cells. Moreover, they cannot present innocuous non-self Ags, such as food Ags or ubiquitous products of commensal flora that may enter the body. Therefore alternative mechanisms must exist to broaden the spectrum of self-Ags presented to developing T cells.
Recent studies suggest that migratory DC populations can transport peripheral Ags to the thymus for central tolerance. Bulk splenic DCs, loaded with peptide Ag and injected intravenously (i.v.), can access the thymus and induce deletion of developing Ag-specific T cells expressing a transgenic T-cell receptor (TCR) (Bonasio et al., 2006). Importantly, up to 50% of thymic DCs arrive from the peripheral circulation (Donskoy and Goldschneider, 2003). Three subtypes of thymic DCs have been characterized: plasmacytoid DCs (pDCs) and two phenotypically and functionally distinct subsets of conventional DCs (cDCs) (Li et al., 2009). The thymic cDC populations are described as CD8αlo CD11b+ Signal-regulatory protein alpha+ (SIRPα+) cDCs, and CD8αhi CD11b− SIRPα− cDCs (Li et al., 2009). The SIRPα− thymic cDC population arises from intrathymic precursors and does not exchange with the circulating peripheral DC pool (Li et al., 2009). The SIRPα+ cDC population on the other hand, migrates into the thymus from the blood (Li et al., 2009), and in vitro co-culture assays have shown that this DC population mediates clonal deletion of as well as regulatory T cell (Treg) induction from developing thymocytes (Proietto et al., 2008). Less is known about the migration and intrathymic function of pDCs. Immature pDCs induce immune tolerance in the periphery through the induction of Tregs (Morelli and Thomson, 2007), but they are also important innate immune cells that produce type I interferons in response to viral infections (Colonna et al., 2004). Thymic pDCs are derived from the blood circulation, as opposed to originating from intrathymic precursors (Li et al., 2009), but unlike migratory cDCs, thymic pDCs do not efficiently induce Tregs from thymocytes in vitro (Proietto et al., 2008). Moreover, they are poor presenters of Ags in the absence of microbial stimulation (Colonna et al., 2004). Plasmacytoid DC-derived type I interferons influence the migration and function of cDCs, but this also requires microbial stimulation (Colonna et al., 2004), which makes their role unclear in the steady state thymus. It has been suggested that the function of pDCs within the thymus, as in other tissues, might simply be to protect the tissue from viral infections (Wu and Shortman, 2005).
We have recently shown that tolerogenic pDCs in lymphoid tissues express the chemokine receptor CCR9 (Hadeiba et al., 2008), a chemoattractant receptor involved in homing of memory and effector lymphocyte populations to the small intestines (Kunkel et al., 2000; Pabst et al., 2004), but also in progenitor T cell homing to the thymus (Uehara et al., 2002). CCR9+ pDCs efficiently induce Tregs from peripheral T cells, inhibiting Ag-specific immune responses and graft-versus-host disease (GVHD). The involvement of CCR9 in T cell progenitor recruitment to the thymus and the known expression of its chemokine ligand CCL25 by thymic epithelial cells, led us to ask whether CCR9 might target peripheral pDCs to the thymus. We present studies here that illustrate the importance of CCR9 in targeting pDCs to the thymus, and demonstrate a CCR9-dependent role for pDCs in central tolerance to extrathymically acquired Ag.
RESULTS
CCR9 regulates pDC homeostasis in the thymus
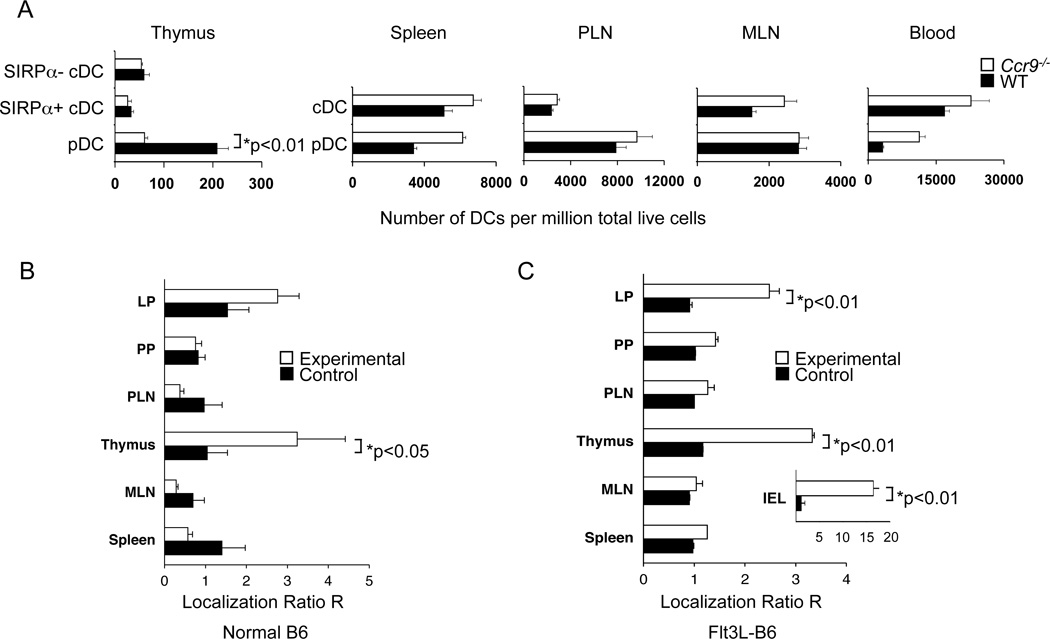
We initially asked whether CCR9 deficiency alters pDC representation in the thymus and, for comparison, in peripheral lymphoid tissues and blood. Ccr9−/− mice had substantially fewer thymic pDCs than wildtype (WT) mice (Figure 1A), wheras CCR9 deficiency had no major impact on pDC numbers in peripheral lymphoid tissues. In contrast Ccr9−/− mice present with higher pDC numbers in the blood compared to WT mice, probably reflecting reduced gut homing. CCR9 deficiency had no significant effect on the representation of cDCs in the blood or peripheral lymphoid tissues, or migratory (SIRPα+) or resident (SIRPα−) cDCs in the thymus (Figure S1A). We used a relatively stringent CD11chi gate to define cDCs (Lineage- B220− PDCA-1−, CD11chi cells), as CD11c intermediate gating included many events with low and abnormal scatter properties (Figure S1A): in comparison to previous studies (Li et al., 2009), our analyses therefore show fewer cDCs than pDCs in the WT thymus.
Figure 1. CCR9 controls pDC representation in the thymus under competitive and non-competitive conditions.
A. Blood leukocytes and cell suspensions from thymi, spleen, peripheral lymph nodes (PLN) and mesenteric lymph nodes (MLN) from untreated C57BL/6 (WT) and Ccr9−/− mice were analyzed by flow cytometry for the number of lineage (lin) (CD3, CD19, NK1.1)neg pDCs (CD11cint B220+ PDCA-1+) and cDCs (CD11chi B220− PDCA-1−) as well as thymic SIRPα+ (CD11b+) and SIRPα− (CD11b−) cDCs. Bar graphs present the mean number of DCs per million live cells (±SEM; 5 mice per group) from one of two experiments. B, C. Bone marrow chimeras (BMC) were generated by reconstituting lethally irradiated WT F1 (CD45.1 × CD45.2) mice with equal numbers of “experimental” Ccr9−/− (CD45.2) bone marrow (BM) cells mixed with congenic WT (CD45.1) BM cells (white bars) or “control” WT (CD45.2) BM cells mixed with congenic WT (CD45.1) BM cells (black bars). Tissues were harvested 8–12 weeks later and cell suspensions analyzed by flow cytometry. To assess BM engraftment efficiency of each donor type, the CD45.1/CD45.2 ratio for BM-derived splenic CD19+ B cells was determined. For each mouse, the CD45.1/CD45.2 ratio among gated (Lin- CD11cint, B220+) pDCs in each tissue was normalized for the engraftment efficiency to yield normalized localization ratios (R): R= (CD45.1/CD45.2)Tissue pDCs/(CD45.1/CD45.2)Splenic B cells. Reconstituted chimeric mice in the left panel (B) were evaluated without further treatment, whereas the pDCs in BMCs in the right panel (C) were expanded by inoculation of mice with Flt3L-secreting B16 tumors. Overall, results are representative of 3 experiments with 4–5 BMC pairs analyzed. See also Figure S1.
To compare the ability of Ccr9−/− versus WT hematopoeitic cells to replenish thymic pDCs in a common host environment, we reconstituted irradiated F1 (CD45.1+ CD45.2+) recipients with equal numbers of congenic WT CD45.1+ and Ccr9−/− CD45.2+ bone marrow (BM) cells. To control for potential CCR9-independent differences between CD45.1 and CD45.2 donor cells, “control chimeras” were also created in which both CD45.1 and CD45.2 BM donors were WT. After 8–12 weeks following BM transfer, when thymic and peripheral lymphocyte and DC pools are fully reconstituted, we determined the ratio of CD45.1+ to CD45.2+ donor-derived cells among pDCs (Lineage- CD11cint B220+ cells) in the thymus and other tissues (Figure 1B) and among thymic migratory and resident cDCs (Figure S1B). To normalize for variations in stem cell numbers from donor mice and stochastic differences in stem cell engraftment between recipients (Baekkevold et al., 2005), these ratios were divided by the ratio of CD45.1+ to CD45.2+ donor-derived splenic B cells, which do not require CCR9 for their development and long-term splenic recruitment. The mean CD45.1/CD45.2 ratio among splenic B cells was not significantly different between control and experimental bone marrow chimeric (BMC) mice, consistent with the hypothesis that CCR9 deficiency does not effect stem cell engraftment (data not shown). In some experiments, recipient mice received a Fms-like tyrosine kinase ligand (Flt3L)-secreting subcutaneous (s.c.) tumor 10–14 days prior to analysis, to expand DC numbers in vivo (Figure 1C).
Compared to control chimeras, where WT CD45.1+ and CD45.2+ DCs were equally represented in all tissues, mixed WT-Ccr9−/− chimeras exhibited predominantly WT pDCs in the reconstituted intraepithelial compartment (IEL), and to a lesser extent in the lamina propria (LP) of the small intestine, consistent with the role of CCL25 in small intestinal pDC homing (Wendland et al., 2007). Moreover, WT-derived BM cells were more efficient than Ccr9−/−-derived BM cells at repopulating the thymic pDC pool. In contrast, WT pDCs had no advantage in populating the peripheral lymphoid tissues studied. Among thymic cDCs, a 2-fold increase in the representation of WT over KO resident SIRPα− cDCs was observed, but with no major effect on the migratory SIRPα+ cDC compartment (Figure S1B): this may reflect the known contribution of CCR9 to thymic recruitment of BM precursors. Results in untreated BMCs were similar to those in which DCs were expanded in vivo after sc injection of the Flt3L-secreting tumor in BMCs (Figure 1B and C). We conclude that CCR9 controls pDC representation in the thymus by either affecting their development or localization to the thymus.
CCR9 mediates peripheral pDC recruitment to the thymus
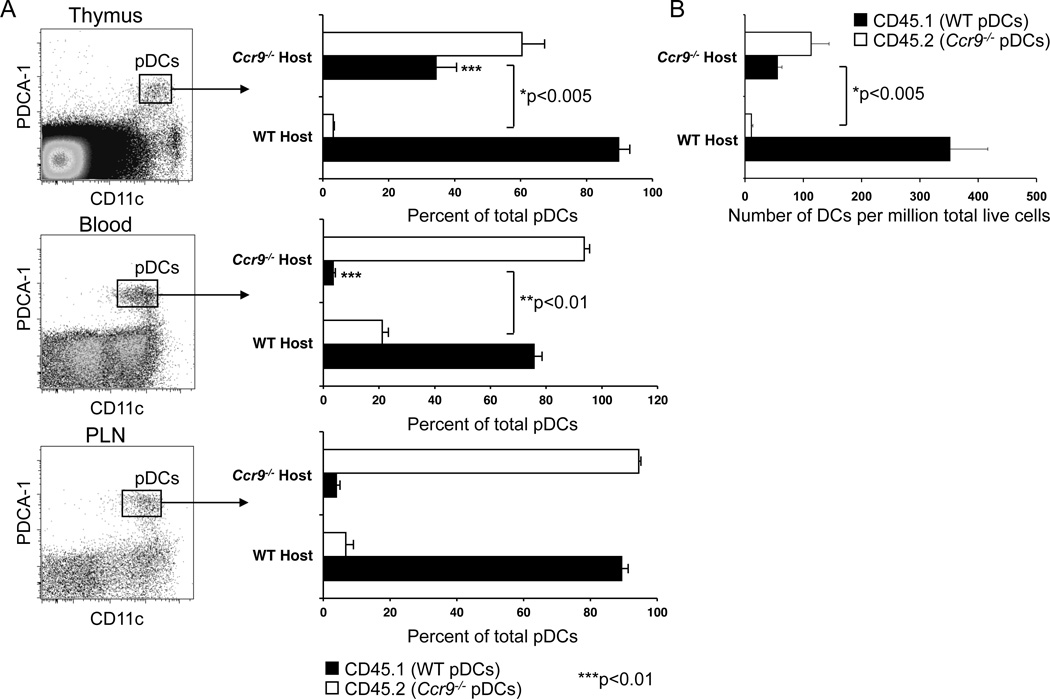
To determine whether CCR9 is required for endogenous pDC recruitment to the thymus, we surgically joined WT (CD45.1) and Ccr9−/− (CD45.2) mice at the flanks to establish a shared circulation. Three weeks after parabiotic union, very few (<0.5%) thymocytes were partner derived, ruling out exchange of hematopoietic precursors (data not shown). More importantly, WT pDCs accessed the Ccr9−/− partner thymus well: in fact, the percentage of WT pDCs in the Ccr9−/− thymus exceeds that in the Ccr9−/− partner’s blood, indicating that WT pDCs have a substantial advantage over host Ccr9−/− pDCs in accessing or surviving in the Ccr9−/− thymus (Figure 2A and Figure S2). In contrast, Ccr9−/− pDCs enter the WT partner thymus poorly: the ratio of Ccr9−/− to WT pDCs is much lower in the WT partner thymus than that of WT to Ccr9−/− pDCs seen in the Ccr9−/− partner thymus. This difference is not attributable to differences in blood exchange, since in the WT parabiont the frequency of partner-derived Ccr9−/− pDCs in blood was larger than that seen in the thymus (Figure 2A and Figure S2). However, the number of pDCs recovered from unmanipulated Ccr9−/− mice is substantially reduced than that in WT mice. Therefore, we also determined total pDC numbers per million live thymocytes (Figure 2B) or per total thymus (data not shown) and found that the number of WT pDCs recovered from Ccr9−/− parabiont thyme is also significantly elevated (p<0.005) compared to the numbers of Ccr9−/− thymic pDCs recovered from WT parabiont thymi, 3 weeks after parabiotic union (Figure 2B). Interestingly, the blood levels of donor Ccr9−/− pDCs in WT parabionts is elevated relative to WT pDCs in Ccr9−/− parabionts, presumably reflecting a decreased ability of the Ccr9−/− pDCs to enter tissues (especially the intestines) of the WT host, whereas the exchange of pDCs in peripheral lymphoid tissues is not affected by CCR9 deficiency (Figure 2A and Figure S2).
Figure 2. Migration of endogenous pDCs to the thymus is CCR9-dependent.
Congenic WT (CD45.1) and Ccr9−/− (CD45.2) mice were surgically joined at the flanks to establish anastomosis. Thymi, peripheral lymph nodes (PLN) and blood were harvested after 3 weeks and analyzed for expression of allelic CD45.1 and CD45.2 markers by flow cytometry among partner-derived, fully differentiated pDCs (Lin- CD11cint PDCA-1+). A. Results show mean percentage of partner-derived pDCs in parabiotic mice with SEM (n=7 mice). B. Bar graphs represent mean number of pDCs per million live cells ±SEM, with 7 mice per group. Data pooled from two experiments. See also Figure S2.
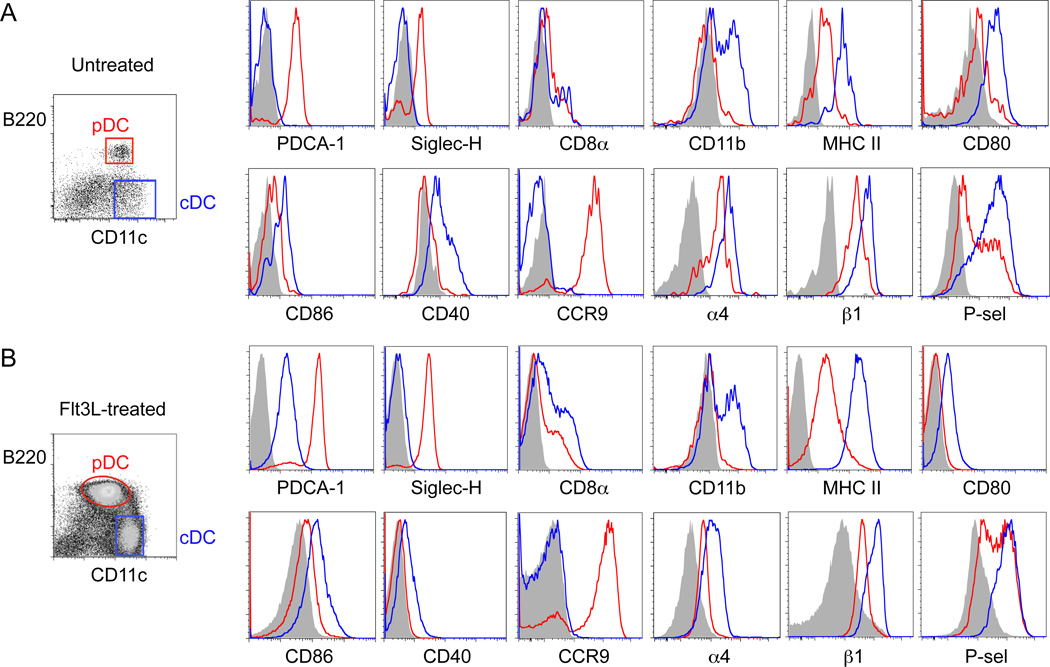
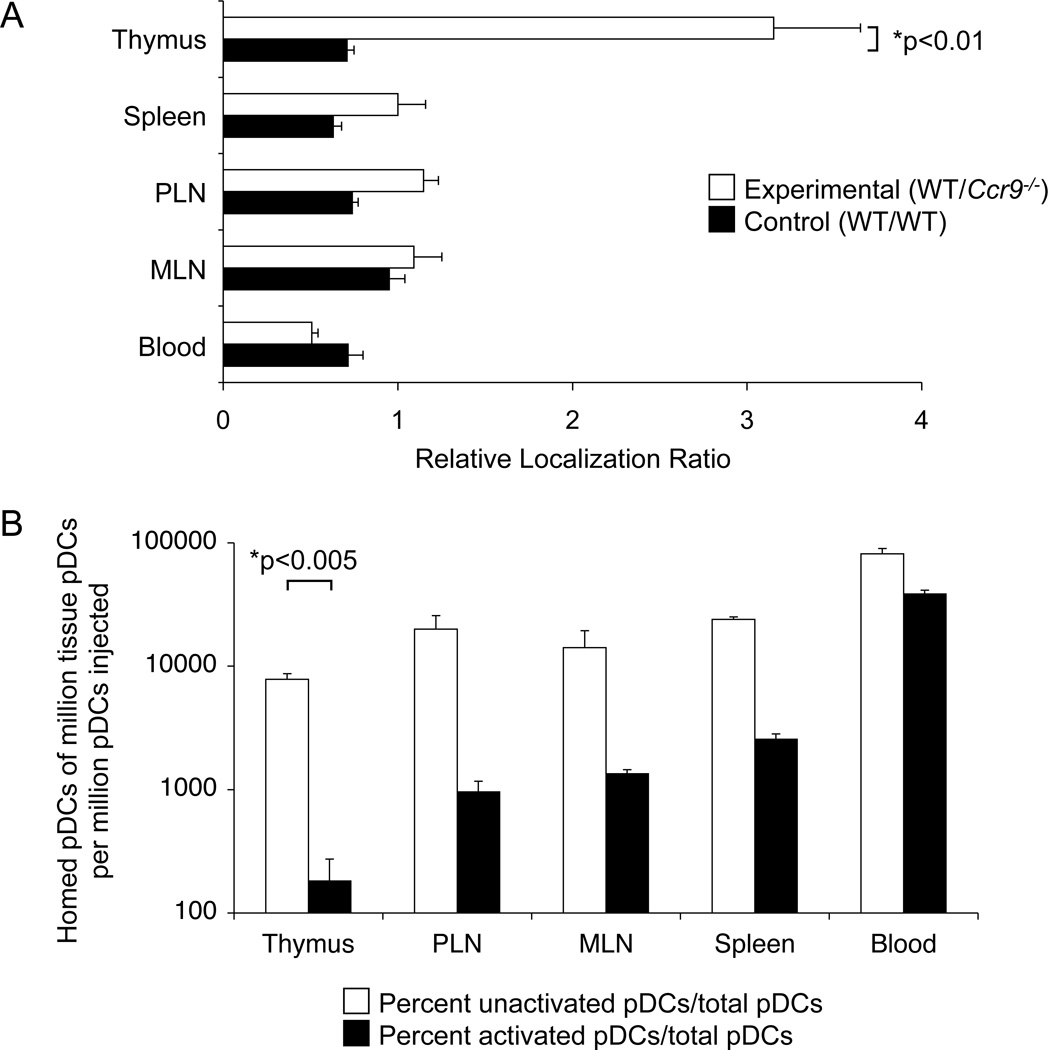
To further confirm a role for CCR9 in pDC recruitment from the blood, we next assessed the ability of Ccr9−/− versus WT pDCs to home into the thymus after i.v. injection. Thymic progenitor cells use a classical multistep adhesion cascade to home to the thymus, involving P selectin-mediated rolling, CCR9- and CCR7-dependent activation, and α4β1-mediated arrest on VCAM-1+ venules in the thymic corticomedullary junction (Krueger et al.; Scimone et al., 2006; Zlotoff et al.). Bulk DC populations from the spleen use the same adhesion molecules to enter the thymus, although the chemoattractant receptor(s) involved are unknown (Bonasio et al., 2006). Flow cytometric studies reveal that CCR9+ pDCs from peripheral lymphoid tissues which express classical lineage markers such as PDCA-1, Siglec-H and low to intermediate expression levels of costimulatory markers (CD40, CD80, CD86 and MHC class II), also display both α4 and β1 integrin chains, and bind chimeric P-selectin Ig (Figure 3A). In comparison, cDCs expressed even higher levels of α4, β1 and P-selectin ligands; but they stain poorly for CCR9. pDCs and cDCs from Flt3L-treated donors displayed the same expression of trafficking receptors and overall surface phenotype as their counterparts from normal donor mice (Figure 3B). 48-hour competitive homing studies were carried out using purified pDC populations from donors exposed to Flt3L to expand DC numbers in vivo, as sufficient DC numbers for homing studies could not be obtained from untreated donor mice. Flt3L has been shown to be a potent growth factor for DCs that does not affect their activation (Gilliet et al., 2002). Purified CCR9-deficient CD45.2+ pDCs, or WT control CD45.2+ pDCs, were mixed with equal numbers of purified congenic WT CD45.1+ pDCs and injected i.v. into normal F1 (CD45.1+ CD45.2+) WT recipients. Flow cytometric analysis of homed pDCs on day 2 showed that a larger ratio of WT (CD45.1+) to Ccr9−/− (CD45.2+) donor pDCs localized to the thymus (Figure 4A and Figure S4), whereas their localization in peripheral lymphoid tissues and blood was similar. In control mice, both CD45.1+ and CD45.2+ donor WT pDCs had comparable thymic homing efficiencies. The results are consistent with a specific role for CCR9 in peripheral pDC recruitment to the thymus.
Figure 3. Immature pDC in peripheral lymphoid tissues express trafficking receptors involved in thymic homing.
Staining of peripheral lymph nodes from normal untreated C57BL/6 mice (A) and Flt3L-B16 inoculated mice (B) for DC lineage markers (PDCA-1, SiglecH, CD8α, CD11b and MHC class II); costimulatory ligands (CD40, CD80 and CD86); and trafficking receptors (CCR9, α4 and β1 integrins and P-selectin binding ligands). DC subsets were analyzed by flow cytometry by gating on pDCs (Lin- CD11cint B220+, red histogram plots) and cDCs (Lin- CD11chi B220−, blue histogram plots). Shaded histogram plots represent isotype controls, or in the case of P-selectin binding, incubation in the presence of 5mM EDTA. Data representative of 5 mice per experiment from a total of 3 experiments. See also Figure S3.
Figure 4. Homing of pDCs to the thymus is CCR9-dependent and is prevented by pDC activation.
Pooled peripheral lymph nodes from B16-Flt3L inoculated WT (CD45.2), congenic WT (CD45.1) and Ccr9−/− (CD45.2) mice were enriched for pDCs using MACS technology. A. Ccr9−/− (CD45.2) pDCs (experimental group) or WT (CD45.2) pDCs (control group) were co-injected i.v. with an equal number of WT (CD45.1) pDCs into WT F1 (CD45.1 × CD45.2) mice. After 48 hours, tissues were harvested and cell suspensions were analyzed by flow cytometry for CD45.1 and CD45.2 markers among gated pDCs (Lin- CD11cint B220+). Bar graphs depict relative localization ratios obtained by dividing the CD45.1/CD45.2 pDC ratio in each tissue by the same ratio in the input (injected) sample. B. Equal numbers of WT unactivated (CD45.1) and CpGA-activated (CD45.2) pDCs were co-injected i.v. (input total of ~ 5 × 106 after overnight harvest) into syngeneic F1 (CD45.1 × CD45.2) recipients. Tissues were analyzed by flow cytometry after 36–48 hours. Results are presented as the number of homed CD45.1+ (unactivated, open bars) or CD45.2+ (activated, black bars) pDCs recovered per million total tissue or blood pDCs (Lin- CD11cint B220+ cells), divided by the number of million pDCs injected. For A and B, data represent means ± SEM from 4–5 recipient mice in one experiment, and are representative of 3 experiments with similar results. See also Figure S4.
Toll-like receptor (TLR) stimulation inhibits pDC homing to the thymus
TLR agonists, which mimic infectious stimuli, activate pDCs altering their phenotype and function from a tolerogenic to an immunostimulatory state (Colonna et al., 2004). To evaluate whether infectious stimuli alter trafficking receptor expression and homing properties of pDCs, we stimulated the cells with the TLR9 ligand CpGA. As expected, activated pDCs expressed higher levels of MHC class II and costimulatory CD86 (B7-2) molecules; they also displayed enhanced P-selectin binding and slightly upregulated α4 and β1 integrin expression levels; but they downregulated CCR9 (Figure S3). In short-term homing assays, resting pDCs localized much more efficiently from the blood to the thymus than CpGA treated pDCs: The ratio of activated to resting pDCs is much lower in the thymus than in the blood at 36–48 hours post-injection (Figure 4B). This is not due to a loss in pDC viability after TLR stimulation, since overnight stimulation with CpGA had no major effect on the number of viable cells recovered (data not shown); and the levels of unactivated (CD45.2+) vs. activated (CD45.1+) donor pDCs were comparable in the blood 36–48 hours after i.v. injection (Figure 4B). Activation also inhibited localization of pDCs from the blood to the peripheral lymph tissues and to a lesser extent to the spleen. In sum, resting pDCs home to the thymus in a CCR9-dependent fashion and thymic homing capacity is compromised upon activation with a potent TLR stimulus.
Ag-loaded pDCs induce central tolerance
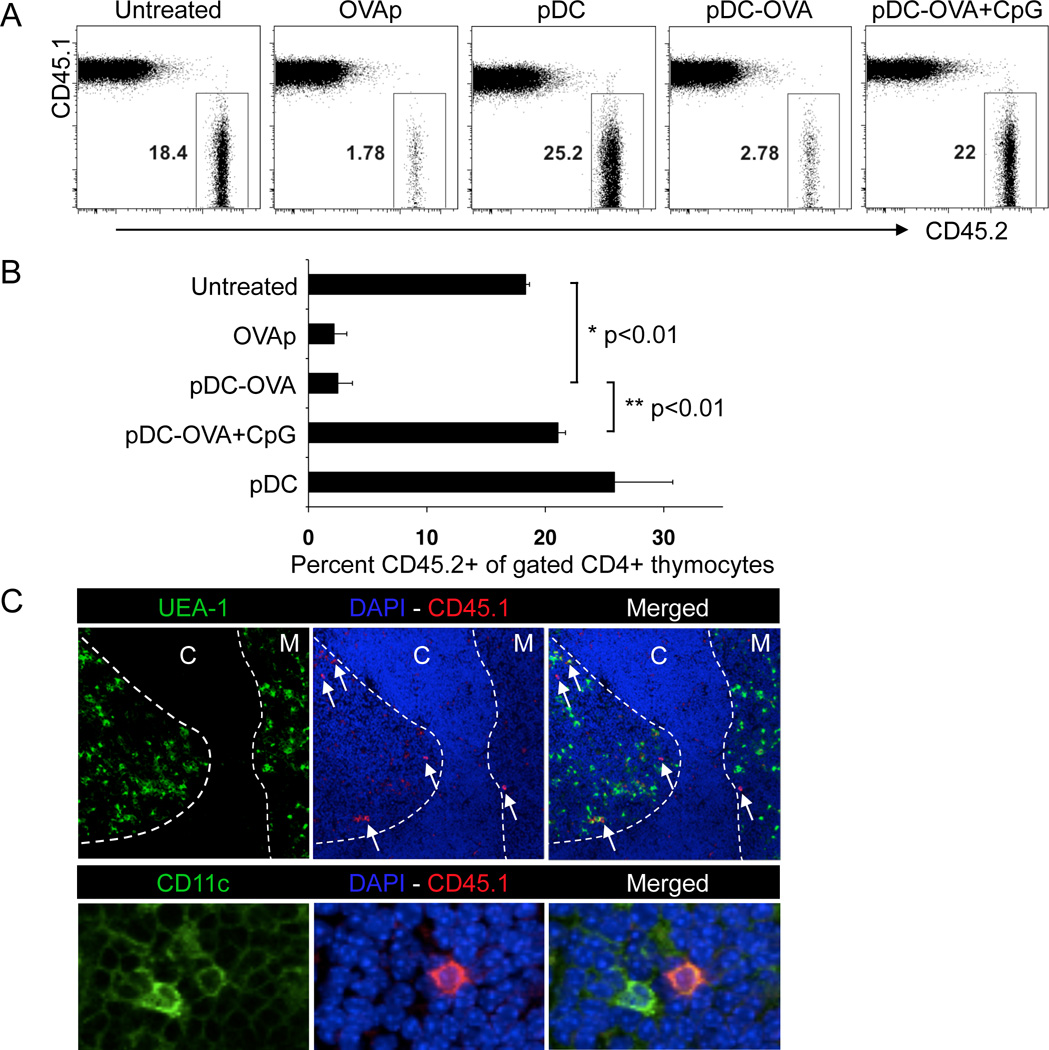
Given that the thymus recruits unactivated pDCs in a CCR9-dependent fashion, we hypothesized that CCR9+ pDCs could efficiently transport peripheral Ags acquired in a tolerogenic context to the thymus and induce negative selection by Ag presentation to developing thymocytes. To evaluate the role of Ag-loaded pDCs on thymic development of Ag-specific thymocytes, lethally irradiated CD45.1 congenic B6 mice were reconstituted with lymphocyte-depleted BM cells from the same CD45.1 congenic B6 mice and CD45.2+ transgenic OT-II Rag1 (recombination-activating gene 1)–deficient mice. Thymocytes developing from the OT-II Rag1−/− BM exclusively express the OT-II TCR, specific for chicken ovalbumin (OVA)-derived peptide (OVAp). This system allows us to compare changes in developing OVA-specific thymocytes (CD45.2-derived) in the context of a diverse polyclonal CD45.1-derived thymocyte pool. Two weeks after BM reconstitution, when intrathymic T cell development is well under way, mice were injected i.v. with either 50 nmoles of OVAp as a positive control, or with 2–5 million purified pDCs that were either untreated or loaded with OVAp followed by extensive washing to eliminate free peptide. OVAp-loaded pDCs were as effective as soluble OVAp at eliminating CD45.2+ OT-II OVA-specific CD4+ CD8− single positive (SP) (Figure 5A and B), but not CD4+ CD8+ double positive (DP) thymocytes (Figure S5A and B) whereas pDCs not loaded with Ag had no effect on either thymocyte population. Furthermore activation of OVAp-loaded pDCs with CpGA abrogated their ability to induce deletion of OT-II SP thymocytes (Figure 5A and B). In separate experiments, we evaluated the intrathymic localization of adoptively transferred peripheral pDCs using immunohistochemistry (Figure 5C). Most pDCs localize in the medulla and corticomedullary junction (Figure 5C). Thus Ag-loaded pDCs injected i.v. localize predominantly in thymic microenvironments associated with negative selection of developing thymocytes; homed pDCs are effective at deleting Ag-specific CD4 thymocytes; and, prior activation of pDCs with TLR ligands abrogates their homing and ability to effect clonal deletion.
Figure 5. Deletion of OVA-specific thymocytes by unactivated, but not CpG-activated, OVA-loaded pDCs.
BMCs were prepared by reconstituting irradiated WT (CD45.1) recipient mice with equal numbers of BM cells from WT (CD45.1) and OT-II Rag1−/− (CD45.2) donor mice, whose T cells express an OVA-specific TCR. 14d after reconstitution, mice were either untreated or injected i.v.daily for 2 consecutive days with 2–5 × 106 unpulsed (pDC) or OVAp-pulsed pDCs that were either unactivated (pDC/OVA) or stimulated with CpGA in vitro (pDC/OVA+CpGA). As a positive control for thymic deletion of OVA-specific T cells, mice were injected i.v. with 50 nmol of soluble OVAp in PBS. Thymocytes were analyzed two days after final pDC or OVA treatment, by gating on Lin- CD4+ CD8− single positive (SP) cells to assess the frequency of the CD45.2+ OVA-specific population. A. Representative FACS plots illustrating deletion of OTII T cells (CD45.2+) by sorted, OVAp-loaded unactivated pDCs, but not by unpulsed or CpGA-activated pDCs. B. Mean percent of OVA-specific CD45.2+ cells among CD4 SP thymocytes, with SEM (N=5). Representative of 3 experiments with similar results. C. Location of donor-derived pDCs in the thymus by immunofluorescence. Normal WT (CD45.2) mice were injected intravenously with purified pDCs from congenic WT (CD45.1) mice. 48 hrs later, thymi were snap frozen and cryosections were stained with Hoechst, a lectin delineating the medulla (UEA-1), and with anti-mouse CD45.1. Colocalization of DAPI and CD45.1 in overlay (middle) panel shows the homed pDCs (white arrows). Merged section with UEA-1 is shown in far right. The cortical (C) and medullary (M) regions are depicted in the top panel. Higher magnification (40X, oil immersion) in lower panel, depicting co-localization of transferred CD45.1+ pDCs (CD45.1 shown in red with cell nuclei shown with DAPI staining in blue and CD11c staining in green). Representative of 7 thymi studied. See also Figure S5.
Thymic clonal deletion by thymotropic pDCs is CCR9-dependent
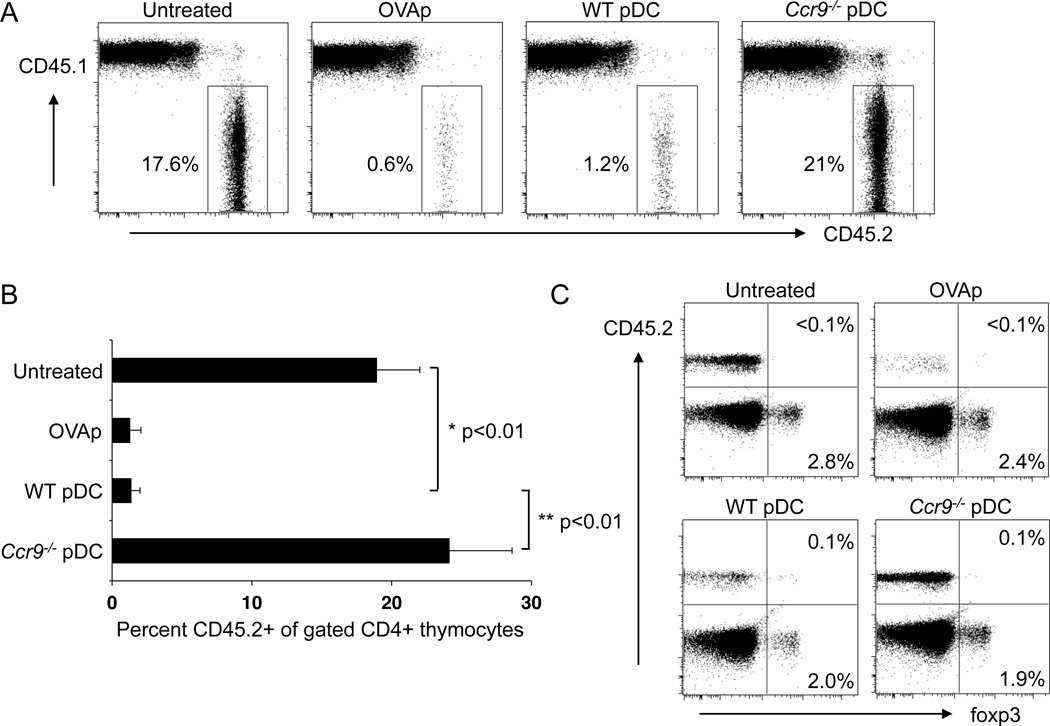
To determine if CCR9 expression on pDCs is required for negative selection of Ag-specific OT-II thymocytes, we sorted pDCs from Flt3L-treated WT and Ccr9−/− mice, loaded them with OVAp and transferred them into the B6 (CD45.1)-OT-II Rag1−/− (CD45.2) BMC mice as described above. In contrast to the efficient deletion of OT-II thymocytes by WT pDCs, Ccr9−/− pDCs had no effect on the Ag-specific thymocyte population (Figure 6A and B). The failure of OVAp-loaded activated pDCs (Figure 5) or peptide-loaded Ccr9−/− pDCs (Figure 6) to mediate clonal deletion rules out the possibility that negative selection is due to OVAp released from loaded DCs. In addition to deletion of Ag-specific thymocytes, central tolerance can also be mediated by the conversion of Ag-specific developing T cells into natural Tregs that express the transcription factor Foxp3. However, we did not observe induction of Foxp3 expression on OVA-specific OT-II T cells (Figure 6C).
Figure 6. Thymic clonal deletion of OVA-specific transgenic T cells by OVA-loaded WT but not by Ccr9−/− pDCs.
BMCs were prepared as in Figure 5, and 14d later, mice were either untreated or injected i.v. for 2 consecutive days with 2–5 × 106 sorted WT (WT pDC) or Ccr9−/− (KO pDC) OVAp-loaded pDCs. Mice treated i.v. with soluble OVAp served as a positive control. Thymocytes were analyzed two days after final pDC or OVAp treatment, by gating on Lin- CD4+ SP cells to assess the frequency of the OVA-specific CD45.2+ population. A. Representative FACS plots illustrating deletion of OVA-specific (CD45.2+) OTII T cells by WT but not Ccr9−/− OVA-loaded pDCs. B. Mean percent of OVA-specific CD45.2+ cells (±SEM) among CD4+ SP thymocytes (N=5). C. Representative FACS plots illustrating lack of Foxp3+ Treg induction among OVA-specific (CD45.2+) thymocytes. Quadrant gates were set based on isotype controls. Representative experiment from a total of three. See also Figure S6.
It remained a formal possibility that CCR9-deficient pDCs obtained from Ccr9−/− donors had compromised tolerogenic potential, similar to TLR-activated pDCs. In direct cellular interaction assays, using in vitro thymic re-aggregation studies, OVAp-loaded WT or Ccr9−/− pDCs were equally potent in deleting OT-II thymocytes 72–98 hours after re-aggregation of OT-II Rag1−/− thymocytes and thymic stromal elements with either Ag-loaded WT or Ccr9−/− pDCs (Figure S6). Loss of Ag-specific CD4+ OT-II thymocytes occurred by apoptosis as evidenced by elevated Annexin V (AnnV) expression levels, and among the few remaining OT-II thymocytes there was no Foxp3 expression. Control DCs that were not loaded with OVAp did not induce thymic deletion of OT-II thymocytes. Thus immature pDCs do not require CCR9 to mediate clonal deletion in direct cellular interaction assays, rather they use CCR9 to home to the thymus, where they induce central tolerance to imported Ags.
Endogenous pDCs transport endocytosed soluble and particulate ‘antigens’ to the thymus
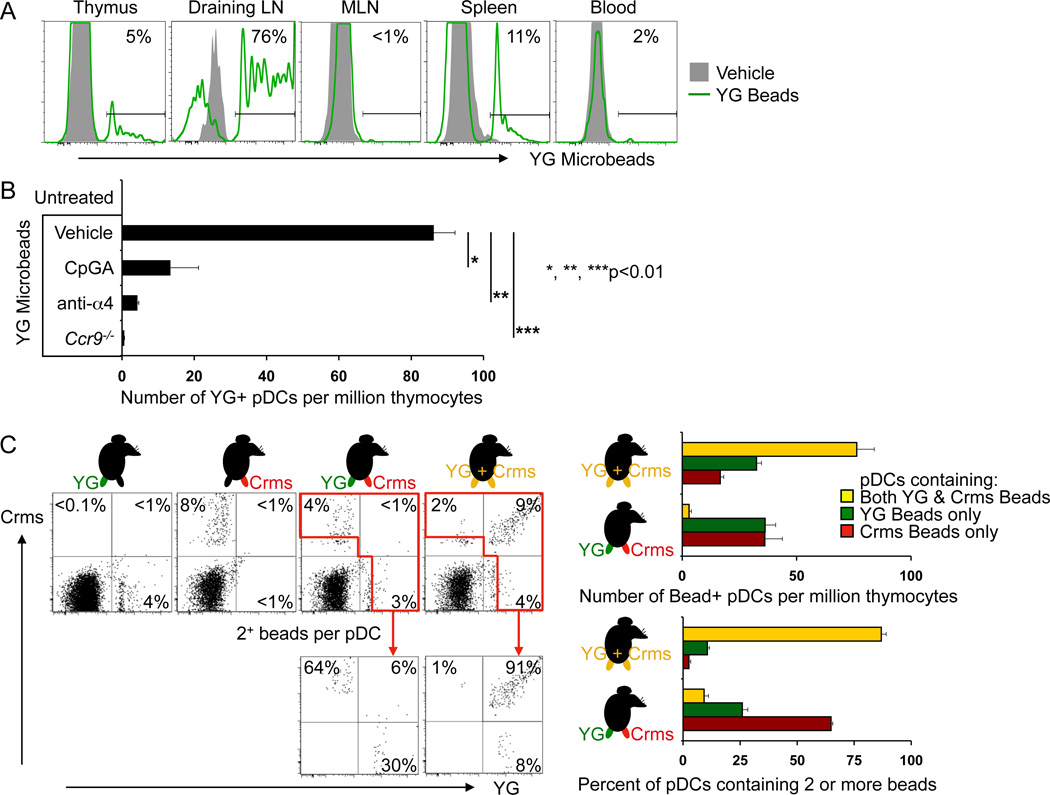
To determine whether endogenous pDC populations have the capacity to transport peripheral Ags to the thymus, we used fluorescence-labeled-dextrans and microspheres (0.2µ in diameter), which are efficiently endocytosed by immature DCs without inducing their maturation, as models of innocuous soluble or particulate tissue molecules. Two days after s.c. injection of Yellow-Green (YG) labeled beads, fluorescent pDCs were readily detected in draining LN, but not in the distal MLN (Figure 7A). More importantly fluorescent pDCs were detected in the thymus after fluorescent dextran or bead injection (Figure 7A and Figure S7B) and soluble ’antigen‘ was incorporated into discrete intracellular vesicles (Figure S7D). A subset of SIRPα+ cDCs, which like pDCs represent a migratory DC subset, also contained transported beads (Figure S7A) or dextran (Figure S7F). The frequency of ‘antigen’ positive SIRPα+ cDCs was variable, but in general somewhat less than that of pDCs in experiments with fluorescent dextrans (Figure S7F). In contrast, resident (non-migratory) thymic SIRPα− cDCs, which have been shown to accumulate soluble fluorescent protein antigens injected i.v. (Li et al., 2009), did not acquire comparable levels of labeled dextran (Figure S7F; or fluorescent beads shown in Figure S7A), even though all thymic DC populations, including resident SIRPα− cDCs efficiently endocytosed labeled dextran ex vivo (Figure S7G).
Figure 7. Endogenous pDCs take up peripheral particulate “Ags” and migrate to the thymus.
WT or Ccr9−/− mice were injected s.c. at the base of the tail with Yellow/Green (YG) fluorescent microspheres. Cohorts of WT mice were also treated with either CpGA in situ, by mixing YG fluorescent microbeads with CpGA and injecting s.c., or with α4 mAb injected i.v. every 12 hours. 48 hrs after bead injection, lymphoid tissues and blood were harvested and analyzed by flow cytometry. A. Representative histograms showing YG bead (FITC channel) signal in gated Lin- CD11cint B220+ PDCA-1+ pDCs in thymi, draining lymph nodes (draining LN), mesenteric lymph nodes (MLN), spleen and blood of WT mice given YG beads s.c.; gray shaded plots are pDCs from mice that received unlabeled microbeads. Results are representative of 3 experiments. B. Mean number of YG bead+ pDCs (Lin- CD11cint B220+) per million live cells in thymi from WT mice injected either with unlabelled microbeads, YG beads, YG beads with CpGA or α4 mAb or in thymi from Ccr9−/− mice injected with YG beads. Error bars represent SEM of results from 5 mice from one experiment of 2 performed with similar results. C. WT mice received s.c. injections of (i) YG beads (YG) alone in the left leg; of (ii) Crimson beads (Crms) alone in the right leg; of (iii) YG beads in the left leg and Crms beads in the right leg; or of (iv) a mixture of YG and Crms beads in both legs. Representative FACS plots of gated thymic pDCs (Lin- CD11cint PDCA-1+) with the % of pDCs containing only YG beads, only Crms beads, or both bead colors. The red gates enclose pDCs that contain 2 or more beads. Pooled data from 4–5 mice per cohort are summarized in the histograms: the top panel presents the number of thymic pDCs that contain 1 or more colored beads (YG only as green bars, Crms only as red bars, or both as yellow bars) in mice receiving either mixed YG+ Crms beads in both legs, or separate beads in each leg. The bottom histogram focuses on thymic pDCs that contain 2 or more colored beads: it shows the % of these pDCs that only contain YG beads (green bars), only Crms beads (red bars), or both (yellow bars) from mice receiving either mixed YG+Crms beads in both legs, or separate beads in each leg. Results are representative of 2 experiments. See also Figures S7.
To confirm that particulate or soluble antigen was transported to the thymus within cells, we inhibited DC recruitment by blocking with α4 mAb (Figure 7B and Figure S7C) (Bonasio et al., 2006). Treatment with α4 mAb decreased the number of fluorescent pDCs found in the thymus two days after either YG-labeled bead (Figure 7B) or FITC-dextran (Figure S7C) injection. Moreover, thymic localization of ’antigen‘-bearing pDCs was dramatically reduced in Ccr9−/− mice (Figure 7B and Figure S7C). Neither α4 mAb treatment, nor CCR9-deficiency altered endocytic uptake by peripheral DCs, as the mean fluorescence intensity (MFI) of FITC signal in pDCs from the draining lymph nodes, blood and spleen was similar after s.c. injection of FITC-dextran (data not shown). Thus peripherally injected soluble (FITC-dextran) or particulate (YG-bead) ‘antigens’ are transported into the thymus by migratory pDCs.
CpGA co-injection prevents pDC transport of peripheral Ags to the thymus
Central tolerance to Ags associated with infectious pathogens is undesirable. To mimic Ag presentation in conjunction with infectious agents, YG beads or FITC-Dextran were co-injected s.c. with CpGA in-situ. CpGA co-injection substantially reduced the numbers of fluorescent bead- or dextran-containing pDCs localizing to the thymus (Figure 7B and Figure S7C), consistent with the effects of CpGA on thymic pDC homing seen in our short-term homing studies (Figure 4B). The number of fluorescent-labeled pDCs in the LN draining the injection site was greatly increased by the treatment (data not shown). Therefore activation of tissue pDCs in situ by infectious signals prevents their localization to the thymus.
Tissue antigens are taken up locally in tissues or draining LN for thymic transport
It remained possible that the injected ’antigens‘ disseminated beyond the local draining LNs and were endocytosed by circulating pDCs in the blood. To address this possibility, we injected YG- and crimson-labeled beads separately into the left and right legs below the knee. Control animals received ‘antigen’ of only one color; or a mixture of the two colors injected in both legs. We reasoned that if particles were taken up primarily in the blood after ‘antigen’ dissemination, then thymic homing pDCs should have endocytosed equal numbers of YG and crimson beads even when the two colors were injected separately into the left and right footpads. Conversely, if ‘antigens’ were primarily endocytosed in the tissues or local draining LNs, then thymic homed pDCs would have a predominance of YG or crimson beads, but not both. Injection of a mixture of YG and crimson bead Ags into the legs provided a control to show that pDCs could in fact incorporate both colors of beads. As illustrated in the FACS plots (Figure 7C), after injection of YG and crimson beads in separate legs, the majority of fluorescent thymic pDCs contain a predominance of beads of one or the other color. In contrast, after injection of mixed beads, many of the fluorescent thymic pDCs contain beads of both colors. Among thymic pDCs containing two or more beads, ~30–60% contained only YG or only crimson beads after injection of a different color in each leg. In contrast, in mice that received the bead mixture, over 90% contained both YG and crimson beads. This experiment clearly demonstrates that locally acquired tissue Ags can be transported to the thymus by peripheral pDCs.
Similar results were obtained when FITC and Alexa Fluor 647 (AF647) fluorescent dextrans were injected into the left and right legs respectively (Figure S7E): Consistent with significant dextran dissemination as expected of a soluble ‘antigen’, all labeled thymic pDCs in this experiment gave both FITC and AF647 fluorescence signals. However, the homed pDCs clearly separated into two distinct subsets, one predominantly AF647+ and the other predominantly FITC+ (Figure S7E). Similarly distinct subsets were seen in blood (not shown) and spleen (Figure S7E). In the spleen, which contained more labeled pDCs, a distinct fraction of pDCs were more equally labeled (had an intermediate AF647/FITC fluorescence ratio); these presumably represent pDCs that had only incorporated systemically disseminated dextrans.
We conclude that endogenous pDCs transport both particulate and soluble tissue Ags to the thymus, and that this transport is mediated by CCR9 and α4 integrins and is inhibited by TLR stimulation.
DISCUSSION
Our studies demonstrate that unactivated pDCs from peripheral lymphoid tissues have the capacity to home to the thymus. CCR9 expression on immature pDCs imparts this thymic homing capacity allowing them to carry innocuous Ags from the periphery to the thymus, where they can delete developing Ag-specific thymocytes and contribute to central tolerance. Infectious signals downregulate CCR9 on pDCs and limit their thymic homing capacity, preventing inappropriate deletion of pathogen-specific T cells. We have previously shown that CCR9 expression defines immature pDCs in lymphoid tissues of mice, with potent immunosuppressive properties both in vitro and in vivo (Hadeiba et al., 2008). The demonstration here that CCR9-expressing pDCs mediate central as well as peripheral tolerance underscores their central role in maintaining immune homeostasis and tolerance.
CCR9 was required for efficient pDC participation in central tolerance in our studies. Compared to WT pDCs, CCR9 deficient pDCs homed poorly to the thymus after iv transfer, and accumulated in reduced numbers in the thymus of mixed BM chimeras. In CCR9-deficient mice, endogenous thymic pDCs were reduced in frequency; and in parabiosis studies, CCR9-deficient pDCs failed to enter the WT thymus well; in fact the number of Ccr9−/− pDCs recovered from the WT thymus (which represented <5% of thymic pDCs) was still lower than the number of WT pDCs recovered from the Ccr9−/− thymus even after prolonged vascular anastomosis, despite higher levels of CCR9-deficient pDCs in the blood of WT parabionts. CCR9-deficiency did not effect the level of pDC exchange in peripheral LNs which, as in other studies, was incomplete: Liu et al (Liu et al., 2007) reported a plateau of 12–18% exchange in LNs even in long-term parabionts, and proposed that LN pDCs are continually replaced by blood-borne precursors that are rapidly cleared from the circulation and do not recirculate, and because of this are poorly exchanged through the limited capillary network joining the parabionts. Ag-loaded CCR9-deficient pDCs failed to mediate clonal deletion of Ag-specific thymocytes in vivo, and this deficiency reflected the role of CCR9 in homing since CCR9-deficient pDCs were fully capable of mediating clonal deletion of thymocytes in ex vivo reconstituted thymic organ cultures. In contrast to our findings, previous studies reported that blockade of the CCR9 ligand CCL25 did not prevent bulk splenic DCs from inducing central tolerance (Bonasio et al., 2006). However, pDCs represent only a minor subset of splenic DCs (Alvarez et al., 2008; Villadangos and Heath, 2005) and, as discussed below, subsets of peripheral cDCs likely participate in central tolerance as well. Interestingly, CCR9 deficiency did not completely abrogate pDC recruitment to the thymus in our experiments, suggesting the potential for additional, overlapping homing mechanisms. Recent studies have established a role for CCR7, in addition to CCR9, in recruitment of T cell precursors to the thymus (Krueger et al.; Zlotoff et al.). However it seems unlikely that thymus-expressed CCR7 ligands are involved in thymic pDC recruitment: CCR9+ pDCs displayed poor chemotactic responses to CCR7 ligands (e.g. CCL21) (Hadeiba et al., 2008), and CCR7 is usually upregulated on activated DC populations, which migrated poorly to the thymus in this and other studies (Proietto et al., 2009).
In agreement with previous studies implicating P-selectin and α4 integrins in thymic DC homing (Bonasio et al., 2006; Scimone et al., 2006), we found that pDCs expressed α4 and functional binding sites for P-selectin, and that endogenous thymic pDC migration was inhibited by α4 integrin blockade. Thus peripheral pDC homing to the thymus likely employs the same thymic recruitment cascade used by T cell progenitors and bulk splenic DCs.
The role of pDCs in peripheral tolerance is well established and usually involves immature or unactivated pDCs mediating the induction of adaptive Tregs in peripheral lymphoid tissues (Hadeiba et al., 2008; Morelli and Thomson, 2007). The results presented here however, suggest that migratory pDCs can also continuously transport peripheral Ags to the thymus and contribute to central tolerance. This mechanism may be an important complement to AIRE-dependent transcription of TSA by mTECs, which are known to express many, but not all peripheral self Ags for central tolerance (Aschenbrenner et al., 2007). In contrast, thymic DCs, including pDCs, lack expression of the transcription factor AIRE and have high endocytic activity (Hubert et al., 2008). Therefore, a primary role of thymic pDCs may be to present peripherally endocytosed TSA such as secreted proteins or Ags shed from apoptotic cells, that are not present in the circulation at tolerogenic levels, and are not adequately expressed or presented by mTECs (Proietto et al., 2009). Alternatively, it is interesting to consider that pDCs may also transport innocuous foreign Ags to the thymus, particularly Ags from food or innocuous flora in the digestive tract. Indeed, as shown here and in a previous study (Wendland et al., 2007), CCR9 mediates pDC localization to the intraepithelial compartment of the gut wall, a site well positioned to sample Ags in the gut lumen. Evidence for thymic tolerance to innocuous fed Ags comes from a recent study, in which oral tolerance to myelin basic protein (MBP) failed if mice were thymectomized prior to MBP feeding (Song et al., 2006). Whether this involves central tolerance mechanisms and if so, whether thymic presentation of fed Ag is mediated by soluble Ag, or instead by gut DCs that transport locally endocytosed mucosal Ags to the thymus, remains to be determined.
In addition to pDCs, a subpopulation of cDCs that express express SIRPα+ and CD11b, also enters the thymus from the blood (Donskoy and Goldschneider, 2003). It is likely that in addition to pDCs, thymus-homing cDCs can transport peripheral Ags and contribute to the control of T cell development. However, CCR9+ pDCs comprise a major fraction of the rapidly exchanging migratory DCs in the thymus and, in our experiments, more thymic pDCs than SIRPα+ cDCs contained transported peripheral Ag (FITC-dextran), suggesting that immature pDCs may play a more dominant role. Interestingly, migratory SIRPα+ cDCs in peripheral lymphoid tissues or in the blood circulation stain poorly for CCR9 (data not shown), indicating that they must use distinct, potentially less efficient, trafficking mechanisms to access the thymus. In fact we find that CCR9-deficiency does not affect the representation of thymic migratory cDC subsets; even in BMC mice, we observed no significant effect of CCR9 on thymic SIRPα+ cDC development or localization.
Thymic DCs can potentially contribute to central tolerance through induction of natural Tregs, as well as by clonal deletion of self-reactive thymocytes. However, OVA-loaded CCR9+ pDCs in our model, and bulk splenic DCs in a previous study (Bonasio et al., 2006), induced central tolerance through clonal deletion without Treg induction, as indicated by lack of detectable Foxp3 expression even among surviving Ag-specific thymocytes. Immature pDCs also effected clonal deletion but not Treg induction in our in vitro Ag-specific thymic re-aggregation culture system. The failure of Treg induction by migratory pDCs may be a function of the OT-II TCR-OVA model system employed in these studies, reflecting unique characteristics of signaling through the transgenic TCR. Alternatively the high concentrations of OVAp-loaded MHC complexes on thymic DCs may dictate the outcome. Several studies have indeed correlated TCR affinity and signal strength with differential effects on clonal deletion versus Treg induction (Atibalentja et al., 2009; Jordan et al., 2001; Relland et al., 2009; Starr et al., 2003). However, it is also possible that pDCs are intrinsically inefficient at inducing Tregs in the thymic environment as shown in a polyclonal thymocyte-DC co-culture system, in which migratory SIRPα+ cDCs, but not thymic pDCs or resident SIRPα− cDCs, induced natural Treg development (Proietto et al., 2008). Thus it is possible that immature migratory pDCs effect central tolerance exclusively through clonal deletion, even though they can induce Tregs in the periphery. Additional studies will be required to determine further the importance of pDC versus migratory or resident cDC populations, and of TCR signaling, in Treg induction versus clonal deletion modalities of central tolerance. Moreover, our studies and those of Bonasio et al (Bonasio et al., 2006) are restricted to CD4 T cell deletion: future studies must address the role of transported peripheral Ag in intrathymic CD8 development as well.
Earlier studies have provided supportive evidence that endogenous DCs, under steady state conditions, can contribute significantly to thymic tolerance to innocuous peripheral Ags. (Proietto et al., 2009); (Bonasio et al., 2006). Here we used fluorescent dextran and particulate beads as model innocuous peripheral Ags to follow Ag transport by endogenous pDCs. Subcutaneous injection labeled a large percentage of pDC and cDC populations in the draining lymph nodes. Importantly, two days after local tissue injection of labeled beads or of FITC-dextran, pDCs and, to a lesser extent, migratory SIRPα+ cDCs containing endocytosed model Ags were readily identified in the thymus. In contrast, resident thymic SIRPα− cDCs remained largely negative ruling out significant accumulation of Ags passively entering the thymus from the blood. Interestingly, in most mice injected peripherally with fluorescent beads, a few thymic SIRPα− cDCs (<1%) did contain one or two beads. These may represent contaminating migrants in the SIRPα− cDC gate, or resident cDCs that have incorporated transported beads released by dying migratory DCs. In the context of the latter possibility, it is also possible that OVA-loaded pDCs may release the antigenic peptide, which could be taken up by cross-presenting thymic cDCs. Tissue-specific Ags released from mTECs have been shown to be cross-presented by thymic DCs and to contribute to central tolerance (Klein et al., 2009). However, because there is little fluorescence in SIRPα− cDCs either after injection of fluorescent beads or dextran, it seems more likely that thymic homed pDCs play the dominant role in clonal deletion by direct presentation of transported Ag. The recruitment of dextran or bead-containing pDCs was reduced in CCR9-deficient mice and was inhibited by blockade of the homing receptor α4, confirming the requirement for thymic homing mechanisms including CCR9 for endogenous pDC transport of peripherally endocytosed Ags to the thymus.
Transport of peripheral Ags into the thymus needs to be regulated to prevent deletion of T cells specific to pathogen-associated molecules. This control may be achieved by modulation of DC homing properties by infectious agents. pDC activation with TLR ligands that mimic infectious signals potently limited thymic homing in our study, and also prevented negative selection of Ag-specific thymocytes by Ag-loaded pDCs transfused i.v. Similarly, activation of bulk splenic DCs with lipopolysaccharide (LPS) inhibited their migration to the thymus (Bonasio et al., 2006). Moreover, co-injection of the potent TLR9 ligand CpGA dramatically inhibited endogenous pDC transport of model antigens from the periphery to the thymus in our experiments, without significantly effecting DC endocytosis in the draining LN. Our in vitro studies showed that peripheral pDC activation downregulated CCR9, without reducing expression of other known DC thymic trafficking receptors (e.g. α4, β1 or P-selectin binding sites). Thus TLR-dependent downregulation of CCR9 may be an important mechanism to prevent pDC-mediated transport of pathogen-associated Ags into the thymus, restricting activated DC populations to the periphery where they can participate in peripheral T cell activation in lymphoid tissues.
The capacity of peripheral pDCs to ‘patrol’ the thymus and to contribute to central tolerance is surprisingly efficient. Our short term homing studies indicate that the number of homed pDCs is low compared to endogenously derived thymic pDCs: After injection of peripheral pDCs, under the same conditions we employed for clonal deletion studies, only 1–2% of thymic pDCs are derived from the injected population. Furthermore, in order to visualize Ag-specific thymocytes, we created a system with an artificially high number of Ag-specific thymocytes using our BM chimeric approach. From our studies, roughly 18% of CD4+ CD8− SP thymocytes in our untreated BMC mice are OVA-specific (CD45.2+) with an average thymic cellularity of 107 cells after irradiation and BM reconstitution. This translates to 1.8 million Ag-specific SP thymocytes being censored by only a very small number of pDCs that home to the thymus (roughly 3,000 as determined from our short-term homing experiments). This ratio of 1 OVA-loaded pDC to 600 OVA-specific SP thymocytes deleted up to 90% of the Ag-specific SP thymocyte population, which suggests highly efficient and dynamic pDC scanning of developing thymocytes as they trickle through the medulla during negative selection. It is interesting to note that thymus-homing OVA-loaded pDCs only delete SP thymocytes, whereas OVAp was shown by us and others (Atibalentja et al., 2009) to drive the deletion of both SP CD4+ thymocytes in the medulla, and also DP thymocytes in the cortex, presumably reflecting OVA presentation by all MHCII+ cells, including cortical thymic epithelial cells (cTECs), mTECs and thymic DCs. Migratory pDCs in our studies localized only in the medulla or the corticomedullary junction, thus potentially limiting their access to DP thymocytes, but facilitating their interactions with developing SP thymocytes. As discussed in a similar context (Bonasio et al., 2006), intravital microscopy of T cell–DC interactions in LNs has shown that single DCs can contact as many as 5,000 T cells per hour (Miller et al., 2004). Assuming similar contact frequencies in the thymic medulla of thymocytes with pDCs, this would allow one Ag-loaded pDC that migrates into the thymus to interact with (and potentially delete up to) 5000 × 24 or 120,000 Ag-specific thymocytes daily. Given about 3000 thymic homed pDCs bearing OVA (and even with repeat scanning events), these calculations suggest that the homed pDCs could scan well over 108 SP thymocytes per day, more than enough to explain our results even if only a fraction of contacts resulted in deletion. Moreover, neither these considerations nor our studies take into account the fact that many innocuous Ags are not encountered in a ‘bolus’ as in our studies, but rather may be continuously present (e.g normal tissue or cell constituents, including normal flora Ags) or frequently encountered (e.g. major food Ags entering the portal system). In these instances pDCs transporting peripheral Ags would be continuously seeding the thymus, allowing prolonged time periods for thymic T cell censoring. In sum, our results suggest that only a small number of Ag-loaded pDCs migrating from the periphery into the thymus may be needed to censor developing thymocytes of rare cognate Ag-specificity.
In conclusion, our results suggest that immature pDCs contribute to immune tolerance to peripheral Ags through CCR9-dependent transport of self- or innocuous Ags to the thymus and subsequent deletion of Ag-specific thymocytes.
EXPERIMENTAL PROCEDURES
Mice
C57BL/6 (CD45.2) and congenic CD45.1 (B6.SJL-Ptprca Pep3b/BoyJ) mice were from obtained the Jackson Laboratory, Bar Harbor, ME. TCR transgenic OT-II Rag1−/− mice (C57BL/6-TgN(OT-II.2a)-RAG1tm1Mom) were obtained from Taconic Farms Inc., Hudson, NY, through the NIAID exchange program (Barnden et al., 1998; Mombaerts et al., 1992). Ccr9−/− were generously provided by Dr. Paul Love (NIH, Bethesda, MD). C57BL/6 F1 (CD45.1 × CD45.2) mice were bred in the VMU facility of the Veterans Affairs Palo Alto Health Care Systems (VAPAHCS). All animal studies were approved by the institutional animal use and care committee and experimentation was conducted in accordance with AAALAC guidelines.
Reagents & Flow Cytometry
For immunfluorescence and FACS staining, Fc receptors were blocked with Fc receptor antibody prior to staining with directly labeled or biotinylated monoclonal antibodies to the Ags indicated in the figures and text. Dead cells were excluded by Propidium Iodide or Ethidium Monoazide staining. The following mAbs were used for staining: CD3-PECy7 (145-2C11), CD4-Pacific Blue (RM4-5), CD4-AlexaFluor 700® (AF700; RM4-5), CD8α-PerCP-Cy5.5 (53-6.7), CD8α-APC (53-6.7), CD11b-PerCP-Cy5.5 (M1/70), CD11b-AF700 (M1/70), CD11c-Pacific Blue (HL3), CD19-PECy7 (1D3), CD25-APC (PC61), CD40-FITC (3/23), CD45.1-PerCP-Cy5.5 (A20), CD45.2-PerCP-Cy5.5 (104), B220-PerCP (RA3-6B2), CD80-biotin (16-10A1), CD86-biotin (GL1), NK1.1-PECy7 (PK136) and CD172a (SIRPα)-PE (P84) from BD Biosciences; and CD8α-AF700 (53-6.7), CD29 (β1-integrin)-PE (HMb1-1), CD45.1-APC (A20), CD45.2-FITC (104), CD45.2-PE (104), CD49d (α4 integrin)-PE (R1-2), SiglecH-FITC (eBio440c), IA/IE-AF700 (M5/114.15.2) and Foxp3-PE (FJK-16s) from eBioscience, San Diego, CA. CCR9-APC (242503) was purchased from R&D Systems, Minneapolis, MN; mAbs to PDCA-1 conjugated to either PE or APC were obtain from Miltenyi Biotec (Bergisch Gladbach, Germany); and B220-Qdot655™ was obtained from Invitrogen, Carlsbad, CA. A recombinant mouse P-selectin-human IgG1 Fc fusion protein (R&D Systems) was used to detect P-selectin ligands. Secondary reagents included Streptavidin-AlexaFluor 700® (eBioscience) for the visualization of biotinylated mAbs, and anti-human IgG1 Fc-FITC (Jackson Immunoresearch, West Grove, PA) for detection of P-selectin-Ig. Tregs, cells were fixed, permeabilized, and stained with anti-Foxp3-PE (FJK-16s) according to the manufacturer’s recommendation (eBioscience). Neutralizing anti-mouse α4 mAb (PS/2) was purified from hybridoma supernatants.
DC isolation and sorting
DCs were isolated from lymphoid tissues of normal mice using Collagenase IV (Worthington Biochemical Corp., Lakewood, NJ) and DNaseI (Sigma-Aldrich, St. Louis, MO) in protein-free media at a final concentration of 2 mg/ml and 1U/ml for 1–2 h at 37 °C. For the isolation of Flt3L-expanded DCs, WT or Ccr9−/− C57BL/6 mice were injected s.c. with 5 × 106 Flt3L-secreting B16 melanoma cells (Flt3L-B16). After 14 days designated lymph nodes were harvested and highly enriched pDC populations (typically >98%) were obtained either by depleting non-DCs using pan DC microbeads (Miltenyi Biotec) followed by sorting Lin- (CD3− CD19− NK1.1−) CD11cint B220+ PDCA-1+ cells; or by density gradient centrifugation over ficoll (Histopaque 1083, Sigma-Aldrich) followed by two rounds of magnetic bead cell sorting using a pDC isolation kit (Miltenyi Biotec).
pDC stimulation with TLR ligands
MACS-purified pDCs from pooled peripheral lymph nodes of Flt3L-B16-injected C57BL/6 mice were cultured at 1–2.5 × 106 cells/ml in complete RPMI 1640 medium supplemented with 10% FCS for 8–12 hrs in the absence or presence of ODN1585 CpG (1 µM) (Invivogen, San Diego, CA). Following stimulation, pDCs were stained for their expression of MHC class II (IA/IE), CD40, CD80, CD86, CCR9, α4, β1, L-selectin, and for P-selectin binding activity; and in some experiments used in short-term homing studies or in clonal deletion assays (see below).
In vivo clonal deletion assay
Bone marrow (BM) cells from OT-II Rag1−/− (CD45.2+) and WT congenic C57BL/6 (CD45.1+) mice, depleted of T- and B-lymphocytes using anti-CD19 and CD90.2 conjugated microbeads and MACS technology, were injected i.v. (2–5 × 106 from each BM donor), into lethally irradiated B6.SJL (CD45.1) recipients. On both days 14 and 15 after irradiation, when thymocyte development from donor BM precursors was underway, mice were treated either with 50 nmol of soluble OVAp (323–339); with 2–5 × 106 WT or Ccr9−/− pDCs pulsed for 1hr at 37°C with 50 µM OVA peptide; with 2–5 × 106 WT pDCs activated in culture with 1 µM CpGA for 8–12 hrs at 37°C followed by pulsing with 50 µM OVA peptide for the last 2 hrs; or with 2–5 × 106 control unpulsed WT pDCs. The mice were sacrificed on day 17 after irradiation for analysis of OVA-specific (OT-II) CD45.2+ thymocytes. In some experiments, thymic Tregs (CD4+ CD8− Foxp3+ CD25hi) were enumerated.
Generation of Bone Marrow Chimeras (BMCs)
BM cells from congenic (B6.SJL; CD45.1) WT mice were mixed with equal numbers of BM cells from either Ccr9−/− (CD45.2) or WT (CD45.2) mice and injected i.v. (2 × 106 BM cells per donor) into lethally irradiated F1 (CD45.1 × CD45.2) C57BL/6 recipients to generate “experimental” or “control” BMCs respectively. In separate experiments, reconstituted mice were injected 2 weeks prior to harvesting tissues, with Flt3L-secreting B16 melanoma cells (as previously described). Tissues and blood were harvested 8–12 wks after BM transplantation and cell suspensions were stained with mAbs for flow cytometric analysis.
pDC Homing Assay
pDCs were purified from pooled peripheral lymph nodes of WT (CD45.2), congenic WT (CD45.1) and Ccr9−/− (CD45.2) mice. In experimental mice, CD45.2+ Ccr9−/− pDCs, and in control mice CD45.2+ WT pDCs, were co-injected (2–5 × 106 DCs) i.v. with the same number of CD45.1+ WT pDCs into syngeneic normal CD45.1+ CD45.2+ F1 WT hosts. After 48 hours, tissues and blood were harvested and cell suspensions analyzed by flow cytometry. In separate experiments, CD45.2+ WT pDCs were also stimulated with CpGA as described above and 5–10 × 106 of these activated DCs were co-injected at a 1:1 ratio with unactivated CD45.1+ pDCs into syngeneic CD45.1+ CD45.2+ F1 mice.
Parabiosis
8- to 10-week-old female WT (CD45.1) and Ccr9−/− (CD45.2) C57BL/6 mice were anesthetized and joined together by surgical union of skin from the shoulder to knee joints as described (Wright et al., 2001). Mice were kept on antibiotic water and tissues were harvested 3 weeks later.
In vivo Fluorescent Microbead administration
Yellow/Green (YG) fluorescent (505/515) carboxylate modified microspheres (FluoSpheres®) or control unlabeled microspheres (both 0.2 µm diameter; Invitrogen), were administered in 200µl (2% suspension) s.c. at the base of the tail into normal C57BL/6 WT or Ccr9−/− mice. In separate cohorts of WT mice, 200µl of YG microspheres were co-injected s.c. with 50 nmols of CpGA (ODN 1585, Invivogen); or mice injected once with YG microspheres s.c. were also treated with 250–500 µg of α4 mAb i.v. or saline every 12 hrs for 48 hrs prior to harvesting the tissues. In separate experiments, mice were injected with up to 150 µl of YG microspheres in the left leg (footpads and legs distal to the knee), and Crimson fluorescent (625/645) carboxylate modified 0.2 µm microspheres (FluoSpheres®; Invitrogen) in the right leg. Controls included injections of YG microspheres into the left leg only, injection of Crimson microspheres into the right leg only and injection of an equal mixture of YG and Crimson microspheres into both the left and right legs. Thymi and peripheral lymphoid tissues were recovered 48 hrs later, and microsphere-containing DCs in cell suspensions were quantified by flow cytometry.
Immunofluorescence localization of homed pDC
Purified pDCs from pooled peripheral lymph nodes of Flt3L-B16-injected congenic B6.SJL (CD45.1) mice were adoptively transferred (2–5 × 106) into normal C57BL/6 (CD45.2) mice, and thymi were recovered 48 hours later. Frozen and fixed 10 µm thymic sections were stained with Hoechst (Invitrogen) to label nuclei, Fluorescein-labeled Ulex Europaeus Agglutinin I UEA-1 (Vector Labs, Burlingame, CA) as a marker of the thymic medulla, anti-mouse CD11c-AlexaFluor 647® (N418, eBioscience) as a pan DC marker and anti-mouse CD45.1 biotin (A20, eBioscience) followed by streptavidin-Alexa Fluor 594 (Invitrogen) to identify homed (donor) pDCs.
Statistical Analysis
Data are presented as mean values ± standard error of the mean (SEM) unless otherwise indicated. Statistical significance between sets of data was assessed using the two-tailed unpaired Student’s t-test.
Supplementary Material
ACKNOWLEDGMENTS
We thank L. Rott for assistance, and G. Dranoff for the Flt3L-expressing B16 melanoma line. H.H. is a recipient of an Investigator Career Award from the Arthritis Foundation and was a fellow under the NIH Training Grant AI07290; K.L. is a fellow of the Deutsche Forschungsgemeinschaft, DFG; C.O. was supported by Fellowships from the Wenner-Gren Foundation and the Crohn's & Colitis Foundation; and A.H. by NIH grant DK085426. The work was supported by NIH grants AI047822 and DK084647 to E.C.B., the Stanford Digestive Disease Center under DK056339, and a Merit Award from the Department of Veterans Affairs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alvarez D, Vollmann EH, von Andrian UH. Mechanisms and consequences of dendritic cell migration. Immunity. 2008;29:325–342. doi: 10.1016/j.immuni.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- Aschenbrenner K, D'Cruz LM, Vollmann EH, Hinterberger M, Emmerich J, Swee LK, Rolink A, Klein L. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat Immunol. 2007;8:351–358. doi: 10.1038/ni1444. [DOI] [PubMed] [Google Scholar]
- Atibalentja DF, Byersdorfer CA, Unanue ER. Thymus-blood protein interactions are highly effective in negative selection and regulatory T cell induction. J Immunol. 2009;183:7909–7918. doi: 10.4049/jimmunol.0902632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baekkevold ES, Wurbel MA, Kivisakk P, Wain CM, Power CA, Haraldsen G, Campbell JJ. A role for CCR4 in development of mature circulating cutaneous T helper memory cell populations. J Exp Med. 2005;201:1045–1051. doi: 10.1084/jem.20041059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- Bonasio R, Scimone ML, Schaerli P, Grabie N, Lichtman AH, von Andrian UH. Clonal deletion of thymocytes by circulating dendritic cells homing to the thymus. Nat Immunol. 2006;7:1092–1100. doi: 10.1038/ni1385. [DOI] [PubMed] [Google Scholar]
- Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- Derbinski J, Gabler J, Brors B, Tierling S, Jonnakuty S, Hergenhahn M, Peltonen L, Walter J, Kyewski B. Promiscuous gene expression in thymic epithelial cells is regulated at multiple levels. J Exp Med. 2005;202:33–45. doi: 10.1084/jem.20050471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donskoy E, Goldschneider I. Two developmentally distinct populations of dendritic cells inhabit the adult mouse thymus: demonstration by differential importation of hematogenous precursors under steady state conditions. J Immunol. 2003;170:3514–3521. doi: 10.4049/jimmunol.170.7.3514. [DOI] [PubMed] [Google Scholar]
- Gilliet M, Boonstra A, Paturel C, Antonenko S, Xu XL, Trinchieri G, O'Garra A, Liu YJ. The development of murine plasmacytoid dendritic cell precursors is differentially regulated by FLT3-ligand and granulocyte/macrophage colony-stimulating factor. J Exp Med. 2002;195:953–958. doi: 10.1084/jem.20020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadeiba H, Sato T, Habtezion A, Oderup C, Pan J, Butcher EC. CCR9 expression defines tolerogenic plasmacytoid dendritic cells able to suppress acute graft-versus-host disease. Nat Immunol. 2008;9:1253–1260. doi: 10.1038/ni.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert FX, Kinkel SA, Webster KE, Cannon P, Crewther PE, Proeitto AI, Wu L, Heath WR, Scott HS. A specific anti-Aire antibody reveals aire expression is restricted to medullary thymic epithelial cells and not expressed in periphery. J Immunol. 2008;180:3824–3832. doi: 10.4049/jimmunol.180.6.3824. [DOI] [PubMed] [Google Scholar]
- Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- Klein L, Hinterberger M, Wirnsberger G, Kyewski B. Antigen presentation in the thymus for positive selection and central tolerance induction. Nat Rev Immunol. 2009;9:833–844. doi: 10.1038/nri2669. [DOI] [PubMed] [Google Scholar]
- Krueger A, Willenzon S, Lyszkiewicz M, Kremmer E, Forster R. CC chemokine receptor 7 and 9 double-deficient hematopoietic progenitors are severely impaired in seeding the adult thymus. Blood. 115:1906–1912. doi: 10.1182/blood-2009-07-235721. [DOI] [PubMed] [Google Scholar]
- Kunkel EJ, Campbell JJ, Haraldsen G, Pan J, Boisvert J, Roberts AI, Ebert EC, Vierra MA, Goodman SB, Genovese MC, et al. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: Epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J Exp Med. 2000;192:761–768. doi: 10.1084/jem.192.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Park J, Foss D, Goldschneider I. Thymus-homing peripheral dendritic cells constitute two of the three major subsets of dendritic cells in the steady-state thymus. J Exp Med. 2009;206:607–622. doi: 10.1084/jem.20082232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Waskow C, Liu X, Yao K, Hoh J, Nussenzweig M. Origin of dendritic cells in peripheral lymphoid organs of mice. Nat Immunol. 2007;8:578–583. doi: 10.1038/ni1462. [DOI] [PubMed] [Google Scholar]
- Miller MJ, Hejazi AS, Wei SH, Cahalan MD, Parker I. T cell repertoire scanning is promoted by dynamic dendritic cell behavior and random T cell motility in the lymph node. Proc Natl Acad Sci U S A. 2004;101:998–1003. doi: 10.1073/pnas.0306407101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol. 2007;7:610–621. doi: 10.1038/nri2132. [DOI] [PubMed] [Google Scholar]
- Pabst O, Ohl L, Wendland M, Wurbel MA, Kremmer E, Malissen B, Forster R. Chemokine receptor CCR9 contributes to the localization of plasma cells to the small intestine. J Exp Med. 2004;199:411–416. doi: 10.1084/jem.20030996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proietto AI, van Dommelen S, Wu L. The impact of circulating dendritic cells on the development and differentiation of thymocytes. Immunol Cell Biol. 2009;87:39–45. doi: 10.1038/icb.2008.86. [DOI] [PubMed] [Google Scholar]
- Proietto AI, van Dommelen S, Zhou P, Rizzitelli A, D'Amico A, Steptoe RJ, Naik SH, Lahoud MH, Liu Y, Zheng P, et al. Dendritic cells in the thymus contribute to T-regulatory cell induction. Proc Natl Acad Sci U S A. 2008;105:19869–19874. doi: 10.1073/pnas.0810268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relland LM, Mishra MK, Haribhai D, Edwards B, Ziegelbauer J, Williams CB. Affinity-based selection of regulatory T cells occurs independent of agonist-mediated induction of Foxp3 expression. J Immunol. 2009;182:1341–1350. doi: 10.4049/jimmunol.182.3.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scimone ML, Aifantis I, Apostolou I, von Boehmer H, von Andrian UH. A multistep adhesion cascade for lymphoid progenitor cell homing to the thymus. Proc Natl Acad Sci U S A. 2006;103:7006–7011. doi: 10.1073/pnas.0602024103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song F, Guan Z, Gienapp IE, Shawler T, Benson J, Whitacre CC. The thymus plays a role in oral tolerance in experimental autoimmune encephalomyelitis. J Immunol. 2006;177:1500–1509. doi: 10.4049/jimmunol.177.3.1500. [DOI] [PubMed] [Google Scholar]
- Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- Uehara S, Grinberg A, Farber JM, Love PE. A role for CCR9 in T lymphocyte development and migration. J Immunol. 2002;168:2811–2819. doi: 10.4049/jimmunol.168.6.2811. [DOI] [PubMed] [Google Scholar]
- Villadangos JA, Heath WR. Life cycle, migration and antigen presenting functions of spleen and lymph node dendritic cells: limitations of the Langerhans cells paradigm. Semin Immunol. 2005;17:262–272. doi: 10.1016/j.smim.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Wendland M, Czeloth N, Mach N, Malissen B, Kremmer E, Pabst O, Forster R. CCR9 is a homing receptor for plasmacytoid dendritic cells to the small intestine. Proc Natl Acad Sci U S A. 2007;104:6347–6352. doi: 10.1073/pnas.0609180104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294:1933–1936. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- Wu L, Shortman K. Heterogeneity of thymic dendritic cells. Semin Immunol. 2005;17:304–312. doi: 10.1016/j.smim.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Zlotoff DA, Sambandam A, Logan TD, Bell JJ, Schwarz BA, Bhandoola A. CCR7 and CCR9 together recruit hematopoietic progenitors to the adult thymus. Blood. 115:1897–1905. doi: 10.1182/blood-2009-08-237784. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.